Abstract
Eukaryotic messenger RNA synthesis is a complex biochemical process requiring the concerted action of multiple “general” transcription factors (TFs) that control the activity of RNA polymerase II at both the initiation1 and elongation2,3 stages of transcription. Because the general transcription factors are present at low levels in mammalian cells, their purification is a formidable undertaking. For this reason we explored the feasibility of using rat liver as a source for purification of the general factors. Rat liver has proven to be an ideal model system for biochemical studies of transcription initiation and elongation by RNA polymerase II (Figs. 1 and 2). In our hands the yield of general transcription factors per gram of rat liver is roughly equivalent to their yield per gram of cultured HeLa cells. Moreover, we have been able to develop convenient and reproducible methods for preparation of rat liver extracts from as much as 1 kg of liver per day. Because it is both technically difficult and expensive to obtain such quantities of cultured cells on a daily basis, rat liver provides a significant logistic advantage for purification of the general transcription factors.
Materials
Reagents
We use male Sprague-Dawley rats (200–300 g) from SASCO, Harlan Sprague-Dawley, and Simonson. Unlabeled ultrapure ribonucleoside 5′-triphosphates and RNAguard are from Pharmacia-LKB Biotechnology (Piscataway, NJ). [α-32P]CTP (>400 Ci/mmol) is from Amersham (Arlington Heights, IL). Phenylmethylsulfonyl fluoride (PMSF) is from Sigma (St. Louis, MO) and is dissolved in dimethyl sulfoxide (DMSO) to 1 M and added to buffers just prior to use. Leupeptin and antipain are from Sigma and are dissolved in water to 25 mg/ml. Bovine serum albumin (BSA) (Pentex, fraction V) is from ICN Immunogiologicals (Costa Mesa, CA). Polyvinyl alcohol (PVA, type II), Torula yeast RNA, and heparin are from Sigma. Schwarz/Mann ultrapure ammonium sulfate and sucrose are from ICN Biomedicals, Inc. Glycerol (spectranalyzed grade) is from Fisher (Pittsburgh, PA). Acetylated BSA and RNasin are from Promega (Madison, WI). Formalin-fixed Staphylococcus aureus is from Bethesda Research Laboratories/Life Technologies (Gaithersburg, MD).
Chromatography Supplies
Phosphocellulose (P11) and DEAE-cellulose (DE-52) are from Whatman (Clifton, NJ). Phosphocellulose is precycled according to manufacturer instructions and washed once before use with three packed column volumes of buffer A (see below) containing 0.1 M KCl and BSA (0.2 mg/ml). Ultrogel AcA 34 is from IBF Biotechnics (Columbia, MD). Phenyl-Sepharose 6 FF (low sub), CM-Sephadex (C-25), and DEAE-Sephadex (A-25) are from Pharmacia. BioGel HTP and HPHT are from Bio-Rad (Richmond, CA). Collodion bags are from Sartorius (Gottingen, Germany). All high-performance liquid chromatography (HPLC) is performed using a Beckman System Gold chromatograph.
Buffers
TMSD is 10 mM Tris–HCl (pH 7.5), 1.5 mM MgCl2, 0.25 M sucrose, 0.5 mM dithiothreitol (DTT), and 0.5 mM PMSF. Buffer A is 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)–NaOH (pH 7.9), 1 mM EDTA, 1 mM DTT, 10% (v/v) glycerol, and 0.5 mM PMSF. Buffer B is 50 mM Tris–HCl (pH 7.9), 0.1 mM EDTA, 1 mM DTT, 10% (v/v) glycerol, and 0.5 mM PMSF. Buffer C is 40 mM Tris–HCl (pH 7.9), 0.5 mM EDTA, 1 mM DTT, and 10% (v/v) glycerol. Buffer D is 40 mM HEPES–NaOH (pH 7.9), 0.5 mM EDTA, 1 mM DTT, and 10% (v/v) glycerol. Buffer E is 40 mM HEPES–NaOH (pH 7.9), 0.1 mM EDTA, 1 mM DTT, and 10% (v/v) glycerol. Buffer G is 40 mM HEPES–NaOH (pH 7.0), 0.5 mM EDTA, 1 mM DTT, and 10% (v/v) glycerol. Buffer I is 10 mM potassium phosphate (pH 7.5) and 1 mM DTT. Buffer P is 10 mM potassium phosphate (pH 7.6), 0.1 mM EDTA, 1 mM DTT, and 10% (v/v) glycerol.
