Abstract
Phytoextraction makes use of trace element-accumulating plants that concentrate the pollutants in their tissues. Pollutants can be then removed by harvesting plants. The success of phytoextraction depends on trace element availability to the roots and the ability of the plant to intercept, take up, and accumulate trace elements in shoots. Current phytoextraction practises either employ hyperaccumulators or fast-growing high biomass plants; the phytoextraction process may be enhanced by soil amendments that increase trace element availability in the soil. This review will focus on the role of plant-associated bacteria to enhance trace element availability in the rhizosphere. We report on the kind of bacteria typically found in association with trace element – tolerating or – accumulating plants and discuss how they can contribute to improve trace element uptake by plants and thus the efficiency and rate of phytoextraction. This enhanced trace element uptake can be attributed to a microbial modification of the absorptive properties of the roots such as increasing the root length and surface area and numbers of root hairs, or by increasing the plant availability of trace elements in the rhizosphere and the subsequent translocation to shoots via beneficial effects on plant growth, trace element complexation and alleviation of phytotoxicity. An analysis of data from literature shows that effects of bacterial inoculation on phytoextraction efficiency are currently inconsistent. Some key processes in plant–bacteria interactions and colonization by inoculated strains still need to be unravelled more in detail to allow full-scale application of bacteria assisted phytoremediation of trace element contaminated soils.
Keywords: Phytoextraction, Rhizosphere bacteria, Endophytes, Plant growth promotion, Trace element mobilization
Highlights
► We discuss how bacteria can affect the efficiency and rate of phytoextraction. ► We review the mechanisms employed by bacteria leading to trace element mobilization. ► Effects of bacterial inoculation on phytoextraction efficiency are inconsistent.
1. Introduction
Phytoextraction is based on the use of pollutant-accumulating plants for trace element removal from soils by concentrating them in the harvestable parts (Salt et al., 1998). An ideal plant for trace element phytoextraction should possess the following characteristics: (a) tolerance to the trace element concentrations accumulated, (b) fast growth and highly effective trace element accumulating biomass, (c) accumulation of trace elements in the above ground parts, (d) easy to harvest (Vangronsveld et al., 2009).
A typical trace element phytoextraction protocol consists of the following steps: (a) cultivation of the appropriate plant/crop species on the contaminated soil; (b) removal of harvestable trace element-enriched biomass from the site; and (c) post harvest treatments (i.e. composting, compacting, thermal treatments) to reduce volume and/or weight of biomass for disposal as a hazardous waste, or for its recycling to reclaim the elements that may have an economic value. If cost is the main advantage, time is the greatest disadvantage of trace element phytoextraction (Vangronsveld et al., 2009). It is known that this process is not fast, but (to be realistic for the practical purpose) time should preferably not exceed 10 years or be even shorter (Robinson et al., 1998; Blaylock and Huang, 2000). It is clear that there is a need for improvement of the natural phytoextraction potential and several studies have addressed this problem (Vassilev et al., 2004; Weyens et al., 2009a, 2009b). The success of phytoextraction depends on several factors including trace element availability to the roots and the ability of the plant to intercept, take up, and accumulate trace elements in shoots (Ernst, 2000). The two main bottle-necks indeed are trace element availability in the soil and trace element uptake and translocation by the plants.
At present, there are three basic strategies of trace element phytoextraction being developed (Vangronsveld et al., 2009): (1) continuous or natural phytoextraction using hyperaccumulators (e.g., Thlaspi spp., Alyssum spp.); (2) continuous or natural phytoextraction using fast-growing high biomass producing plants (e.g., Salix or Populus sp.); (3) induced or chemically-assisted phytoextraction introducing soil amendments (e.g., metal chelators or acidifying amendments) to increase trace element mobility in the soil.
Metalliferous plants are able to grow on trace element enriched soils and rocks without any symptoms of toxicity. In an evolutionary perspective, trace element enriched soils might be considered as “ecological islands” (Lefèbvre and Vernet, 1990), inhabited by particular, often endemic, taxa. Regarding their behaviour towards the transfer of trace elements from soil to plant tissue, the metalliferous plants may be divided into three groups: excluders, indicators and (hyper)accumulators (Baker, 1981; Baker and Brooks, 1989). Trace element-tolerant excluders are able to grow on metalliferous soils without distinct bioaccumulation of the trace elements. The trace elements are mainly excluded from uptake into plant tissues and trace element concentrations in the shoots are lower than in the roots. Indicator plants “mirror” the soil trace element concentration in their shoots. Hyperaccumulator plants are able to accumulate large amounts of trace elements in their aerial tissues without showing any symptoms of toxicity. Plants accumulating >100 mg kg−1 of Cd, >1000 mg kg−1 of Cu, Co, Cr, Ni or Pb, or >10,000 mg kg−1 of Mn or Zn have been defined as hyperaccumulator species (Baker and Brooks, 1989). An extended definition was provided by Baker and Whiting (2002), who claimed that the shoot/root or leaf/root ratio has to be >1, indicating a clear translocation of trace elements to the shoots. More than 400 plant species have been identified as hyperaccumulators, of which 75% are Ni hyperaccumulators growing on ultramafic soils (Baker et al., 2000). Hyperaccumulation of trace elements is found throughout the whole plant kingdom in temperate as well as tropical climates, but is typically restricted to endemic species growing on mineralized soils and related rock types. The most important types of metalliferous soils hosting hyperaccumulator plants are (1) serpentine soils (enriched in Ni, Cr, Co), (2) “calamine” soils (enriched in Zn, Cd, Pb), (3) Se-rich soils and (4) Cu- and Co-containing soils (Reeves and Baker, 2000). Related to the soil habitat, the hyperaccumulators are classified into accumulators of (1) Ni, (2) Zn, Cd, Pb, (3) Se, (4) Co, Cu and (5) other elements (Al, As, Cr, Mn, Tl) (Reeves and Baker, 2000).
Trace element tolerance mechanisms were reviewed by Schat et al. (2000) and by Clemens (2001). Briefly, the simplest trace element tolerance strategy, termed “avoidance”, consists in limited uptake into the plant body. However, a clear evidence for this mechanism was only found for some arsenite-tolerant plants. In most trace element-tolerant higher plants, trace elements are strongly retained in root tissues. Regardless of whether trace elements are mainly accumulated in roots or in shoots, internal tolerance mechanisms are the basis for efficient detoxification of the trace elements. This internal detoxification is based on (i) sequestration of the trace elements, i.e. transport to cell components not involved in physiological processes (vacuole, cell wall), and (ii) complexation with metal-binding peptides, i.e. metallothioneins and phytochelatins.
Excessive trace element uptake by hyperaccumulators has been found to be associated with partial depletion of labile, easily bio-accessible trace element pools in the rhizosphere (e.g., Fitz et al., 2003; Hammer and Keller, 2002; Puschenreiter et al., 2003, 2005; Whiting et al., 2001a, 2001b) and active root proliferation towards contaminated soil areas (Schwartz et al., 1999). Depletion of labile trace element pools in the rhizosphere of hyperaccumulator plants often has been found to be associated with sustained or even enhanced solubility (i.e. soil solution concentration; e.g., Wenzel et al., 2003). Puschenreiter et al. (2005) found evidence for enhanced dissolution of Ni-bearing silicates in the rhizosphere of the Ni hyperaccumulator Thlaspi goesingense, likely induced by root exudates (e.g., low molecular weight organic acids). Mineralogical studies showed the presence of smectite in the rhizosphere of the Ni-hyperaccumulator Alyssum serpyllifolium subsp. lusitanicum, which was associated with a more intense weathering of Ni-rich ferromagnesium minerals (chlorite, serpentine) and an increase in labile Ni (Kidd et al., 2009). Arsenic mobilization by root exudates (in particular oxalic acid) of the As hyperaccumulating fern Pteris vittata was found by Tu et al. (2004).
Despite the long history of interest in metalliferous plants, the attention of microbiologists towards bacteria and in particular plant-associated bacteria from trace element enriched habitats is more recent (Mengoni et al., 2010). A better understanding of the growth-promoting mechanisms of bacteria from trace element enriched environments and their natural capacity to cope with these contaminants could be exploited for a sustainable growth of crops, biomass for biofuel production, and feedstocks for industrial processes on trace element contaminated land (Rajkumar et al., 2009; Weyens et al., 2009a, 2009b). Furthermore, plant-associated bacteria can be exploited to improve the efficiency of phytoremediation processes (Kuffner et al., 2008, 2010; Sessitsch and Puschenreiter, 2008; Sheng et al., 2008a, 2008b; van der Lelie et al., 2000; Weyens et al., 2009a, 2009b; Glick, 2010).
This review will concentrate on how plant-associated bacteria can contribute to improve trace element uptake by plants and thus the efficiency and rate of phytoextraction. Three specific mechanisms of how microorganisms may increase plant trace element uptake have been suggested: they may (1) increase root surface area and hair production, (2) increase element availability, and/or (3) increase soluble element transfer from the rhizosphere to the plant (Weyens et al., 2009a). Further, an increased biomass production can enhance the efficiency of trace element phytoextraction. Attention will be paid both to hyperaccumulating metalliferous plants and fast-growing high biomass producing plants.
2. Plant-associated microbial communities under elevated trace element exposure
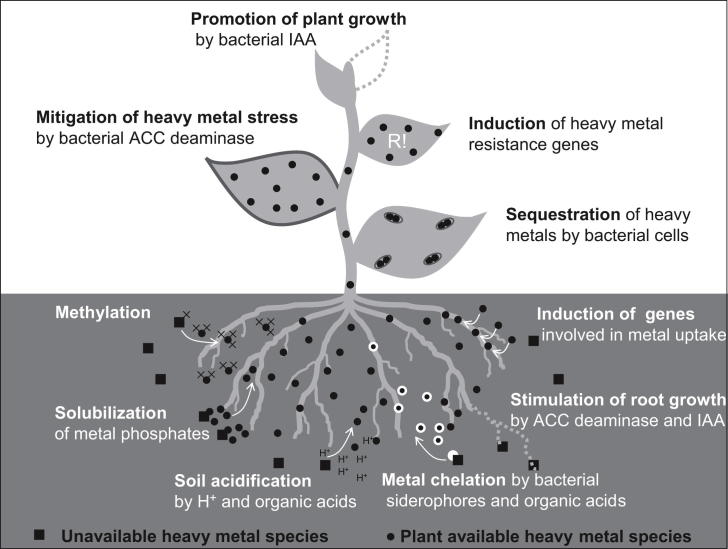
Plant–microbe interactions in relation to trace elements have been mostly addressed in the context of phytoremediation. For phytoremediation applications, (hyper)accumulating plant species are of interest as well as plants which produce high biomass and tolerate trace elements to a certain degree. A plant with high biomass production and application potential is Phragmites, which accumulates trace elements in moderate amounts. The various mechanisms how microorganisms may interact with plants in relation to trace elements accumulation or resistance are summarized in Fig. 1.
Fig. 1.
Putative plant–microbe interactions influencing trace element accumulation in plants (after Pilon-Smits, 2005).
Kunito et al. (2001) compared the characteristics of bacterial communities in the rhizosphere of Phragmites with those of non-rhizosphere soil in a highly Cu-contaminated area near a copper mine in Japan. Higher bacterial numbers were detected in the rhizosphere, which may be due to the lower Cu concentrations and/or due to the availability of root exudates, that can serve as a carbon source for bacteria. Nevertheless, the percentage of highly resistant strains was higher in the rhizosphere than in non-rhizosphere soil. Cu toxicity was found to be lower in rhizosphere soil. The study by Kunito et al. (2001) also indicated that Cu toxicity lowered the frequency of r-strategists (bacteria capable of rapid growth and utilization of resources) as these are more sensitive to toxic substances (Kozdroj, 1995). In addition, rhizosphere and non-rhizosphere isolates behaved very differently regarding exopolymer production (Kunito et al., 2001). Exopolymers produced by bacteria were shown to strongly bind trace elements (Bitton and Freihofer, 1978), leading to the formation of organic–metal complexes, which are difficult to degrade (Francis et al., 1992; Hattori, 1996; Huysman et al., 1994). Furthermore, trace element concentrations induced the production of exopolymers (Chao and Chen, 1991; Kidambi et al., 1995) and trace element resistance due to exopolymer production has been shown (Bitton and Freihofer, 1978).
Most studies have been performed with either Zn or Ni-hyperaccumulating plants. Already in 1991 Schlegel and co-authors detected higher numbers of tolerant bacteria in the rhizosphere of Ni accumulating plants. Similarly, Idris et al. (2004) identified a high number of different Ni-resistant bacteria in the rhizosphere of the Ni-hyperaccumulator T. goesingense. Cultivation of bacteria on Ni-containing medium resulted mostly in the isolation of Methylobacterium spp., an alphaproteobacterial genus, as well as Rhodococcus spp. and Okibacterium spp., belonging to the Actinobacteria (or Gram-positive bacteria with a high G + C content) (Idris et al., 2004). Different communities were identified in the rhizosphere or in the root vicinity of other Ni-hyperaccumulators, like Alyssum bertolonii (Mengoni et al., 2001). Mengoni et al. (2001) sampled rhizosphere as well as bulk soil at three serpentine sites and Ni-resistant strains were isolated. In the rhizosphere the cultivable population was dominated by Ni-resistant Pseudomonas strains, whereas in soil samples mostly Ni-resistant Streptomyces strains were found. In general, isolates obtained in this study showed co-resistance to Cr and Co, although other tolerance combinations (e.g., Ni, Zn and Cu) or single tolerances were also found, indicating independent evolution of heavy trace element resistance determinants (Mengoni et al., 2001). Becerra-Castro et al. (2009) also found a higher proportion of Ni-tolerant bacteria in the rhizosphere of two populations of the Ni-hyperaccumulator A. serpyllifolium subsp. lusitanicum compared to non-hyperaccumulators growing at the same site or to non-vegetated soil. Densities of Ni-resistant rhizobacteria were positively correlated with water-soluble Ni concentrations. Becerra-Castro et al. (2011) isolated and characterized Ni-resistant rhizosphere bacteria from two Ni-hyperaccumulating subspecies of A. serpyllifolium (subsp. lusitanicum and subsp. malacitanum). The most Ni-resistant bacteria (tolerating up to 10 mM Ni in plate assays) were mainly represented by members of the genera Arthrobacter spp. and Streptomyces spp. Kuffner et al. (2010) isolated and characterized Zn-resistant endophytes and rhizosphere bacteria of Zn-accumulating willows (Salix caprea). A high number of bradyrhizobia, various Betaproteobacteria, Actinobacteria and members of the Bacteroidetes/Chlorobi group were found in the rhizosphere, whereas endophytes comprised mostly Sphingomonas spp., Methylobacterium spp. and various Actinobacteria. With regard to Cu-contaminated soil, Kunito et al. (1997) reported a dominance of Cu-resistant Bacillus spp. in the rhizosphere of Phragmites, whereas non-rhizosphere soil was dominated by Methylobacterium spp.
