Abstract
MRL/MpJ-Faslpr/lpr/J (MRLlpr) mice develop lupus-like disease manifestations in an IL-21–dependent manner. IL-21 is a pleio-tropic cytokine that can influence the activation, differentiation, and expansion of B and T cell effector subsets. Notably, auto-reactive CD4+ T and B cells spontaneously accumulate in MRLlpr mice and mediate disease pathogenesis. We sought to identify the particular lymphocyte effector subsets regulated by IL-21 in the context of systemic autoimmunity and, thus, generated MRLlpr mice deficient in IL-21R (MRLlpr.IL-21R−/−). Lymphadenopathy and splenomegaly, which are characteristic traits of the MRLlpr model were significantly reduced in the absence of IL-21R, suggesting that immune activation was likewise decreased. Indeed, spontaneous germinal center formation and plasma cell accumulation were absent in IL-21R–deficient MRLlpr mice. Correspondingly, we observed a significant reduction in autoantibody titers. Activated CD4+ CD44+ CD62Llo T cells also failed to accumulate, and CD4+ Th cell differentiation was impaired, as evidenced by a significant reduction in CD4+ T cells that produced the pronephritogenic cytokine IFN-γ. T extrafollicular helper cells are a recently described subset of activated CD4+ T cells that function as the primary inducers of autoantibody production in MRLlpr mice. Importantly, we demonstrated that T extrafollicular helper cells are dependent on IL-21R for their generation. Together, our data highlighted the novel observation that IL-21 is a critical regulator of multiple pathogenic B and T cell effector subsets in MRLlpr mice.
MRL/MpJ-Faslpr/lpr/J (MRLlpr) mice spontaneously develop systemic autoimmunity that is characterized by generalized activation of B and T cells and pathologic features resembling those observed in patients with systemic lupus erythematosus (SLE). Hallmark features of disease include the development of glomerulonephritis and cutaneous lesions and production of somatically mutated autoantibodies that recognize nuclear components (1, 2). Multiple lymphocyte effector populations contribute to disease pathogenesis in MRLlpr mice. Both B cells and CD4+ T cells are critical for the development of renal pathology and autoantibody production (3–7). B cells promote the development of disease features via both Ab-dependent and -independent mechanisms and are required for the accumulation of activated memory CD4+ and CD8+ T cells (7, 8). The predominant source of somatically mutated autoantibodies in MRLlpr mice derives from extrafollicular foci that require CD4+ T extra- follicular helper (Thef) cells for their formation (9, 10). CD4+ Thef cells develop via an ICOS-dependent mechanism in MRLlpr mice, and MRLlpr.ICOS−/− mice develop reduced autoantibody titers (10–12). Nevertheless, development of renal pathology is only modestly impacted in MRLlpr.ICOS−/− mice, consistent with an Ab-independent role for the development of nephritis, and accumulation of activated lymphocyte populations still occurs (7, 11, 12). In addition, activated CD4+ T cells from MRLlpr mice produce high levels of IFN-γ, which is a cytokine that is necessary for the development of renal pathology, suggesting that CD4+ T cell-derived IFN-γ may promote the development of renal pathology in MRLlpr mice (13–16). Thus, multiple lymphocyte populations exhibit spontaneous activation and accumulation in MRLlpr mice; however, the molecular mechanisms underlying these processes, which ultimately contribute to disease pathogenesis, remain poorly understood.
IL-21 is the most recently discovered member of the type I cy-tokine family. IL-21 binds the IL-21R, which is a heterodimer composed of IL-21Rα and the common γ-chain that is shared with receptors specific for IL-2, IL-4, IL-7, IL-9, and IL-15 (17–19). IL-21R is expressed by lymphoid cells, including B cells and activated CD4+ T cells (17, 20). Its expression is also found on myeloid cells, such as monocytes and dendritic cells (21, 22). IL-21 is produced by activated CD4+ T cells and NKT cells and exhibits complex context-dependent biologic activity. For instance, in vitro IL-21 induces apoptosis of resting B cells but drives enhanced proliferation of B and T cells stimulated via their AgRs (20, 23). In vivo IL-21 is required for the normal accumulation of early Ab-secreting cells in response to immunization with T-dependent Ags (24, 25). Consistent with this observation, costimulation of B cells via their BCR and IL-21 promotes plasma cell differentiation in vitro (26, 27). IL-21 is also required for normal germinal center (GC) formation via a B cell-intrinsic pathway (25, 28–30). IL-21 was also recently shown to be produced by T follicular helper (Tfh) cells, which are a specialized subset of activated CD4+ T cells that promote GC-dependent Ab responses (31, 32). In some experimental systems, IL-21 is required for the differentiation of Tfh cells (33–35). Thef cells produce IL-21 and promote Ab production in an IL-21–dependent manner; however, it still remains unclear whether IL-21–derived signals are required for their formation (10). In addition, Th17 cells, which have recently been associated with SLE in human patients and mouse models, can also use IL-21 for their differentiation (32, 36–38).
Recent candidate gene studies identified polymorphisms in both the IL-21 and IL-21R genes that associate with development of SLE (39–41). Moreover, IL-21 promotes disease pathogenesis in preclinical lupus models, such as the BXSB-Yaa and MRLlpr mice (42, 43). We sought to identify the particular lymphocyte effector subsets regulated by IL-21 in the context of systemic autoimmunity and, thus, generated MRLlpr mice deficient in IL-21R (MRLlpr.IL-21R−/−). Lymphadenopathy and splenomegaly were significantly reduced and corresponded to dramatic reductions in the absolute numbers of CD4+ T, double-negative (DN) T, and B cells. Notably, the spontaneous accumulation of GC B cells and plasma cells is completely abrogated in MRLlpr.IL-21R−/− mice. Similarly, activated CD44hi CD62lo CD4+ T cells accumulate in an IL-21R–dependent manner. Multiple T effector subsets are correspondingly impacted by loss of IL-21R signaling. Notably, the frequency of IFN-γ–producing CD4+ T cells was significantly reduced in IL-21R–deficient MRLlpr mice. In addition, we demonstrated that Thef cells require IL-21R for their development in MRLlpr mice. Overall, these studies highlighted that IL-21R signaling is necessary for the spontaneous accumulation of multiple B and T cell effector populations in MRLlpr mice.
Materials and Methods
Mice
IL-21R knockout (KO) mice were generated, as previously described (44). MRLlpr mice deficient in IL-21R were generated by backcrossing C57BL/ 6. IL-21R−/− mice onto the MRLlpr background (The Jackson Laboratory). Congenic MRLlpr mice were generated using a speed-congenic MaxBax backcrossing strategy (Charles River Laboratories). Mice were considered fully backcrossed after obtaining 99% MRLlpr genomic sequence, as assessed by 81 microsatellite markers covering the entire murine genome. Age- and sex-matched MRL/MpJ mice were obtained from The Jackson Laboratory. All animals were housed in a pathogen-free animal facility at Pfizer. Mice were used according to protocols approved by the Pfizer Animal Care and Use Committee.
Evaluation of disease progression
To evaluate the role of IL-21R in the development of lupus-like disease in MRLlpr.IL-21R−/− and wild-type (WT) MRLlpr.IL-21R+/+ mice, littermate animals were scored blindly for proteinuria, skin lesions, lymphadenopa-thy, and splenomegaly. Proteinuria was measured using Albustix (Bayer) and scored on a scale of 0–5; 0, none; 1, trace; 2, 30 mg/dl; 3, 100 mg/dl; 4, 300 mg/dl; and 5, ≥2000 mg/dl. Lymph nodes were palpated, and lymphadenopathy was scored on a scale of 0–3; 0, none; 1, small; 2, moderate, at two different sites; and 3, large, at three or more different sites. Skin lesions were assessed by gross pathology and scored on a scale of 0–3; 0, none; 1, small (face, ears); 2, moderate (<2 cm face, ears, and back); and 3, severe (>2 cm face, ears, and back). Spleens were weighed and splenocytes were counted to assess splenomegaly.
Flow cytometry
Single-cell suspensions of splenocytes were prepared, treated with RBC lysis buffer (Sigma), and then washed with FACS buffer (PBS plus 1% FCS). Cells were then incubated with purified anti-CD16/32 (Fc block; clone 2.4G2; BD Pharmingen), washed, and stained with primary Abs diluted in FACS buffer for 30 min at 4°C. Intracellular staining of cytokines was performed using the BD intracellular FACS kit, according to the manufacturer’s protocol (BD Biosciences). Abs used in these studies included anti-T and B cell-activating Ag “GL7” (clone GL7), CD4 (clone GK1.5), CD3 (clone 145-2C11), CD8α (clone 53-6.7), CD138 (clone 281-2), B220 (clone RA3-6B2), CD19 (clone 1D3), CD44 (clone IM7), CD62L (clone MEL-14), IL-21R (4A9), TCRβ (clone H57-587), PSGL1 (clone 4RA10), PD1 (clone J43), CXCR5 (clone 2G8), CXCR4 (clone 2B11), CD38 (clone 90), CD278/ICOS (clone C398.4a), IL-17 (clone TC11-18H10), IL-4 (clone 11B11), and IFN-γ (clone XMG1.2). Unlabeled anti-CXCR5 mAb was visualized using allophycocyanin-goat anti–rat-Ig (BD Biosciences). Cells were also stained with live/dead Fixable Aqua dead cell stain (Invitrogen), as per the manufacturer’s directions. At least 100,000 events were counted using either a FACSCalibur or LSRII (Becton Dickinson) and analyzed using FlowJo software (Tree Star).