Methods
Runoff Transcription Assay of General Initiation Factors
The general initiation factors are purified as described below. Reaction mixtures (60 μl) containing 20 mM Tris–HCl (pH 7.9), 20 mM HEPES–NaOH (pH 7.9), 60 mM KCl, 0.1 mM EDTA, 1 mM DTT, 3% (v/v) glycerol, BSA (0.5 mg/ml), 2% (w/v) PVA, 6 units of RNasin, 200 ng of NdeI-digested pDN-AdML plasmid DNA4 [which contains adenovirus ML (AdML) core promoter sequences from −50 to +10 and directs synthesis of a 254-nucleotide runoff transcript], and approximately 10 ng of TFIIA, 5 ng of TFIIB, 60 ng of TFIID, 20 ng of TFIIE, 10 ng of TFIIF, 40 ng of TFIIH, and 0.003 unit of RNA polymerase IIA,5 are preincubated for 30 min at 28°. Transcription is initiated by addition of 50 μM ATP, 50 μM UTP, 10 μM CTP, 10 μCi of [α-32P]CTP, and 7 mM MgCl2. After 3 min, heparin (10 μg/ml) or 0.5% (w/v) Sarkosyl is added to prevent further initiations, and 50 μM GTP is added to allow synthesis of full-length runoff transcripts. After incubation for 30 min at 28°, reaction mixtures are diluted with an equal volume of 0.2 M Tris–HCl (pH 7.5), 25 mM EDTA, 0.3 M NaCl, 2% (w/v) sodium dodecyl sulfate (SDS), digested with proteinase K at 1 mg/ml for 10 min at room temperature, and ethanol precipitated with 20 μg of torula yeast RNA as carrier. Runoff transcripts are suspended in 9 M urea, 0.025% (w/v) bromphenol blue, and 0.025% (w/v) xylene cyanol FF and analyzed by electrophoresis through 6% (w/v) polyacrylamide, 7 M urea gels.
Assay of SII
SII is an RNA polymerase II-binding protein that activates a nascent RNA cleavage activity in elongation complexes and permits readthrough of intrinsic arrest sites by RNA polymerase II.6 Readthrough can be assayed in standard runoff transcription assays using templates containing transcription arrest sites. The cleavage activity is more difficult to detect unless elongation complexes are washed free of ribonucleoside triphosphates to prevent renewed elongation of truncated RNA chains. Preparation of washed elongation complexes allows both assays to be conducted side by side by withholding or restoring ribonucleoside triphosphates with SII.
Assembly of Arrested RNA Polymerase II Elongation Complexes
The plasmid pADTerm-2 contains a TaqI fragment from the human histone H3.3 gene, which bears a transcription arrest site for RNA polymerase II (site Ia).7 The fragment was inserted into the unique AccI site of pDN-AdML. When cleaved with NdeI this plasmid yields a runoff transcript of 530 nucleotides and a transcript of 205 nucleotides when arrest occurs at site Ia. Transcription typically employs partially purified RNA polymerase II and general transcription factors isolated from rat liver7 by a modification of the methods of Conaway et al.8 One hundred nanograms of DNA template is incubated with rat liver RNA polymerase II (0.5 μg; DEAE-Sephadex fraction) and fraction D (TFIIH and TFIID; 2 μg, CM-Sephadex fraction) in 20 μl of 20 mM HEPES–NaOH (pH 7.9), 20 mM Tris–HCl (pH 7.9), 2% (w/v) PVA, acetylated BSA (0.4 mg/ml), 12 units of RNAguard, 0.15 M KCl, 2 mM DTT, and 7% (v/v) glycerol for 30 min at 28°. Thirty-three microliters of a solution containing TFIIF/E (1 μg; BioGel HTP fraction) and TFIIB (3 ng; TSK phenyl-5PW fraction) in the same buffer without KCl is then added, and the incubation is continued for another 20 min. MgCl2, ATP, UTP, and [α-32P]CTP are added in 6 μl to final concentrations of 7 mM, 20 μM, 20 μM, and approximately 0.6 μM, respectively. In the absence of GTP, ternary complexes containing a 14-nucleotide transcript are synthesized because the first G residue appears in the transcript at position +15. After 20 min, heparin (10 μg/ml) is added to ternary complexes followed by more of each ribonucleoside triphosphate (800 μM), and incubation is continued for 15 min at 28°.
Immunoprecipitation and Washing of Elongation Complexes for SII-Mediated Cleavage and Readthrough Assays
Elongation complexes are immunoprecipitated with a monoclonal antibody (D44) against RNA developed by Eliat et al.9 Elongation complexes are made 10 μg/ml in protein A-purified D44 IgG and incubated at 4° for 5 min. Ten microliters of formalin-fixed S. aureus washed in reaction buffer containing 20 mM Tris–HCl (pH 7.9), 3 mM HEPES–NaOH (pH 7.9), 62 mM KCl, 2.2% (w/v) PVA, 3% (v/v) glycerol, 2 mM DTT, 0.5 mM EDTA, and acetylated BSA (0.3 mg/ml) is added to each reaction equivalent and incubated at 4° for 5 min. Complexes are collected by centrifugation at 16,000 g for 2 min at 4° in a microcentrifuge. Complexes are washed in 1.2 vol of reaction buffer by gentle resuspension in reaction buffer and centrifugation. The final resuspension is in 55 μl of reaction buffer. Three cycles of resuspension and centrifugation are sufficient to reduce nucleotide concentrations to submicromolar levels. SII activity can be followed by assaying the nascent RNA cleavage reaction where SII is mixed with elongation complexes and 7 mM MgCl2 at 28°. The extent of cleavage is a function of SII concentration and incubation time. The propensity of elongation complexes to hydrolyze their RNA chains varies between different elongation complexes, with arrested (SII-dependent) complexes cleaving more rapidly at a given concentration of SII, or requiring lower SII concentrations to achieve half-maximal cleavage, than complexes stalled at other template locations (SII-independent) by nucleotide starvation. Hence, kinetic experiments are required to assess the activity of an SII preparation. Readthrough activity of an SII preparation can be determined using arrested complexes that have not been isolated using the above-described anti-RNA immunoselection or by restoring ribonucleoside triphosphates and MgCl2 to such washed complexes.