Cultivation-independent 16S rRNA gene-based community analysis has become state-of-the-art to address questions of microbial diversity and population changes. Mostly, community analysis is performed with DNA, which does not give any information on the activity of cells, and even dead cells may be detected insofar as their DNA has not been destroyed by nuclease activity. One approach to detect metabolically active cells rather than resting or dormant cells is to base community analysis on isolated rRNA instead of DNA as active cells usually contain a far higher amount of ribosomes. This approach was used by Gremion et al. (2003) to characterize microbial communities in Zn-contaminated bulk soil and in the rhizosphere of the Zn-hyperaccumulator Thlaspi caerulescens. Active populations included mostly members of the Rubrobacteria subdivision, belonging to the high G + C Gram-positives. Active bacteria were only a minor part of the “DNA-based” communities (Gremion et al. (2003)). Cultivation-independent analysis confirmed the high abundance of Proteobacteria in the rhizosphere of A. bertolonii (Mengoni et al., 2004). Mengoni et al. (2004) also showed that the plant had a more pronounced effect on rhizosphere microbial communities than the soil environment. In a later study, Mengoni et al. (2009) used terminal-restriction fragment length polymorphism (T-RFLP) to study the leaf-associated bacterial communities of the same hyperaccumulator, and found a high plant-by-plant variability, even from the same population. These authors highlighted the large reservoir of biodiversity that these plant-associated bacterial communities offer.
In addition to the rhizosphere, endophyte populations inhabiting accumulating plants were investigated. The Ni hyperaccumulator T. goesingense hosted a range of different Methylobacterium strains, mostly belonging to the M. extorquens and M. mesophilicum (Idris et al., 2004). In addition, one strain was isolated, characterized and described as a novel species, Methylobacterium goesingense (Idris et al., 2006). Methylobacterium strains in the rhizosphere and inside plants belonged to the same species, but consisted of different strains indicating that these plant habitats provide different conditions for bacteria (Idris et al., 2004). Most strains were highly resistant to Ni, but were also shown to be resistant against different combinations of trace elements, indicating the independent evolution of resistance traits. Despite the fact that trace element determinants are frequently located on plasmids, horizontal transfer of plasmids between Methylobacterium spp. isolated from T. goesingense shoots and rhizosphere was found to be unlikely (Idris et al., 2006). Similar to the findings for T. goesingense, a large number of trace element tolerating methylobacteria were also isolated from shoots of the Zn-hyperaccumulator Thlaspi caerulescens, but they were not found in association with roots (Lodewyckx et al., 2002). T. goesingense endophytes were also investigated by cultivation-independent approaches revealing a high diversity (Idris et al., 2004). The cultivable endophytic community of A. bertolonii was predominantly represented by taxa related to the genera Staphylococcus, Curtobacterium, Bacillus, and Microbacterium, while the contribution to diversity from Proteobacteria (mainly Pseudomonas) was less important (Barzanti et al., 2007). In contrast, cultivation-independent analysis indicated a dominance of Alpha- and Gammaproteobacteria in the leaf-associated community of A. bertolonii from three serpentine outcrops in Central Italy (Mengoni et al., 2009). These results confirm that cultivable and culture-independent methods capture distinct ”windows” of bacterial diversity and can give complementary information.
Bacteria belonging to all major bacterial phyla could be found, including Acidobacteria. The phylum Holophaga/Acidobacterium has been found to be dominant in many soils world-wide (e.g., Sessitsch et al., 2001) and acidobacteria may also colonize the rhizosphere (e.g., Idris et al., 2004; Mengoni et al., 2004). However, usually these bacteria do not colonize the apoplast of plants. In the highly toxic environment represented by the apoplast of a Ni-hyperaccumulating plant, the diversity of endophytes was higher (Idris et al., 2004) than that which is usually observed among endophytes from other plants (e.g., Rasche et al., 2006; Reiter and Sessitsch, 2006). Lodewyckx et al. (2002) compared root and rhizoplane isolates with endophytes isolated from Thlaspi caerulescens shoots. Similar species were found in both compartments. However, shoot endophytes showed higher resistance to Zn and Cd than strains isolated from roots and rhizoplane. Most isolates from the Zn-hyperaccumulators were affiliated to the genera Methylobacterium and Sphingomonas, which were also found to be represented in high numbers in shoots of the Ni-hyperaccumulator T. goesingense (Idris et al., 2004) as well as in Zn accumulating willows (Kuffner et al., 2010). Endophytic bacteria isolated from both the root and shoot tissues of the Cd/Zn-hyperaccumulator Sedum alfredii were closely related to Pseudomonas, Bacillus, Stenotrophomonas and Acinetobacter (Long et al., 2011). Apart from showing a high resistance to Zn and Cd, some endophytic isolates were also able to effectively solubilize ZnCO3 and Zn(PO4)2 (Long et al., 2011). In this case, endophytic bacteria isolated from the leaves and roots showed a higher resistance to metals (Zn and Cd) than those isolated from the stems. Members belonging to the genera Bacillus were also predominant among Cu-resistant-endophytic bacteria of the Cu-accumulator Elsholtzia splendens (Sun et al., 2010).
3. Mechanisms involved in trace element mobilization by microbes
Microbial influence on trace element speciation and mobility is an important component of biogeochemical cycles of trace elements. Processes such as chemical transformation, chelation and protonation will lead to mobilization of trace elements, whereas precipitation or sorption decrease trace element availability. The influence of bacterial and fungal activity on trace element mobility and its use for bioremediation has been reviewed by Gadd (2004). This review summarizes the current knowledge on potential mechanisms of trace element mobilization by rhizosphere bacteria.
Sorbed, precipitated and occluded trace elements can be solubilized by acidification, chelation and ligand-induced dissolution. Upon soil acidification protons replace trace element cations at sorption sites and dissolve trace element containing minerals such as phosphates. Bacteria can acidify their environment by the export of protons for maintenance of charge balance. Upon chelation, organic chelator compounds scavenge trace element ions from sorption sites and mineral lattices and protect them from resorption (Gadd, 2004). To date two groups of bacterially produced natural chelators are known. These are carboxylic acid anions and siderophores.
3.1. Carboxylic acid anions
Among a large variety of C compounds, oxalate, malate, and citrate are some of the most important organic acids identified in root and microbial exudates (Jones, 1998; Ehrlich, 1998). As the pKa1 values of most carboxylates are below 3.5 and the cytosolic pH of root cells typically ranges from 7.1 to 7.5, carboxylic acids are typically present in soil solution as fully or partially dissociated anions (Ryan et al., 2001). In plant cells, complexation with carboxylic acids, particularly malate, citrate but also with the basic amino acid histidine is a mechanism of trace element detoxification (Küpper et al., 2004; Salt et al., 1999). Bacteria producing trace element-chelating organic acids, such as citric, oxalic or acetic acid have been shown to mobilize various trace elements in soil (Li et al., 2009). Li and coworkers also demonstrated that the release of carboxylic acids from bacterial cells was stimulated by the presence of trace elements. Acid-producing rhizosphere bacteria have been intensely studied, regarding their plant growth-promoting capacity of releasing phosphorus from insoluble trace element phosphates. Therefore, they are often referred to as phosphate solubilizers (Gupta et al., 2002; Nautiyal, 1999). Increased trace element uptake in various plants after inoculation with acid producers or phosphate solubilizers has been reported (Ma et al., 2011). Recently, it has been demonstrated that oxalic acid is involved in the solubilization of Ni from serpentine rocks (Becerra-Castro et al., Unpublished).
3.2. Bacterial siderophores
Siderophore “iron carriers” are iron-chelating secondary metabolites, which various organisms release under iron-limiting conditions. Iron-limiting habitats for bacteria are soil, seawater, plants and animal hosts. In seawater the concentration of iron is generally low, whereas in soil iron bioavailability is low, as iron prevails in insoluble Fe(III)-hydroxides at neutral pH. In plants and animals iron is transported and stored in complexes with high affinity ligands. Therefore, siderophore production is widespread among bacteria and siderophores are perhaps the most common bacterial secondary metabolites. Five hundred different bacterial siderophores have been described, with molecular sizes varying between 500 and 1500 Da. Siderophores can derive from several biosynthetic pathway types and are diverse in molecular structure (Barry and Challis, 2009). Many siderophores are relatively stable biomolecules, protected from environmental peptidases and lytic enzymes, by alternating composition of d- and l-amino acids. Their ability to form high affinity complexes with Fe(III) relies on bidentate functional groups with negatively charged oxygens. These are in most cases catecholate, hydroxamate or alpha-hydroxycarboxylate groups.
Siderophores are secreted and Fe(III)-siderophore complexes are recognized and scavenged from the environment by membrane receptor proteins. They are too large to pass membrane porins. In gram-negative bacteria, transport through the cytoplasmatic membrane is mediated by ABC-transporters and the TonB protein transfers the necessary metabolic energy from the cytoplasmatic to the outer membrane. In gram-positive bacteria this process is less understood, and they lack TonB. Many bacteria produce more than one siderophore, and different molecules could be produced under different conditions.
All siderophores possess higher affinity for Fe(III) than for Fe(II) or any other trace element ion. However, complexes of lower stability are also formed with other trace elements (Hernlem et al., 1996; Hider and Kong, 2010). Divalent cations (e.g., Fe2+, Zn2+, Cu2+, Cd2+) form less stable complexes due to their reduced charge density (charge/size ratio). Addition of trace elements to bacterial cultures induces siderophore synthesis and leads to the formation of siderophore–metal complexes (Dimkpa et al., 2008a, 2008b, 2009). Extracellular complexation by siderophores is considered to be a mechanism of bacterial trace element resistance (Nies, 1999; van der Lelie et al., 2000). Siderophore-bound divalent trace elements cannot compete for membrane passage with nutrient cations and are likely not recognized by siderophore receptors due to conformational mismatching (Greenwald et al., 2008). Siderophore synthesis was shown to simultaneously increase iron uptake and to reduce Cd uptake in Streptomycetes (Dimkpa et al., 2009). In contrast, siderophore-mediated uptake of trivalent trace element cations (Al3+) has been demonstrated in iron-depleted cultures of Bacillus megaterium (Hu and Boyer, 1996) and Pseudomonas aeruginosa (Greenwald et al., 2008). Synthesis of several siderophores varying in trace element affinity, preferences and inductivity may convey competitive advantage in trace element contaminated environments (Dimkpa et al., 2009).
Siderophore-producing bacteria have been shown to enhance chlorophyll content and growth of various crop plants in contaminated soil by selectively supporting iron uptake from the pool of trace element cations competing for import (Burd et al., 1998, 2000; Dimkpa et al., 2009). Moreover complexation of trace elements by bacterial siderophores in the rhizosphere likely prevents generation of free radicals and oxidative stress. Dimkpa et al. (2008a) have employed siderophore-containing filtrates of Streptomycetes cultures as natural biodegradable chelators instead of synthetic compounds for chelator-assisted phytoextraction. This strategy bears certain advantages over inoculation with living cells, as it does not require establishment of a population in the rhizosphere, does not raise biosafety issues and guarantees siderophore synthesis under controlled conditions. Culture filtrate treatments improved iron uptake and growth in all experiments conducted by Dimkpa et al. (2008a, 2009), suggesting that the siderophores produced by these Streptomycetes are sufficiently stable for application as biochelators. In addition the culture filtrates partly enhanced trace element uptake (Dimkpa et al., 2009). There are still conflicting theories, whether complexation by bacterial siderophores makes trace elements more or less available for plants (Abou-Shanab et al., 2003; Glick, 2003; van der Lelie et al., 2000). Inoculation of plants with siderophore producing bacteria has been observed to both promote and reduce heavy metal uptake, depending on the combination of plant, bacterium and metal (Ma et al., 2011).
3.3. Other bacterial trace element chelators
There is little information about bacterially produced trace element ligands else than carboxylic acids and siderophores. Certain nitrogen-fixing bacteria produce molybdate binding tetradentate catecholates, which also function as siderophores (Hider and Kong, 2010). The pigment melanin, which is produced by many fungi and Streptomycetes can bind trace elements to its carboxylic groups and was shown to be involved in trace element sorption and trace element tolerance of S. scabies (Haferburg and Kothe, 2010). Molecular structures and functional groups of many antibiotics and other bacterial secondary metabolites suggest a potential to complex trace elements (Gräfe and Radics, 1986). In trace element tolerant bacteria the addition of trace elements to culture media can trigger synthesis of various secondary metabolites (Haferburg et al., 2009; Sprocati et al., 2006). Haferburg et al. (2009) postulated that many metabolites induced upon trace element stress may be involved in trace element detoxification by chelation. In addition to secondary metabolites, proteins such as phytochelatins, metallothioneins and metallohistins may play a role in trace element complexation in the rhizosphere. They are small and cysteine- and or histidine-rich proteins with high affinity to trace element cations, which plants synthesize in response to trace element stress. Phytochelatins, metallothioneins and metallohistins are also produced by certain bacteria, with particularly high occurrence in Actinomycetes from metalliferous environments (Gadd, 2004; Haferburg and Kothe, 2010).