Flow cytometric analysis of kidney infiltrates was performed as follows. Mice were perfused with 30 ml HBSS containing 2 mM EDTA. Kidneys were isolated, capsules were removed, and the tissues were processed using a gentleMACS tissue dissociator (Miltenyi Biotec). Tissue homogenates were filtered through a 70-μm nylon mesh filter, and leukocytic infiltrates were isolated by centrifugation over a Percoll gradient. Cells were then washed with FACS buffer, counted, and stained, as above.
In vitro B cell stimulation
Spleens from untreated 8-wk-old mice were dissociated into single-cell suspensions and lysed with RBC lysis buffer (Sigma). B lymphocytes were isolated using magnetic cell-sorting CD19 MicroBeads (Miltenyi Biotec), according to the manufacturer’s instructions. Purified B cells were cultured in RPMI 1640 medium plus 10% FBS in a 96-well plate and incubated with 2.5 μg/ml purified anti-mouse CD40 (BD Pharmingen) and 10 μg/ml F(ab′)2 goat anti-mouse IgM (Jackson ImmunoResearch Laboratories) in the presence or absence of 50 ng/ml IL-21 (R&D Systems). Supernatants were collected after 96 h of incubation and analyzed for IgG content using an IgG ELISA kit (Bethyl).
Serum Ig ELISAs
Serum Ig was measured by ELISA. Briefly, Costar plates (Thermolab Systems) were coated overnight with 1 μg/ml goat anti-mouse Ig(H+L) (Southern Biotech). Plates were blocked the next day with PBS plus 1% BSA, serially diluted serum samples were added (1:100 starting dilution), and bound Ab was detected with HRP-conjugated goat Abs directed against mouse IgM, IgG1, IgG2a, IgG2b, or IgG3 (Southern Biotech). Purified unlabeled mouse IgM, IgG1, IgG2a, IgG2b, or IgG3 Ab (Southern Biotech) was used as a standard to quantify serum Ig concentration. Plates were developed using 3,3′,5,5′-tetramethylbenzidine (KPL), and reactions were stopped with 2 N sulfuric acid. Absorbances were read at 450 nm using a SpectraMax Plus 384 microplate reader (Molecular Devices) and SoftMax Pro software.
Serum anti-dsDNA ELISA
Serum anti-dsDNA IgG Abs were measured by ELISA. Briefly, Immulon 1B plates (Thermolab Systems) were UV irradiated overnight and then coated with 2 μg/ml calf thymus DNA (Sigma) for 1 h at room temperature. Plates were blocked with PBS plus 1% BSA, diluted serum samples were added (1:100 dilution), and bound Ab was detected with HRP-conjugated goat Abs directed against mouse IgG1, IgG2a, IgG2b, or IgG3 Ab (Southern Biotech). Plates were developed using 3,3′,5,5′-tetramethyl-benzidine (KPL), and reactions were stopped with 2 N sulfuric acid. Absorbances were read at 450 nm using a SpectraMax Plus 384 microplate reader (Molecular Devices) and SoftMax Pro software.
Anti-nuclear Ab assay
Serum anti-nuclear Abs (ANAs) were measured using a HEp-2 ANA kit (Antibodies), according to the manufacturer’s protocol. Briefly, serum samples were diluted to 1:100 in sample-dilution buffer, bound Ab was detected using a FITC–anti-mouse IgG Ab (Southern Biotech), and images were captured using a Nikon Eclipse E800 fluorescent microscope. To quantitate serum ANA, mean fluorescence intensity (MFI) was measured for ≥10 cells/sample using ImagePro Plus software, and average MFI was determined.
Histopathological evaluation of H&E- and periodic acid-Schiff–stained tissue sections
Routine H&E and periodic acid-Schiff (PAS) staining was performed on formalin-fixed, paraffin-embedded sections of kidney. To assess inflammation, the total numbers of inflammatory foci were counted in the kidneys and salivary glands in H&E-stained tissue sections. If foci had coalesced, an estimate was made of the numbers of individual foci present. To assess glomeruli, morphometry was performed on PAS-stained tissue sections of kidney using a commercial image-analysis software package (Image-Pro Plus v.5.1; Media Cybernetics, Silver Spring, MD). Ten glomeruli from each kidney were photographed at 40× magnification in 24-bit color at 1388 ×1040 resolution using a Zeiss Axio Imager.A1 microscope and a Zeiss AxioCam HRc digital microscope camera (Carl Zeiss Microimaging, Thornwood, NY). Using Image-Pro Plus, glomeruli captured in digital photomicrographs were evaluated morphometrically by manually tracing the circumference of the glomerular tuft and measuring the total area of the traced glomerular tuft. The total areas of PAS-positive mesangium (as a measure of immune complex deposition and basement membrane thickening) and nuclei (as an estimate of cellularity) in the traced glomerular tuft were also measured in Image-Pro Plus using color segmentation.
Immunohistochemistry and immunofluorescent Ab staining
Immunohistochemical staining for IL-21R (polyclonal Ab; cat. #14-6469 eBioscience), IgD (clone 11-26c.2a; BD Biosciences), biotinylated peanut agglutinin (PNA; Vector Labs), IgG and IgM (polyclonal Abs; Jackson ImmunoResearch, West Grove, PA), and complement C3 (ICN/Cappel) was performed using acetone-fixed cryosections of kidney specimens and a Nemesis 7200 autostainer (Biocare Medical, Concord, CA). TBST was used for washes between each step (except after the protein block). Endogenous peroxidase was quenched with 3% H2O2 in 70% methanol for 30 min or with dual endogenous peroxidase block (DakoCytomation) for 10 min. A protein block (3% nonfat dry milk powder, 10% goat serum, and 0.01% Tween) was applied for 30 min to PNA-, IgD-, IgG-, IgM-, and C3-stained sections, and Rodent Block M (Biocare Medical) was applied to IL-21R–stained sections. To detect Ig or C3 deposition, sections were incubated with 1:200 dilutions of goat anti-mouse IgM-HRP, goat anti-mouse IgG-HRP, goat anti-mouse complement C3-HRP, or negative control Chromo Pure Goat IgG for 1 h. Anti–IL-21R Ab was used at 1 μg/ml diluted in Rodent Block M. IgD and PNA staining was performed at 1 and 4 μg/ml, respectively, in TBST. Secondary Ab staining for IL-21R was performed using an HRP EnVisionTM+ anti-rabbit (DakoCytomation) kit. Streptavidin:HRP (eBioscience) was incubated with PNA-stained sections prior to development. Diaminobenzidine substrate chromogen (DakoCytomation) was applied to HRP-labeled sections, and Permanent Red Chromagen (DakoCytomation) was applied for alkaline phosphatase-labeled sections. Sections were then counterstained in hematoxylin, rinsed in water, dehydrated through graded ethanol, cleared in xylene, and coverslipped.
Immunohistochemical staining for IgG, IgM, and C3 within glomeruli was subjectively evaluated by a veterinary pathologist in a blinded manner as none (0), slight (1), mild (2), moderate (3), or severe (4). To confirm results of immunohistochemical staining for IgG, immunofluorescent Ab staining for IgG was also performed on 4% paraformaldehyde-fixed cryosections of kidney specimens from a subset of animals. Sections were permeabilized in methanol at −20°C for 10 min. Sections were washed in TBST, and a protein block (0.75% normal BSA Fraction V; Invitrogen) was applied for 30 min. Goat anti-mouse IgG Alexa Fluor 488 (10 μg/ml; Invitrogen, Carlsbad, CA) was applied for 1 h in a dark, humid chamber. Sections were rinsed in TBST, mounted with VECTASHIELD DAPI mounting media (Vector Laboratories, Burlingame, CA), and examined using a Nikon E800 microscope and a 40× plan Apo objective (Nikon, Melville, NY).