Assay of SIII
Transcription reaction conditions are the same as those used for assays of the general initiation factors except that (1) 50 ng of recombinant yeast TATA-binding protein (TBP; AcA 44 fraction)10 replaces TFIID; (2) transcription is initiated by addition of 50 μM ATP, 1 μM UTP, 10 μM CTP, 50 μM GTP, and 10 μCi of [α-32P]CTP; and (3) heparin or Sarkosyl are not added to reaction mixtures. Note that SIII increases the rate of elongation of runoff transcripts, so the optimal length of reaction incubations must be determined by kinetic measurements.
Protein Determination
Protein concentrations are measured using the protein dye assay (Bio-Rad) according to manufacturer instructions. Bovine serum albumin is the standard.
Resolution and Purification of RNA Polymerase II General Transcription Factors
Preparation of Rat Liver Homogenate
Fifty to 60 male Sprague-Dawley rats weighing 200–300 g each are fasted overnight (with water ad libitum) to reduce glycogen stores, which can complicate subcellular fractionation. Rats are anesthetized with ether or carbon dioxide and decapitated using a small rodent guillotine (Harvard Apparatus, South Natick, MA). Livers are rapidly excised and immersed in ice-cold TMSD. We note that failure to excise livers within 1–3 min after decapitation, or freezing livers at this stage, will result in significantly lower yields of general transcription factors.
All further operations are carried out at 4° in a cold room. Livers (~500 g total) are minced into small pieces using scalpels, suspended in TMSD to a final volume of 1400 ml, and homogenized by two passes through a Ziegler–Pettit continuous-flow homogenizer.11
Preparation of Cytosolic and Nuclear Ammonium Sulfate Fractions
Subcellular fractionation of rat liver8 is carried out by a modification of the methods of Fleischer and Kervina.12 The homogenate is distributed to six 250-ml conical bottom polypropylene bottles (Corning, Corning, NY) and centrifuged for 10 min at 2000 rpm (800 g) in a J-6 centrifuge. The supernatants (cytosol) are pooled and centrifuged for 90 min at 13,500 rpm (28,000 g) in a JA-14 rotor. Solid (NH4)2SO4 is then added slowly with stirring to the resulting postlysosomal supernatant to 38% saturation [0.213 g of (NH4)2SO4 per milliliter]. Thirty minutes after the ammonium sulfate has dissolved, the pH is adjusted by addition of NaOH [1 μl of 1 N NaOH per gram of (NH4)2SO4 added], and the suspension is centrifuged for 45 min at 9500 rpm (16,000 g) in a JA-10 rotor. The resulting pellets (cytosolic 0–38% ammonium sulfate fraction), which contain RNA polymerase II, can be quick-frozen in liquid nitrogen and stored at −80° without significant loss of activity. Solid (NH4)2SO4 is then added slowly with stirring to the supernatant to 65% saturation [0.153 g of (NH4)2SO4 per milliliter]. Thirty minutes after the ammonium sulfate has dissolved, the pH is adjusted by addition of NaOH [1 μl of 1 N NaOH per gram of (NH4)2SO4 added], and the suspension is centrifuged for 45 min at 9500 rpm (16,000 g) in a JA-10 rotor. The resulting pellets (cytosolic 40–65% ammonium sulfate fraction), which contain TFIIB, TFIIF, and SII, can be quick-frozen in liquid nitrogen and stored at −80° without significant loss of activity.
The pellets (nuclei) of the first centrifugation step are washed twice more by resuspension in TMSD and centrifugation at 2000 rpm (800 g) in the J-6 centrifuge. Crude nuclei are then resuspended in TMSD to a final volume of 2000 ml and extracted with 0.32 M (NH4)2SO4 by dropwise addition of 175 ml of saturated (NH4)2SO4 with gentle stirring.13 Thirty minutes after addition of ammonium sulfate, the extract is centrifuged for 90 min at 9500 rpm (16,000 g) in a JA-10 rotor. Solid (NH4)2SO4 is then added slowly with stirring to the resulting postnuclear supernatant to 40% saturation [0.186 g of (NH4)2SO4 per milliliter]. Thirty minutes after the ammonium sulfate has dissolved, the pH is adjusted by addition of NaOH [1 μl of 1 N NaOH per gram of (NH4)2SO4 added], and the suspension is centrifuged for 45 min at 9500 rpm (16,000 g) in a JA-10 rotor. The resulting pellets (nuclear 0–40% ammonium sulfate fraction), which contain TFIIA, TFIID, TFIIE, TFIIH, and SIII, can be quick-frozen in liquid nitrogen and stored at −80° for at least 2 years without significant loss of activity.