4. Effect of microbially induced plant growth promotion and metal extraction capacity of plants
Trace element-resistant microorganisms can enhance plant establishment and growth under the stress conditions of toxic trace element concentrations. Plant growth-promoting bacteria (PGPB) (such as phosphate and potassium solubilisers, the free living N2-fixing bacteria, rhizobia etc.) and fungi such as arbuscular mycorrhizal fungi (AMF) have been shown to have beneficial effects. Most studies attribute a stimulation in plant growth and biomass to the production of phytohormones (such as indoleacetic acid, IAA), suppression of stress ethylene production (due to ACC deaminase activity), or improvement in plant nutritive status due to the presence of N2 fixers, PO4-solubilisers, or siderophore-producers. Plant growth promotion plays a major role in the extraction and removal of trace elements since a simple improvement in biomass results in an increase in the overall trace element yield (phytoextracted trace elements). Numerous studies have isolated and characterized rhizosphere or endophytic bacteria associated with trace element-tolerant or trace element-(hyper)accumulating plants as a means of identifying interesting strains for phytoextraction purposes. Most of these strains were characterized for plant growth promotion traits and when the host plants were re-inoculated plant growth was frequently improved. Examples of microbial-induced plant growth promotion in a phytoextraction context can be found in crop plants, hyperaccumulators and woody tree species. In addition to plant growth promotion, bacteria were reported to have a beneficial effect on plant stress tolerance. This may be achieved by the enzyme ACC deaminase leading to a reduction of stress-induced ethylene levels in the plant (Burd et al., 1998, 2000; Glick, 2004). This stimulation in plant tolerance and growth is often concomitant with above-mentioned microbial-induced improvement in plant nutritive status (Ghosh et al., 2011; Ma et al., 2011). Bacterial siderophores can indirectly alleviate trace element toxicity by supplying the plant with iron and eliminating iron deficiency (Burd et al., 1998, 2000). However, in many studies no clear connection was found between the microbial strain's PGP traits (determined in vitro) and the observed growth enhancement (Becerra-Castro et al., 2012; Cabello-Conejo et al., 2011; Luo et al., 2011).
Enhanced plant growth and biomass production has been observed in several hyperaccumulating plants after inoculating with rhizosphere or endophytic bacterial strains (Abou-Shanab et al., 2003, 2006; Cabello-Conejo et al., 2011; Ghosh et al., 2011; Li et al., 2007; Whiting et al., 2001b). Zaidi et al. (2006) reported that a Bacillus subtilis strain was able to promote the growth of Brassica juncea and thereby increased Ni accumulation. The strain was shown to produce IAA, which might have been responsible for plant growth promotion. Similarly, a Cr6+-resistant Pseudomonas strain producing IAA was able to promote growth of B. juncea and thereby increased trace element extraction (Rajkumar et al., 2005). In that study a Bacillus sp. strain, which did not produce IAA, was also able to increase plant growth and to enhance Cr6+-extraction, although to a lower extent. Similarly, Dell'Amico et al. (2008) observed IAA, ACC deaminase and siderophore producing rhizosphere isolates to promote growth of Brassica napus in presence of cadmium. Mastretta et al. (2009) found that the inoculation of Nicotiana tabacum with Cd-resistant seed endophyte Sanguibacter sp. S_d2 increased shoot Cd concentrations compared to non-inoculated plants. The AMF Glomus intraradices was shown to enhance growth of Helianthus annuus, and as a result also the total Ni extracted in certain soil treatments (Ker and Charest, 2010). The AM colonization also significantly increased the activity of glutamine synthetase, indicating an enhanced Ni tolerance.
Enhanced plant growth and biomass production has been observed in several hyperaccumulating plants after inoculating with rhizosphere or endophytic bacterial strains. Examples in the literature can be found for As-, Cd/Zn-, or Ni-hyperaccumulators (Abou-Shanab et al., 2003, 2006; Cabello-Conejo et al., 2011; Ghosh et al., 2011; Li et al., 2007; Whiting et al., 2001b). Jankong et al. (2007) reported the importance of rhizobacteria for the biomass production of silverback fern (Pityrogramma calomelanos) on arsenic contaminated soil. Higher biomass production correlated with enhanced remediation. The effect of rhizosphere bacteria on Zn accumulation by a hyperaccumulator (Thlaspi caerulescens) and a non-accumulator (Thlaspi arvense) was compared (Whiting et al., 2001b). The authors could show that the rhizosphere microflora facilitated biomass production and Zn uptake of the hyperaccumulator, whereas T. arvense was not affected. Interestingly, the rhizosphere of T. caerulescens hosted higher bacterial numbers than that of T. arvense. Data suggested that the bacteria enhanced the availability of water-soluble Zn in the soil, which overcame a major rate-limiting step for Zn uptake by T. caerulescens in soils with low concentrations of labile Zn (Whiting et al., 2001b). In both hydroponically- and soil-grown plants, inoculating the Cd/Zn-hyperaccumulator S. alfredii with trace element-tolerant rhizobacterial strains belonging to the genera Burkholderia improved plant trace element tolerance, biomass production and Cd (and Zn) uptake and extraction (Guo et al., 2011; Li et al., 2007). In some cases, plant P content was improved as well as translocation of Cd and Zn from the root to shoot. The Ni hyperaccumulator A. serpyllifolium subsp. lusitanicum grown in ultramafic soil showed a significantly higher shoot Ni concentration and improved root to shoot Ni transport after inoculation with a Ni-resistant rhizosphere bacteria Arthrobacter nitroguajacolicus (Cabello-Conejo et al., 2011). Abou-Shanab et al. (2003, 2006) similarly considered rhizobacteria as highly important for the mobilization of nickel in soil and for its uptake by the hyperaccumulating plant Alyssum murale.
Hyperaccumulators were frequently considered non-mycorrhizal until relatively recently. Berkheya coddii was one of the first hyperaccumulating plants in which arbuscular mycorrhizal symbiosis was reported (Turnau and Mesjasz-Przbylowicz, 2003). Since then several hyperaccumulators have been shown to form symbiosis with AMF, and AM colonization enhanced plant growth and tolerance to trace elements. A stimulation in the biomass of B. coddii led to a higher total Ni content (and hence extraction) in mycorrhizal plants (Orlowska et al., 2011). Likewise, inoculation of the As-hyperaccumulating fern Pteris vittata with indigenous AM fungi resulted in the increase of dry mass and As uptake (Al Agely et al., 2005).
Trace element-accumulating trees such as Salix can be ideal phytoextractors due to their high biomass and massive root system. Kuffner et al. (2008) revealed a positive effect of rhizosphere bacteria on accumulated trace element contents in willows (Salix caprea). Microbial inoculants increased Cd and Zn translocation factors from roots to leaves of Salix caprea grown in a moderately contaminated soil (De Maria et al., 2011). Total Cd extraction by Populus canadensis was significantly increased when in association with the ectomycorrhizal fungus Paxillus involutus (Sell et al., 2005). Rhizosphere bacterial strains did not affect Cd and Zn accumulation in the leaves of Salix viminalis but the microbial-induced increase in biomass production resulted in an increase in the total trace element phytoextracted (Becerra-Castro et al., 2012).
Most studies evaluated the effects of re-inoculating host plants with their associated isolated strains (Abou-Shanab et al., 2003, 2006; Cabello-Conejo et al., 2011; Ghosh et al., 2011; Li et al., 2007). The specificity of these plant-microbial combinations is unclear. For example, rhizosphere bacteria originally isolated from hyperaccumulating plants have been shown to promote growth of diverse plant species (in many cases belonging to distinct botanical groups). The Ni-resistant PGPB strain Psychrobacter sp. SRS8 originally isolated from the rhizosphere of the Ni-hyperaccumulator A. serpyllifolium was found to effectively promote the growth and phytoextraction potential of the energy crops Ricinus communis and Helianthus annuus in artificially Ni-contaminated soils (Ma et al., 2011). Grandlic et al. (2008) found the effect of bacterial strains on the biomass of Atriplex lentiformis and Buchloe dactyloides was both plant- and substrate-dependent. Becerra-Castro et al. (2012) evaluated the influence of fourteen strains of Cd/Zn-resistant rhizosphere bacteria (isolated from Gramineae and woody trees/shrubs) on growth and trace element accumulation of Festuca pratensis and Salix viminalis. While almost all strains promoted growth of the grass, the majority of strains had a negative effect on Salix, and only five strains (identified as Massilia sp., Pseudomonas sp., Rhodococcus sp. and Streptomyces sp.) had a positive effect on both plant species. In contrast, Whiting et al. (2001b) could show that the rhizosphere microflora facilitated biomass production and Zn uptake of the hyperaccumulator T. caerulescens, whereas the non-accumulator T. arvense was not affected. These studies highlight the complex nature of these plant-microbial interactions, and need to optimize the soil-plant-microbial system on a site-by-site basis. Many factors will need to be taken into consideration and effects will depend on the plant species, soil properties and trace element concentration, as well as the microbial species and strains used. However, given the diversity of soil, rhizosphere, and endophytic trace element-tolerant microorganisms the opportunity to find beneficial plant-microbial partnerships is considerable.
5. Effect of microbially induced trace element plant-availability and metal-extraction capacity by plants
As shown above, in many cases, trace element extraction was increased due to the enhancement in shoot biomass production. On the other hand, microorganisms have also been shown to influence the bioavailability of trace elements. Plants exude organic compounds into the rhizosphere supporting the growth and metabolic activities of plant-associated microorganisms. Microbial processes and metabolites can, like plants, strongly affect trace element behaviour and bioavailability, which in turn contribute to an increase in plant trace metal uptake and accumulation. Microbial activity was associated with an enhanced release of Co and Ni in ultramafic soils of New Caledonia (Amir and Pineau, 2003). Quantin et al. (2002) showed that bacterial reduction of oxides led to the solubilization of Fe, Mn, Ni and Co and modified soil metal distribution. Trace element mobilization by rhizosphere bacteria and simultaneous promotion of uptake has been observed in experiments with crop plants. Cadmium and lead mobilizing strains enhanced the uptake of these trace elements in tomato (Jiang et al., 2008) and a zinc mobilizer promoted Zn accumulation in Ricinus communis (Rajkumar and Freitas, 2008a).
Amir and Pineau (2003) detected a positive correlation between microbial activity and bioavailability of trace elements in ultramafic soils. In agreement with this, inoculation of an autoclaved soil with a small portion of the same, non-heated soil, resulted in the release of trace elements (Amir and Pineau, 2003). In addition, the presence of a trace element-tolerant plant as well as the addition of compost stimulated the trace element mobilization process, whereas the pH had a minor effect. Amir and Pineau (2003) suggested that the rhizosphere effect on trace element release is due to the stimulation of chemoorganotrophic microorganisms rather than to the direct effect of root secretions. It was also suggested that plant roots may also partly absorb trace elements. de Souza et al. (1999) showed that rhizosphere bacteria are necessary to achieve optimum rates of selenium accumulation and volatilization by Indian mustard. In that study, the tested rhizosphere bacteria increased root hair production of Indian mustard and enhanced trace element uptake was observed. However, from the different experiments performed the authors concluded that stimulation of root hair production was not responsible for the enhanced accumulation of Se. A heat-labile compound was shown to enhance Se accumulation in axenic plants, but it was not clear whether it was produced by rhizosphere bacteria or by bacterized roots (de Souza et al., 1999). The authors postulated that the heat-labile compound stimulated the selenate transporter in plants.
Numerous authors have shown bacterial-induced trace element mobilization or solubilization of trace element-rich minerals using in vitro testing. Trace element-mobilizing metabolites from rhizosphere and endophytic bacteria can increase soil trace element extractability. This mobilization has frequently been attributed to the action of bacterial siderophores or to the release of organic acids (Lodewyckx et al., 2002; Abou-Shanab et al., 2003; Braud et al., 2009). Culture filtrates of rhizobacterial strains isolated from hyperaccumulating subspecies of Alyssum serpyllifolium increased Ni extraction from ultramafic soils. Surprisingly, cell-free cultures of rhizobacteria isolated from the closely related, non-hyperaccumulator (subsp. serpyllifolium) was also able to enhance Ni extractability. Trace element-mobilizing metabolites of various Actinobacteria mobilized soil Zn and/or Cd (Kuffner et al., 2010). However, in many of these studies there was no clear connection between isolate characteristics (e.g. capacity to produce siderophores or organic acids) and their ability to mobilize soil trace metals. For example, several bacterial strains which did not produce siderophores mobilized high amounts of trace elements, probably due the production of other secondary metabolites (Kuffner et al., 2008, 2010). Moreover, in some cases siderophore-producers have actually led to a decrease in Zn and Cd (Kuffner et al., 2008, 2010), or Ni (Becerra-Castro et al., 2011), bioavailability. In addition to the potential effect of siderophores it has been suggested that bacterial exopolymers may complex trace elements leading to reduced availability for plants (Diels et al., 1995; Kunito et al., 2001).