Immunofluorescent staining for F4/80, B220, CD4, and IgM was performed on acetone-fixed cryosections of spleen specimens. Sections were protein blocked (3% BSA; Sigma) for 20 min, and primary rat anti-mouse Abs were applied for 2 h at 1:200 in blocking solution; F4/80 Alexa Fluor 647 (clone: BM8; eBiosciences), B220 Biotin (clone: RA3-6B2; BD Pharmingen), CD4 Pacific Blue (BD Pharmingen), and IgM FITC (BD Pharmingen). Sections were rinsed in washing solution (1% BSA), and secondary reagents, which included rabbit IgG anti-FITC Alexa Fluor 488 (1:200, Invitrogen) or streptavidin Alexa Fluor 555 (1:500; Invitrogen), were applied for 1 h. Sections were rinsed with washing solution, followed by 1× PBS, and examined using a Leica TCS SP5 Spectral Confocal Microscope, 405UV and a 40× Apo objective. The area (mm2) of extra-follicular regions was measured using ImageJ 1.38x software. Plasmablasts localized within the extrafollicular region were blindly manually counted, and numbers of plasmablasts/mm2 of extrafollicular region were calculated, using all regions examined.
Ex vivo intracellular cytokine analysis
For the detection of Th1, Th2, and Th17 cells, ex vivo, freshly isolated splenocytes were stimulated with PMA (50 ng/ml) and ionomycin (1 μg/ml) in the presence of 1 μl/ml GolgiStop (BD Biosciences) for 4 h and then examined for production of IFN-γ, IL-17, and IL-4 by intracellular flow cytometry. Cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences).
Results
Nephritis and skin lesions develop in an IL-21R–dependent manner in MRLlpr mice
We sought to better understand the impact of IL-21R deficiency on immune activation in the MRLlpr mouse model of lupus. To these ends, we generated MRLlpr.IL-21R−/− mice and first assessed the development of disease features in both MRLlpr.IL-21R+/+ and MRLlpr.IL-21R−/− animals. By 3 mo of age, MRLlpr.IL-21R+/+ mice developed proteinuria, lymphadenopathy, and splenomegaly, which increased dramatically in severity with age (Fig. 1A, 1B, data not shown). Also, 86% of 5-mo-old MRLlpr.IL-21R+/+ mice exhibited severe skin lesions around the face, back, and neck (Fig. 1C, data not shown). Notably, the severity of these disease features was significantly reduced in aged MRLlpr.IL-21R−/− mice compared with IL-21R+/+ littermates (Fig. 1C). Three-month-old MRLlpr.IL-21R−/− mice exhibited reduced levels of lymphade-nopathy, splenomegaly, and proteinuria; interestingly, the severity of these features did not increase from 3 to 5 mo of age in MRLlpr. IL-21R−/− mice (Fig. 1A, 1B, data not shown). In addition, only 69% of MRLlpr.IL-21R−/− mice developed skin disease by 5 mo of age, which was limited to small lesions and hair loss on the nose and face (Fig. 1C, data not shown).
FIGURE 1.
IL-21R–dependent pathology in MRLlpr mice. Female MRLlpr.IL-21R+/+ and MRLlpr. IL-21R−/− mice were examined for proteinuria (A), lymphadenopathy (B), and skin lesions (C). *p = 0.024, **p ≤ 0.01, ***p = 0.01, Student t test. D, Photomicrographs (left panels) show glomerular IgG deposits in kidney sections prepared from MRLlpr.IL-21R+/+ (WT) and MRLlpr.IL-21R−/− (KO) mice. Scale bars, 50 μm. Bar graph (right panel) shows the degree of anti-IgG labeling in stained kidney sections. *p ≤ 0.01, Student t test. E, Graphs show immuno-histochemical-derived scores for kidney IgM (left panel) and C3 (right panel) deposition. *p = 0.015, Student t test. F, Photomicrographs (left panels) show H&E-stained kidney sections prepared from WT and KO mice. Bar graph (right panel) shows the number of inflammatory foci. **p < 0.0001, Student t test. Scale bars, 500 μm. G, PAS staining was performed to measure thickening of the GBM. Bars indicate the average of 10 mice/group, and error bars indicate the SEM. *p = 0.012, Student t test.
We also assessed the degree of renal pathology present in kidneys obtained from MRLlpr.IL-21R+/+ and IL-21R−/− mice. Deposition of immune complexes in the glomeruli is a prominent disease feature of lupus nephritis and is a critical mediator of kidney damage in MRLlpr mice (45, 46). IgM, IgG, and C3 deposits were readily observed in the glomeruli of 5-mo-old IL-21R WT MRLlpr mice (Fig. 1D, 1E). Kidneys obtained from MRLlpr.IL-21R+/+ mice also contained numerous mononuclear inflammatory cell infiltrates and had thickening of the glomerular basement membrane (GBM), which is consistent with the development of lupus nephritis (Fig. 1F, 1G). By contrast, kidneys obtained from MRLlpr.IL-21R−/− mice had significantly reduced levels of IgG and C3 deposition in their glomeruli (Fig. 1D, 1E). Moreover, inflammatory cell infiltrates were dramatically reduced in the kidneys of MRLlpr.IL-21R−/− mice, as was GBM thickening (Fig. 1F, 1G). The decreased renal pathology observed in IL-21R−/− mice correlated well with reduced proteinuria in 5-mo-old MRLlpr.IL-21R−/− mice relative to IL-21R+/+ littermates (Fig. 1A). Deposition of IgM was not affected in the IL-21R−/− background; however, this isotype does not appear to play a pathogenic role in the MRLlpr model (Fig. 1E) (47). We also examined salivary glands, because inflammatory infiltrates also accumulate in this organ as MRLlpr mice age; we found that the number of inflammatory foci were significantly reduced in MRLlpr.IL-21R−/− mice (data not shown).
Because loss of IL-21R signaling in MRLlpr mice dramatically limited the development of renal pathology, we subsequently examined the expression levels of IL-21R in kidneys obtained from aged MRLlpr mice to determine whether IL-21R signaling locally might contribute to renal pathology. By quantitative RT-PCR analysis, IL-21R expression levels were significantly increased in MRLlpr mice compared with MRLWT mice (Fig. 2A). As expected, expression of IL-21R was undetectable in kidneys obtained from MRLlpr.IL-21R−/− mice (Fig. 2A). To determine the localization of IL-21R–expressing cells, we next performed immunohistochemical analysis on kidney sections. IL-21R expression was observed on a fraction of cells present in the peri-vascular infiltrates of MRLlpr mice (Fig. 2B). Staining for IL-21R was not observed in kidney sections prepared from MRLlpr.IL-21R−/− mice (Fig. 2B). Interestingly, IL-21R+ cells appeared lymphocytic and tended to localize in small clusters that were adjacent to or near glomeruli (Fig. 2B).
FIGURE 2.
IL-21R is expressed on inflammatory infiltrates in MRLlpr kidneys. A, Graph shows relative expression levels of IL-21R in kidneys obtained from MRL/MpJ (MpJ), MRLlpr.IL-21R+/+ (WT), and MRLlpr.IL-21R−/− (KO) mice. Symbols represent values obtained from individual mice, and lines indicate the mean ± SEM. **p = 0.009, Student t test. B, Photomicrographs show IL-21R staining on kidney sections prepared from MRLlpr.IL-21R+/+ (left panels) and MRLlpr.IL-21R−/− (right panels). Original magnifica-tion ×20 (top panels), ×40 (bottom panels). Boxed areas in top panels are shown in lower photomicrographs. Scale bars, 50 μm (top panels) and 25 μm (bottom panels). Arrow indicates an additional region staining for IL-21R. A glomerulus is present in the upper right corner of the ×20 MRLlpr.IL-21R+/+ section. C, Number of leukocytes, CD4+ T cells (CD4+ CD3+ CD8− CD19−), CD8+ T cells (CD8+ CD3+ CD4− CD19−), DN T cells (CD3+ B220+ CD4− CD8− CD19−) and B cells (CD19+ B220+ CD3−) obtained from the kidneys of WT and KO mice. Symbols represent values obtained from individual mice, and lines indicate the mean ± SEM. **p ≤ 0.003, Student t test. D, IL-21R levels are shown for MRLlpr.IL-21R+/+ mice (black line), MRLlpr.IL-21R−/− mice (dashed line), and “fluorescence minus one” stained controls (gray line) on the cell types indicated, as in C. All populations were also CD45+ “live” singlet gated.
To identify the cell types that express IL-21R, we performed flow cytometric analysis on leukocytes enriched from MRLlpr.IL-21R+/+ kidneys. Consistent with the increased numbers of inflammatory foci observed in kidneys obtained from MRLlpr.IL-21R+/+ mice, intrarenal leukocyte counts were significantly elevated in MRLlpr. IL-21R+/+ mice compared with MRLlpr.IL-21R−/− mice (Fig. 2C). The majority of the cells isolated from MRLlpr.IL-21R+/+ kidneys were CD4+ T cells (67 ± 2.1%; n = 10), with CD19+ B cells (0.9 ± 0.1%; n = 10), CD8+ T cells (9.0 ± 0.9%; n = 10), and DN T cells (6.5 ± 1.1%; n = 10) making up the remaining minority population. Kidneys from MRLlpr.IL-21R−/− mice contained nearly 20-fold fewer CD4+ T cells than were present in kidneys prepared from MRLlpr.IL-21R+/+ mice (Fig. 2C). By contrast, the number of CD8+ and DN T cells was reduced by ~2.5-fold in kidneys obtained from MRLlpr.IL-21R+/+ mice, and B cell numbers were unchanged between these strains. Thus, intrarenal CD4+ T cell accumulation occurs via an IL-21R–dependent pathway in MRLlpr mice.