Purification of TFIIB, TFIIF, and SII
TFIIB,8 TFIIF,14 and SII can be purified to homogeneity from the cytosolic 38–65% ammonium sulfate fraction from as few as 50 rats. Here we describe purification of TFIIB, TFIIF, and SII from 1 kg of rat liver (~100 rats).
The 38–65% ammonium sulfate fraction from ~100 rats is dissolved in 200 ml of buffer A containing leupeptin and antipain at 10 μg/ml each. The solution is dialyzed 2–4 hr against buffer A, diluted with buffer A to a conductivity equivalent to that of buffer A containing 0.2 M KCl, and centrifuged for 10 min at 7000 rpm (7500 g) in a JA-14 rotor. The resulting supernatant is mixed with phosphocellulose (~30 mg of protein per milliliter packed column bed volume) equilibrated in buffer A containing 0.2 M KCl for 1 hr with occasional stirring in a column with the following dimensions: diameter < height < 2× diameter. The slurry is then filtered at one to two packed column volumes per hour and washed at the same flow rate with buffer A containing 0.33 M KCl until the eluate reaches a conductivity equivalent to that of buffer A containing 0.33 M KCl and contains < 50 μg of protein per milliliter. TFIIB, TFIIF, and SII are eluted stepwise from phosphocellulose at the same flow rate with buffer A containing 0.6 M KCl. One-fifth column volume fractions are collected. Note that active fractions can be quick-frozen at this and all subsequent steps if necessary and stored at −80° for at least 2 years without significant loss of activity.
Active fractions from phosphocellulose are dialyzed briefly against buffer A to reduce the concentration of KCl to 0.1–0.3 M. Solid (NH4)2SO4 is then added slowly with stirring to this fraction to 65% saturation [0.4 g of (NH4)2SO4 per milliliter]. Thirty minutes after the ammonium sulfate has dissolved, the pH is adjusted by addition of NaOH [1 μl of 1 N NaOH per gram of (NH4)2SO4 added], and the suspension is centrifuged for 45 min at 13,500 rpm (28,000 g) in a JA-14 rotor. The ammonium sulfate precipitate is then dissolved in 15 ml of buffer A containing leupeptin (10 μg/ml) and antipain (10 μg/ml). The resulting solution is dialyzed in collodion bags against buffer A to a conductivity approximately equivalent to that of 1 M (NH4)2SO4 and then centrifuged for 15 min at 10,000 rpm (12,000 g) in a JA-20 rotor. The resulting supernatant is applied at 35 ml/hr to an Ultrogel AcA 34 column (2.6 × 100 cm) equilibrated in buffer A containing 0.4 M KCl but lacking PMSF. The column is eluted at the same flow rate, and 10-ml fractions are collected.
Purification of TFIIB and SII
Active TFIIB and SII fractions, which elute from AcA 34 with an apparent native molecular mass of ~40 kDa, are applied directly at three packed column volumes per hr to a BioGel HTP column (~1 mg of protein per milliliter packed column bed volume) that is equilibrated in buffer P and that has the following dimensions: diameter < height < 2× diameter. The column is washed at the same flow rate with buffer P containing 150 mM potassium phosphate (pH 7.6) until the eluate contains <50 μg of protein per milliliter. TFIIB and SII are eluted stepwise at the same flow rate with buffer P containing 400 mM potassium phosphate (pH 7.6). One-fifth column volume fractions are collected. Active fractions are diluted with an equal volume of buffer A containing 3.0 M (NH4)2SO4 and centrifuged for 20 min at 35,000 rpm (100,000 g) in a 50 Ti rotor. The supernatant is applied at 1 ml/min to a TSK phenyl-5PW column (7.5 × 75 mm) equilibrated in buffer A containing 1.5 M (NH4)2SO4 but lacking PMSF. TFIIB and SII are eluted at the same flow rate with a 30-ml linear gradient from buffer A containing 1.5 M (NH4)2SO4 to buffer A. One-milliliter fractions are collected. TFIIB elutes from TSK phenyl-5PW at ~0.5 M (NH4)2SO4. Active TFIIB fractions are then rechromatographed on TSK phenyl-5PW under the same conditions. At this stage TFIIB is >95% pure.
Active SII fractions, which elute from TSK phenyl-5PW at ~1.2 M (NH4)2SO4, are dialyzed against buffer A containing 0.1 M KCl but lacking PMSF and applied at 1 ml/min to a TSK SP-5PW column (7.5 × 75 mm) equilibrated in the same buffer. SII is eluted at the same flow with a 30-ml linear gradient from 0.1 to 0.5 M KCl in buffer A lacking PMSF. One-milliliter fractions are collected. SII elutes from TSK SP-5PW at ~0.17 M KCl. At this stage SII is >95% pure.
