Abstract
Many neurodegenerative diseases demonstrate abnormal mitochondrial morphology and biochemical dysfunction. Alterations are often systemic rather than brain-limited. Mitochondrial dysfunction may arise as a consequence of abnormal mitochondrial DNA, mutated nuclear proteins that interact directly or indirectly with mitochondria, or through unknown causes. In most cases it is unclear where mitochondria sit in relation to the overall disease cascades that ultimately causes neuronal dysfunction and death, and there is still controversy regarding the question of whether mitochondrial dysfunction is a necessary step in neurodegeneration. In this chapter we highlight and catalogue mitochondrial perturbations in some of the major neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD). We consider data that suggest mitochondria may be critically involved in neurodegenerative disease neurodegeneration cascades.
Keywords: cybrid, mitochondria, mitochondrial DNA, neurodegenerative disease
1. The quintessential neurodegenerative diseases
Neurodegenerative diseases are characterized by gradually progressive, selective loss of anatomically or physiologically related neuronal systems. The clinical syndromes associated with particular neuroanatomical patterns of cell dysfunction and loss are typically categorized by whether they initially affect cognition, movement, strength, coordination, sensation, vision, or autonomic control. Prototypical examples include AD, PD, ALS, and HD. HD is strictly an autosomal dominant disorder. With AD, PD, and ALS most cases are age-related and show sporadic epidemiology, although rare Mendelian variants do occur. As life expectancy continues to advance in developed countries the incidence of these disorders increases and will continue to do so.
Mitochondrial dysfunction is a common theme in these diseases. Mitochondria are known to play a central role in many cell functions including ATP generation, intracellular Ca2+ homeostasis, reactive oxygen species (ROS) formation, and apoptosis. Neurons are particularly dependent on mitochondria because of their high energy demands. It seems reasonable to hypothesize neurons are relatively intolerant of mitochondrial dysfunction. This assumption is supported by the fact that maternally inherited diseases with known homoplasmic or near-homoplasmic mitochondrial DNA (mtDNA) mutations tend to affect the central nervous system and muscle, the body’s two most aerobic tissues.
2. Alzheimer’s Disease
AD is the most common neurodegenerative disease and the most frequent cause of dementia. By far the greatest risk factor for AD is ageing, and approximately one in ten persons over 65 and nearly half of those over 85 have AD (Antuono and Beyer, 1999). With such high prevalence rates among the oldest old it is difficult to not consider AD pathology from outside the context of aging itself (Swerdlow, 2007a).
AD can be divided into early versus late onset forms as well as sporadic and autosomal-dominant variants. Autosomal dominant AD represents the minority of AD cases and typically presents before the age of 65. It is caused by mutations in genes encoding for either the amyloid precursor protein (APP), presenilin 1 (PS1), or presenilin (PS2), and these mutations appear to alter processing of APP towards the 42 amino acid beta amyloid (Aβ) derivative (Scheuner et al., 1996). Aβ is the major constituent of amyloid plaques observed in particular brain regions of AD patients, including neocortex, hippocampus, and other subcortical regions essential for cognitive function. In 1992 the “amyloid cascade hypothesis” was proposed (Hardy and Higgins, 1992). This hypothesis states altered processing of APP or changes in Aβ stability result in a chronic imbalance between Aβ production and clearance. Gradual accumulation of aggregated Aβ initiates a complex, multistep process that includes gliosis, inflammatory changes, neuritic/synaptic change, neurofibrillary tangles, reductions in neurotransmitters, and finally neurodegeneration and neuronal cell death.
However, it is not quite clear how Aβ might induce neurodegeneration. One possible mechanism is that Aβ interferes with mitochondrial function. When maintained in the presence of Aβ, isolated mitochondria show diminished respiratory capacity in general, and specifically inhibition of several key enzymes including cytochrome oxidase, α-ketoglutarate dehydrogenase, and pyruvate dehydrogenase (Pereira et al., 1998; Canevari et al., 1999; Casley et al., 2002). Brief exposure of cultured rat hippocampal neurons to sub-lethal Aβ concentrations resulted in rapid and severe impairment of mitochondrial transport without inducing apparent cell death (Rui et al., 2006). At concentrations insufficient to kill cells, Aβ appears to induce an increase in mitochondrial DNA (mtDNA) levels and reduces the number of normal appearing mitochondria (Diana et al., 2008). Cells depleted of endogenous mtDNA (ρ0) cells, which lack functional electron transport chains (ETC), are impervious to Aβ (Cardoso et al., 2001). A further study reports a positive correlation between levels of soluble Aβ and hydrogen peroxide in brain mitochondria isolated from APP transgenic mice (Manczak et al., 2006), which supports the view that mutant APP or soluble Aβ impairs mitochondrial metabolism. Physical associations between mitochondria and APP as well as between mitochondria and Aβ have been reported in transgenic mice (Manczak et al., 2006). Aβ binds to a mitochondrial protein called Aβ-binding alcohol dehydrogenase (ABAD), and it has been demonstrated that blocking the interaction of Aβ and ABAD can suppress Aβ-induced apoptosis and free-radical generation in neurons (Lustbader et al., 2004). These physical associations have also been supported by human AD studies (Lustbader et al., 2004; Anandatheerthavarada et al., 2003; Crouch et al., 2005; Caspersen et al., 2005; Devi et al., 2006). Physical associations between PS1 and mitochondria are also reported (Hansson et al., 2004).
Besides functional changes, extensive literature indicates mitochondrial structural dynamics are also altered in AD patients. Quantitative ultrastructural morphometric analysis shows that compared to age-matched control group brains AD brains contain a significantly lower percentage of normal mitochondria (de la Monte et al., 2000) and a significantly higher percentage of mitochondria with broken cristae (Hirai et al., 2001). Also, in fibroblasts from sporadic AD patients mitochondria are longer, with two or more mitochondria often joined together, while those of age-matched normal human fibroblasts are much shorter and appear sausage-shaped or rounded (Wang et al., 2008a). Similar morphological changes are also found in neurons over-expressing wild-type APP. APP over-expressing cells actually show mitochondria with heterogeneous morphologies; approximately 50% of cells contain fragmented, punctiform mitochondria and the mitochondria in some cells show elongated, net-like structures (Wang et al., 2008b).
It is known that the activities of several mitochondrial enzymes including complex IV (cytochrome c oxidase; COX), pyruvate dehydrogenase complex, and α-ketoglutarate dehydrogenase complex are reduced in AD (Swerdlow and Kish, 2002). COX is the last enzyme in the respiratory ETC of mitochondria and receives electrons from cytochrome c. It contains several metal prosthetic sites and 13 protein subunits of which ten are encoded by nuclear and three by mtDNA genes. In 1990, deficient COX activity was found in platelets of AD patients. A similar finding was made in AD brains in 1992 (Parker et al., 1990a; Kish et al., 1992). Subsequently, the finding of reduced COX activity in AD patients has been replicated in platelets (Parker et al., 1994; Bosetti et al., 2002; Cardoso et al., 2004a), fibroblasts (Curti et al., 1997), focal brain regions (Bosetti et al., 2002), and large parts of the brain (Mutisya et al., 1994; Wong-Riley et al., 1997). These reports indicate mitochondrial dysfunction occurs in AD and that AD mitochondrial dysfunction is systemic rather than brain-limited.
COX reduction has also been reported at all stages of the disease, including mild cognitive impairment (MCI) (Swerdlow and Kish, 2002; Valla et al., 2006). APP transgenic mice also develop early signs of mitochondrial perturbation; expression of mitochondrial genes is altered when these mice are only two months old, which precedes by months the appearance of cognitive signs (Manczak et al., 2006).