IL-21R expression levels were assessed on each of these lymphocyte populations. The anti–IL-21R Ab used for these studies appeared specific for IL-21R, based on its lack of staining of lymphocytes obtained from both the spleen and kidney of MRLlpr.IL-21R−/− mice compared with WT controls (Fig. 2D, data not shown). Notably, CD4+, CD8+, DN T cells, and B cells each had detectable expression of IL-21R. However, B cells (1580 ± 300 MFI; n = 9) expressed IL-21R at nearly 5-fold higher levels than did CD4+ T cells (328 ± 8.1 MFI; n = 9) in the kidney, and CD8+ (640 ± 29.5 MFI; n = 9) and DN T (540 ± 26.2 MFI; n = 9) cells expressed intermediate levels (Fig. 2D). Therefore, the limited distribution of IL-21R staining seen by immunohisto-chemical staining may reflect detection of the highest surface levels of IL-21R, which was observed on B cells (Fig. 2D). Collectively, these results indicated that IL-21R is required for the pathologic accumulation of intrarenal CD4+ T cells and that IL-21R–expressing cells contribute to the inflammatory infiltrates present in the diseased kidneys of MRLlpr mice.
IL-21R is required for B and T cell accumulation in MRLlpr mice
Splenomegaly develops in MRLlpr mice as a consequence of accumulation of DN (CD4− CD8− CD3+) T cells and activated CD4+ and CD8+ T and B cells (1). Notably, IL-21 was shown to enhance both the proliferation and survival of B and T cells (48). To understand the potential contribution of IL-21 to the lympho-proliferative phenotype observed in MRLlpr mice, we examined the cellular composition of spleens obtained from adult MRLlpr. IL-21R+/+ and MRLlpr.IL-21R−/− mice. MRLlpr.IL-21R−/− spleens contained half as many total splenocytes as did IL-21R WT MRLlpr spleens, indicating that IL-21R contributes to the development of splenomegaly in MRLlpr mice (Fig. 3). Interestingly, the relative frequency of B cells, CD4+ T cells, and DN T cells was similar in spleens obtained from MRLlpr.IL-21R+/+ and MRLlpr.IL-21R−/− mice (data not shown). Consequently, the absolute number of B cells, CD4+ T cells, and DN T cells was reduced by roughly half in MRLlpr.IL-21R−/− spleens (Fig. 3). CD8+ T cells numbers were unaffected by IL-21R deficiency in the MRLlpr background (Fig. 3). However, it is noteworthy that the DN T cells that accumulate in MRLlpr mice derive from activated CD8+ T cells, which suggests that IL-21R signaling in CD8+ T cells is involved in the accumulation of DN T cells (6, 49, 50). Because IL-21 supports accumulation of chronically activated CD8+ T cells in response to viral infection, our observations suggested that IL-21 may play a similar role in autoim-mune disease settings (51–53). Collectively, these results indicated that IL-21R signaling is critical for the systemic accumulation of B, CD4+ T, and DN T cells subsets in MRLlpr mice.
FIGURE 3.

Lymphocyte accumulation is reduced in IL-21R–deficient MRLlpr mice. Graphs show the number of splenocytes, B cells (B220+ CD3−), CD4+ T cells (CD4+ CD3+ B220−), CD8+ T cells (CD8+ CD3+ B220−), and DN T cells (CD4− CD8− B220+ CD3+). Bars represent the mean, and error bars indicate the SEM for at least four mice/group. Results are representative of three independent experiments. *p = 0.016, **p ≤ 0.009, Student t test.
IL-21R regulates spontaneous B cell activation and autoantibody responses in MRLlpr mice
IL-21 promotes the differentiation of B cells to plasma cells and isotype switching (26, 27). Because autoantibody production by B cells contributes to renal pathology in MRLlpr mice, this suggested that signaling via IL-21R on B cells could promote B cell-activation pathways in MRLlpr mice (7). GC-independent and -dependent B cell responses were reported to be activated in MRLlpr mice (9, 54). We assessed the extent of B cell activation by determining the absolute numbers of splenic GC B cells and plasma cells. We included MRL/MpJ mice in these analyses to better assess the background level of B cell activation in the absence of the lpr mutation. GC B cells were identified using a combination of cell surface markers that included GL7, PNA, and CD38. Indeed, the percentage and absolute number of splenic GC GL7+ PNA+ B cells was significantly reduced in MRLlpr.IL-21R−/− mice compared with MRLlpr.IL-21R+/+ mice (Fig. 4A, 4B). These GL7+ PNA+ B cells also expressed low levels of CD38, which is consistent with B cells bearing a GC phenotype (55) (data not shown). Immunohistochemical staining for PNA revealed GCs localized to the B cell follicles in MRLlpr.IL-21R+/+ mice (Fig. 4E). GCs were not observed in spleen sections prepared from MRLlpr.IL-21R−/− mice (Fig. 4E). The percentage and absolute number of splenic plasma cells were also significantly reduced in MRLlpr.IL-21R−/− mice compared with MRLlpr.IL-21R+/+ mice (Fig. 4C, 4D). Strikingly, the absolute numbers of GC B cells and plasma cells were similar to those present in MRL/MpJ mice, which lack the lpr mutation. In MRLlpr mice, plasmablasts accumulate in the extrafollicular space and play a critical role in production of autoantibody (9). Immunofluorescence staining identified large plasmablastic foci in the extra-follicular space in MRLlpr.IL-21R+/+ mice (Fig. 4F). By contrast, extrafollicular plasmablasts were significantly reduced in MRLlpr. IL-21R−/− mice, consistent with the flow cytometric results (Fig. 4F, 4G). Collectively, these results highlighted that signaling via IL-21R is critical for the spontaneous accumulation of activated B cell subsets in MRLlpr mice.
FIGURE 4.
Spontaneous GC formation and plasma cell accumulation is IL-21R dependent in MRLlpr mice. A, Dot plots show expression levels of PNA and GL7 on B220+ CD3− “live” splenocytes obtained from MRL/MpJ (MpJ), MRLlpr.IL-21R+/+ (WT), and MRLlpr.IL-21R−/− (KO) mice. B, Percentage (left panel) and absolute number (right panel) of B220+ CD3− splenocytes that coexpress GL7 and PNA in MpJ, WT, and KO mice. Symbols represent values obtained from individual mice, and lines indicate the mean ± SEM. Left panel, **p = 0.0014, Student t test. Right panel, **p = 0.0079, Mann–Whitney U test. C, Gating scheme for plasma cells that were identified as CD138+ B220int/lo CD3−. “Live” CD3− singlet splenocytes are shown. Percentages indicate the frequency of plasma cells in total live leukocytes. D, Absolute number of plasma cells in spleens obtained from MpJ, WT, and KO mice. **p = 0.0079, Mann–Whitney U test. E, PNA (brown) and IgD (red) staining of spleen sections prepared from MRLlpr.IL-21R+/+ (left panel) and MRLlpr.IL-21R−/− (right panel) mice. Scale bars, 100 μm. F, Immunofluorescent staining of splenic sections from MRLlpr mice either intact (left panel) or deficient (right panel) for IL-21R (n ≥ 7/genotype). Panels show extrafollicular regions stained for F4/80 (white), B220 (red), CD4 (blue), and IgM (green). Plasmablasts were identified as B220− IgM+ cells. G, Number of B220− IgM+ plasmablasts/unit area of the extrafollicular region. Symbols represent individual animals and the average of 10 samples/individual. **p = 0.006, Student t test
The significant reduction in total plasma cell numbers in MRLlpr. IL-21R−/− mice suggested that Ab production may be similarly affected. Indeed, IgG1, IgG2a, IgG3, and IgM serum titers were all significantly reduced in MRLlpr.IL-21R−/− mice (Fig. 5A, Supplemental Fig. 1). Levels of anti-nuclear and anti-dsDNA autoantibodies were likewise reduced in MRLlpr.IL-21R−/− mice (Fig. 5B–D, Supplemental Fig. 2). To further corroborate these observations, we next examined the ability of IL-21R WT and MRLlpr.IL-21R−/− B cells to secrete Ig following stimulation in vitro. Notably, even in the absence of exogenous stimulation, IL-21R WT MRLlpr B cells secreted detectable levels of IgG Ab, consistent with the increased numbers of plasma cells observed in the spleens of these animals (Fig. 5E). Stimulation of IL-21R WT MRLlpr B cells with anti-IgM and anti-CD40 resulted in an increase in detectable levels of Ab, which could be enhanced by addition of exogenous IL-21 (Fig. 5E). By contrast, IgG was barely detectable in culture supernatants when MRLlpr.IL-21R−/− B cells were stimulated under any of these conditions (Fig. 5E). Collectively, these data demonstrated that IL-21R plays a critical role in regulating the spontaneous B cell activation and autoantibody production in MRLlpr mice.