Purification of TFIIF
Active TFIIF fractions, which elute from AcA 34 near the void volume, are dialyzed against buffer C to a conductivity equivalent to that of buffer C containing 90 mM KCl and centrifuged for 20 min at 30,000 rpm (100,000 g) in a 45 Ti rotor. The resulting supernatant is applied at 5 ml/min to a TSK DEAE-5PW column (21.5 × 150 mm) equilibrated in buffer C containing 90 mM KCl. TFIIF is eluted at the same flow rate with a 500-ml linear gradient from 90 to 320 mM KCl in buffer C. Ten-milliliter fractions are collected. The active fractions, which elute from TSK DEAE-5PW at ~0.2 M KCl, are dialyzed against buffer D to a conductivity equivalent to that of buffer D containing 0.1 M KCl and centrifuged for 20 min at 30,000 rpm (100,000 g) in a 45 Ti rotor. The resulting supernatant is applied at 1 ml/min to a TSK SP-5PW column (7.5 × 75 mm) equilibrated in buffer D containing 0.1 M KCl. TFIIF is eluted at the same flow rate with a 30-ml linear gradient from 0.1 to 0.5 M KCl in buffer D. One-milliliter fractions are collected. The active fractions, which elute from TSK SP-5PW at ~0.2 M KCl, are diluted with an equal volume of buffer E containing 3.0 M (NH4)2SO4 and centrifuged for 20 min at 35,000 rpm (100,000 g) in a 50 Ti rotor. The resulting supernatant is applied at 1 ml/min to a TSK phenyl-5PW column (7.5 × 75 mm) equilibrated in buffer E containing 1.5 M (NH4)2SO4. TFIIF is eluted at the same flow rate with a 30-ml linear gradient from buffer E containing 1.5 M (NH4)2SO4 to buffer E. One-milliliter fractions are collected. At this stage TFIIF is >95% pure.
Purification of TFIIA, TFIID, TFIIE, TFIIH, and SIII
TFIIA,15 TFIID,16 TFIIE,10 TFIIH,13,17 and SIII18 as well as RNA polymerase IIA5 can be purified from the nuclear extract. Here we describe purification of TFIIA, TFIID, TFIIE, TFIIH, and SIII from 1 kg of rat liver (~100 rats).
The nuclear 0–40% ammonium sulfate fraction from ~100 rats is dissolved in ~200 ml of buffer B containing leupeptin and antipain at 10 μ/ml each. The solution is then diluted with buffer B to a conductivity equivalent to that of buffer B containing 0.1 M (NH4)2SO4 and centrifuged for 10 min at 7000 rpm (7500 g) in a JA-14 rotor. The resulting supernatant is mixed with DEAE cellulose (~10 mg of protein per milliliter packed column bed volume) for 1 hr with occasional stirring in a column that is equilibrated in buffer B containing 0.1 M (NH4)2SO4 and that has the following dimensions: diameter < height < 2× diameter. The slurry is then filtered at one to two packed column volumes per hour and washed at the same flow rate with buffer B containing 0.1 M (NH4)2SO4 until the eluate contains < 50 μg of protein per milliliter. The eluate contains TFIID, TFIIH, and SIII. TFIIA, TFIIE, and RNA polymerase IIA are eluted stepwise from DEAE-cellulose at the same flow rate with buffer B containing 0.5 M (NH4)2SO4. One-fifth column volume fractions are collected.
For further purification of TFIID, TFIIH, and SIII, the 0.1 M (NH4)2SO4 eluate from DEAE-cellulose is dialyzed briefly against buffer A to a conductivity equivalent to that of buffer A containing 0.15 M KCl and centrifuged for 10 min at 7000 rpm (7500 g) in a JA-14 rotor. The resulting supernatant is mixed with phosphocellulose (~15 mg of protein per milliliter packed column bed volume) for 1 hr with occasional stirring in a column that is equilibrated in buffer A containing 0.15 M KCl and that has the following dimensions: diameter < height < 2× diameter. The slurry is then filtered at one to two packed column volumes per hour and washed at the same flow rate with buffer A containing 0.15 M KCl until the eluate contains < 50 μg of protein per milliliter. TFIIH is then eluted stepwise from phosphocellulose at the same flow rate with buffer A containing 0.5 M KCl. One-fifth column volume fractions are collected. TFIID and SIII are eluted stepwise at the same flow rate with buffer A containing 1.0 M KCl. One-fifth column volume fractions are collected.
Purification of TFIIH
Active TFIIH fractions, which elute from phosphocellulose with 0.5 M KCl, are diluted with an equal volume of buffer A containing 2.0 M (NH4)2SO4 and centrifuged for 10 min at 7000 rpm (7500 g) in a JA-14 rotor. The supernatant is applied at one to two packed column volumes per hour to a phenyl-Sepharose 6FF column (~15 mg of protein per milliliter packed column bed volume) that is equilibrated in buffer A containing 1.0 M (NH4)2SO4 and that has the following dimensions: height = 2–3× diameter. TFIIH is eluted at the same flow rate with a 10× column volume linear gradient from buffer A containing 1.0 M (NH4)2SO4 to buffer A. One-third column volume fractions are collected. Active fractions, which elute from phenyl-Sepharose at ~0.1 M (NH4)2SO4, are dialyzed against buffer C to a conductivity equivalent to that of buffer C containing 50 mM KCl and centrifuged for 20 min at 30,000 rpm (100,000 g) in a 45 Ti rotor. The resulting supernatant is applied at 5 ml/min to a TSK DEAE-5PW column (21.5 × 150 mm) equilibrated in buffer C containing 50 mM KCl. TFIIH is eluted at the same flow rate with a 500-ml linear gradient from 50 to 300 mM KCl in buffer C. Ten-milliliter fractions are collected. The active fractions, which elute from TSK DEAE-5PW at ~0.2 M KCl, are dialyzed against buffer D to a conductivity equivalent to that of buffer D containing 0.1 M KCl and centrifuged for 20 min at 30,000 rpm (100,000 g) in a 45 Ti rotor. The resulting supernatant is applied at 1 ml/min to a TSK SP-5PW column (7.5 × 75 mm) equilibrated in buffer D containing 0.1 M KCl. TFIIH is eluted at the same flow rate with a 30-ml linear gradient from 0.1 to 0.4 M KCl in buffer D. One-milliliter fractions are collected. At this stage TFIIH, which elutes from TSK SP-5PW at ~0.23 M KCl, is >90% pure.