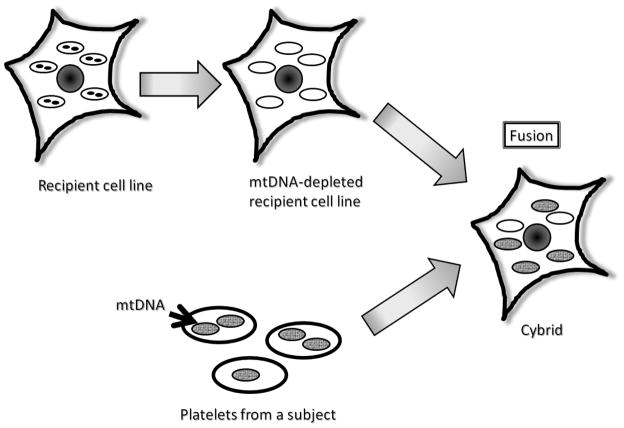
Cytoplasmic hybrid (cybrid) studies suggest mtDNA is at least partly responsible for the reduced activity of COX in AD patients (Swerdlow et al., 1997). A diagram that provides an overview of the cybrid technique is shown in Figure 1. When platelet mtDNA from AD patients is expressed within neuronal cell lines grown in culture (cytoplasmic hybrid cell lines, or cybrids), the resulting cells continue to manifest reduced COX activity and this specific biochemical defect persists over time in the cybrid lines (Swerdlow et al., 1997; Swerdlow, 2007b). It also has been observed that AD cybrid cell lines containing AD subject mitochondria/mtDNA overproduce free radicals, accumulate Aβ, and have decreased ATP levels (Swerdlow, 2007b; Khan et al., 2000; Cardoso et al., 2004b). Since three of the 13 COX subunits are encoded by mtDNA, this phenomenon suggests mtDNA differs between AD patients and control subjects, and indirectly supports the view that mtDNA contributes to the AD-associated COX activity reduction.
Figure 1. The cybrid technique.
The black circles represent nuclei in parental cells. The ovals represent mitochondria. The black dots within the ovals represent mitochondrial DNA.
It remains unclear how mtDNA from AD subjects specifically differs from that of control subjects. Several studies show oxidative modification of both nuclear DNA and mtDNA are increased in AD brains (Gabbita et al., 1998; Mecocci et al., 1994; Wang et al., 2005). Levels of 8-hydroxy-2-deoxyguanosine (8-OHdG) are widely considered to reflect levels of oxidative stress (Valavanidis et al., 2009), and mtDNA 8-OHdG is increased in AD patient cortical brain regions (Mecocci et al., 1994). It is known that mtDNA with large mtDNA deletions (including a 4977 base-pair deletion that involves mtDNA cytochrome oxidase subunit genes) preferentially accumulates in human AD brains compared to control aged brain (Corral-Debrinski et al., 1994; Hambleta and Castora, 1997) and the frequency of point mutations are also higher in several brain regions including parietal gyrus, hippocampus, and cerebellum of AD subjects (Chang et al., 2000). Although AD mtDNA sequences contain a higher number of substitutions in tRNA genes, without a corresponding biochemical analysis it is hard to know whether these mtDNA mutations constitute a major etiological factor in sporadic AD (Elson et al., 2006).
Mitochondrial genes contain frequent polymorphic variations, and mtDNA gene products function in the context of nuclear-encoded proteins that also contain polymorphic variations. It is possible that polymorphism-defined ETC subunit combinations do not function identically. If so, this could explain why epidemiologic associations between mtDNA polymorphisms and AD risk are difficult to establish (Swerdlow and Kish, 2002).
Although clear mtDNA features contributing to the pathogenesis of AD are still not known, the possibility that maternal mitochondrial inheritance may influence disease risk and pathology has been considered. While several studies actually conclude there is no evidence of a maternal effect in AD, or even that there is predominant paternal transmission (Ehrenkrantz et al., 1999; Payami and Hoffbuhr, 1993), other epidemiological studies find maternal inheritance strongly influences AD risk (Duara et al., 1993; Edland et al., 1996). Among AD patients with one affected parent, the ratio of mothers to fathers affected is 3:1. For cases in which affected proband relations include one affected parent and at least sibling, the mother to father ratio increases to 9:1 (Edland et al., 1996). Recently, a genetic study indentified new possible regions of linkage on chromosome 10 and 12 only among families with maternal transmission of late-onset AD (Bassett et al., 2002). Brain imaging techniques also provide evidence of maternal transmission of AD risk. Positron emission tomography (PET) imaging, when using 2-[18F] fluoro-2-deoxy-D-glucose (FDG) as the tracer, can be used to determine the cerebral metabolic rate of glucose (CMRglc). It has been demonstrated that in AD patients, CMRglc is reduced in several neuroanatomic areas including the parietotemporal, posterior cingulate, and to a smaller extent frontal cortex and medial temporal lobe regions (Mosconi, 2005). These reductions occur years before AD symptom onset. One FDG-PET study reported that cognitively intact subjects (aged from 46–80) with AD mothers but not AD fathers had AD-like patterns of CMRglc reduction even after accounting for other possible AD risk factors (Mosconi et al., 2007; Mosconi et al., 2009).
The amyloid cascade hypothesis, which assumes AD is always a primary amyloidosis, has dominated thinking in the AD research field for decades but other etiologic hypotheses have been formulated. The “mitochondrial cascade hypothesis” was proposed in 2004 (Swerdlow and Khan, 2004). In the mitochondrial cascade hypothesis, mitochondria sit at the apex of AD histopathology and neurodegeneration. It assumes AD mitochondrial dysfunction drives amyloidosis, tau phosphorylation, and cell cycle re-entry (Swerdlow and Khan, 2009; Swerdlow, 2007c). As mentioned above, since AD mitochondrial dysfunction is systemic altered mitochondrial function in AD cannot simply represent a consequence of neurodegeneration. Although many investigators believe that mitochondrial dysfunction is a downstream event in the development of AD and may play a minor role in the disease, the results of several studies including cell culture and transgenic mouse studies support that brain mitochondrial bioenergetic defects (such as oxidative damage, COX activity, oxygen consumption, and H2O2 production) precedes or drives Aβ production/deposition and plaque formation (Khan et al., 2000; Manczak et al., 2006; Praticò et al., 2001; Yao et al., 2009). The mitochondrial cascade hypothesis also takes aging phenomena into account. It postulates inheritance determines mitochondrial baseline function and durability, which in turn influences how mitochondria change with age. It is presumed more durable mitochondria adequately function for more decades than less durable mitochondria. When mitochondrial change reaches a threshold and bioenergetic homeostasis can no longer be maintained, AD histopathology and symptoms may ensue (Swerdlow, 2007c).
In summary, mounting evidence indicates altered mitochondrial function associates with AD. If mitochondrial dysfunction is critical for the initiation and progression of AD, the susceptibility of mitochondrial to environmental and genetic risk factors should play a role in the development of AD and mitochondria need to be considered in late-onset, sporadic AD prevention and treatment development efforts.
3. Parkinson’s Disease
PD is the most common neurodegenerative movement disorder. It affects ~1% of the population above the age of 60 (Abou-Sleiman et al., 2006) and 1–3% of those over 80 years of age (Tanner and Goldman, 1996). PD is clinically characterized by rigidity, resting tremor, bradykinesia and postural instability. The key symptoms and signs arise from a preferential loss of dopaminergic neurons of the substantia nigra pars compacta, although early neurodegenration also occurs in other discrete brainstem and basal forebrain nuclei. Another hallmark is that surviving nigral neurons may contain Lewy bodies, intracytoplasmic inclusions that are mainly composed of fibrillar α-synuclein protein (Spillantini et al., 1997). The presence of nigral Lewy Bodies establishes the histological diagnosis of PD.
Like AD, PD is clinically partitioned into early versus late onset variants and Mendelian versus non-Mendelian forms. With advancing age the percentage of cases caused by Mendelian gene mutations declines. Most PD (~90%) is sporadic and does not show Mendelian inheritance (Trimmer and Bennett, 2009).
Mitochondrial dysfunction has long been implicated in the pathogenesis of PD. Evidence first emerged in the 1980’s that drug abusers developed an acute and irreversible parkinsonian syndrome after using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). The active metabolite of MPTP, 1-methyl-4-phenylpyridinium (MPP+), is transported intracellularly by the dopamine transporter (DAT). Perhaps because of DAT uptake it accumulates in dopaminergic neurons and inhibits complex I (Nicklas et al., 1985). MPP+-induced complex I inhibition further leads to increased free radical production/oxidative stress, decreased ATP production, increased intracellular calcium concentration, excitotoxicity, nitric oxide-related cellular damage, and ultimately the death of dopaminergic neurons (Beal, 1998; Hantraye et al., 1996; Mizuno et al., 1988; Ng et al., 1996; Sheehan et al., 1997; Smith et al., 1994; Ali et al., 1994). MPTP has been extensively for PD cell culture and animal modeling.
In 1989, several groups reported that Complex I activity was reduced in the substantia nigra, platelets, and skeletal muscle of patients with idiopathic PD (Parker et al., 1989; Schapira et al., 1989; Bindoff et al., 1989). Since then altered complex I activity was also reported in fibroblasts and frontal cortex (Mytilineou et al., 1994; Parker et al., 2008 ). It has been hypothesized that this PD systemic complex I activity may be a consequence of exposure to exogenous inhibitors, systemic endogenous production of an inhibitory factor, or mtDNA-encoding of Complex I subunits (Swerdlow, 2000). Data supporting all of these possibilities are published. For example, the complex I inhibitor rotenone has been used to model PD in rats. Rats administered rotenone develop a PD-like syndrome characterized by loss of substantia nigra neurons and the formation of α-synuclein-rich inclusion bodies (Betarbet et al., 2000; Cannon et al., 2009).