Several studies have observed an actual increase in soil trace element extractability or re-distribution of soil trace elements pools after bioaugmentation. Braud et al. (2006) found an increase in the exchangeable Pb fraction, and simultaneous decrease in the carbonate-bound fraction, after inoculation with P. aeruginosa and Pseudomonas fluorescens. In a soil bioaugmented with the ectomycorrhizal fungus Paxillus involutus, the concentrations of NH4NO3-extractable Cd, Cu, Pb and Zn were higher than those recorded in the non-bioaugmented soil depending on the soil composition (Baum et al., 2006). Inoculating ultramafic soils with the actinobacterial Microbacterium arabinogalactanolyticum AY509224 increased soil Ni extractability (Abou-Shanab et al., 2003, 2006). The authors suggested that the production of siderophores and carboxylic acids, as well as phosphate solubilization, by the bacterial isolates facilitated Ni solubility. The same strain promoted Ni accumulation and yield in A. murale, but the success of phytoaugmentation was dependent upon the soil Ni concentration. Inoculating B. juncea with the Ni-mobilizing rhizobacteria, Psychrobacter sp. SRA1 and Bacillus cereus SRA10, led to a significant increase in root and shoot Ni accumulation (Ma et al., 2009a). A Zn/Cd-mobilizing, endophytic Actinobacteria strain enhanced trace element accumulation in leaves of Salix caprea (Kuffner et al., 2010).
Finally, a few studies can be found in which plant growth and/or trace element accumulation has been improved using combinations of plant-associated microorganisms. In a hydroponic study, a combination of seven As-resistant rhizobacteria (identified as Pseudomonas sp., Comamonas sp. and Stenotrophomonas sp. and tolerating 10 mM arsenate) enhanced plant As uptake in the fronds of the As-hyperaccumulator Pteris vittata. Microbial exudation of pyochelin-type siderophores, together with root exudates, solubilized As from the growth media spiked with insoluble FeAsO4 and AlAsO4 minerals (Ghosh et al., 2011). In soil-grown plants, inoculation of Salix caprea with Streptomyces sp. (isolated from a Pb-mining area in Austria) in combination with the fungus Cadophora finlandica led to an increase in phytoextraction of Cd and Zn (De Maria et al., 2011). Similarly, plant-associated bacteria (Micrococcus luteus and Sphingomonas sp.) were shown to enhance ectomycorrhiza formation (fungal strain Hebeloma crustuliniforme) and growth of Salix viminalis x caprea, and consequently total Cd and Zn accumulation in shoot biomass was also increased in these fungal–bacterial inoculant combinations (Zimmer et al., 2009).
6. Meta-analysis of inoculation experiments
We performed a meta-analysis of 73 publications containing 738 individual cases (treatments) to reveal most frequently found effects of inoculation experiments on shoot biomass production, trace element concentration and content in shoots. Table 1 shows the results for different trace elements, whereas Table 2 summarizes the effects of experimental conditions, i.e. mode of soil sterilization, initial soil pH, types of microbes used, types of soil contamination, mode of inoculation and source of the inoculants. Both tables summarize cases of significant increases, decreases or no change compared to the non-inoculated controls.
Table 1.
Relative changes of trace element concentration in shoots (lines) vs. relative changes of shoot biomass (columns) for individual and for all trace elements.a
| Concentration in shoots | Shoot biomass |
|||
|---|---|---|---|---|
| Increase | No change | Decrease | Sum | |
| Zinc | ||||
| Increase | 15.4 | 15.4 | 1.71 | 32.5 |
| No change | 20.5 | 33.3 | 2.56 | 56.4 |
| Decrease | 8.55 | 2.56 | 0.00 | 11.1 |
| Sum | 44.4 | 51.3 | 4.27 | 100 |
| Lead | ||||
| Increase | 25.8 | 1.03 | 0.00 | 26.8 |
| No change | 41.2 | 19.6 | 5.15 | 66.0 |
| Decrease | 2.06 | 4.12 | 1.03 | 7.22 |
| Sum | 69.1 | 24.7 | 6.19 | 100 |
| Nickel | ||||
| Increase | 20.3 | 10.5 | 0.00 | 30.8 |
| No change | 31.6 | 16.5 | 1.50 | 49.6 |
| Decrease | 16.5 | 2.26 | 0.75 | 19.5 |
| Sum | 68.4 | 29.3 | 2.26 | 100 |
| Copper | ||||
| Increase | 8.62 | 27.6 | 5.17 | 41.4 |
| No change | 36.2 | 10.3 | 0.00 | 46.6 |
| Decrease | 8.62 | 3.45 | 0.00 | 12.1 |
| Sum | 53.4 | 41.4 | 5.17 | 100 |
| Cadmium | ||||
| Increase | 22.7 | 10.9 | 0.78 | 34.4 |
| No change | 19.5 | 23.4 | 2.34 | 45.3 |
| Decrease | 16.4 | 3.91 | 0.00 | 20.3 |
| Sum | 58.6 | 38.3 | 3.13 | 100 |
| As concentration in shoot | ||||
| Increase | 13.2 | 5.66 | 0.00 | 18.9 |
| No change | 22.6 | 13.2 | 9.43 | 45.3 |
| Decrease | 24.5 | 11.3 | 0.00 | 35.8 |
| Sum | 60.4 | 30.2 | 9.43 | 100 |
| All metals | ||||
| Increase | 18.5 | 11.0 | 1.00 | 30.5 |
| No change | 29.7 | 20.5 | 3.00 | 53.2 |
| Decrease | 12.2 | 3.83 | 0.33 | 16.3 |
| Sum | 60.3 | 35.3 | 4.33 | 100 |
Values in the table indicate the percentage of studies documenting a given observation. Data were obtained from: Abou-Shanab et al. (2003), Abou-Shanab et al. (2006), Al Agely et al. (2005), Andreazza et al. (2010), Arriagada et al. (2007), Azcón et al. (2009), Bai et al. (2008); Baum et al. (2006), Braud et al. (2009), Brunetti et al. (2011), Burd et al. (1998, 2000), Chen et al. (2003, 2004, 2006, 2007a, 2007b, 2010), Citterio et al. (2005), Dary et al. (2010), Davies et al. (2001), De Maria et al. (2011), Di Gregorio et al. (2006), Dimkpa et al. (2009), Dos Santos Utmazian et al. (2007), Duponnois et al. (2006), Farwell et al. (2006, 2007), Ganesan (2008), Gao et al. (2010), He et al. (2009, 2010), Ike et al. (2007), Jankong et al. (2007), Janoušková et al. (2005), Jiang et al. (2008), Kuffner et al. (2008, 2010), Kumar et al. (2008, 2009), Leung et al. (2006), Liao et al. (2003), Liu et al. (2005), Ma et al. (2009a, 2009b, 2009c), Malcova et al. (2003), Marques et al. (2006), Medina et al. (2006), Nie et al. (2002), Rai et al. (2004), Rajkumar and Freitas (2008a, 2008b), Rajkumar et al. (2006), Reed and Glick (2005), Rodriguez et al. (2008), Safronova et al. (2006), Sell et al. (2005), Sheng and Xia (2006), Sheng et al. (2008a, 2008b, 2008c), Shilev et al. (2001, 2006), Sudova et al. (2007), Tank and Saraf (2009), Trotta et al. (2006), Vivas et al. (2003a, 2003b, 2006), Wang et al. (2005, 2007a, 2007b), Wani et al. (2007), Whiting et al. (2001b), Wu et al. (2006), Xu et al. (2008), Zaidi et al. (2006).
Table 2.
Effect of different experimental conditions on relative changes of trace element concentration in shoots, shoot biomass, and trace element content in shoots.a
| Percentage | Trace element concentration |
Shoot biomass |
Trace element content |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Decrease | No effect | Increase | nb | Decrease | No effect | Increase | nb | Decrease | No effect | Increase | nb | ||
| Soil pH | |||||||||||||
| <5.0 | 0.3 | 50.0 | 0.0 | 50.0 | 2 | 0.00 | 0.0 | 100 | 2 | 50.0 | 0.0 | 50.0 | 2 |
| 5.0–5.9 | 16.4 | 5.9 | 57.4 | 36.6 | 101 | 0.00 | 13.2 | 86.8 | 121 | 33.3 | 33.3 | 33.3 | 3 |
| 6.0–6.9 | 21.0 | 8.4 | 56.6 | 35.0 | 143 | 1.29 | 56.1 | 42.6 | 155 | 9.0 | 47.8 | 43.3 | 67 |
| 7.0–7.9 | 33.6 | 25.0 | 48.4 | 26.6 | 188 | 5.65 | 27.8 | 66.5 | 249 | 11.0 | 41.2 | 47.8 | 137 |
| > 8.0 | 8.9 | 22.7 | 57.6 | 19.7 | 66 | 8.00 | 18.0 | 74.0 | 50 | 20.0 | 36.7 | 43.3 | 30 |
| Large range of pH values | 3.3 | 12.5 | 79.2 | 8.3 | 24 | 0.00 | 14.3 | 85.7 | 21 | 0.0 | 33.3 | 66.7 | 21 |
| not indicated | 16.5 | 16.5 | 46.4 | 37.1 | 97 | 10.3 | 47.4 | 42.2 | 116 | 22.2 | 33.3 | 44.4 | 27 |
| Metal source of experimental soil | |||||||||||||
| Uncontaminated | 16.1 | 9.9 | 66.2 | 23.9 | 71 | 5.3 | 40.7 | 54.0 | 113 | 10.0 | 35.0 | 55.0 | 20 |
| Contaminated (geogenic) | 3.1 | 13.0 | 39.1 | 47.8 | 23 | 0.0 | 91.3 | 8.7 | 23 | n.i. | n.i. | n.i. | 0 |
| Polluted (anthropogenic) | 31.3 | 13.0 | 62.5 | 24.5 | 216 | 6.4 | 43.4 | 50.2 | 219 | 13.3 | 54.7 | 32.0 | 128 |
| Spiked | 44.3 | 22.5 | 40.4 | 37.1 | 275 | 3.7 | 23.7 | 72.6 | 321 | 13.7 | 28.2 | 58.1 | 117 |
| Amended (with cont. material) | 5.1 | 0.0 | 83.3 | 16.7 | 36 | 0.0 | 2.6 | 97.4 | 38 | 0.0 | 27.3 | 72.7 | 22 |
| Soil sterilization treatment | |||||||||||||
| Heat sterilized | 37.3 | 14.4 | 50.2 | 35.3 | 215 | 4.0 | 28.4 | 67.6 | 275 | 8.6 | 35.5 | 55.9 | 93 |
| γ-radiated + heat sterilized | 0.8 | 0 | 100 | 0 | 6 | 66.7 | 33.3 | 0.0 | 6 | 100 | 0.0 | 0.0 | 6 |
| γ-radiated | 11.4 | 42.3 | 46.2 | 11.5 | 78 | 9.5 | 51.2 | 39.3 | 84 | 20.3 | 64.4 | 15.3 | 59 |
| No sterilization treatment | 50.5 | 11.2 | 56.5 | 32.3 | 322 | 2.6 | 33.2 | 64.2 | 349 | 7.0 | 34.9 | 58.1 | 129 |
| Taxonomic group of inoculant | |||||||||||||
| Arbuscular mycorrhizal fungi | 28.5 | 29.0 | 45.0 | 26.0 | 169 | 8.6 | 39.0 | 52.4 | 210 | 22.4 | 38.3 | 39.3 | 107 |
| Ectomycorrhizal fungi | 3.5 | 0.0 | 88.5 | 11.5 | 26 | 7.7 | 30.8 | 61.5 | 26 | 20.0 | 50.0 | 30.0 | 10 |
| Unspecified mycorrhizol fungi | 1.6 | 0.0 | 41.7 | 58.3 | 12 | 8.3 | 25.0 | 66.7 | 12 | n.i. | n.i. | n.i. | 0 |
| Other fungal organisms | 1.6 | 25.0 | 66.7 | 8.3 | 12 | 0.0 | 91.7 | 8.3 | 12 | 0.0 | 100 | 0.0 | 3 |
| Arbuscular mycorrhizal fungi + other fungal organism | 2.2 | 0.0 | 50.0 | 50.0 | 16 | 0.0 | 0.0 | 100 | 16 | n.i. | n.i. | n.i. | 0 |
| Rhizobacteria | 53.3 | 12.3 | 57.0 | 30.7 | 342 | 1.4 | 34.4 | 64.2 | 369 | 2.4 | 50.8 | 46.8 | 126 |
| Endophytes | 1.9 | 0.0 | 0.0 | 100 | 10 | 0.0 | 14.3 | 85.7 | 14 | n.i. | n.i. | n.i. | 0 |
| Co-inoculation of different micr. strains | 7.5 | 17.6 | 50.0 | 32.4 | 34 | 10.9 | 10.9 | 78.2 | 55 | 18.2 | 9.1 | 72.7 | 33 |
| Mode of inoculation | |||||||||||||
| Single strains | 79.4 | 17.4 | 52.2 | 30.4 | 500 | 4.4 | 37.2 | 58.4 | 562 | 13.3 | 44.5 | 42.2 | 211 |
| Mix of strains | 20.6 | 10.7 | 58.7 | 30.6 | 121 | 4.6 | 19.7 | 75.7 | 152 | 9.2 | 28.9 | 61.8 | 76.0 |
| Source of inoculant | |||||||||||||
| Non-polluted soils | 2.17 | 0 | 56.3 | 43.8 | 16.0 | n.i. | n.i. | n.i. | 0 | n.i. | n.i. | n.i. | 0 |
| Polluted soils | 71.3 | 16.4 | 53.6 | 30.0 | 450 | 2.12 | 30.3 | 67.6 | 518 | 10.2 | 32.4 | 57.4 | 176 |
| Indigenous from polluted soils | 9.8 | 12.3 | 70.2 | 17.5 | 57.0 | 12.5 | 56.9 | 30.6 | 72.0 | 17.3 | 63.5 | 19.2 | 52.0 |
| Not indicated | 16.8 | 19.4 | 42.9 | 37.8 | 98.0 | 9.7 | 33.1 | 57.3 | 124.0 | 13.6 | 44.1 | 42.4 | 59.0 |
| All cases | 100 | 16.1 | 53.5 | 30.4 | 621 | 4.48 | 33.5 | 62.0 | 714 | 12.2 | 40.4 | 47.4 | 287 |
Values in the table indicate the percentage of studies documenting a given observation. Data were obtained from: Abou-Shanab et al. (2003), Abou-Shanab et al. (2006), Al Agely et al. (2005), Andreazza et al. (2010), Arriagada et al. (2007), Azcón et al. (2009), Bai et al. (2008); Baum et al. (2006), Braud et al. (2009), Brunetti et al. (2011), Burd et al. (1998, 2000), Chen et al. (2003, 2004, 2006, 2007a, 2007b, 2010), Citterio et al. (2005), Dary et al. (2010), Davies et al. (2001), De Maria et al. (2011), Di Gregorio et al. (2006), Dimkpa et al. (2009), Dos Santos Utmazian et al. (2007), Duponnois et al. (2006), Farwell et al. (2006, 2007), Ganesan (2008), Gao et al. (2010), He et al. (2009, 2010), Ike et al. (2007), Jankong et al. (2007), Janoušková et al. (2005), Jiang et al. (2008), Kuffner et al. (2008, 2010), Kumar et al. (2008, 2009), Leung et al. (2006), Liao et al. (2003), Liu et al. (2005), Ma et al. (2009a, 2009b, 2009c), Malcova et al. (2003), Marques et al. (2006), Medina et al. (2006), Nie et al. (2002), Rai et al. (2004), Rajkumar and Freitas (2008a, 2008b), Rajkumar et al. (2006), Reed and Glick (2005), Rodriguez et al. (2008), Safronova et al. (2006), Sell et al. (2005), Sheng and Xia (2006), Sheng et al. (2008a, 2008b, 2008c), Shilev et al. (2001, 2006), Sudova et al. (2007), Tank and Saraf (2009), Trotta et al. (2006), Vivas et al. (2003a, 2003b, 2006), Wang et al. (2005, 2007a, 2007b), Wani et al. (2007), Whiting et al. (2001b), Wu et al. (2006), Xu et al. (2008), Zaidi et al. (2006).