Purification of TFIID and SIII
Active TFIID and SIII fractions, which elute from phosphocellulose with 1.0 M KCl, are dialyzed against buffer A containing 0.5 M (NH4)2SO4 for 2–3 hr to reduce the concentration of KCl to 0.1–0.3 M. Solid (NH4)2SO4 is then added slowly with stirring to this fraction to ~60% saturation [0.3 g of (NH4)2SO4 per milliliter]. Thirty minutes after the ammonium sulfate has dissolved, the pH is adjusted by addition of NaOH [1 μl of 1 N NaOH per gram of (NH4)2SO4 added], and the suspension is centrifuged for 45 min at 13,500 rpm (28,000 g) in a JA-14 rotor. The ammonium sulfate precipitate is then dissolved in 4–5 ml of buffer G. The resulting solution is dialyzed briefly against buffer G to a conductivity equivalent to that of buffer G containing 0.5 M (NH4)2SO4 and then centrifuged for 20 min at 35,000 rpm (100,000 g) in a 50 Ti rotor. The resulting supernatant is applied at 4 ml/min to a TSK SW4000 column (21.5 × 600 mm) equilibrated in buffer G containing 0.4 M KCl. The column is eluted at the same flow rate, and 5-ml fractions are collected. Active TFIID fractions, which elute from TSK SW4000 with an apparent native molecular mass of ~1300 kDa, are dialyzed against buffer A to a conductivity equivalent to that of buffer A containing 35 mM (NH4)2SO4 and centrifuged for 15 min at 10,000 rpm (12,000 g) in a JA-20 rotor. The resulting supernatant is applied at one or two packed column volumes to a CM-Sephadex (C-25) column (~4 mg of protein per milliliter packed column bed volume) that is equilibrated in buffer A containing 35 mM (NH4)2SO4 and that has the following dimensions: height = 2–3 × diameter. The column is washed at the same flow rate until the eluate contains <50 μg of protein per milliliter. TFIID is eluted stepwise at the same flow rate with buffer A containing 140 mM (NH4)2SO4. One-fifth column volume fractions are collected.
Purification of SIII
Active SIII fractions, which elute from TSK SW4000 with an apparent native molecular mass of ~140 kDa, are diluted with an equal volume of buffer E containing 2.0 M (NH4)2SO4 and centrifuged for 20 min at 30,000 rpm (100,000 g) in a 45 Ti rotor. The resulting supernatant is applied at 5 ml/min to a TSK phenyl-5PW column (21.5 × 150 mm) equilibrated in buffer E containing 1.0 M (NH4)2SO4. SIII is eluted at the same flow rate with a 500-ml linear gradient from buffer E containing 1.0 M (NH4)2SO4 to buffer E. Ten-milliliter fractions are collected. The active fractions, which elute from TSK phenyl-5PW at ~0.4 M (NH4)2SO4, are dialyzed against buffer D containing 50 mM KCl to a conductivity equivalent to that of buffer D containing 100 mM KCl and centrifuged for 20 min at 30,000 rpm (100,000 g) in a 45 Ti rotor. The resulting supernatant is applied at 1 ml/min to a TSK SP-5PW column (7.5 × 75 mm) equilibrated in buffer D containing 100 mM KCl. SIII is eluted at the same flow rate with a 50-ml linear gradient from 100 to 800 mM KCl in buffer D. One-milliliter fractions are collected. SIII elutes at ~350 mM KCl. At this stage SIII is >95% pure.
Purification of TFIIE and TFIIA
For further purification of TFIIE and TFIIA, the 0.5 M (NH4)2SO4 fraction from DEAE cellulose is precipitated with ammonium sulfate. Solid (NH4)2SO4 is added slowly with stirring to this fraction to ~60% saturation [0.35 g of (NH4)2SO4 per milliliter]. Thirty minutes after the ammonium sulfate has dissolved, the pH is adjusted by addition of NaOH [1 μl of 1 N NaOH per gram of (NH4)2SO4 added], and the suspension is centrifuged for 45 min at 13,500 rpm (28,000 g) in a JA-14 rotor. The ammonium sulfate precipitate is then dissolved in 10 ml of buffer A containing leupeptin (10 μg/ml) and antipain (10 μg/ml). The resulting solution is dialyzed in collodion bags against buffer A to a conductivity approximately equivalent to that of 1 M (NH4)2SO4 and then centrifuged for 15 min at 10,000 rpm (12,000 g) in a JA-20 rotor. The resulting supernatant is applied at 35 ml/hr to an Ultrogel AcA 34 column (2.6 × 100 cm) equilibrated in buffer A containing 0.4 M KCl but lacking PMSF. The column is eluted at the same flow rate, and 10-ml fractions are collected.