FIGURE 5.
Autoantibody production and hypergammaglobulinemia develop in an IL-21R–dependent manner in MRLlpr mice. A, Circulating levels of IgG1 Ab present in serum obtained from WT (black bars) and KO (white bars) mice at 6, 12, and 20 wk of age. Bars indicate the mean for 10 mice/group. **p ≤ 0.0016, Student t test. B, IgG ANA-staining patterns of MRLlpr.IL-21R+/+ (top panel) and MRLlpr.IL-21R−/− (bottom panel) sera in the HEp-2 assay. Original magnification ×20. C, Mean MFI values obtained from individual WT and KO mice. **p < 0.0001, Mann–Whitney U test. D, Circulating levels of IgG1 anti-dsDNA Abs in serum obtained from WT (black bars) and KO (white bars) mice at 6, 12, and 20 wk of age. Bars indicate the mean of 10 mice/group. **p ≤ 0.0012, Student t test. E, In vitro Ab production by B cells obtained from MRLlpr.IL-21R+/+ and MRLlpr.IL-21R−/− mice following coculture with medium alone, anti-IgM and anti-CD40, or anti-IgM, anti-CD40, and IL-21. *p ≤ 0.017, Student t test.
IL-21R is required for the development of Thef cells responses in MRLlpr mice
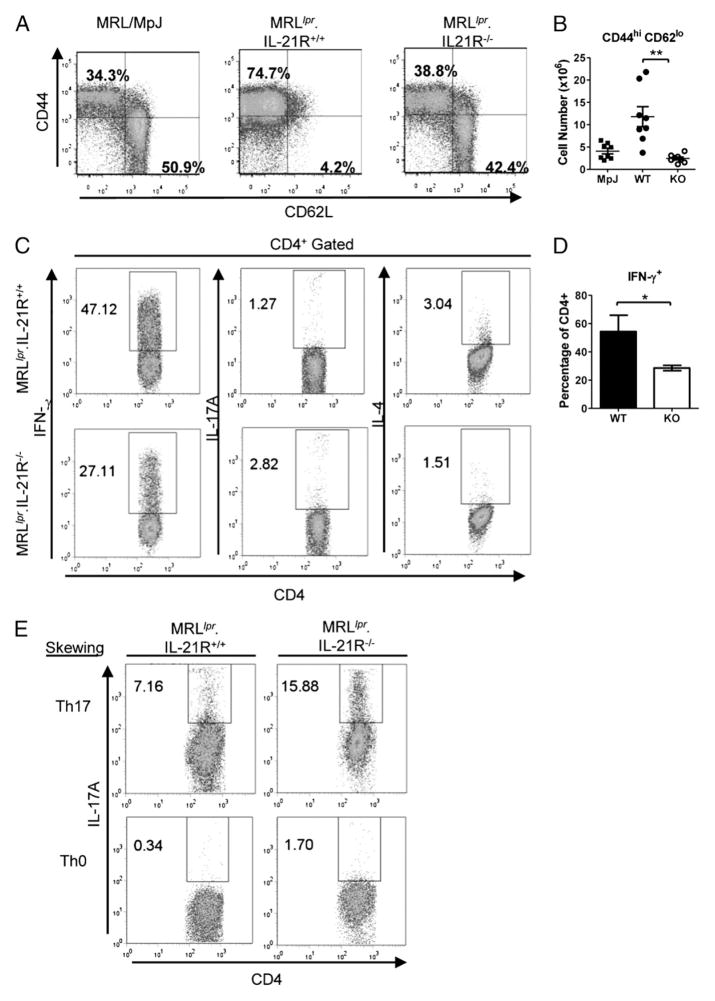
IL-21 can also induce proliferation and differentiation of CD4+ T cells. Notably, CD4+ T cells are required for the development of lupus nephritis and autoantibody production in MRLlpr mice (5, 6). Moreover, accumulation of activated CD4+ T cells, including IFN-γ–producing CD4+ T cells and Thef cells that produce IL-21, is a prominent feature in MRLlpr mice (10, 13). We next assessed CD4+ T cell activation in MRLlpr mice lacking IL-21R by first examining cell surface levels of CD44 and CD62L. Approximately 75% of CD4+ T cells in MRLlpr.IL-21R+/+ mice appeared activated, bearing a CD44hi CD62Llo cell surface phenotype (Fig. 6A). By contrast, 39% of CD4+ T cells present in spleens obtained from MRLlpr.IL-21R−/− mice expressed a similar phenotype (Fig. 6A). Remarkably, the percentage of activated CD4+ T cells in control MRL/MpJ mice, which lack the lpr mutation, was indistinguishable from that of MRLlpr.IL-21R−/− mice (Fig. 6A). Absolute numbers of CD44+ CD62Llo CD4+ T cells were also significantly reduced in MRLlpr.IL-21R−/− mice compared with MRLlpr.IL-21R+/+ mice and reached values similar to those observed in MRL/MpJ mice, indicating that activated CD4+ T cells also accumulate in MRLlpr mice via an IL-21R–dependent process (Fig. 6A, 6B).
FIGURE 6.
IFN-γ–producing CD4+ T cells are reduced in MRLlpr.IL-21R−/− mice. A, Cell surface expression of CD44 and CD62L on CD4+ B220− splenocytes from MRL/MpJ (left panel), MRLlpr.IL-21R+/+ (middle panel), and MRLlpr.IL-21R−/− (right panel) mice. B, Absolute numbers of CD4+ B220− splenocytes that are CD44hi CD62lo from MRL/MpJ (MpJ), MRLlpr.IL-21R+/+ (WT), and MRLlpr.IL-21R−/− (KO) mice. Symbols represent individual mice. Lines show the mean ± SEM. **p = 0.0003, Mann–Whitney U test. C, Expression levels of IFN-γ, IL-17A, and IL-4 by CD4+ T cells stimulated directly ex vivo. D, Percentage of CD4+ splenocytes that express IFN-γ following ex vivo stimulation of splenocytes obtained from MRLlpr.IL-21R+/+ and MRLlpr.IL-21R−/− mice. Bars show the mean value obtained from four mice/group, and error bars indicate the SEM. *p = 0.02, Mann–Whitney U test. E, Expression levels of IL-17 by CD4+ T cells from MRLlpr.IL-21R+/+ (left panels) or MRLlpr.IL-21R−/− (right panels) mice stimulated under Th0-skewing (bottom row) or Th17-skewing (top row) conditions. Percentage of cells falling in the indicated gate is shown in C and E.
Cytokine production by Th1, Th2, and Th17 CD4+ Th subsets has also been implicated in the development of pathologic features in MRLlpr mice (56, 57). To determine whether loss of IL-21R signaling in MRLlpr mice may impact cytokine production by CD4+ T cells, we examined the ex vivo cytokine profiles of splenic CD4+ T cells. Indeed, IFN-γ–producing CD4+ T cells constituted nearly 50% of total CD4+ cells in MRLlpr mice, and this population was reduced by 50% in spleens obtained from MRLlpr.IL-21R−/− mice (Fig. 6C, 6D). IL-17 and IL-4 were produced by <5% of total CD4+ T cells in MRLlpr.IL-21R+/+ and MRLlpr.IL-21R−/− mice (Fig. 6C). Interestingly, the percentage of IL-17–producing CD4+ T cells was increased by 2-fold in MRLlpr. IL-21R−/− mice compared with MRLlpr.IL-21R+/+ mice; however, similar absolute numbers of these cells were present in the two strains as a consequence of CD4+ T cell accumulation in the MRLlpr.IL-21R+/+ mice (Fig. 6C, data not shown). This suggested that Th17 cells in MRLlpr mice do not require IL-21R for their formation and that they are not required for disease progression. Consistent with this observation, IL-17 transcripts could not be detected in mRNA isolated from either kidneys or ears from diseased MRLlpr.IL-21R+/+ or MRLlpr.IL-21R−/− mice (data not shown). Moreover, CD4+ T cells from MRLlpr.IL-21R−/− spleens also produced IL-17 under Th17-skewing conditions (Fig. 6E). The percentage of IL-4–producing CD4+ T cells was reduced by 50% in MRLlpr.IL-21R−/− mice compared with MRLlpr.IL-21R+/+ mice (Fig. 5C). Collectively, these data indicated that IL-21R supports the accumulation of IFN-γ– and IL-4–producing Th subsets in MRLlpr mice.