Number of studies evaluated.
Table 1 shows that for all trace elements except Zn in the majority of cases an increase of shoot biomass was observed due to inoculation. For all investigated cases, 60.3% showed an increased shoot biomass, whereas for 35.3 no significant change was found. Only for 4.3% a decrease in biomass production was observed. In contrast, for the majority of all cases (and also for all individual trace elements) no significant change of trace element concentration in shoots was found (53.2%). Only 30.5% of the investigated cases showed a significant increase of trace element concentration in shoots compared to the non-inoculated control. For 16.3% a decrease of trace element concentration was observed. The results of the meta-analysis suggest that inoculation with microbes increases the shoot biomass concentration more frequently than the shoot trace element concentration. For 18.8% of all cases, an increase of both shoot biomass and shoot trace element concentration was found. These plant-microbe combinations are the most promising regarding the aim to maximize the phytoextraction efficiency. However, these combined positive effects might be restricted to the specific experimental conditions, under which the experiment has been performed.
The effect of several experimental conditions on the outcome of inoculation is shown in Table 2. The overview shows that increased trace element accumulation was less frequently reported at high soil pH (>7) compared to lower pH values (<7). This result is surprising, since the bioavailability of cationic trace elements is lower at high pH values and it might be expected that trace element mobilization activities are more pronounced at higher soil pH. In contrast to trace element concentrations, there is no clear effect of soil pH on the effect of inoculation on biomass production. Regarding the source of the experimental soils, inoculation had the greatest effects on trace element concentration for plants grown and inoculated on geogenically contaminated soils. On the other hand, biomass production was rather low under these conditions. However, only 23 cases were reported, therefore these results are less representative. Most experiments were carried out on either anthropogenically polluted or spiked soils. Interestingly, increased trace element concentration, increased biomass and increased trace element content was more frequently observed on spiked soils compared to polluted soils. Regarding soil sterilization, it is interesting to note that the effect of inoculation is similar for heat-sterilized and non-sterilized soils. Only for γ-sterilized soils the percentage of inoculation trials with increased trace element content and biomass production was clearly lower. Arbuscular mycorrhiza and rhizosphere bacteria were the most frequently inoculated soil microorganisms. The frequency of increased trace element concentrations and biomass production was higher for rhizosphere bacteria. Compared to single strain inoculation, the application of mixed strains did not change the frequency of increased trace element concentrations, but clearly increased the percentage of cases with increased biomass production and trace element content in shoots.
Our meta-analysis merely gives an indication on which effects microbial inoculants might have on trace element uptake and biomass production under trace element polluted conditions. However, data have to be treated with care as experimental conditions were very variable and in most cases microorganisms were already selected, which showed a high potential to have specific, desirable effects. Overall, microorganisms may either increase, decrease or have no effect on trace element accumulation, whereas negative effects on biomass production are rarely observed. Furthermore, endophytes seem to have a high potential to increase trace element accumulation and might be involved in the translocation of trace elements, although these findings are only supported so far by very few reported cases. One major problem related to the observation of no effects due to inoculation lies in the fact that often inoculated microorganisms, particularly when applied under non-sterile conditions, can hardly be traced. Therefore, colonization of the inoculants might have been unsuccessful leading to non-significant results. Furthermore, very little is known, whether strains showing either mobilizing or stabilizing effects have the same type of effects under distinct conditions, e.g. when inoculated on different plant genotypes or different soil conditions. In particular, when the production of secondary metabolites such as siderophores or other chelators is involved, the environmental conditions inducing their production have to be considered.
7. Contribution of rhizosphere microbes to soil remediation (or phytoextraction) technologies
Bacterial populations associated with plants growing in metalliferous soils and potentially accumulating metals are characterized by a high diversity. These communities might also have important functions in relation to plant growth under these adverse conditions as well as trace element uptake. Plant growth-promoting effects by associated bacteria can greatly improve plant performance and also result in higher amounts of accumulated trace elements. An important role of the microflora associated with metal-accumulating plants seems to be the capacity to mobilize trace elements in soils thereby increasing the bioavailable fraction. However, the involved mechanisms are poorly understood.
On the basis of this review we suggest that specific strains showing good activity and colonization potential will be useful in enhancing phytoextraction applications. In particular, the application of genetically engineered plant-associated microorganisms may be a promising approach in enhancing phytoextraction procedures. However, the performance of these microorganisms under natural conditions has to be investigated in detail. Although these strains are likely to be superior in terms of trace element resistance and mobilization, they might face competition problems similar to promising natural strains. Furthermore, biosafety aspects have to be considered and their release depends on national legislation. Addressing the issue of persistence and competition capacity of inoculant strains, while developing their potential for plant growth promotion, stress resistance and trace element accumulation, represent promising strategies for improving current phytoremediation techniques.
Acknowledgements
This work was supported by the Austrian Science Foundation (FWF grant no. L561-B17) and by the 7th Framework Program of the European Commission (FP7-KBBE-266124, GREENLAND).
References
- Abou-Shanab R.A., Angle J.S., Delorme T.A., Chaney R.L., van Berkum P., Moawad H., Ghanem K., Ghozlan H.A. Rhizobacterial effects on nickel extraction from soil and uptake by Alyssum murale. New Phytologist. 2003;158:219–224. [Google Scholar]
- Abou-Shanab R.A., Angle J.S., Chaney R.L. Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high Ni soils. Soil Biology and Biochemistry. 2006;38:2882–2889. [Google Scholar]
- Al Agely A., Sylvia D.M., Ma L.Q. Mycorrhizae increase arsenic uptake by the hyperaccumulator Chinese brake fern (Pteris vittata L.) Journal of Environmental Quality. 2005;6:2181–2186. doi: 10.2134/jeq2004.0411. [DOI] [PubMed] [Google Scholar]
- Amir H., Pineau R. Release of Ni and Co by microbial activity in New Caledonian ultramafic soils. Canadian Journal of Microbiology. 2003;49:288–293. doi: 10.1139/w03-039. [DOI] [PubMed] [Google Scholar]
- Andreazza R., Okeke B.C., Lambais M.R., Bortolon L., Bastos de Melo G.W., Camargo F.A.O. Bacterial stimulation of copper phytoaccumulation by bioaugmentation with rhizosphere bacteria. Chemosphere. 2010;81:1149–1154. doi: 10.1016/j.chemosphere.2010.09.047. [DOI] [PubMed] [Google Scholar]
- Arriagada C.A., Herrera M.A., Ocampo J.A. Beneficial effect of saprobe and arbuscular mycorrhizal fungi on growth of Eucalyptus globulus co-cultured with Glycine max in soil contaminated with heavy metals. Journal of Environmental Management. 2007;84:93–99. doi: 10.1016/j.jenvman.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Azcón R., Medina A., Roldán A., Biró B., Vivas A. Significance of treated agrowaste residue and autochthonous inoculates (Arbuscular mycorrhizal fungi and Bacillus cereus) on bacterial community structure and phytoextraction to remediate soils contaminated with heavy metals. Chemosphere. 2009;75:327–334. doi: 10.1016/j.chemosphere.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Bai J., Lin X., Yin R., Zhang H., Junhua W., Xueming C., Yongming L. The influence of arbuscular mycorrhizal fungi on As and P uptake by maize (Zea mays L.) from As-contaminated soils. Applied Soil Ecology. 2008;38:137–145. [Google Scholar]
- Baker A.J.M. Accumulators and excluders – strategies in the response of plants to heavy metals. Journal of Plant Nutrition. 1981;3:643–654. [Google Scholar]
- Baker A.J.M., Brooks R.R. Terrestrial higher plants which accumulate metallic elements – a review of their distribution, ecology and phytochemistry. Biorecovery. 1989;1:81–126. [Google Scholar]
- Baker A.J.M., McGrath S.P., Reeves R.D., Smith J.A.C. Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Terry N., Bañuelos G.S., editors. Phytoremediation of Contaminated Soil and Water. CRC Press Inc.; 2000. pp. 85–107. [Google Scholar]
- Baker A.J.M., Whiting S.N. In search of the Holy Grail – a further step in understanding metal hyperaccumulation? New Phytologist. 2002;155:1–4. doi: 10.1046/j.1469-8137.2002.00449_1.x. [DOI] [PubMed] [Google Scholar]
- Barry S.M., Challis G.L. Recent advances in siderophore biosynthesis. Current Opinion in Chemical Biology. 2009;13:205–215. doi: 10.1016/j.cbpa.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Barzanti R., Ozino F., Bazzicalupo M., Gabbrielli R., Galardi F., Gonnelli C., Mengoni A. Isolation and characterization of endophytic bacteria from the nickel hyperaccumulator plant Alyssum bertolonii. Microbial Ecology. 2007;53:306–316. doi: 10.1007/s00248-006-9164-3. [DOI] [PubMed] [Google Scholar]
- Baum C., Hrynkiewicz K., Leinweber P., Meissner R. Heavy metal mobilization and uptake by mycorrhizal and nonmycorrhizal willows (Salix x dasyclados) Journal of Plant Nutrition and Soil Science. 2006;169:516–522. [Google Scholar]
- Becerra-Castro C., Monterroso C., Garcia-Leston M., Prieto-Fernandez A., Acea M.J., Kidd P.S. Rhizosphere microbial densities and trace element tolerance of the nickel hyperaccumulator Alyssum serpyllifolium subsp. lusitanicum. International Journal of Phytoremediation. 2009;11:525–541. doi: 10.1080/15226510902717549. [DOI] [PubMed] [Google Scholar]
- Becerra-Castro C., Kidd P.S., Prieto-Fernandez A., Weyens N., Acea M.-J., Vangronsveld J. Endophytic and rhizoplane bacteria associated with Cytisus striatus growing on hexachlorocyclohexane-contaminated soil: isolation and characterization. Plant and Soil. 2011;340:413–433. [Google Scholar]
- Becerra-Castro C., Monterroso C., Prieto-Fernández A., Rodríguez-Lamas L., Loureiro-Viñas M., Acea M.J., Kidd P.S. Pseudometallophytes colonising Pb/Zn mine tailings: a description of the plant-microorganism-rhizosphere soil system and isolation of metal-tolerant bacteria. Journal of Hazardous Materials. 2012;217–218:350–359. doi: 10.1016/j.jhazmat.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Bitton G., Freihofer V. Influence of extracellular polysaccharide on the toxicity of copper and cadmium toward Klebsiella aerogenes. Microbial Ecology. 1978;4:119–125. doi: 10.1007/BF02014282. [DOI] [PubMed] [Google Scholar]
- Blaylock M., Huang J. Phytoextraction of metals. In: Raskin I., Ensley B., editors. Phytoremediation of Toxic Metals: Using Plants to Clean up the Environment. Wiley; New York: 2000. pp. 53–69. [Google Scholar]
- Braud A., Jezequel K., Vieille E., Tritter A., Lebeau T. Changes in the extractability of Cr and Pb in a polycontaminated soil after bioaugmentation with microbial producers of biosurfactants, organic acids and siderophores. Water Air and Soil Pollution: Focus. 2006;6:261–279. [Google Scholar]
- Braud A., Jezequel K., Bazot S., Lebeau T. Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere. 2009;74:280–286. doi: 10.1016/j.chemosphere.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Brunetti G., Farrag K., Rovira P.S., Nigro F., Senesi N. Greenhouse and field studies on Cr, Cu, Pb and Zn phytoextraction by Brassica napus from contaminated soils in the Apulia region, Southern Italy. Geoderma. 2011;160:517–523. [Google Scholar]
- Burd G.I., Dixon D.G., Glick B.R. A plant growth promoting bacterium that decreases nickel toxicity in seedlings. Applied and Environmental Microbiology. 1998;64:3663–3668. doi: 10.1128/aem.64.10.3663-3668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd G.I., Dixon D.G., Glick B.R. Plant growth promoting bacteria that decrease heavy metal toxicity in plants. Canadian Journal of Microbiology. 2000;46:237–245. doi: 10.1139/w99-143. [DOI] [PubMed] [Google Scholar]
- Cabello-Conejo, M.I., Becerra-Castro, C., Monterroso, C., Prieto-Fernández, A., Mench, M., Kidd, P.S., 2011. Effects of rhizobacterial inoculation on biomass and nickel concentration in Alyssum pintodasilvae. In: Proceedings of the 11th International Conference on the Biogeochemistry of Trace Elements (ICOBTE), Florence, Italy, 4–7th July 2011.