Several nuclear gene mutations associated with autosomal dominant and recessive forms of Mendelian PD have also been identified (Table 1). Examples of such genes are α-synuclein, Parkin, phosphate and tensin homologue-induced kinase 1 (PINK1), DJ1, leucine-rich repeat kinase 2 (LRRK2), and Htr A serine peptidase 2 (HTRA2). Genetically modified organisms based on knock-out, over-expression, or mutant versions of these genes have since been generated for purposes of PD animal modeling. Interestingly, many of these nuclear genes also implicate a role for mitochondria in PD pathogenesis.
Table 1.
Interactions between mitochondria and proteins encoded by genes that are mutated in Mendelian Parkinson’s Disease.
| Locus | Gene product | Inheritance & comments | Direct or indirect interaction with mitochondria |
|---|---|---|---|
| PARK1/4 | α-Synuclein | AD | Mutant α-synuclein sensitizes neurons to oxidative stress and damage. |
| PARK2 | Parkin | AR, most common cause of recessive juvenile PD | Parkin mutations lead to increased oxidative stress and in turn mitochondrial dysfunction can affect parkin function. |
| PARK6 | PINK1 | AR, second most common cause of recessive juvenile PD | A mitochondria-localized kinase; its deficiency sensitizes mitochondria to rotenone and induces degeneration of dopaminergic neurons. |
| PARK7 | DJ-1 | AR | A possible redox sensor; binds to mitochondrial complex I and maintain its activity. |
| PARK8 | LRRK2 | AD, most common cause of dominant PD | Associates with the outer mitochondrial membrane and can bind parkin. |
| PARK13 | OMI/HTRA2 | * AD? | A mitochondrial protease; acts downstream of PINK1; loss of HtrA2 results in the accumulation of unfolded proteins in the mitochondria and increased production of ROS. |
AD=autosomal dominant; AR=autosomal recessive.
Not uniformly accepted.
In transgenic mice over-expressing α-synuclein, mitochondrial function is impaired, oxidative stress increases, and in the face of complex I inhibitors the threshold for nigral degeneration is reduced (Song et al., 2004). In another study of mice that over-express mutant α-synuclein, α–synuclein immunostaining suggested this protein directly affects mitochondria (Martin et al., 2006).
Parkin, a ubiquitin ligase, is believed to protect neuron mitochondria (Palacino et al., 2004). It has been reported in drosophila and mouse models that parkin deficiency or mutations lead to increased oxidative stress and mitochondrial impairment (Palacino et al., 2004; Pesah et al., 2004). It is important to note that mitochondrial dysfunction and oxidative stress also affect parkin function and exacerbate the consequences of parkin mutations (Chung et al., 2004).
PINK1, a mitochondria-localized kinase, appears to protect against cell death (Silvestri et al., 2005). This protective effect is abrogated by PD-related mutations that disable its kinase function (Petit et al., 2005). PINK deficiency increases the sensitivity of mitochondria to rotenone and induces degeneration of dopaminergic neurons in drosophila (Yang et al., 2006). These reports and others provide strong evidence that mitochondrial dysfunction plays an important role in the pathogenesis of Mendelian PD, and are consistent with an important role for mitochondrial function in sporadic PD.
As mentioned above, reduced complex I activity is a systemic event in PD. Complex I is a large multimeric enzyme containing 46 known protein subunits. At least seven of these subunits are encoded by genes on mtDNA. Because mtDNA makes such an important contribution to the structure and function of complex I and mtDNA abnormalities can produce sporadic disease, in 1989 it was hypothesized that mtDNA alteration might constitute a key risk factor for the development of idiopathic PD (Parker et al., 1989). An early study found levels of the common mtDNA deletion were increased in PD brains, but this study did not use age-matched controls (Ikebe et al., 1990). Other studies using DNA isolated from brain homogenates found that relative to age-matched controls, mtDNA deletions were not increased (Schapira et al., 1990; Lestienne et al., 1991). More recently it was shown that mtDNA deletion burdens increase with advancing age and are further increased in nigral neurons from PD subjects (Bender et al., 2006; Kraytsberg et al., 2006).
Multiple groups have used the cybrid technique to show transfer of mitochondria and mtDNA from sporadic PD subject platelets produces cell lines with persistently reduced complex I activity(Swerdlow et al., 1996a; Gu et al., 1998; Esteves et al., 2008; Esteves et al., 2010). PD cybrid cell lines also have increased reactive oxygen species production, reduced mitochondrial calcium storage, less ATP production, depolarized mitochondria, and higher caspase 3 activity. PD cybrid cell lines generate Lewybody-like inclusions without the need from exogenous protein expression or toxin-mediated inhibition of mitochondrial or proteasomal function (Trimmer et al., 2004). Mitochondrial respiration and pathways influenced by aerobic metabolism are also altered in PD cybrid cell lines. A recent study reported PD cybrid mitochondria have an increased proton leak and decreased respiratory reserve capacity. In these cybrid cell lines levels of the transcriptional co-activator PGC1-α, which coordinates mitochondrial biogenesis, were reduced (Esteves et al., 2010).
Although the actual mtDNA alterations that account for these findings are still unknown, these results strongly suggest mtDNA contributes to reduced complex I activity in sporadic PD. This mtDNA contribution could derive from inherited or somatic mtDNA mutations. Several lines of investigation support a role for mtDNA inheritance. Epidemiologic studies suggest for non-Mendelian cases who nevertheless have a PD-affected parent, the affected parent is more likely to be the mother (Wooten et al., 1997; Swerdlow et al., 2001). Mitochondrial haplogroup and polymorphism association studies demonstrate mtDNA variations alter PD risk as well (Swerdlow, 2000; van der Walt et al., 2003). The systemic nature of the PD complex I defect, in conjunction with the fact that expression of PD subject platelet mtDNA probably accounts for the results of PD cybrid studies, also suggests mtDNA inheritance is more likely to play a key role than somatic mutation acquisition (Swerdlow, 2009).
As discussed above, the use of PD tissues and a number of experimental PD models has contributed to our recognition and understanding of how mitochondria are important to PD pathogenesis. In vivo human studies also contribute to this knowledge base. Proton and phosphorus magnetic resonance spectroscopy (1H and 31P MRS) are powerful, noninvasive techniques that facilitate quantitative in vivo measurements of metabolism pathway intermediates. 31P MRS allows quantitative measurements of high energy phosphates such as adenosine triphosphate and phosphocreatine, and can be used to provide an indication of brain energy stores (Henchcliffe et al., 2008). One study using these techniques found high-energy phosphates were reduced in the putamen and midbrain of both early and advanced PD patient groups (Hattingen et al., 2009).
Most would agree mitochondria play an important role in PD pathogenesis. Abundant evidence supports this view. Although identifying the actual mtDNA features that associate with sporadic PD warrants further investigation, at this point targeting mitochondrial function in PD treatment development efforts is well-justified.
4. Amyotrophic lateral sclerosis
ALS is a neurodegenerative disease that primarily affects strength. It is characterized by upper and lower motor neuron degeneration. Weakness and muscle atrophy usually begin asymmetrically and distally in a single limb, spreads within the neuro-axis to involve contiguous muscle groups innervated by nearby motor neurons, and eventually also affects more rostral motor neurons. Approximately 10% of ALS cases are familial and the rest are sporadic. Similar to AD and PD, the incidence of ALS increases with increasing age, and the older the age of onset the less likely Mendelian inheritance is responsible. Among familial cases, the most common mutations occur in the copper-zinc superoxide dismutase (SOD1) gene on chromosome 21. SOD1 mutations account for about 20% of the familial cases and 2% of all cases. More recently, mutations in two RNA processing proteins, TDP-43 and FUS/TLW(Kabashi et al., 2008; Sreedharan et al., 2008; Vance et al., 2009; Kwiatkowski et al., 2009), have also been found in kindreds with familial ALS variants.