We next quantified the numbers of Tfh and Thef cells present in MRLlpr.IL-21R+/+ and MRLlpr.IL-21R−/− mice. Tfh and Thef cells are CD4+ T cell effector subsets that are specialized for promoting GC-dependent Ab responses and extrafollicular Ab responses, respectively. The relative contribution of Tfh and Thef cells to the autoantibody production observed in MRLlpr mice remains controversial, with Thef cells reported to be the predominant inducer of Ab production in MRLlpr mice (10). However, the presence of GC B cells in MRLlpr mice in our study suggested that Tfh CD4+ T cells may also contribute (Fig. 4B, 4E). Tfh and Thef CD4+ T cells can be identified by their cell surface phenotypes. Both populations belong to a subset of activated CD4+ T cells that bear a CD4+ TCRβ+ B220− CD44hi CD62Llo PD1+ and PSGL1lo phenotype (10, 30). We observed that MRLlpr mice had an increased percentage of CD4+ T cells bearing a CD62Llo CD44hi PSGL1lo phenotype compared with MRL/MpJ and MRLlpr.IL-21R−/− mice, which suggested that IL-21R may contribute to an increased representation of Tfh or Thef cells in MRLlpr mice (Fig. 7A). We then assessed the numbers of Tfh and Thef cells based on their unique patterns of chemokine receptor expression, because Tfh cells bear a CXCR5+ CXCR4− cell surface phenotype, and Thef cells express CXCR5− CXCR4+ (10). Indeed, MRLlpr mice contained a population of Tfh cells that constituted roughly 0.75% of CD4+ T cells (Fig. 7B, 7C). Notably, this percentage did not differ between strains (Fig. 7B, 7C). Nevertheless, the absolute number of Tfh cells was significantly reduced in MRLlpr mice, consistent with the profound reduction in GC B cells seen by flow cytometry- and immunohistochemical-based analyses (Figs. 4A, 4E, 7D). This result suggested that the accumulation of Tfh cells in MRLlpr mice is also IL-21R dependent (Fig. 7D). In agreement with previous reports, Thef cells constituted a much larger per-centage of total CD4+ T cells in MRLlpr mice: ~2.5% versus 0.75% of CD4+ T cells were Thef and Tfh cells, respectively (Fig. 7E). Strikingly, MRLlpr.IL-21R−/− mice exhibited a >5-fold reduction in the percentage of CD4+ T cells bearing a Thef phenotype (Fig. 7E). Moreover, the absolute number of Thef cells was reduced by >17-fold in MRLlpr.IL-21R−/− mice compared with MRLlpr.IL-21R+/+ mice and by 2-fold compared with MRL/MpJ mice (Fig. 7F). Thus, Thef cell accumulation in MRLlpr mice is IL-21R dependent.
FIGURE 7.
IL-21R–dependent accumulation of Thef cells. A, Expression levels of CD62L and PSGL1 on CD4+ CD44hi TCRβ+ B220− splenocytes. Percentages indicate the mean value obtained from five animals. B, Percentage of CD4+ B220− splenocytes that are CXCR4− CXCR5+ PD1+ PSGL1lo CD44hi. C, Expression levels of PD1 and CXCR5 on CD4+ CXCR4− CD44hi PSGL1lo B220− splenocytes from MRL/MpJ, MRLlpr.IL-21R+/+, and MRLlpr.IL-21R−/− mice. Percentages indicate the mean value of total CD4+ cells for five mice/group. D, Absolute number of Tfh cells present in spleens obtained from MRL/MpJ (MpJ), MRLlpr.IL-21R+/+ (WT), and MRLlpr.IL-21R−/− (KO) mice. *p = 0.0159, Mann–Whitney U test. E, Expression levels of PD1 and CXCR4 on CD4+ CD44hi PSGL1lo B220− splenocytes from MRL/MpJ (left panel), MRLlpr.IL-21R+/+ (middle panel), and MRLlpr. IL-21R−/− (right panel) mice. Percentages indicate the mean value of total CD4+ cells for five mice/group. F, Absolute number of Thef cells present in spleens obtained from MRL/MpJ (MpJ), MRLlpr.IL-21R+/+ (WT), and MRLlpr.IL-21R−/− (KO) mice. **p = 0.0079, Mann–Whitney U test. Results are representative of three independent experiments.
Discussion
Our studies highlighted the novel finding that IL-21R is critical for the characteristic lymphoaccumulation and activation observed in MRLlpr mice. Specifically, we demonstrated that spontaneous GC formation and plasma cell accumulation are completely abrogated in MRLlpr mice. Consequently, total Ig levels and serum autoantibody titers are significantly reduced. We also showed that accumulation of activated CD4+ T cells is dependent on IL-21R. Thef cells were reported to be the primary inducers of Ab production in MRLlpr mice. Our studies highlighted that this unique Th population requires IL-21R for its development. Consistent with the pleiotropic abilities described for IL-21, our studies highlighted that IL-21R–derived signals are vital for activation of multiple disease-associated effector pathways in MRLlpr mice.
We previously showed that neutralizing the activity of IL-21 by therapeutic administration of IL-21R:Fc abrogated renal and serologic disease manifestations in MRLlpr mice (43). In this study, we extended these observations by demonstrating that congenital deficiency of IL-21R similarly limited the development of renal pathology and autoantibody production, firmly establishing the IL-21/IL-21R pair as key inducer of the pathologic features observed in MRLlpr mice. Notably, the primary source of IL-21 is reported to be Thef cells; however, the factors regulating the development and maintenance of this population remain poorly understood (10). Our results demonstrated that Thef cells require IL-21R for their accumulation in MRLlpr mice. Cognate interactions with Ag-specific B cells are required for the accumulation of Tfh cells, and an analogous process may support Thef cells in this model (58). Indeed, B cells promote accumulation of activated CD4+ T cells in MRLlpr mice; thus, the accumulation of Thef cells seen in MRLlpr mice may occur indirectly via the effects of IL-21 on B cells (7). Alternatively, a positive feedback loop may promote the development of these cells, as was reported for Tfh cells (34, 35).
Thef cells require ICOS for their development, because ICOS-deficient MRLlpr mice fail to accumulate Thef cells, similar to MRLlpr.IL-21R−/− mice (10). Disease development in MRLlpr. ICOS−/− mice was reduced, but it exhibited notable differences from that seen in MRLlpr.IL-21R−/− mice (11, 12). Proteinuria and cutaneous lesions were unaffected in the absence of ICOS; additionally, accumulation of B cells, DN T cells, and activated CD4+ T cells still occurred (11, 12). This suggested that Thef cells are not the sole source of IL-21 in this model. We did identify a small population of Tfh cells in MRLlpr mice, which was not observed by investigators examining MRLlpr.ICOS+/+ mice; this may have resulted from environmental variation between laboratories and/or differences in the sensitivities of the staining protocols used (10). Notably, disease features were maintained in the absence of Thef and Tfh cells in MRLlpr.ICOS−/− mice, suggesting that IL-21 derived from alternative cellular sources, including the Th1, Th2, and Th17 populations present in MRLlpr mice, may support lymphocyte accumulation, development of proteinuria, and skin lesions. Further studies to identify potential cellular sources of IL-21 will be dependent on the generation of IL-21 reporter animals or validated intracellular staining reagents.
Expression of IL-21R is increased in the kidneys of MRLlpr mice, and immunohistochemical staining revealed IL-21R expression on a subset of cells in the perivascular infiltrates. By flow cytometric analysis, we determined that CD19+ B cells made up a small fraction of intrarenal leukocytes that expressed the highest levels of IL-21R, possibly explaining the limited distribution of IL-21R staining observed by immunohistochemistry. Nevertheless, all intrarenal lymphocyte populations examined, which also included CD4+, CD8+, and DN T cells, expressed IL-21R at varying levels. These observations suggested that IL-21 may promote pathology locally via effects on renal infiltrates. Notably, activated CD4+ T cells accumulate in the kidneys of MRLlpr mice, and a subset of these cells bear a Tfh-like phenotype and, thus, may function as a local source of IL-21 (11, 59). We observed that CD4+ T cells made up the predominant infiltrating cell type in kidneys of MRLlpr mice, and, notably, IL-21R was critical for their pathologic accumulation. Using quantitative RT-PCR analysis, we were unable to detect IL-21 message in kidneys obtained from MRLlpr mice. However, this is not necessarily indicative of local IL-21 protein levels, because secretion of IL-21 by Thef cells that accumulate in secondary lymphoid organs might also lead to elevated circulating levels of cytokine (10).