Purification of TFIIE
Active TFIIE fractions, which elute from AcA 34 near the void volume, are dialyzed against buffer C to a conductivity equivalent to that of buffer C containing 70 mM KCl and then centrifuged for 20 min at 30,000 rpm (100,000 g) in a 45 Ti rotor. The resulting supernatant is applied at 5 ml/min to a TSK DEAE-5PW column (21.5 × 150 mm) equilibrated with buffer C containing 70 M KCl. TFIIE is eluted at the same flow rate with a 500-ml linear gradient from 70 to 375 mM KCl in buffer C. Ten-milliliter fractions are collected. The active fractions, which elute from TSK DEAE-5PW at ~0.2 M KCl, are dialyzed against buffer D to a conductivity equivalent to that of buffer D containing 40 mM KCl and then centrifuged for 20 min at 30,000 rpm (100,000 g) in a 45 Ti rotor. The resulting supernatant is applied at 1 ml/min to a TSK SP-5PW column (7.5 × 75 mm) equilibrated in buffer D containing 40 mM KCl. TFIIE is eluted at the same flow rate with a 40-ml linear gradient from 40 to 400 mM KCl in buffer D. One-milliliter fractions are collected. The active fractions, which elute from TSK SP-5PW at ~250 mM KCl, are diluted with an equal volume of buffer D containing 3.0 M (NH4)2SO4 and then centrifuged for 20 min at 35,000 rpm (100,000 g) in a 50 Ti rotor. The resulting supernatant is applied at 1 ml/min to a TSK phenyl-5PW column (7.5 × 75 mm) equilibrated in buffer D containing 1.5 M (NH4)2SO4. TFIIE is eluted at the same flow rate with a 50-ml linear gradient from buffer D containing 1.5 M (NH4)2SO4 to buffer D. One-milliliter fractions are collected. TFIIE elutes at ~0.3 M (NH4)2SO4. At this stage TFIIE is >90% pure.
Purification of TFIIA
Active TFIIA fractions, which elute from AcA 34 with an apparent native molecular mass of ~160 kDa, are dialyzed against buffer C to a conductivity equivalent to that of buffer C containing 70 mM KCl and then centrifuged for 20 min at 30,000 rpm (100,000 g) in a 45 Ti rotor. The resulting supernatant is applied at 5 ml/min to a TSK DEAE-5PW column (21.5 × 150 mm) equilibrated in buffer C containing 70 mM KCl. TFIIA is eluted at the same flow rate with a 500-ml linear gradient from 70 to 375 mM KCl in buffer C. Ten-milliliter fractions are collected. The active fractions, which elute from TSK DEAE-5PW at ~250 mM KCl, are dialyzed against buffer D to a conductivity equivalent to that of buffer D containing 50 mM KCl and then centrifuged for 20 min at 30,000 rpm (100,000 g) in a 45 Ti rotor. The resulting supernatant is applied at 1 ml/min to a TSK SP-5PW column (7.5 × 75 mm) equilibrated in buffer D containing 50 mM KCl. TFIIA does not bind to TSK SP-5PW under these conditions. The active fractions are diluted with an equal volume of buffer D containing 2.0 M (NH4)2SO4 and then centrifuged for 20 min at 35,000 rpm (100,000 g) in a 50 Ti rotor. The resulting supernatant is applied at 1 ml/min to a TSK phenyl-5PW column (7.5 × 75 mm) equilibrated in buffer D containing 1.0 M (NH4)2SO4. TFIIA is eluted at the same flow rate with a 30-ml linear gradient from buffer D containing 1.0 M (NH4)2SO4 to buffer D. One-milliliter fractions are collected. The active fractions, which elute from TSK phenyl-5PW at ~0.2 M (NH4)2SO4, are dialyzed in collodion bags against buffer I to a conductivity equivalent to that of buffer I and then centrifuged for 20 min at 35,000 rpm (100,000 g) in a 50 Ti rotor. The resulting supernatant is applied at 0.5 ml/min to a BioGel HPHT column (7.8 × 100 mm) equilibrated in buffer I. TFIIA is eluted at the same flow rate with a 27-ml linear gradient from 10 to 600 mM potassium phosphate in buffer I. One-milliliter fractions are collected. TFIIA elutes at ~0.2 M potassium phosphate.
Fig. 1.