- Chao W.L., Chen C.L.F. Role of exopolymer and acid-tolerance in the growth of bacteria in solutions with high copper ion concentrations. Journal of General and Applied Microbiology. 1991;37:363–370. [Google Scholar]
- Chen B., Shen H., Li X., Feng G., Christie P. Effects of EDTA application and arbuscular mycorrhizal colonization on growth and zinc uptake by maize (Zea mays L.) in soil experimentally contaminated with zinc. Plant and Soil. 2004;261:219–229. [Google Scholar]
- Chen B.D., Li X.L., Tao H.Q., Christie P., Wong M.H. The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of zinc. Chemosphere. 2003;50:839–846. doi: 10.1016/s0045-6535(02)00228-x. [DOI] [PubMed] [Google Scholar]
- Chen B.D., Zhu Y.G., Smith F.A. Effects of arbuscular mycorrhizal inoculation on uranium and arsenic accumulation by Chinese brake fern (Pteris vittata L.) from a uranium mining-impacted soil. Chemosphere. 2006;62:1464–1473. doi: 10.1016/j.chemosphere.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Chen B.D., Zhu Y.G., Duan J., Xiao X.Y., Smith S.E. Effects of the arbuscular mycorrhizal fungus Glomus mosseae on growth and metal uptake by four plant species in copper mine tailings. Environmental Pollution. 2007;147:374–380. doi: 10.1016/j.envpol.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Chen B., Xiao X., Zhu Y.-G., Smith F.A., Miao Xie Z., Smith S.E. The arbuscular mycorrhizal fungus Glomus mosseae gives contradictory effects on phosphorus and arsenic acquisition by Medicago sativa Linn. Science of the Total Environment. 2007;379:226–234. doi: 10.1016/j.scitotenv.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Chen L., Luo S., Xiao X., Guo H., Chen J., Wan Y., Li B., Xu T., Xi Q., Rao C., Liu C., Zeng G. Application of plant growth-promoting endophytes (PGPE) isolated from Solanum nigrum L. for phytoextraction of Cd-polluted soils. Applied Soil Ecology. 2010;46:383–389. [Google Scholar]
- Citterio S., Prato N., Fumagalli P., Aina R., Massa N., Santagostino A., Sgorbati S., Berta G. The arbuscular mycorrhizal fungus Glomus mosseae induces growth and metal accumulation changes in Cannabis sativa L. Chemosphere. 2005;59:21–29. doi: 10.1016/j.chemosphere.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Clemens S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta. 2001;212:475–486. doi: 10.1007/s004250000458. [DOI] [PubMed] [Google Scholar]
- Davies J.F.T., Puryear J.D., Newton R.J., Egilla J.N., Saraiva Grossi J.A. Mycorrhizal fungi enhance accumulation and tolerance of chromium in sunflower (Helianthus annuus) Journal of Plant Physiology. 2001;158:777–786. [Google Scholar]
- Dary M., Chamber-Pérez M.A., Palomares A.J., Pajuelo E. “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. Journal of Hazardous Materials. 2010;177:323–330. doi: 10.1016/j.jhazmat.2009.12.035. [DOI] [PubMed] [Google Scholar]
- de Souza M.P., Chu D., Zhao M., Zayed A.M., Ruzin S.E., Schichnes D., Terry N. Rhizosphere bacteria enhance selenium accumulation and volatilization by Indian mustard. Plant Physiology. 1999;119:565–573. doi: 10.1104/pp.119.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Amico E., Cavalca L., Andreoni V. Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biology and Biochemistry. 2008;40:74–84. [Google Scholar]
- De Maria S., Rivelli A.R., Kuffner M., Sessitsch A., Wenzel W.W., Gorfer M., Strauss J., Puschenreiter M. Interactions between accumulation of trace elements and major nutrients in Salix caprea after inoculation with rhizosphere microorganisms. Chemosphere. 2011;84:1256–1261. doi: 10.1016/j.chemosphere.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gregorio S., Barbafieri M., Lampis S., Sanangelantoni A.M., Tassi E., Vallini G. Combined application of Triton X-100 and Sinorhizobium sp Pb002 inoculum for the improvement of lead phytoextraction by Brassica juncea in EDTA amended soil. Chemosphere. 2006;63:293–299. doi: 10.1016/j.chemosphere.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Diels L., Dong Q.H., van der Lelie D., Baeyens W., Mergeay M. The czc operon of Alcaligenes eutrophus CH34: from resistance mechanism to the removal of heavy metals. Journal of Indian Microbiology. 1995;14:142–153. doi: 10.1007/BF01569896. [DOI] [PubMed] [Google Scholar]
- Dimkpa C.O., Svatos A., Merten D., Buchel G., Kothe E. Hydroxamate siderophores produced by Streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Canadian Journal of Microbiology. 2008;54:163–172. doi: 10.1139/w07-130. [DOI] [PubMed] [Google Scholar]
- Dimkpa C.O., Svatos A., Dabrowska P., Schmidt A., Boland W., Kothe E. Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere. 2008;74:19–25. doi: 10.1016/j.chemosphere.2008.09.079. [DOI] [PubMed] [Google Scholar]
- Dimkpa C.O., Merten D., Svatos A., Büchel G., Kothe E. Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. Journal of Applied Microbiology. 2009;107:1687–1696. doi: 10.1111/j.1365-2672.2009.04355.x. [DOI] [PubMed] [Google Scholar]
- Dos Santos Utmazian M.N., Schweiger P., Sommer P., Gorfer M., Strauss J., Wenzel W.W. Influence of Cadophora finlandica and other microbial treatments on cadmium and zinc uptake in willows grown on polluted soil. Plant Soil Environment. 2007;53:158–166. [Google Scholar]
- Duponnois R., Kisa M., Assigbetse K., Prin Y., Thioulouse J., Issartel M., Moulin P., Lepage M. Fluorescent pseudomonads occurring in Macrotermes subhyalinus mound structures decrease Cd toxicity and improve its accumulation in sorghum plants. Science of the Total Environment. 2006;370:391–400. doi: 10.1016/j.scitotenv.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Ehrlich H.L. Geomicrobiology: its significance for geology. Earth-Science Reviews. 1998;45:45–60. [Google Scholar]
- Ernst W.H.O. Evolution of metal hyperaccumulation and phytoremediation hype. New Phytologist. 2000;146:357–358. [Google Scholar]
- Farwell A.J., Vesely S., Nero V., Rodriguez H., Shah S., Dixon D.G., Glick B.R. The use of transgenic canola (Brassica napus) and plant growth-promoting bacteria to enhance plant biomass at a nickel-contaminated field site. Plant and Soil. 2006;288:309–318. [Google Scholar]
- Farwell A.J., Vesely S., Nero V., McCormack K., Rodriguez H., Shah S., Dixon D.G., Glick B.R. Tolerance of transgenic canola (Brassica napus) amended with ACC deaminase-containing plant growth-promoting bacteria to flooding stress at a metal-contaminated field site. Environmental Pollution. 2007;147:540–545. doi: 10.1016/j.envpol.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Fitz W.J., Wenzel W.W., Zhang H., Nurmi J., Stipek K., Fischerova Z., Schweiger P., Köllensperger G., Ma L.Q., Stingeder G. Rhizosphere characteristics of the arsenic hyperaccumulator Pteris vittata L. and monitoring of phytoremoval efficiency. Environmental Science and Technology. 2003;37:5008–5014. doi: 10.1021/es0300214. [DOI] [PubMed] [Google Scholar]
- Francis A.J., Dodge C.J., Gillow J.B. Biodegradation of metal citrate complexes and implications for toxic-metal mobility. Nature. 1992;356:140–142. [Google Scholar]
- Gadd G.M. Microbial influence on metal mobility and application for bioremediation. Geoderma. 2004;122:109–119. [Google Scholar]
- Gao Y., Miao C., Mao L. Improvement of phytoextraction and antioxidative defense in Solanum nigrum L. under cadmium stress by application of cadmium-resistant strain and citric acid. Journal of Hazardous Materials. 2010;181:771–777. doi: 10.1016/j.jhazmat.2010.05.080. [DOI] [PubMed] [Google Scholar]
- Ganesan V. Rhizoremediation of cadmium soil using a cadmium-resistant plant growth-promoting rhizopseudomonad. Current Microbiology. 2008;56:403–407. doi: 10.1007/s00284-008-9099-7. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Rathinasabapathi B., Ma L.Q. Arsenic-resistant bacteria solubilized arsenic in the growth media and increased growth of arsenic hyperaccumulator Pteris vittata L. Bioresource Technology. 2011;102:8756–8761. doi: 10.1016/j.biortech.2011.07.064. [DOI] [PubMed] [Google Scholar]
- Glick B.R. Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnology Advances. 2003;21:383–393. doi: 10.1016/s0734-9750(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Glick B.R. Teamwork in phytoremediation. Nature Biotechnology. 2004;22:526–527. doi: 10.1038/nbt0504-526. [DOI] [PubMed] [Google Scholar]
- Glick B.R. Using soil bacteria to facilitate phytoremediation. Biotechnology Advances. 2010;28:367–374. doi: 10.1016/j.biotechadv.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Gräfe U., Radics L. Isolation and structure elucidation of 6-(3′methylbuten-2′-yl)isatin, an unusual metabolite from Streptomyces albus. Journal of Antibiotics. 1986;39:162–163. doi: 10.7164/antibiotics.39.162. [DOI] [PubMed] [Google Scholar]
- Greenwald J., Zeder-Lutz G., Hagege A., Celia H., Pattus F. The metal dependence of pyoverdine interactions with its outer membrane receptor FpvA. Journal of Bacteriology. 2008;190:6548–6558. doi: 10.1128/JB.00784-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandlic M., Mendez M.O., Chorover J., Machado B., Maier R. Plant growth-promoting bacteria for phytostabilization of mine tailings. Environmental Science and Technology. 2008;42:2079–2084. doi: 10.1021/es072013j. [DOI] [PubMed] [Google Scholar]
- Gremion F., Chatzinotas A., Harms H. Comparative 16S rDNA and 16S rRNA sequence analysis indicates that Actinobacteria might be a dominant part of the metabolically active bacteria in heavy metal-contaminated bulk and rhizosphere soil. Environmental Microbiology. 2003;5:896–907. doi: 10.1046/j.1462-2920.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- Guo J., Tang S., Ju X., Ding Y., Liao S., Song N. Effects of inoculation of a plant growth promoting rhizobacterium Burkholderia sp. D54 on plant growth and metal uptake by a hyperaccumulator Sedum alfredii Hance grown on multiple metal contaminated soil. World Journal of Microbiology and Biotechnology. 2011;27:2835–2844. [Google Scholar]
- Gupta A., Meyer J.M., Goel R. Development of heavy metal-resistant mutants of phosphate-solubilizing Pseudomonas sp. NBRI 4014 and their characterization. Current Microbiology. 2002;45:323–327. doi: 10.1007/s00284-002-3762-1. [DOI] [PubMed] [Google Scholar]
- Haferburg G., Groth I., Mollmann U., Kothe E., Sattler I. Arousing sleeping genes: shifts in secondary metabolism of metal tolerant actinobacteria under conditions of heavy metal stress. Biometals. 2009;22:225–234. doi: 10.1007/s10534-008-9157-4. [DOI] [PubMed] [Google Scholar]
- Haferburg G., Kothe E. Metallomics: lessons for metalliferous soil remediation. Applied Microbiology and Biotechnology. 2010;87:1271–1280. doi: 10.1007/s00253-010-2695-z. [DOI] [PubMed] [Google Scholar]
- Hammer D., Keller C. Changes in the rhizosphere of metal-accumulating plants evidenced by chemical extractants. Journal of Environmental Quality. 2002;31:1561–1569. doi: 10.2134/jeq2002.1561. [DOI] [PubMed] [Google Scholar]
- Hattori H. Decomposition of organic matter with previous cadmium adsorption in soils. Soil Science and Plant Nutrition. 1996;42:745–752. [Google Scholar]
- He L.Y., Chen Z.J., Ren G.D., Zhang Y.F., Qian M., Sheng X.F. Increased cadmium and lead uptake of a cadmium hyperaccumulator tomato by cadmium-resistant bacteria. Exotoxicology and Environmental Safety. 2009;72:1343–1348. doi: 10.1016/j.ecoenv.2009.03.006. [DOI] [PubMed] [Google Scholar]
- He C.Q., Tan G.E., Liang X., Du W., Chen Y.L., Zhi G.Y., Zhu Y. Effect of Zn-tolerant bacterial strains on growth and Zn accumulation in Orychophragmus violaceus. Applied Soil Ecology. 2010;44:1–5. [Google Scholar]
- Hernlem B.J., Vane L.M., Sayles G.D. Stability constants for complexes of the siderophore desferrioxamine B with selected heavy metal cations. Inorganica Chimica Acta. 1996;244:179–184. [Google Scholar]
- Hider R.C., Kong X. Chemistry and biology of siderophores. Natural Products Report. 2010;27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- Hu X., Boyer G.L. Siderophore-mediated aluminium uptake by Bacillus megaterium ATCC19213. Applied and Environmental Microbiology. 1996;62:4044–4048. doi: 10.1128/aem.62.11.4044-4048.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysman F., Verstraate W., Brookes P.C. Effect of manuring practises and increased copper concentrations on soil microbial populations. Soil Biology and Biochemistry. 1994;26:103–110. [Google Scholar]