Autoantibodies to nuclear Ags, particularly those reactive with dsDNA, are thought to play a primary role in the pathogenesis of SLE (60, 61). In MRLlpr mice, these autoantibodies are often somatically mutated and affinity matured (62–64). Recent studies identified autoreactive B cells at extrafollicular sites that are somatically mutated and clonally related, suggesting that autoanti-body responses can also develop via an extrafollicular pathway in MRLlpr mice (9, 65). We showed that spontaneous GC formation and plasma cell accumulation requires IL-21R in MRLlpr mice. Consistent with this observation, early extrafollicular Ab-secreting cell responses and GC formation are impaired in IL-21R/IL-21–deficient animals following immunization with T-dependent Ags (24, 25, 28–30). By contrast, the spontaneous formation of GCs in the lupus-prone sanroque model occurs in an IL-21R–independent manner, thus indicating that both IL-21-dependent and -independent mechanisms can regulate GC activity (66). GC B cells were shown to express IL-21R, thus suggesting that the profound loss of GC B cells in MRLlpr.IL-21R−/− mice is mediated via direct effects of IL-21 on these cells (31). Interestingly, immunization of IL-21R–deficient animals with the T-dependent Ag NP-CGG resulted in impaired GC formation, as well as accelerated memory B cell accumulation (25). Moreover, it is noteworthy that the af-finity of the Abs produced by post-GC B cells was significantly reduced in IL-21R−/− mice immunized with a T-dependent Ag (25). Therefore, it seems possible that loss of IL-21R signaling in MRLlpr mice may not only impact the spontaneous accumulation of GC B cells, it may also restrict the quality of GC-derived class-switched autoantibodies, which would have important implications for therapeutic blockade of IL-21/IL-21R in B cell-mediated autoimmune diseases. Whether IL-21R could function similarly in the extrafollicular autoantibody response observed in MRLlpr mice remains to be determined.
Activated CD44+ CD62Llo CD4+ T cells accumulated in an IL-21R–dependent manner in MRLlpr mice. Previous studies showed that activated CD4+ T cells express elevated levels of IL-21R, and in vitro IL-21 provides a comitogenic stimulus for anti-CD3–treated T cells (23). In addition, treatment of NOD mice with IL-21 resulted in enhanced division and accumulation of CD4+ CD44+ T cells (67). These observations suggested that IL-21 may mediate accumulation of activated CD4+ T cells in MRLlpr mice by promoting proliferation. IL-21 was reported to impact the differentiation of Th1 and Th17 cells (32, 38, 68, 69). Consistent with these observations, we found that IL-21R–deficient MRLlpr mice had fewer IFN-γ–producing T cells. Elevated levels of plasma IFN-γ correlate with disease activity in both SLE patients and murine models of lupus (70–73). In addition, MRLlpr mice deficient in IFN-γ have delayed mortality, reduced anti-dsDNA Ab titers, and decreased severity of lymphadenopathy and glomerulonephritis (15, 16). This suggests that the decreased renal pathology observed in MRLlpr.IL-21R−/− mice may also result from impaired generation of the Th1 response.
IL-21 was also demonstrated as an autocrine growth factor for Th17 cells; however, the role of IL-17 in the pathogenesis of SLE remains unclear. Serum levels of IL-17 are elevated in SLE patients, and an increased frequency of IL-17–producing Th cells in SLE patients has been reported (74–77). Similarly, in the recombinant inbred autoimmune-prone BXD2 strain of mice, it was noted that Th17 cells are present in elevated numbers and that IL-17R deficiency resulted in a significant reduction in autoanti-body titers (37). Interestingly, CD4+ T cells from MRLlpr mice also produce IL-17, and the development of renal pathology is IL-23R dependent, suggesting that IL-17 may be required for the development of lupus nephritis in C57BL/6lpr mice (78, 79). Surprisingly, we observed a small population of IL-17–producing CD4+ T cells in WT MRLlpr mice that appeared increased in the IL-21R–deficient background. This result indicated that Th17 cell formation is not dependent on IL-21R in MRLlpr mice, which was further confirmed by the observation that MRLlpr.IL-21R−/− splenic T cells could differentiate into IL-17–producing cells in vitro when stimulated under Th17-skewing culture conditions. IL-17 transcripts were undetectable in diseased kidneys and skin from MRLlpr mice (data not shown). Similarly, Bubier et al. (42) recently reported that neither IL-17–producing T cells nor IL-17 transcripts could be detected in kidneys of diseased lupus-prone BSXB-Yaa mice, suggesting that IL-17 is not required for the development of SLE-like disease features in these particular models.
In summary, we showed that the IL-21 pathway drives systemic autoimmunity in the MRLlpr model of SLE by promoting both pathogenic B and T cell responses in these mice. Only a single new therapy has been approved by the U.S. Food and Drug Administration in the past 47 y, which is due, in part, to the clinical heterogeneity of the disease. B cells are thought to play a central role in the pathogenesis of SLE, and many recently developed experimental therapeutics have focused on either eliminating or limiting the activity of these cells (80). However, T cells are also major contributors to the disease processes via B cell-dependent and -independent pathways (81). The profound dampening of lymphocyte effector activation by inhibition of IL-21 signaling suggests that blockade of the IL-21 pathways may be a promising strategy for the treatment of B and T cell-mediated autoimmune diseases, such as SLE.
Supplementary Material
Acknowledgments
We thank Dr. Terrie Cunliffe-Beamer and Kim Muzzi for assistance with backcrossing and breeding IL-21R–deficient and IL-21R WT MRLlpr mice and Jameel Syed for expert technical assistance.
Abbreviations used in this article
- ANA
anti-nuclear autoantibody
- DN
double negative
- GBM
glomerular basement membrane
- GC
germinal center
- KO
knockout
- MFI
mean fluorescence intensity
- MRLlpr
MRL/MpJ-Faslpr/lpr/J
- PAS
periodic acid-Schiff
- PNA
peanut agglutinin
- SLE
systemic lupus erythematosus
- Tfh
T follicular helper
- Thef
T extrafollicular helper
- WT
wild-type
Footnotes
Disclosures
A.L.R., H.G., D.H., T.A.D., Y.C., S.K., N.S., M.R., L.B., Q.M., M.C., C.N.-N., D.Y., and K.D.-J. are current or former employees of Pfizer Inc. A.L.R., T.A.D., Y.C., M.S., M.R., Q.M., M.C., C.N.-N., D.Y., and K.D.-J. have equity interests in Pfizer Inc. S.A.B. and J.C. have no financial conflicts of interest.
References
- 1.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 2.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jevnikar AM, Grusby MJ, Glimcher LH. Prevention of nephritis in major histocompatibility complex class II-deficient MRL-lpr mice. J Exp Med. 1994;179:1137–1143. doi: 10.1084/jem.179.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabs DA, Burek CL, Hu Q, Kuppers RC, Lee B, Prendergast RA. Anti-CD4 monoclonal antibody therapy suppresses autoimmune disease in MRL/Mp-lpr/lpr mice. Cell Immunol. 1992;141:496–507. doi: 10.1016/0008-8749(92)90166-m. [DOI] [PubMed] [Google Scholar]
- 6.Koh DR, Ho A, Rahemtulla A, Fung-Leung WP, Griesser H, Mak TW. Murine lupus in MRL/lpr mice lacking CD4 or CD8 T cells. Eur J Immunol. 1995;25:2558–2562. doi: 10.1002/eji.1830250923. [DOI] [PubMed] [Google Scholar]
- 7.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng GM, Liu L, Kyttaris VC, Tsokos GC. Lupus serum IgG induces skin inflammation through the TNFR1 signaling pathway. J Immunol. 2010;184:7154–7161. doi: 10.4049/jimmunol.0902514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 10.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odegard JM, DiPlacido LD, Greenwald L, Kashgarian M, Kono DH, Dong C, Flavell RA, Craft J. ICOS controls effector function but not trafficking receptor expression of kidney-infiltrating effector T cells in murine lupus. J Immunol. 2009;182:4076–4084. doi: 10.4049/jimmunol.0800758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tada Y, Koarada S, Tomiyoshi Y, Morito F, Mitamura M, Haruta Y, Ohta A, Nagasawa K. Role of inducible costimulator in the development of lupus in MRL/lpr mice. Clin Immunol. 2006;120:179–188. doi: 10.1016/j.clim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Budd RC, Schumacher JH, Winslow G, Mosmann TR. Elevated production of interferon-gamma and interleukin 4 by mature T cells from au-toimmune lpr mice correlates with Pgp-1 (CD44) expression. Eur J Immunol. 1991;21:1081–1084. doi: 10.1002/eji.1830210435. [DOI] [PubMed] [Google Scholar]
- 14.Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol. 1998;161:494–503. [PubMed] [Google Scholar]
- 15.Haas C, Ryffel B, Le Hir M. IFN-gamma is essential for the development of autoimmune glomerulonephritis in MRL/Ipr mice. J Immunol. 1997;158:5484–5491. [PubMed] [Google Scholar]
- 16.Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101:364–371. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci USA. 2000;97:11439–11444. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 19.Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, Sugamura K. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–665. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 21.Brandt K, Bulfone-Paus S, Foster DC, Rückert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–4098. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- 22.Pelletier M, Bouchard A, Girard D. In vivo and in vitro roles of IL-21 in inflammation. J Immunol. 2004;173:7521–7530. doi: 10.4049/jimmunol.173.12.7521. [DOI] [PubMed] [Google Scholar]