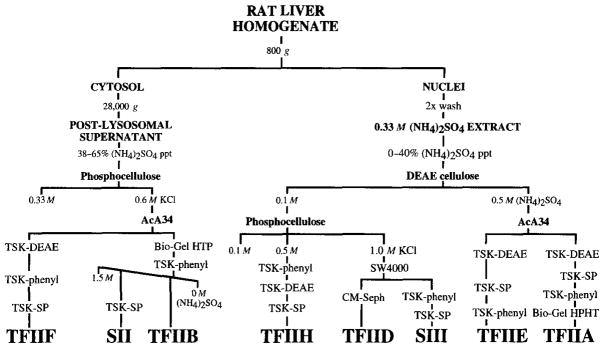
Resolution and purification of RNA polymerase II general transcription factors from rat liver. TFIIF (rat βγ)14; TFIIB (rat α)8; TFIIH (rat δ)13; TFIID (rat τ)16; SII18; TFIIE (rat ε)10; TFIIA15; SIII (Elongin).17
Fig. 2.
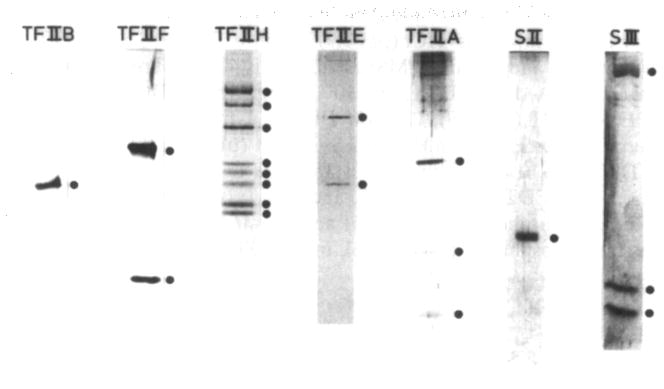
Sodium dodecyl sulfate-polyacrylamide gel analysis of RNA polyacrylamide gel analysis of RNA polymerase II general transcription factors from rat liver. Subunits of purified general transcription factors are indicated by black circles.
Acknowledgments
Work in the authors’ laboratories is supported by NIH Grant GM41628 and funds provided to the Oklahoma Medical Research Foundation by the H. A. and Mary K. Chapman Charitable Trust (J.W.C. and R.C.C) and by NIH Grant GM46331 and American Cancer Society Grant JFRA-394 (D.R).
References
- 1.Conaway RC, Conaway JW. Annu Rev Biochem. 1993;62:161. doi: 10.1146/annurev.bi.62.070193.001113. [DOI] [PubMed] [Google Scholar]
- 2.Kane CM. In: Transcription: Mechanisms and Regulation. Conaway RC, Conaway JW, editors. Raven; New York: 1994. p. 279. [Google Scholar]
- 3.Kerppola TK, Kane CM. FASEB J. 1991;5:2833. doi: 10.1096/fasebj.5.13.1916107. [DOI] [PubMed] [Google Scholar]
- 4.Conaway RC, Conaway JW. J Biol Chem. 1988;263:2962. [PubMed] [Google Scholar]
- 5.Serizawa H, Conaway RC, Conaway JW. Proc Natl Acad Sci USA. 1992;89:7476. doi: 10.1073/pnas.89.16.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reines D. In: Transcription: Mechanisms and Regulation. Conaway RC, Conaway JW, editors. Raven; New York: 1994. p. 263. [Google Scholar]
- 7.Reines D, Chamberlin MJ, Kane CM. J Biol Chem. 1989;264:10799. [PubMed] [Google Scholar]
- 8.Conaway JW, Bond MW, Conaway RC. J Biol Chem. 1987;262:8293. [PubMed] [Google Scholar]
- 9.Eilat D, Hochberg M, Fischel R, Laskov R. Proc Natl Acad Sci USA. 1982;79:3818. doi: 10.1073/pnas.79.12.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conaway JW, Hanley JP, Garrett KP, Conaway RC. J Biol Chem. 1991;266:7804. [PubMed] [Google Scholar]
- 11.Ziegler DM, Pettit FH. Biochemistry. 1966;5:2932. doi: 10.1021/bi00873a024. [DOI] [PubMed] [Google Scholar]
- 12.Fleischer S, Kervina M. Methods Enzymol. 1974;XXXI:6. doi: 10.1016/0076-6879(74)31005-1. [DOI] [PubMed] [Google Scholar]
- 13.Conaway RC, Conaway JW. Proc Natl Acad Sci USA. 1989;86:7356. doi: 10.1073/pnas.86.19.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conaway JW, Conaway RC. J Biol Chem. 1989;264:2357. [PubMed] [Google Scholar]
- 15.Aso T, Serizawa H, Conway RC, Conaway JW. EMBO J. 1994;13:435. doi: 10.1002/j.1460-2075.1994.tb06278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conaway JW, Reines D, Conaway RC. J Biol Chem. 1990;265:7552. [PMC free article] [PubMed] [Google Scholar]
- 17.Conaway JW, Bradsher JN, Conaway RC. J Biol Chem. 1992;267:10142. [PubMed] [Google Scholar]
- 18.Bradsher JN, Jackson KW, Conaway RC, Conaway JW. J Biol Chem. 1993;268:25587. [PubMed] [Google Scholar]