Mitochondrial alterations have been described in sporadic ALS as well as in models of familial ALS. Mitochondrial morphological changes, such as bizarre giant mitochondria and spiny or stubby mitochondria, are found at greater than normal frequencies (Hirano et al., 1984; Masui et al., 1985; Nakano et al., 1987). Abnormal mitochondria accumulate in the axon hillock and initial segment of axons (Sasaki and Iwata, 1996). Changes are observed in both neural and non-neural tissues (Swerdlow et al., 2000). Changes in mitochondrial electron transport chain activities have been noted by several groups using biopsies from patients with ALS and animal models of ALS. While the overall results of many different studies support the overall view that mitochondria and mitochondrial function are altered in ALS, particular results from these studies are not homogeneous. In one study, complex I activity was increased in postmortem brain tissue from a patient with familial ALS (Bowling et al., 1993). Reduced complex IV activity was shown in patients with sporadic ALS (Fujita et al., 1996). Complex I and II–III deficiencies were observed in patients with familial ALS due to SOD1 mutations and also in an SOD1 transgenic mouse model (Browne et al., 1998).
When the cybrid technique was used to study the function of mitochondria obtained from ALS subject platelets, ALS cybrids produced on a neuroblastoma nuclear background showed a significant reduction in complex I activity and non-significant trends towards reduced complex III and IV activities (Swerdlow et al., 1996b; Swerdlow et al., 1998). In another study that used spinal cord tissue from patients with ALS, it was reported that activity of citrate synthase, which is often used as a marker of mitochondrial mass, was significantly lower than it was in control subjects. Along with the decreased activities of respiratory chain complexes I + III, II+ III, and IV this paper reported, low citrate synthase activity suggests there is a loss of mitochondria from spinal cords of ALS patients (Wiedemann et al., 2002).
Cell ROS levels may increase when mitochondrial respiration is impaired, although ROS itself may impair mitochondrial function (Bacman et al., 2006). There is certainly abundant evidence that indicates oxidative stress in increased in ALS. In sporadic ALS cases both lipid and protein oxidation are enhanced in spinal cord motor neurons and glia (Shibata et al., 2001). Also, the percentage of oxidized CoQ10 in sporadic ALS subject cerebrospinal fluid exceeds that of age-matched controls and positively correlates with illness duration (Murata et al., 2008). Markers of immune system activation are significantly elevated in ALS postmortem CNS tissue (Simpson et al., 2004), and increased blood ROS and lactate production levels suggests a close relationship between mitochondrial function and oxidative stress in ALS (Siciliano et al., 2002). Some propose oxidation-induced DNA damage contributes to sporadic ALS pathogenesis (Murata et al., 2008).
As to whether alterations in mtDNA are associated with ALS, diminished levels of mtDNA were observed in skeletal muscle of patients with sporadic ALS (Vielhaber et al., 2000). Mitochondrial DNA haplogroups also appear to influence ALS risk (Mancuso et al., 2004). Other studies suggest levels of the 4977-base pair mtDNA common deletion are elevated in sporadic ALS (Ro et al., 2003; Dhaliwal and Grewal, 2000). Since correlation does not establish causality, though, further investigation is needed to determine whether mtDNA somatic mutations play a causal role in sporadic ALS or are merely a byproduct of upstream events.
Most ALS laboratory modeling is accomplished using transgenic rodents that express an ALS-associated SOD1 mutation. The SOD1 gene was the first gene recognized to cause autosomal dominant ALS, and more than 100 different mutations have been mapped to it (Bacman et al., 2006). SOD1 protein functions as a ubiquitous antioxidant enzyme that catalyzes the dismutation of superoxide radicals to hydrogen peroxide, which can be converted to molecular oxygen by additional antioxidant enzymes such as catalase and glutathione peroxidase. It localizes predominantly to the cytoplasm, but both wild type and mutant SOD1 protein have been found in the intermembrane space, matrix and outer membrane of mitochondria of ALS-affected tissues (Higgins et al., 2002; Velde et al., 2008; Vijayvergiya et al., 2005; Liu et al., 2004). It is postulated that mutant SOD1 accumulates and aggregates in the outer mitochondrial membrane, that this impairs mitochondrial protein import, and disrupting mitochondrial protein import perturbs mitochondrial function (Liu et al., 2004).
Extensive mitochondrial fragmentation occurs in cell models of mutant SOD1 overexpression (Raimondi et al., 2006; Menzies et al., 2002). Mitochondrial vacuolation is another abnormal morphologic feature characteristic of SOD1 ALS models. This is seen in spinal motor neurons from these mice, and it occurs in conjunction with expansion of the intermembrane space and the mitochondrial outer membrane (Higgins et al., 2003). A transient explosive increase in vacuoles is observed in mutant SOD1-expressing transgenic mice just prior to motor neuron demise (Kong and Xu, 1998), which suggests mitochondrial dysfunction may trigger ALS cell death cascades.
SOD1-induced mitochondrial membrane damage discharges the mitochondrial membrane potential, impairs mitochondrial respiration, and reduces the ability of mitochondria to buffer cytosolic calcium (Borthwick et al., 1999; Jung et al., 2002; Carri et al., 1997). In SOD1 mice these changes precede the onset of motor signs (Damiano et al., 2006).
Substantial evidence suggests mitochondrial dysfunction plays a crucial role in ALS motor neuron degeneration. Where mitochondrial dysfunction sits in the ALS pathologic cascade is unclear and where mitochondria sit in the degeneration cascade hierarchy Mendelian and sporadic ALS may differ. In the Mendelian forms mitochondrial dysfunction certainly must occur downstream of the causative mutation, but even in Mendelian ALS mitochondrial dysfunction may play a fairly upstream role. In sporadic ALS it is possible that mitochondrial dysfunction occupies the apex of the ALS pathology pyramid, but this remains unproven (Beal, 1995).
5. Huntington’s Disease
HD is a degenerative movement disorder clinically characterized by choreiform movements, psychiatric disturbances, and dementia. Symptoms may develop in childhood or young adulthood but usually manifest in middle age. Clinical changes reflect neuron dysfunction and loss that preferentially affects GABAergic medium spiny striatal neurons (Vonsattel and DiFiglia, 1998). The disease becomes less neuroanatomically specific during later stages as it extends to other brain regions. HD is strictly an autosomal dominant disorder and it is caused by a CAG triplet repeat expansion (>35 CAGs) in the first exon of the Huntingtin (HTT) gene on chromosome 4 (Huntington’s Disease Collaborative Research Group, 1993).
Impaired cell energy production and metabolism in HD were recognized before the responsible gene mutation was identified. Energy metabolism-related deficits were predicted in the early 1980’s following observations of excessive weight loss and deficient brain FDG uptake on PET (Sanberg et al., 1981; Kuhl et al., 1982). In the early 1990’s proton nuclear magnetic resonance spectroscopy further revealed increased lactate in the cortex and basal ganglia of HD subjects (Jenkins et al., 1993).
Several electron transport chain enzyme activities are deficient in HD tissues. Complex II, III and IV activities are significantly reduced in HD subject brains (Gu et al., 1996; Browne et al., 1997). Additional data suggest the complex II defect is particularly relevant to the demise of neuron populations affected in HD (Benchoua et al., 2006). Complex II inhibitors have successfully been used to model HD; systemic administration of the complex II inhibitors 3-nitropropionic acid and malonate to rodents and primates recreates an HD-like pattern of neurodegeneration and an HD-consistent behavioral phenotype (Beal et al., 1993; Brouillet et al., 1995). Surprisingly, though, for two non-brain tissues (platelets and muscle) complex I activity is reduced but complex II, III, and IV activities are not (Arenas et al., 1998; Parker et al., 1990b).
Since HTT polyglutamine repeat expansion is the primary cause of HD, the question arises as to how and why mitochondrial dysfunction arises in HD. This could conceivably result from direct or indirect effects that HTT may have on mitochondria. Another question that requires consideration is whether mitochondrial dysfunction plays an important intermediary role in HD dysfunction and neurodegeneration cascades. These questions have been studied using transgenic mice that express all or part of the mutant huntingtin gene, but despite considerable efforts decisive conclusions remain elusive.
Available data do indicate polyglutamine-expanded HTT directly associates with mitochondria. A study of mice expressing a 72 glutamine-long expansion found brain mitochondria had lower mitochondrial membrane potentials and depolarized at lower calcium exposures than did mitochondria from control mouse brains. These biochemical defects preceded the onset of structural and behavioral abnormalities by months (Panov et al., 2002). This study further found that when normal mitochondria were incubating with a fusion protein containing an abnormally long polyglutamine repeat, the mitochondria developed calcium handling deficits consistent with those seen in human HD subject tissues and HD transgenic animal models. A different study also found mitochondria from HD transgenic mice were overly sensitive to calcium-induced mitochondria permeability transitions. This phenomena was also observed in normal mitochondria exposed to mutant HTT (Choo et al., 2004).