- Idris R., Trivonova R., Puschenreiter M., Wenzel W.W., Sessitsch A. Bacterial communities associated with flowering plants of the Ni-hyperaccumulator Thlaspi goesingense. Applied and Environmental Microbiology. 2004;70:2667–2677. doi: 10.1128/AEM.70.5.2667-2677.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris R., Kuffner M., Bodrossy L., Puschenreiter M., Monchy S., Wenzel W.W., Sessitsch A. Characterization of Ni-tolerant methylobacteria associated with the hyperaccumulating plant Thlaspi goesingense and description of Methylobacterium goesingense sp. nov. Systematic and Applied Microbiology. 2006;29:634–644. doi: 10.1016/j.syapm.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Ike A., Sriprang R., Ono H., Murooka Y., Yamashita M. Bioremediation of cadmium contaminated soil using symbiosis between leguminous plant and recombinant rhizobia with the MTL4 and the PCS genes. Chemosphere. 2007;66:1670–1676. doi: 10.1016/j.chemosphere.2006.07.058. [DOI] [PubMed] [Google Scholar]
- Jankong P., Visoottiviseth P., Khokiattiwong S. Enhanced phytoremediation of arsenic contaminated land. Chemosphere. 2007;68:1906–1912. doi: 10.1016/j.chemosphere.2007.02.061. [DOI] [PubMed] [Google Scholar]
- Janoušková M., Pavlíková D., Macek T., Vosátka M. Arbuscular mycorrhiza decreases cadmium phytoextraction by transgenic tobacco with inserted metallothionein. Plant and Soil. 2005;272:29–40. [Google Scholar]
- Jiang C.Y., Sheng X.F., Qian M., Wang Q.Y. Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere. 2008;72:157–164. doi: 10.1016/j.chemosphere.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Jones D.L. Organic acids in the rhizosphere – a critical review. Plant and Soil. 1998;205:25–44. [Google Scholar]
- Ker K., Charest C. Nickel remediation by AM-colonized sunflower. Mycorrhiza. 2010;20:399–406. doi: 10.1007/s00572-009-0293-7. [DOI] [PubMed] [Google Scholar]
- Kidambi S.P., Sundin G.W., Palmer D.A., Chakrabarty A.M., Bender C.L. Copper as a signal for alginate synthesis in Pseudomonas syringae pv. syringae. Applied and Environmental Microbiology. 1995;61:2172–2179. doi: 10.1128/aem.61.6.2172-2179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd P., Barcelo J., Bernal M.P., Navari-Izzo F., Poschenrieder C., Shilev S., Clemente R., Monteroso C. Trace element behaviour at the root-soil interface: implications in phytoremediation. Environmental and Experimental Botany. 2009;67:243–259. [Google Scholar]
- Kozdroj J. Microbial responses to single or successive soil contamination with Cd or Cu. Soil Biology and Biochemistry. 1995;27:1459–1465. [Google Scholar]
- Kuffner M., Puschenreiter M., Wieshammer G., Gorfer M., Sessitsch A. Rhizosphere bacteria affect growth and metal uptake of heavy metal accumulating willows. Plant and Soil. 2008;304:35–44. [Google Scholar]
- Kuffner M., De Maria S., Puschenreiter M., Fallmann K., Wieshammer G., Gorfer M., Strauss J., Rivelli A.M., Sessitsch A. Bacteria associated with Zn and Cd-accumulating Salix caprea with differential effects on plant growth and heavy metal availability. Journal of Applied Microbiology. 2010;108:1471–1484. doi: 10.1111/j.1365-2672.2010.04670.x. [DOI] [PubMed] [Google Scholar]
- Kumar K.V., Singh N., Behl H.M., Srivastava S. Influence of plant growth promoting bacteria and its mutant on heavy metal toxicity in Brassica juncea grown in fly ash amended soil. Chemosphere. 2008;72:678–683. doi: 10.1016/j.chemosphere.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Kumar K.V., Srivastava S., Singh N., Behl H.M. Role of metal resistant plant growth promoting bacteria in ameliorating fly ash to the growth of Brassica juncea. Journal of Hazardous Materials. 2009;170:51–57. doi: 10.1016/j.jhazmat.2009.04.132. [DOI] [PubMed] [Google Scholar]
- Kunito T., Nagaoka K., Tada N., Saeki K., Senoo K., Oyaizu H., Matsumoto S. Characterization of Cu-resistant bacterial communities in Cu-contaminated soils. Soil Science and Plant Nutrition. 1997;43:709–717. [Google Scholar]
- Kunito T., Saeki K., Nagaoka K., Oyaizu H., Matsumoto S. Characterization of copper-resistant bacterial community in rhizosphere of highly copper-contaminated soil. European Journal Soil Biology. 2001;37:95–102. [Google Scholar]
- Küpper H., Mijovilovich A., Meyer-Klaucke W., Kroneck P.M.H. Tissue- and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiology. 2004;134:748–757. doi: 10.1104/pp.103.032953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H.M., Ye Z.H., Wong M.H. Interactions of mycorrhizal fungi with Pteris vittata (As hyperaccumulator) in As-contaminated soils. Environmental Pollution. 2006;139:1–8. doi: 10.1016/j.envpol.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Lefèbvre C., Vernet P. Microevolutionary processes on contaminated deposits. In: Shaw A.J., editor. Heavy Metal Tolerance in Plants: Evolutionary Aspects. CRC Press Inc.; Boca Raton, FL: 1990. pp. 286–297. [Google Scholar]
- Li W.C., Ye Z.H., Wong M.H. Effects of bacteria on enhanced metal uptake of the Cd/Zn hyperaccumulating plant, Sedum alfredii. Journal of Experimental Botany. 2007;58:4173–4182. doi: 10.1093/jxb/erm274. [DOI] [PubMed] [Google Scholar]
- Li W., Ye Z., Wong M. Metal mobilization and production of short-chain organic acids by rhizosphere bacteria associated with a Cd/Zn hyperaccumulating plant, Sedum alfredii. Plant Soil. 2009;326:453–467. [Google Scholar]
- Liao J.P., Lin X.G., Cao Z.H., Shi Y.Q., Wong M.H. Interactions between arbuscular mycorrhizae and heavy metals under sand culture experiment. Chemosphere. 2003;50:847–853. doi: 10.1016/s0045-6535(02)00229-1. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhu Y.G., Chen B.D., Christie P., Li X.L. Influence of the arbuscular mycorrhizal fungus Glomus mosseae on uptake of arsenate by the as hyperaccumulator fern Pteris vittata L. Mycorrhiza. 2005;15(3):187–192. doi: 10.1007/s00572-004-0320-7. [DOI] [PubMed] [Google Scholar]
- Lodewyckx C., Mergeay M., Vangronsveld J., Clijsters H., van der Lelie D. Isolation, characterization, and identification of bacteria associated with the zinc hyperaccumulator Thlaspi caerulescens subsp. calaminaria. International Journal of Phytoremediation. 2002;4:101–115. doi: 10.1080/15226510208500076. [DOI] [PubMed] [Google Scholar]
- Long X.X., Chen X.M., Chen Y.G., Woon-Chung W.J., Wei Z.B., Wu Q.T. Isolation and characterization endophytic bacteria from hyperaccumulator Sedum alfredii Hance and their potential to promote phytoextraction of zinc polluted soil. World Journal of Microbiology and Biotechnology. 2011;27:1197–1207. [Google Scholar]
- Luo S.L., Chen L., Chen J.L., Xiao X., Xu T.Y., Wan Y., Rao C., Liu C.B., Liu Y.T., Lai C., Zeng G.M. Analysis and characterization of cultivable heavy metal-resistant bacterial endophytes isolated from Cd-hyperaccumulator Solanum nigrum L. and their potential use for phytoremediation. Chemosphere. 2011;85:1130–1138. doi: 10.1016/j.chemosphere.2011.07.053. [DOI] [PubMed] [Google Scholar]
- Ma Y., Rajkumar M., Freitas H. Isolation and characterization of Ni mobilizing PGPB from serpentine soils and their potential in promoting plant growth and Ni accumulation by Brassica spp. Chemosphere. 2009;75:719–725. doi: 10.1016/j.chemosphere.2009.01.056. [DOI] [PubMed] [Google Scholar]
- Ma Y., Rajkumar M., Freitas H. Inoculation of plant growth promoting bacterium Achromobacter xylosoxidans strain Ax10 for the improvement of copper phytoextraction by Brassica juncea. Journal of Environmental Management. 2009;90:831–837. doi: 10.1016/j.jenvman.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Ma Y., Rajkumar M., Freitas H. Improvement of plant growth and nickel uptake by nickel resistant-plant growth promoting bacteria. Journal of Hazardous Materials. 2009;166:1154–1161. doi: 10.1016/j.jhazmat.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Ma Y., Prasad M.N., Rajkumar M., Freitas H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnology Advances. 2011;29:248–258. doi: 10.1016/j.biotechadv.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Malcova R., Vosatka M., Gryndler M. Effects of inoculation with Glomus intraradices on lead uptake by Zea mays L. and Agrostis capillaris L. Applied Soil Ecology. 2003;23:55–67. [Google Scholar]
- Marques A.P.G.C., Oliveira R.S., Rangel A.O.S.S., Castro P.M.L. Zinc accumulation in Solanum nigrum is enhanced by different arbuscular mycorrhizal fungi. Chemosphere. 2006;65:1256–1263. doi: 10.1016/j.chemosphere.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Mastretta C., Taghavi S., van der Lelie D., Mengoni A., Galardi F., Gonnelli C., Barac T., Boulet J., Weyens N., Vangronsveld J. Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. International Journal of Phytoremediation. 2009;11:251–267. [Google Scholar]
- Medina A., Vassileva M., Barea J.-M., Azcon R. The growth-enhancement of clover by Aspergillus-treated sugar beet waste and Glomus mosseae inoculation in Zn contaminated soil. Applied Soil Ecology. 2006;33:87–98. [Google Scholar]
- Mengoni A., Barzanti A., Gonnelli C., Gabrielli R., Bazzicalupo M. Characterization of nickel-resistant bacteria isolated from serpentine soil. Environmental Microbiology. 2001;3:691–698. doi: 10.1046/j.1462-2920.2001.00243.x. [DOI] [PubMed] [Google Scholar]
- Mengoni A., Grassi E., Brazanti A., Biondi E.G., Gonnelli C., Kim C.K., Bazzicalupo M. Genetic diversity of microbial communities of serpentine soil and of rhizosphere of the Ni-hyperaccumulator plant Alyssum bertolonii. Microbial Ecology. 2004;48:209–217. doi: 10.1007/s00248-003-0149-1. [DOI] [PubMed] [Google Scholar]
- Mengoni A., Pini F., Shu W.S., Huang L.N., Bazzicalupo M. Plant-by-plant variations of leaf-associated bacterial communities in the nickel-hyperaccumulator Alyssum bertolonii Desv. Microbial Ecology. 2009;58:660–667. doi: 10.1007/s00248-009-9537-5. [DOI] [PubMed] [Google Scholar]
- Mengoni A., Schat H., Vangronsveld J. Plants as extreme environments? Ni-resistant bacteria and Ni-hyperaccumulators of serpentine flora. Plant and Soil. 2010;331:5–16. [Google Scholar]
- Nautiyal C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- Nie L., Shah S., Burd G.I., Dixon D.G., Glick B.R. Phytoremediation of arsenate contaminated soil by transgenic canola and the plant growth-promoting bacterium Enterobacter cloacae CAL2. Plant Physiology and Biochemistry. 2002;40:355–361. [Google Scholar]
- Nies D.H. Microbial heavy metal resistance. Applied Microbiology and Biotechnology. 1999;51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- Orlowska E., Przybylowicz W., Orlowski D., Turnau K., Mesjasz-Przybylowicz J. The effect of mycorrhiza on the growth and elemental composition of Ni-hyperaccumulating plant Berkheya coddii Roessler. Environmental Pollution. 2011;159:3730–3738. doi: 10.1016/j.envpol.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits E. Phytoremediation. Annual Review of Plant Biology. 2005;56:15–39. doi: 10.1146/annurev.arplant.56.032604.144214. [DOI] [PubMed] [Google Scholar]
- Puschenreiter M., Wieczorek S., Horak O., Wenzel W.W. Chemical changes in the rhizosphere of metal hyperaccumulator and excluder Thlaspi species. Journal of Plant Nutrition and Soil Science. 2003;166:579–584. [Google Scholar]
- Puschenreiter M., Schnepf A., Molina Millán I., Fitz W.J., Horak O., Klepp J., Schrefl T., Lombi E., Wenzel W.W. Changes of Ni biogeochemistry in the rhizosphere of the hyperaccumulator Thlaspi goesingense. Plant and Soil. 2005;271:205–218. [Google Scholar]
- Quantin C., Becquer T., Rouiller J.H., Berthelin J. Redistribution of metals in a New Caledonia ferralsol after microbial weathering. Soil Science Society American Journal. 2002;66:1797–1804. [Google Scholar]
- Rai U.N., Pandey K., Sinha S., Singh A., Saxena R., Gupta D.K. Revegetating fly ash landfills with Prosopis juliflora L.: impact of different amendments and Rhizobium inoculation. Environment International. 2004;30:293–300. doi: 10.1016/S0160-4120(03)00179-X. [DOI] [PubMed] [Google Scholar]
- Rajkumar M., Lee K.J., Lee W.H., Banu J.R. Growth of Brassica juncea under chromium stress: influence of siderophores and indole-3-acetic acid-producing rhizosphere bacteria. Journal of Environmental Biology. 2005;26:693–699. [PubMed] [Google Scholar]
- Rajkumar M., Nagendran R., Lee K.J., Lee W.H., Kim S.Z. Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere. 2006;62:741–748. doi: 10.1016/j.chemosphere.2005.04.117. [DOI] [PubMed] [Google Scholar]
- Rajkumar M., Freitas H. Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere. 2008;71:834–842. doi: 10.1016/j.chemosphere.2007.11.038. [DOI] [PubMed] [Google Scholar]