- 23.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 24.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, III, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 25.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 27.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 28.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bessa J, Kopf M, Bachmann MF. Cutting edge: IL-21 and TLR signaling regulate germinal center responses in a B cell-intrinsic manner. J Immunol. 2010;184:4615–4619. doi: 10.4049/jimmunol.0903949. [DOI] [PubMed] [Google Scholar]
- 30.Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 32.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 33.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 34.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Pan HF, Ye DQ, Li XP. Type 17 T-helper cells might be a promising therapeutic target for systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2008;4:352–353. doi: 10.1038/ncprheum0815. [DOI] [PubMed] [Google Scholar]
- 37.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in au-toimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 38.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawalha AH, Kaufman KM, Kelly JA, Adler AJ, Aberle T, Kilpatrick J, Wakeland EK, Li QZ, Wandstrat AE, Karp DR, et al. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Ann Rheum Dis. 2008;67:458–461. doi: 10.1136/ard.2007.075424. [DOI] [PubMed] [Google Scholar]
- 40.Hughes T, Kim-Howard X, Kelly JA, Kaufman KM, Langefeld CD, Ziegler J, Sanchez E, Kimberly RP, Edberg JC, Ramsey-Goldman R, et al. BIOLUPUS Network. Fine-mapping and transethnic genotyping establish IL2/IL21 genetic association with lupus and localize this genetic effect to IL21. Arthritis Rheum. 2011;63:1689–1697. doi: 10.1002/art.30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webb R, Merrill JT, Kelly JA, Sestak A, Kaufman KM, Langefeld CD, Ziegler J, Kimberly RP, Edberg JC, Ramsey-Goldman R, et al. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum. 2009;60:2402–2407. doi: 10.1002/art.24658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, III, Leonard WJ, Roopenian DC. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci USA. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol. 2007;178:3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- 44.Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, Johnson KA, Witek JS, Senices M, Konz RF, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–569. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 45.Vlahakos D, Foster MH, Ucci AA, Barrett KJ, Datta SK, Madaio MP. Murine monoclonal anti-DNA antibodies penetrate cells, bind to nuclei, and induce glomerular proliferation and proteinuria in vivo. J Am Soc Nephrol. 1992;2:1345–1354. doi: 10.1681/ASN.V281345. [DOI] [PubMed] [Google Scholar]
- 46.Vlahakos DV, Foster MH, Adams S, Katz M, Ucci AA, Barrett KJ, Datta SK, Madaio MP. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 1992;41:1690–1700. doi: 10.1038/ki.1992.242. [DOI] [PubMed] [Google Scholar]
- 47.Jiang C, Foley J, Clayton N, Kissling G, Jokinen M, Herbert R, Diaz M. Abrogation of lupus nephritis in activation-induced deaminase-deficient MRL/lpr mice. J Immunol. 2007;178:7422–7431. doi: 10.4049/jimmunol.178.11.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habib T, Nelson A, Kaushansky K. IL-21: a novel IL-2-family lymphokine that modulates B, T, and natural killer cell responses. J Allergy Clin Immunol. 2003;112:1033–1045. doi: 10.1016/j.jaci.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 49.Merino R, Fossati L, Iwamoto M, Takahashi S, Lemoine R, Ibnou-Zekri N, Pugliatti L, Merino J, Izui S. Effect of long-term anti-CD4 or anti-CD8 treatment on the development of lpr CD4− CD8− double negative T cells and of the autoimmune syndrome in MRL-lpr/lpr mice. J Autoimmun. 1995;8:33–45. doi: 10.1006/jaut.1995.0003. [DOI] [PubMed] [Google Scholar]
- 50.Mehal WZ, I, Crispe N. TCR ligation on CD8+ T cells creates double-negative cells in vivo. J Immunol. 1998;161:1686–1693. [PubMed] [Google Scholar]
- 51.Fröhlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 52.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luzina IG, Atamas SP, Storrer CE, daSilva LC, Kelsoe G, Papadimitriou JC, Handwerger BS. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001;70:578–584. [PubMed] [Google Scholar]
- 55.Oliver AM, Martin F, Kearney JF. Mouse CD38 is down-regulated on germinal center B cells and mature plasma cells. J Immunol. 1997;158:1108–1115. [PubMed] [Google Scholar]
- 56.Peng SL, Moslehi J, Craft J. Roles of interferon-gamma and interleukin-4 in murine lupus. J Clin Invest. 1997;99:1936–1946. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinmetz OM, Turner JE, Paust HJ, Lindner M, Peters A, Heiss K, Velden J, Hopfer H, Fehr S, Krieger T, et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol. 2009;183:4693–4704. doi: 10.4049/jimmunol.0802626. [DOI] [PubMed] [Google Scholar]
- 58.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barber DF, Bartolomé A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, Camps M, Rückle T, Schwarz MK, Rodríguez S, et al. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005;11:933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- 60.Slack JH, Hang L, Barkley J, Fulton RJ, D’Hoostelaere L, Robinson A, Dixon FJ. Isotypes of spontaneous and mitogen-induced autoanti-bodies in SLE-prone mice. J Immunol. 1984;132:1271–1275. [PubMed] [Google Scholar]
- 61.Okamura M, Kanayama Y, Amastu K, Negoro N, Kohda S, Takeda T, Inoue T. Significance of enzyme linked immunosorbent assay (ELISA) for antibodies to double stranded and single stranded DNA in patients with lupus nephritis: correlation with severity of renal histology. Ann Rheum Dis. 1993;52:14–20. doi: 10.1136/ard.52.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kita Y, Sumida T, Ichikawa K, Maeda T, Yonaha F, Iwamoto I, Yoshida S, Koike T. V gene analysis of anticardiolipin antibodies from MRL-lpr/ lpr mice. J Immunol. 1993;151:849–856. [PubMed] [Google Scholar]
- 63.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171:265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in au-toimmunity. Nature. 1987;328:805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- 65.William J, Euler C, Shlomchik MJ. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol. 2005;174:6879–6887. doi: 10.4049/jimmunol.174.11.6879. [DOI] [PubMed] [Google Scholar]
- 66.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 68.Suto A, Wurster AL, Reiner SL, Grusby MJ. IL-21 inhibits IFN-gamma production in developing Th1 cells through the repression of Eomesodermin expression. J Immunol. 2006;177:3721–3727. doi: 10.4049/jimmunol.177.6.3721. [DOI] [PubMed] [Google Scholar]
- 69.Fina D, Sarra M, Caruso R, Del Vecchio Blanco G, Pallone F, MacDonald TT, Monteleone G. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut. 2008;57:887–892. doi: 10.1136/gut.2007.129882. [DOI] [PubMed] [Google Scholar]
- 70.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with au-toimmune disease. N Engl J Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 71.al-Janadi M, Aal-Dalaan Sal-Balla, Raziuddin S. Cytokine profile in systemic lupus erythematosus, rheumatoid arthritis, and other rheumatic diseases. J Clin Immunol. 1993;13:58–67. doi: 10.1007/BF00920636. [DOI] [PubMed] [Google Scholar]
- 72.Prud’homme GJ, Kono DH, Theofilopoulos AN. Quantitative polymerase chain reaction analysis reveals marked overexpression of interleukin-1 beta, interleukin-1 and interferon-gamma mRNA in the lymph nodes of lupus-prone mice. Mol Immunol. 1995;32:495–503. doi: 10.1016/0161-5890(95)00024-9. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi S, Fossati L, Iwamoto M, Merino R, Motta R, Kobayakawa T, Izui S. Imbalance towards Th1 predominance is associated with acceleration of lupus-like autoimmune syndrome in MRL mice. J Clin Invest. 1996;97:1597–1604. doi: 10.1172/JCI118584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus eryth-ematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 75.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 76.Chen XQ, Yu YC, Deng HH, Sun JZ, Dai Z, Wu YW, Yang M. Plasma IL-17A is increased in new-onset SLE patients and associated with disease activity. J Clin Immunol. 2010;30:221–225. doi: 10.1007/s10875-009-9365-x. [DOI] [PubMed] [Google Scholar]
- 77.Crispín JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biswas PS, Gupta S, Chang E, Song L, Stirzaker RA, Liao JK, Bhagat G, Pernis AB. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest. 2010;120:3280–3295. doi: 10.1172/JCI42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC. Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J Immunol. 2010;184:4605–4609. doi: 10.4049/jimmunol.0903595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tieng AT, Peeva E. B-cell-directed therapies in systemic lupus erythematosus. Semin Arthritis Rheum. 2008;38:218–227. doi: 10.1016/j.semarthrit.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 81.Hoffman RW. T cells in the pathogenesis of systemic lupus erythematosus. Clin Immunol. 2004;113:4–13. doi: 10.1016/j.clim.2004.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.