Other data indicate mutant HTT may indirectly influence mitochondrial function by altering mitochondria-relevant transcription events. HTT appears to interact with several transcription factors, including p53, CREB-binding protein, Sp1, and PGC1- α (Bae et al., 2005; Sugars and Rubinsztein, 2003; Weydt et al., 2006; Cui et al., 2006). p53 is a tumor suppressor protein that also regulates genes involved in mitochondrial function and oxidative stress. A recent study reported mutant HTT binds p53, upregulates nuclear p53 levels and transcriptional activity, and through these effects causes mitochondrial membrane depolarization. p53 suppression prevented mitochondrial depolarization and HTT-induced cytotoxicity(Bae et al., 2005). PGC-1α is a transcription coactivator that regulates mitochondrial biogenesis and metabolic pathways relevant to cell bioenergetics. PGC-1α knock-out mice have an HD-like phenotype (Lin et al., 2004), and reduced expression of PGC-1α target genes is seen in HD patient and HD transgenic mouse striatum (Weydt et al., 2006). Crossing PGC-1α knock-out mice with HD transgenic mice exacerbates striatal neurodegeneration and motor abnormalities, while lentivirus-mediated delivery of PGC-1α to the striatum is neuroprotective in HD transgenic mice (Cui et al., 2006).
Resveratrol, an activator of the sirtuin Sir2 homolog 1 (SIRT1) may also protect against mutant HTT-induced metabolic dysfunction (Parker et al., 2005). SIRT1 deacetylates and activates PGC-1α (Nemoto et al., 2005; Rodgers et al., 2005). PGC-1α activation is under consideration for its potential as an HD therapeutic target.
6. Conclusions
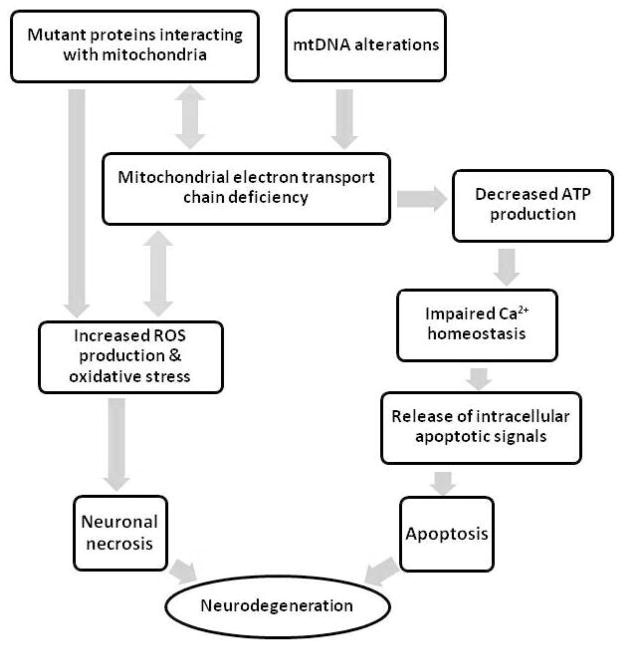
Depicting the hierarchical cascades that drive and mediate neuron dysfunction and death in neurodegenerative diseases is extremely complex (Figure 2). Identifying individual pathologies is easier than defining how they interact. Strong evidence acquired over decades shows mitochondrial abnormalities occur in persons with various neurodegenerative diseases, and further shows distinct mitochondrial abnormalities are characteristic of particular disorders. This is the case for very rare neurodegenerative diseases and also for very common age-related disorders such as AD and PD. It has been considered for some time that mitochondria might play a quite upstream role in sporadic neurodegenerations. It is now also known that a remarkable number of proteins that cause neurodegeneration in their mutant forms interact with mitochondria or affect mitochondrial function. It is important that studies of the mitochondria-neurodegeneration nexus continue for many reasons. Such studies could yield insights into and treatments for diseases that devastate millions of people.
Figure 2.
Attempt to summarize relationships between mitochondria and other characteristic neurodegeneration features.
References
- Abou-Sleiman PM, Muqit MMK, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nature Reviews Neuroscience. 2006;7:13. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Ali SF, David SN, Newport GD, Cadet JL, Slikker W., Jr MPTP-induced oxidative stress and neurotoxicity are age-dependent: evidence from measures of reactive oxygen species and striatal dopamine levels. Synapse. 1994;18:27–34. doi: 10.1002/syn.890180105. [DOI] [PubMed] [Google Scholar]
- Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. The Journal of Cell Biology. 2003;161:14. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antuono P, Beyer J. The burden of dementia. A medical and research perspective. Theoretical Medicine and Bioethics. 1999;20:11. doi: 10.1023/a:1009915605467. [DOI] [PubMed] [Google Scholar]
- Arenas J, Campos Y, Ribacoba R, Martin MA, Rubio JC, Ablanedo P, Cabello A. Complex I defect in muscle from patients with Huntington’s disease. Ann Neurol. 1998;43:397–400. doi: 10.1002/ana.410430321. [DOI] [PubMed] [Google Scholar]
- Bacman SR, Bradley WG, Moraes CT. Mitochondrial Involvement in Amyotrophic Lateral Sclerosis: Trigger or Target? Molecular Neurobiology. 2006;33:19. doi: 10.1385/MN:33:2:113. [DOI] [PubMed] [Google Scholar]
- Bae BI, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward SD, Moran TH, Montell C, Ross CA, Snyder SH, Sawa A. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron. 2005;47:13. doi: 10.1016/j.neuron.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Bassett SS, Avramopoulos D, Fallin D. Evidence for parent of origin effect in late-onset Alzheimer disease. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2002;114:8. doi: 10.1002/ajmg.10648. [DOI] [PubMed] [Google Scholar]
- Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Annals of neurology. 1995;38:10. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- Beal MF. Excitotoxicity and nitric oxide in Parkinson’s disease pathogenesis. Ann Neurol. 1998;44:S110–4. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. The Journal of Neuroscience. 1993;13:12. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchoua A, Trioulier Y, Zala D, Gaillard MC, Lefort N, Dufour N, Saudou F, Elalouf JM, Hirsch E, Hantraye P, Déglon N, Brouillet E. Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Molecular Biology of the Cell. 2006;17:12. doi: 10.1091/mbc.E05-07-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nature Genetics. 2006;38:3. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, Mackenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature Neuroscience. 2000;3:6. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bindoff LA, Birch-Machin M, Cartlidge NEF, Parker WD, Turnbull DM. Mitochondrial function in Parkinson’s disease. Lancet. 1989;2:1. doi: 10.1016/s0140-6736(89)90291-2. [DOI] [PubMed] [Google Scholar]
- Borthwick GM, Johnson MA, Ince PG, Shaw PJ, Turnbull DM. Mitochondrial enzyme activity in amyotrophic lateral sclerosis: implications for the role of mitochondria in neuronal cell death. Annals of Neurology. 1999;46:4. doi: 10.1002/1531-8249(199911)46:5<787::aid-ana17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiology of Aging. 2002;23:6. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Bowling AC, Schulz JB, Brown RH, Beal MF. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. Journal of Neurochemistry. 1993;61:4. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Brouillet E, Hantraye P, Ferrante RJ, Dolan R, Leroy-Willig A, Kowall NW, Beal MF. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5. doi: 10.1073/pnas.92.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SE, Bowling AC, Baik MJ, Gurney M, Brown RH, Beal MF. Metabolic dysfunction in familial, but not sporadic, amyotrophic lateral sclerosis. Journal of Neurochemistry. 1998;71:7. doi: 10.1046/j.1471-4159.1998.71010281.x. [DOI] [PubMed] [Google Scholar]
- Browne SE, Bowling AC, Macgarvey U, Baik MJ, Berger SC, Muqit MM, Bird ED, Beal MF. Oxidative damage and metabolic dysfunction in Huntington’s disease: selective vulnerability of the basal ganglia. Annals of Neurology. 1997;41:8. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- Canevari L, Clark JB, Bates TE. β-Amyloid fragment 25–35 selectively decreases complex IV activity in isolated mitochondria. FEBS Letters. 1999;457:4. doi: 10.1016/s0014-5793(99)01028-5. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson’s disease. Neurobiology of disease. 2009;34:12. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso SM, Proença MT, Santos S, Santana I, Oliveira CR. Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurobiology of Aging. 2004a;25:6. doi: 10.1016/s0197-4580(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Santana I, Swerdlow RH, Oliveira CR. Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Abeta toxicity. Journal of Neurochemistry. 2004b;89:10. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Santos S, Swerdlow RH, Oliveira CR. Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB Journal. 2001;15:3. doi: 10.1096/fj.00-0561fje. [DOI] [PubMed] [Google Scholar]
- Carri MT, Ferri A, Battistoni A, Famhy L, Gabbianelli R, Poccia F, Rotilio G. Expression of a Cu,Zn superoxide dismutase typical of familial amyotrophic lateral sclerosis induces mitochondrial alteration and increase of cytosolic Ca2+ concentration in transfected neuroblastoma SH-SY5Y cells. FEBS Letters. 1997;414:4. doi: 10.1016/s0014-5793(97)01051-x. [DOI] [PubMed] [Google Scholar]
- Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. β-Amyloid inhibits integrated mitochondrial respiration and key enzyme activities. Journal of Neurochemistry. 2002;80:10. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, Mckhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. The FASEB Journal. 2005;19:23. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- Chang SW, Zhang D, Chungb HD, Zassenhaus HP. The frequency of point mutations in mitochondrial DNA is elevated in the Alzheimer’s brain. Biochemical and Biophysical Research Communications. 2000;273:6. doi: 10.1006/bbrc.2000.2885. [DOI] [PubMed] [Google Scholar]