- Rajkumar M., Freitas H. Effects of inoculation of plant-growth promoting bacteria on Ni uptake by Indian mustard. Bioresource Technology. 2008;99:3491–3498. doi: 10.1016/j.biortech.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Rajkumar M., Prasad M.N.V., Freitas H., Ae N. Biotechnological applications of serpentine soil bacteria for phytoremediation of trace elements. Critical Reviews of Biotechnology. 2009;29:120–130. doi: 10.1080/07388550902913772. [DOI] [PubMed] [Google Scholar]
- Rasche F., Velvis H., Zachow C., Berg G., van Elsas J.D., Sessitsch A. Impact of transgenic potatoes expressing antibacterial agents on bacterial endophytes is comparable to effects of wild type potatoes and changing environmental conditions. Journal of Applied Ecology. 2006;43:555–566. [Google Scholar]
- Reed M.L.E., Glick B.R. Growth of canola (Brassica napus) in the presence of plant growth promoting bacteria and either copper or polycyclic aromatic hydrocarbons. Canadian Journal of Microbiology. 2005;51:1061–1069. doi: 10.1139/w05-094. [DOI] [PubMed] [Google Scholar]
- Reeves R.D., Baker A.J.M. Metal-accumulating plants. In: Raskin I., Ensley B.D., editors. Phytoremediation of Toxic Metals. John Wiley & Sons; 2000. pp. 193–229. [Google Scholar]
- Reiter B., Sessitsch A. The bacterial microflora in association with the wildflower Crocus albiflorus. Canadian Journal of Microbiology. 2006;52:1–10. doi: 10.1139/w05-109. [DOI] [PubMed] [Google Scholar]
- Robinson B.H., Leblanc M., Petit D., Brooks R.R., Kirkman J.H., Gregg P.E.H. The potential of Thlaspi caerulescenss for phytoremediation of contaminated soils. Plant and Soil. 1998;203:47–56. [Google Scholar]
- Rodriguez H., Vessely S., Shah S., Glick B.R. Isolation and characterization of nickel resistant Pseudomonas strains and their effect on the growth of non-transformed and transgenic canola plants. Current Microbiology. 2008;57:170–174. doi: 10.1007/s00284-008-9181-1. [DOI] [PubMed] [Google Scholar]
- Ryan P.R., Delhaize E., Jones D.L. Function and mechanism of organic anion exudation from plant roots. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:527–560. doi: 10.1146/annurev.arplant.52.1.527. [DOI] [PubMed] [Google Scholar]
- Safronova V., Stepanok V., Engqvist G., Alekseyev Y., Belimov A. Root-associated bacteria containing 1-aminocyclopropane-1-carboxylate deaminase improve growth and nutrient uptake by pea genotypes cultivated in cadmium supplemented soil. Biology and Fertility of Soils. 2006;42:267–272. [Google Scholar]
- Salt D.E., Smith R.D., Raskin I. Phytoremediation. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Salt D.E., Benhamou N., Leszczyniecka M., Raskin I. A possible role for rhizobacteria in water treatment by plant roots. International Journal of Phytoremediation. 1999;1:67–69. [Google Scholar]
- Schat H., Llugany M., Bernhard R. Metal-specific patterns of tolerance, uptake, and transport of heavy metals in hyperaccumulating and nonhyperaccumulating metallophytes. In: Terry N., Bañuelos G.S., editors. Phytoremediation of Contaminated Soil and Water. CRC Press; Boca Raton: 2000. pp. 174–188. [Google Scholar]
- Schwartz C., Morel J.L., Saumier S., Whiting S.N., Baker A.J.M. Root development of the Zinc-hyperaccumulator plant Thlaspi caerulescens as affected by metal origin, content and localization in soil. Plant and Soil. 1999;208:103–115. [Google Scholar]
- Sessitsch A., Weilharter A., Gerzabek M.H., Kirchmann H., Kandeler E. Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Applied and Environmental Microbiology. 2001;67:4215–4224. doi: 10.1128/AEM.67.9.4215-4224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessitsch A., Puschenreiter M. Endophytes and rhizosphere bacteria of plants growing in heavy metal contaminated soil. In: Dion P., Nautiyal C.S., editors. Microbiology of Extreme Soils. Springer; Berlin Heidelberg: 2008. [Google Scholar]
- Sell J., Kayser A., Schulin R., Brunner I. Contribution of ectomycorrhizal fungi to cadmium uptake of poplars and willows from a heavily polluted soil. Plant Soil. 2005;277:245–253. [Google Scholar]
- Sheng X.-F., Xia J.-J. Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere. 2006;64:1036–1042. doi: 10.1016/j.chemosphere.2006.01.051. [DOI] [PubMed] [Google Scholar]
- Sheng X.F., He L.Y., Wang Q.Y., Ye H.S., Jiang C. Effects of inoculation of biosurfactant producing Bacillus sp. J119 on plant growth and cadmium uptake in a cadmium amended soil. Journal of Hazardous Materials. 2008;155:17–22. doi: 10.1016/j.jhazmat.2007.10.107. [DOI] [PubMed] [Google Scholar]
- Sheng X.F., Xia J.J., Jiang C.Y., He L.Y., Qian M. Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environmental Pollution. 2008;156:1164–1170. doi: 10.1016/j.envpol.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Sheng X.F., Jiang C.Y., He L.Y. Characterization of plant growth-promoting Bacillus edaphicus NBT and its effect on lead uptake by Indian mustard in a lead-amended soil. Canadian Journal of Microbiology. 2008;54:417–422. doi: 10.1139/w08-020. [DOI] [PubMed] [Google Scholar]
- Shilev S.I., Ruso J., Puig A., Benlloch M., Jorrin J., Sancho E. Rhizospheric bacteria promote sunflower (Helianthus annus L.) plant growth and tolerance to heavy metals. Minerva Biotechnology. 2001;13:37–39. [Google Scholar]
- Shilev S., Fernandez A., Benlloch M., Sancho E.D. Sunflower growth and tolerance to arsenic is increased by the rhizospheric bacteria Pseudomonas fluorescens. In: Morel J.-L., editor. Phytoremediation of Metal Contaminated Soils. Springer; Netherlands: 2006. pp. 315–326. [Google Scholar]
- Sprocati A.R., Alisi C., Segre L., Tasso F., Galletti M., Cremisini C. Investigating heavy metal resistance, bioaccumulation and metabolic profile of a metallophile microbial consortium native to an abandoned mine. Science of the Total Environment. 2006;366:649–658. doi: 10.1016/j.scitotenv.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Sudova R., Pavlikova D., Macek T., Vosatka M. The effect of EDDS chelate and inoculation with the arbuscular mycorrhizal fungus Glomus intraradices on the efficacy of lead phytoextraction by two tobacco clones. Applied Soil Ecology. 2007;35:163–173. [Google Scholar]
- Sun L.N., Zhang Y.F., He L.Y., Chen Z.J., Wang Q.Y., Qian M., Sheng X.F. Genetic diversity and characterization of heavy metal-resistant-endophytic bacteria from two copper-tolerant plant species on copper mine wasteland. Bioresource Technology. 2010;101:501–509. doi: 10.1016/j.biortech.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Tank N., Saraf M. Enhancement of plant growth and decontamination of nickel-spiked soil using PGPR. Journal of Basic Microbiology. 2009;48:1–10. doi: 10.1002/jobm.200800090. [DOI] [PubMed] [Google Scholar]
- Trotta A., Falaschi P., Cornara L., Minganti V., Fusconi A., Drava G., Berta G. Arbuscular mycorrhizae increase the arsenic translocation factor in the as hyperaccumulating fern Pteris vittata L. Chemosphere. 2006;65:74–81. doi: 10.1016/j.chemosphere.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Tu S., Ma L., Luongo T. Root exudates and arsenic accumulation in arsenic hyperaccumulating Pteris vittata and non-hyperaccumulating Nephrolepis exaltata. Plant and Soil. 2004;258:9–19. [Google Scholar]
- Turnau K., Mesjasz-Przbylowicz J. Arbuscular mycorrhiza of Berkheya coddii and other Ni-hyperaccumulating members of Asteraceae from ultramafic soils in South Africa. Mycorrhiza. 2003;13:185–190. doi: 10.1007/s00572-002-0213-6. [DOI] [PubMed] [Google Scholar]
- Vangronsveld J., Herzig R., Weyens N., Boulet J., Adriaensen K., Ruttens A., Thewys T., Vassilev A., Meers E., Nehnelajova E., van der Lelie D., Mench M. Phytoremediation of contaminated soils and groundwater: lessons from the field. Environmental Science and Pollution Research. 2009;16:765–794. doi: 10.1007/s11356-009-0213-6. [DOI] [PubMed] [Google Scholar]
- van der Lelie D., Corbisier P., Diels L., Gilis A., Lodewyckx C., Mergeay M., Taghavi S., Spelmans N., Vangronsveld J. The role of bacteria in the phytoremediation of heavy metals. In: Terry N., Bañuelos G.S., editors. Phytoremediation of Contaminated Soil and Water. Lewis Publishers (CRC Press); Boca Raton, FL: 2000. pp. 265–281. [Google Scholar]
- Vassilev A., Schwitzguébel J.P., Thewys T., van der Lelie D., Vangronsveld J. The use of plants for remediation of metal contaminated soils. Scientific World Journal. 2004;4:9–34. doi: 10.1100/tsw.2004.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivas A., Biro B., Ruiz-Lozano J.M., Barea J.M., Azcon R. Two bacterial strains isolated from a Zn-polluted soil enhance plant growth and mycorrhizal efficiency under Zn-toxicity. Chemosphere. 2006;62:1523–1533. doi: 10.1016/j.chemosphere.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Vivas A., Voros I., Biro B., Campos E., Barea J.M., Azcon R. Symbiotic efficiency of autochthonous arbuscular mycorrhizal fungus (G. mosseae) and Brevibacillus sp. isolated from cadmium polluted soil under increasing cadmium levels. Environmental Pollution. 2003;126:179–189. doi: 10.1016/s0269-7491(03)00195-7. [DOI] [PubMed] [Google Scholar]
- Vivas A., Azcón R., Biró B., Barea J.M., Ruiz-Lozano J.M. Influence of bacterial strains isolated from lead-polluted soil and their interactions with arbuscular mycorrhizae on the growth of Trifolium pratense L. under lead toxicity. Canadian Journal of Microbiology. 2003;49:577–588. doi: 10.1139/w03-073. [DOI] [PubMed] [Google Scholar]
- Wang F., Lin X., Yin R. Heavy metal uptake by arbuscular mycorrhizas of Elsholtzia splendens and the potential for phytoremediation of contaminated soil. Plant and Soil. 2005;269:225–232. [Google Scholar]
- Wang F.Y., Lin X.G., Yin R. Inoculation with arbuscular mycorrhizal fungus Acaulospora mellea decreases Cu phytoextraction by maize from Cu-contaminated soil. Pedobiologia. 2007;51:99–109. [Google Scholar]
- Wang F.Y., Lin X.G., Yin R. Role of microbial inoculation and chitosan in phytoextraction of Cu, Zn, Pb and Cd by Elsholtzia splendens – a field case. Environmental Pollution. 2007;147:248–255. doi: 10.1016/j.envpol.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Wani P.A., Khan M.S., Zaidi A. Effect of metal tolerant plant growth promoting Bradyrhizobium sp. (vigna) on growth, symbiosis, seed yield and metal uptake by greengram plants. Chemosphere. 2007;70:36–45. doi: 10.1016/j.chemosphere.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Wenzel W.W., Bunkowski M., Puschenreiter M., Horak O. Rhizosphere characteristics of indigenous growing nickel hyperaccumulator and excluder plants on serpentine soil. Environmental Pollution. 2003;123:131–138. doi: 10.1016/s0269-7491(02)00341-x. [DOI] [PubMed] [Google Scholar]
- Weyens N., van der Lelie D., Taghavi S., Vangronsveld J. Phytoremediation: plant-endophyte partnerships take the challenge. Current Opinion in Biotechnology. 2009;20:248–254. doi: 10.1016/j.copbio.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Weyens N., van der Lelie D., Taghavi S., Newman L., Vangronsveld J. Exploiting plant–microbe partnerships for improving biomass production and remediation. Trends in Biotechnology. 2009;27:591–598. doi: 10.1016/j.tibtech.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Whiting S.N., Leake J.R., McGrath S.P., Baker A.J.M. Assessment of Zn mobilization in the rhizosphere of Thlaspi caerulescens by bioassay with non-accumulator plants and soil extraction. Plant and Soil. 2001;237:147–156. [Google Scholar]
- Whiting S.N., de Souza S.P., Terry N. Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens. Environment, Science and Technology. 2001;35:3144–3150. doi: 10.1021/es001938v. [DOI] [PubMed] [Google Scholar]
- Wu S.C., Cheung K.C., Luo Y.M., Wong M.H. Effects of inoculation of plant growth-promoting rhizobacteria on metal uptake by Brassica juncea. Environmental Pollution. 2006;140:124–135. doi: 10.1016/j.envpol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Xu P.L., Christie P., Liu Y., Zhang J.L., Li X.L. The arbuscular mycorrhizal fungus Glomus mosseae can enhance arsenic tolerance in Medicago truncatula by increasing plant phosphorus status and restricting arsenate uptake. Environmental Pollution. 2008;156:215–220. doi: 10.1016/j.envpol.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zaidi S., Usmani S., Singh B.R., Musarrat J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere. 2006;64:991–997. doi: 10.1016/j.chemosphere.2005.12.057. [DOI] [PubMed] [Google Scholar]
- Zimmer D., Baum C., Leinweber P., Hrynkiewicz K., Meissner R. Associated bacteria increase the phytoextraction of cadmium and zinc from a metal-contaminated soil by mycorrhizal willows. International Journal of Phytoremediation. 2009;11:200–213. doi: 10.1080/15226510802378483. [DOI] [PubMed] [Google Scholar]