- Choo YS, Johnson GV, Macdonald M, Detloff PJ, Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Human Molecular Genetics. 2004;13:14. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- Chung KKK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science. 2004;304:4. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Mckee AC, Beal MF, Graham BH, Wallace DC. Marked changes in mitochondrial DNA deletion levels in Alzheimer brains. Genomics. 1994;23:471–6. doi: 10.1006/geno.1994.1525. [DOI] [PubMed] [Google Scholar]
- Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1–42. The Journal of Neuroscience. 2005;25:8. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:11. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Curti D, Rognoni F, Gasparini L, Cattaneo A, Paolillo M, Racchi M, Zani L, Bianchetti A, Trabucchi M, Bergamaschi S, Govoni S. Oxidative metabolism in cultured fibroblasts derived from sporadic Alzheimer’s disease (AD) patients. Neuroscience Letters. 1997;236:4. doi: 10.1016/s0304-3940(97)00741-6. [DOI] [PubMed] [Google Scholar]
- Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, Beal MF, Manfredi G. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. Journal of Neurochemistry. 2006;96:13. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- De La Monte SM, Luong T, Neely TR, Robinson D, Wands JR. Mitochondrial DNA damage as a mechanism of cell loss in Alzheimer’s disease. Laboratory Investigation. 2000;80:13. doi: 10.1038/labinvest.3780140. [DOI] [PubMed] [Google Scholar]
- Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. The Journal of Neuroscience. 2006;26:12. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal GK, Grewal RP. Mitochondrial DNA deletion mutation levels are elevated in ALS brains. Neuro Report. 2000;11:3. doi: 10.1097/00001756-200008030-00032. [DOI] [PubMed] [Google Scholar]
- Diana A, Simic G, Sinforiani E, Orru N, Pichiri G, Bono G. Mitochondria morphology and DNA content upon sublethal exposure to beta-amyloid(1-42) peptide. Collegium antropologicum. 2008;32:8. [PubMed] [Google Scholar]
- Duara R, Lopez-Alberola RF, Barker WW, Loewenstein DA, Zatinsky M, Eisdorfer CE, Weinberg GB. A comparison of familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1377–84. doi: 10.1212/wnl.43.7.1377. [DOI] [PubMed] [Google Scholar]
- Edland SD, Silverman JM, Peskind ER, Tsuang D, Wijsman E, Morns JC. Increased risk of dementia in mothers of Alzheimer’s disease cases: evidence for maternal inheritance. Neurology. 1996;47:3. doi: 10.1212/wnl.47.1.254. [DOI] [PubMed] [Google Scholar]
- Ehrenkrantz D, Silverman JM, Smith CJ, Birstein S, Marin D, Mohs RC, Davis KL. Genetic epidemiological study of maternal and paternal transmission of Alzheimer’s disease. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 1999;88:5. doi: 10.1002/(sici)1096-8628(19990820)88:4<378::aid-ajmg15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Elson JL, Herrnstadt C, Preston G, Thal L, Morris CM, Edwardson JA, Beal MF, Turnbull DM, Howell N. Does the mitochondrial genome play a role in the etiology of Alzheimer’s disease? Human Genetics. 2006;119:14. doi: 10.1007/s00439-005-0123-8. [DOI] [PubMed] [Google Scholar]
- Esteves AR, Lu J, Rodova M, Onyango I, Lezi E, Dubinsky R, Lyons KE, Pahwa R, Burns JM, Cardoso SM, Swerdlow RH. Mitochondrial Respiration and Respiration Associated Proteins in Cell Lines Created through Parkinson’s Subject Mitochondrial Transfer. Journal of Neurochemistry. 2010 doi: 10.1111/j.1471-4159.2010.06631.x. [DOI] [PubMed] [Google Scholar]
- Esteves ARF, Domingues F, Ferreira IL, Januário C, Swerdlow RH, Oliveira CR, Cardoso SM. Mitochondrial function in Parkinson’s disease cybrids containing an nt2 neuron-like nuclear background. Mitochondrion. 2008;8:10. doi: 10.1016/j.mito.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Fujita K, Yamauchi M, Shibayama K, Ando M, Honda M, Nagata Y. Decreased cytochrome c oxidase activity but unchanged superoxide dismutase and glutathione peroxidase activities in the spinal cords of patients with amyotrophic lateral sclerosis. Journal of Neuroscience Research. 1996;45:6. doi: 10.1002/(SICI)1097-4547(19960801)45:3<276::AID-JNR9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. Journal of Neurochemistry. 1998;71:7. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- Gu M, Cooper JM, Taanman JW, Schapira AHV. Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Annals of Neurology. 1998;44:10. doi: 10.1002/ana.410440207. [DOI] [PubMed] [Google Scholar]
- Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AH. Mitochondrial defect in Huntington’s disease caudate nucleus. Annals of Neurology. 1996;39:5. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- Hambleta NS, Castora FJ. Elevated levels of the Kearns-Sayre syndrome mitochondrial DNA deletion in temporal cortex of Alzheimer’s patients. Mutation Research. 1997;379:10. doi: 10.1016/s0027-5107(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Hansson CA, Frykman S, Farmery MR, Tjernberg LO, Nilsberth C, Pursglove SE, Ito A, Winblad B, Cowburn RF, Thyberg J, Ankarcrona M. Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria. The Journal of Biological Chemistry. 2004;279:7. doi: 10.1074/jbc.M404500200. [DOI] [PubMed] [Google Scholar]
- Hantraye P, Brouillet E, Ferrante R, Palfi S, Dolan R, Matthews RT, Beal MF. Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat Med. 1996;2:1017–21. doi: 10.1038/nm0996-1017. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:2. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hattingen E, Magerkurth J, Pilatus U, Mozer A, Seifried C, Steinmetz H, Zanella F, Hilker R. Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain. 2009;132:13. doi: 10.1093/brain/awp293. [DOI] [PubMed] [Google Scholar]
- Henchcliffe C, Shungu DC, Mao X, Huang C, Nirenberg MJ, Jenkins BG, Beal MF. Multinuclear magnetic resonance spectroscopy for in vivo assessment of mitochondrial dysfunction in Parkinson’s disease. Annals of the New York Academy of Sciences. 2008;1147:15. doi: 10.1196/annals.1427.037. [DOI] [PubMed] [Google Scholar]
- Higgins CM, Jung C, Xu Z. ALS-associated mutant SOD1G93A causes mitochondrial vacuolation by expansion of the intermembrane space and by involvement of SOD1 aggregation and peroxisomes. BMC Neuroscience. 2003;4:14. doi: 10.1186/1471-2202-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CMJ, Jung C, Ding H, Xu Z. Mutant Cu, Zn superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. The Journal of Neuroscience. 2002;22:6. doi: 10.1523/JNEUROSCI.22-06-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PLR, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. The Journal of Neuroscience. 2001;21:7. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A, Donnenfeld H, Sasaki S, Nakano I. Fine structural observations of neuroWlamentous changes in amyotrophic lateral sclerosis. Journal of Neuropathology and Experimental Neurology. 1984;43:10. doi: 10.1097/00005072-198409000-00001. [DOI] [PubMed] [Google Scholar]
- Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:13. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Ikebe S, Tanaka M, Ohno K, Sato W, Hattori K, Kondo T, Mizuno Y, Ozawa T. Increase of deleted mitochondrial DNA in the striatum in Parkinson’s disease and senescence. Biochemical and Biophysical Research Communications. 1990;170:5. doi: 10.1016/0006-291x(90)90497-b. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Koroshetz WJ, Beal MF, Rosen BR. Evidence for impairment of energy metabolism in vivo in Huntington’s disease using localized 1H NMR spectroscopy. Neurology. 1993;43:7. doi: 10.1212/wnl.43.12.2689. [DOI] [PubMed] [Google Scholar]
- Jung C, Higgins CMJ, Xu Z. A quantitative histochemical assay for activities of mitochondrial electron transport chain complexes in mouse spinal cord sections. Journal of Neuroscience Methods. 2002;114:8. doi: 10.1016/s0165-0270(01)00524-6. [DOI] [PubMed] [Google Scholar]
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, Mcconkey BJ, Vande Velde C, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, Pradat PF, Camu W, Meininger V, Dupre N, Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nature Genetics. 2008;40:3. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, Krebs CT, Bennett JC, Parks JK, Swerdlow RH, Parker WD, Bennett JP. Alzheimer’s disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Annals of Neurology. 2000;48:8. [PubMed] [Google Scholar]
- Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, Distefano LM, Nobrega JN. Brain cytochrome oxidase in Alzheimer’s disease. Journal of neurochemistry. 1992;59:4. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. The Journal of Neuroscience. 1998;18:10. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, Mckee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nature Genetics. 2006;38:3. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- Kuhl DE, Phelps ME, Markham CH, Metter EJ, Riege WH, Winter J. Cerebral metabolism and atrophy in Huntington’s disease determined by 18FDG and computed tomographic scan. Annals of Neurology. 1982;12:10. doi: 10.1002/ana.410120504. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, De Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, Mckenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:4. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Lestienne P, Nelson I, Riederer P, Reichmann H, Jellinger K. Mitochondrial DNA in postmortem brain from patients with Parkinson’s disease. Journal of neurochemistry. 1991;56:1. doi: 10.1111/j.1471-4159.1991.tb02087.x. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:15. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Liu J, Lillo C, Jonsson PA, Velde CV, Ward CM, Miller TM, Subramaniam JR, Rothstein JD, Marklund S, Andersen PM, Brännström T, Gredal O, Wong PC, Williams DS, Cleveland DW. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004;43:9. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:5. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Conforti FL, Rocchi A, Tessitore A, Muglia M, Tedeschi G, Panza D, Monsurrò M, Sola P, Mandrioli J, Choub A, Delcorona A, Manca ML, Mazzei R, Sprovieri T, Filosto M, Salviati A, Valentino P, Bono F, Caracciolo M, Simone IL, Bella VL, Majorana G, Siciliano G, Murri L, Quattrone A. Could mitochondrial haplogroups play a role in sporadic amyotrophic lateral sclerosis? Neuroscience Letters. 2004;371:5. doi: 10.1016/j.neulet.2004.08.060. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Human Molecular Genetics. 2006;15:13. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. The Journal of Neuroscience. 2006;26:10. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y, Mozai T, Kakehi K. Functional and morphometric study of the liver in motor neuron disease. Journal of Neurology. 1985;232:5. doi: 10.1007/BF00314034. [DOI] [PubMed] [Google Scholar]
- Mecocci P, Macgarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Annals of neurology. 1994;36:5. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- Menzies FM, Cookson MR, Taylor RW, Turnbull DM, Chrzanowska-Lightowlers ZMA, Dong L, Figlewicz DA, Shaw PJ. Mitochondrial dysfunction in a cell culture model of familial amyotrophic lateral sclerosis. Brain. 2002;125:12. doi: 10.1093/brain/awf167. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Suzuki K, Sone N, Saitoh T. Inhibition of mitochondrial respiration by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mouse brain in vivo. Neurosci Lett. 1988;91:349–53. doi: 10.1016/0304-3940(88)90705-7. [DOI] [PubMed] [Google Scholar]
- Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. European Journal of Nuclear Medicine and Molecular Imaging. 2005;32:25. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, Tsui W, Santi SD, Leon MJD. Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proceedings of the National Academy of Sciences of the United States of America,; 2007. p. 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, Pirraglia E, Tsui W, Santi SD, Leon MJD. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72:8. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Ohtsuka C, Terayama Y. Increased mitochondrial oxidative damage and oxidative DNA damage contributes to the neurodegenerative process in sporadic amyotrophic lateral sclerosis. Free Radical Research. 2008;42:5. doi: 10.1080/10715760701877262. [DOI] [PubMed] [Google Scholar]
- Mutisya EM, Bowling AC, Beal MF. Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. Journal of Neurochemistry. 1994;63:6. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- Mytilineou C, Werner P, Molinari S, Di-Rocco A, Cohen G, Yahr MD. Impaired oxidative decarboxylation of pyruvate in fibroblasts from patients with Parkinson’s disease. Journal of neural transmission Parkinson’s disease and dementia section. 1994;8:6. doi: 10.1007/BF02260943. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Hirayama K, Terao K. Hepatic ultrastructural changes and liver dysfunction in amyotrophic lateral sclerosis. Archives of Neurology. 1987;44:4. doi: 10.1001/archneur.1987.00520130079022. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} The Journal of Biological Chemistry. 2005;280:5. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- Ng MC, Iacopino AM, Quintero EM, Marches F, Sonsalla PK, Liang CL, Speciale SG, German DC. The neurotoxin MPTP increases calbindin-D28k levels in mouse midbrain dopaminergic neurons. Brain Res Mol Brain Res. 1996;36:329–36. doi: 10.1016/0169-328x(95)00266-u. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life sciences. 1985;36:6. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. The Journal of biological chemistry. 2004;279:9. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nature Neuroscience. 2002;5:6. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Néri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nature Genetics. 2005;37:2. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- Parker WD, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Annals of neurology. 1989;26:5. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Parker WD, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Annals of neurology. 1989;26:5. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Parker WD, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990a;40:12. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Boyson SJ, Luder AS, Parks JK. Evidence for a defect in NADH: ubiquinone oxidoreductase (complex I) in Huntington’s disease. Neurology. 1990b;40:1231–4. doi: 10.1212/wnl.40.8.1231. [DOI] [PubMed] [Google Scholar]
- Parker WD, Mahr NJ, Filley CM, Parks JK, Hughes D, Young DA, Cullum CM. Reduced platelet cytochrome c oxidase activity in Alzheimer’s disease. Neurology. 1994;44:5. doi: 10.1212/wnl.44.6.1086. [DOI] [PubMed] [Google Scholar]
- Parker WD, Parksa JK, Swerdlow RH. Complex I deficiency in Parkinson’s disease frontal cortex. Brain Research. 2008;1189:4. doi: 10.1016/j.brainres.2007.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payami H, Hoffbuhr K. Lack of evidence for maternal effect in familial Alzheimer’s disease. Genetic epidemiology. 1993;10:4. doi: 10.1002/gepi.1370100622. [DOI] [PubMed] [Google Scholar]
- Pereira C, Santos MS, Oliveira C. Mitochondrial function impairment induced by amyloid peptide on PC12 cells. Neuropharmacology and Neurotoxicology. 1998;9:7. doi: 10.1097/00001756-199806010-00015. [DOI] [PubMed] [Google Scholar]
- Pesah Y, Pham T, Burgess H, Middlebrooks B, Verstreken P, Zhou Y, Harding M, Bellen H, Mardon G. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:12. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M, Chen F, Gu Y, Hasegawa H, Salehi-Rad S, Wang L, Rogaeva E, Fraser P, Robinson B, George-Hyslop PS, Tandon A. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. The Journal of biological chemistry. 2005;280:8. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- Praticò D, Uryu K, Leight S, Trojanoswk JQ, Lee VMY. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. The Journal of Neuroscience. 2001;21:5. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi A, Mangolini A, Rizzardini M, Tartari S, Massari S, Bendotti C, Francolini M, Borgese N, Cantoni L, Pietrini G. Cell culture models to investigate the selective vulnerability of motoneuronal mitochondria to familial ALS-linked G93ASOD1. European Journal of Neuroscience. 2006;24:13. doi: 10.1111/j.1460-9568.2006.04922.x. [DOI] [PubMed] [Google Scholar]
- Ro LS, Lai SL, Chen CM, Chen ST. Deleted 4977-bp mitochondrial DNA mutation is associated with sporadic amyotrophic lateral sclerosis: a hospital-based case-control study. Muscle & Nerve. 2003;28:7. doi: 10.1002/mus.10504. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:6. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute Impairment of Mitochondrial Trafficking by beta–Amyloid Peptides in Hippocampal Neurons. Journal of Neuroscience. 2006;26:8. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanberg PR, Fibiger HC, Mark RF. Body weight and dietary factors in Huntington’s disease patients compared with matched controls. The Medical Journal of Australia. 1981;1:3. doi: 10.5694/j.1326-5377.1981.tb135681.x. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Iwata M. Impairment of fast axonal transport in the proximal axons of anterior horn neurons in amyotrophic lateral sclerosis. Neurology. 1996;47:6. doi: 10.1212/wnl.47.2.535. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Holt IJ, Sweeney M, Harding AE, Jenner P, Marsden CD. Mitochondrial DNA analysis in Parkinson’s disease. Movement Disorders. 1990;5:4. doi: 10.1002/mds.870050406. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird T, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nature medicine. 1996;2:7. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Sheehan JP, Swerdlow RH, Parker WD, Miller SW, Davis RE, Tuttle JB. Altered calcium homeostasis in cells transformed by mitochondria from individuals with Parkinson’s disease. J Neurochem. 1997;68:1221–33. doi: 10.1046/j.1471-4159.1997.68031221.x. [DOI] [PubMed] [Google Scholar]
- Shibata N, Nagai R, Uchida K, Horiuchi S, Yamada S, Hirano A, Kawaguchi M, Yamamoto T, Sasaki S, Kobayashi M. Morphological evidence for lipid peroxidation and protein glycoxidation in spinal cords from sporadic amyotrophic lateral sclerosis patients. Brain Research. 2001;917:8. doi: 10.1016/s0006-8993(01)02926-2. [DOI] [PubMed] [Google Scholar]
- Siciliano G, D’avino C, Del Corona A, Barsacchi R, Kusmic C, Rocchi A, Pastorini E, Murri L. Impaired oxidative metabolism and lipid peroxidation in exercising muscle from ALS patients. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. 2002;3:6. doi: 10.1080/146608202760196011. [DOI] [PubMed] [Google Scholar]
- Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, Casari G. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Human Molecular Genetics. 2005;14:16. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- Simpson EP, Henry YK, Henkel JS, Smith RG, Appel SH. Increased lipid peroxidation in sera of ALS patients: a potential biomarker of disease burden. Neurology. 2004;62:8. doi: 10.1212/wnl.62.10.1758. [DOI] [PubMed] [Google Scholar]
- Smith TS, Swerdlow RH, Parker WD, Jr, Bennett JP., Jr Reduction of MPP(+)-induced hydroxyl radical formation and nigrostriatal MPTP toxicity by inhibiting nitric oxide synthase. NeuroReport. 1994;5:2598–600. doi: 10.1097/00001756-199412000-00048. [DOI] [PubMed] [Google Scholar]
- Song DD, Shults CW, Sisk A, Rockenstein E, Masliah E. Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Experimental Neurology. 2004;186:15. doi: 10.1016/S0014-4886(03)00342-X. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;288:2. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, De Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:5. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugars KL, Rubinsztein DC. Transcriptional abnormalities in Huntington disease. Trends in Genetics. 2003;19:6. doi: 10.1016/S0168-9525(03)00074-X. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Molecular Mechanisms of Neurodegenerative Diseases. New Jersey: Humana Press Inc; 2000. Role of Mitochondria in Parkinson’s Disease. [Google Scholar]
- Swerdlow RH. Is aging part of Alzheimer’s Disease, or is Alzheimer’s Disease part of aging? Neurobiology of Aging. 2007a;28:16. doi: 10.1016/j.neurobiolaging.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. Journal of Neuroscience Research. 2007b;85:13. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Pathogenesis of Alzheimer’s disease. Clinical Interventions in Aging. 2007c;2:13. [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. The neurodegenerative mitochondriopathies. Journal of Alzheimer’s Disease. 2009;17:15. doi: 10.3233/JAD-2009-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Medical Hypotheses. 2004;63:13. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Experimental neurology. 2009;218:8. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Kish SJ. Mitochondria in Alzheimer’s disease. International Review of Neurobiology. 2002;53:45. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parker WD, Currie LJ, Bennett JP, Harrison MB, Trugman JM, Wooten GF. Gender ratio differences between Parkinson’s disease patients and their affected parents. Parkinsonism & Related Disorders. 2001;7:5. doi: 10.1016/s1353-8020(00)00029-8. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Davis RE, Parker WD. Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology. 1997;49:8. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Cassarino DS, Trimmer PA, Miller SW, Maguire DJ, Sheehan JP, Maguire RS, Pattee G, Juel VC, Phillips LH, Tuttle JB, Bennett JP, Davis RE, Parker WD. Mitochondria in sporadic amyotrophic lateral sclerosis. Experimental Neurology. 1998;153:8. doi: 10.1006/exnr.1998.6866. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Miller SW, Davis RE, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Parker WD. Origin and functional consequences of the complex I defect in Parkinson’s disease. Annals of Neurology. 1996a;40:9. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Miller SW, Pattee G, Davis RE, Parker WD. Evidence of genetic mitochondrial pathology in sporadic Amyotrophic Lateral Sclerosis. Society for Neuroscience Abstract. 1996b;22:1. [Google Scholar]
- Swerdlow RH, Parks JK, Pattee G, Parker WD. Role of mitochondria in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. 2000;1:6. doi: 10.1080/14660820050515179. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Goldman SM. Epidemiology of Parkinson’s disease. Neurologic clinics. 1996;14:19. doi: 10.1016/S0733-8619(05)70259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer PA, Bennett JP. The cybrid model of sporadic Parkinson’s disease. Experimental Neurology. 2009;218:6. doi: 10.1016/j.expneurol.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer PA, Borland MK, Keeney PM, Bennett JP, Parker WD. Parkinson’s disease transgenic mitochondrial cybrids generate Lewy inclusion bodies. Journal of Neurochemistry. 2004;88:13. doi: 10.1046/j.1471-4159.2003.02168.x. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. Journal of Environmental Science and Health, Part C. 2009;27:20. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, Ahern GL, Baxter L, Alexander G, Walker DG, Reiman EM. Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion. 2006;6:8. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Walt JM, Nicodemus KK, Martin ER, Scott WK, Nance MA, Watts RL, Hubble JP, Haines JL, Koller WC, Lyons K, Pahwa R, Stern MB, Colcher A, Hiner BC, Jankovic J, Ondo WG, Allen FH, Jr, Goetz CG, Small GW, Mastaglia F, Stajich JM, Mclaurin AC, Middleton LT, Scott BL, Schmechel DE, Pericak-Vance MA, Vance JM. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am J Hum Genet. 2003;72:804–11. doi: 10.1086/373937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, De Belleroche J, Gallo JM, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:4. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velde CV, Miller TM, Cashman NR, Cleveland DW. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6. doi: 10.1073/pnas.0712209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielhaber S, Kunz D, Winkler K, Wiedemann FR, Kirches E, Feistner H, Heinze HJ, Elger CE, Schubert W, Kunz WS. Mitochondrial DNA abnormalities in skeletal muscle of patients with sporadic amyotrophic lateral sclerosis. Brain. 2000;123:10. doi: 10.1093/brain/123.7.1339. [DOI] [PubMed] [Google Scholar]
- Vijayvergiya C, Beal MF, Buck J, Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. The Journal of Neuroscience. 2005;25:8. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel JP, Difiglia M. Huntington disease. Journal of Neuropathology & Experimental Neurology. 1998;57:16. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. Journal of Neurochemistry. 2005;93:10. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Fujioka H, Zhu X. Dymanin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s Disease patients. American Journal of Pathology. 2008a;173:13. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008b;105:6. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, Gilbert ML, Morton GJ, Bammler TK, Strand AD, Cui L, Beyer RP, Easley CN, Smith AC, Krainc D, Luquet S, Sweet IR, Schwartz MW, La Spada AR. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metabolism. 2006;4:14. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Wiedemann FR, Manfredi G, Mawrin C, Beal MF, Schon EA. Mitochondrial DNA and respiratory chain function in spinal cords of ALS patients. Journal of Neurochemistry. 2002;80:10. doi: 10.1046/j.0022-3042.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M, Antuono P, Ho KC, Egan R, Hevner R, Liebl W, Huang Z, Rachel R, Jones J. Cytochrome oxidase in Alzheimer’s disease: biochemical, histochemical, and immunohistochemical analyses of the visual and other systems. Vision Research. 1997;37:16. doi: 10.1016/S0042-6989(96)00210-6. [DOI] [PubMed] [Google Scholar]
- Wooten GF, Currie LJ, Bennett JP, Harrison MB, Trugman JM, Parker WD. Maternal inheritance in Parkinson’s disease. Annals of Neurology. 1997;41:4. doi: 10.1002/ana.410410218. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]