Abstract
Background
Lubiprostone has been used to treat constipation through its effects to stimulate Cl− secretion, resulting in water and electrolyte secretion.
Aim
Potential associated changes in intestinal mucus and the colonizing bacteria (microbiome) have not been studied. As mucus obstructions may play a role in cystic fibrosis, the hypothesis that lubiprostone alters intestinal mucus and the microbiome was investigated.
Methods
Ion transport studies were performed ex vivo. For mucus and microbiome studies, mice were gavaged daily with lubiprostone or vehicle. Mucin from intestinal sections was analyzed in Carnoy’s fixed tissues stained with Alcian blue. Microbiome composition was analyzed by 16S rRNA gene-based sequencing.
Results
Lubiprostone stimulated short circuit current in all mouse intestinal segments after both serosal and mucosal additions, albeit at lower concentrations in the latter. Current was Cl-dependent and blocked by mucosal diphenylcarboxylic acid, serosal bumetanide, and serosal Ba++. The CFTR inhibitor CFTRinh172 had a marginal effect. Mucus near epithelial cells (inner layer mucus) was not present in the small intestine of any mice. Proximal colon inner mucus layer was thicker in ΔF/ΔF compared with +/ΔF and +/+ mice. Lubiprostone decreased inner mucus layer thickness in both proximal and distal colon of all mice. Furthermore, lubiprostone altered the intestinal microbiome by increasing abundance of Lactobacillus and Alistipes.
Conclusions
Lubiprostone activates non-CFTR Cl− secretion and alters the colonic inner mucus layer, which is associated with changes in the composition of the enteric microbiome.
Keywords: Intestinal mucus, Intestinal microbiota, Cl− secretion, Lubiprostone
Introduction
Cystic fibrosis (CF) is the most common fatal autosomal recessive disease in Caucasians, caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Of these mutations, ΔF508 is the most prevalent [1]. Because CFTR is a cAMP-regulated anion channel critical for epithelial Cl− and bicarbonate secretion, CF patients have thickened mucus secretions that can result in many of the associated complications of this disease.
The mucus layer of the intestinal tract is an important first line of defense against microbes and physical injury. It exists in two layers, an inner firmly adherent mucus layer and an outer loosely adherent layer [2]. Commensal microbes are associated with the outer mucus layer, but both are principally comprised of mucin glycoproteins secreted by goblet cells. Thickened intestinal mucus secretions in CF patients lead to a variety of intestinal disorders including meconium ileus, rectal prolapse, volvulus, and distal obstructive syndrome [3]. CF patients also suffer from abdominal pain, steatorrhea, poor nutritional state, and constipation [3]. Capsule endoscopy studies have even demonstrated specific signs of small bowel inflammation including edema, erythema, mucosal breaks, and frank ulceration [4].
Altered secretions of the respiratory epithelium in CF patients are associated with chronic and abnormal changes in bacterial colonization patterns, particularly with abundant Pseudomonas aeruginosa [5]. Patients with CF have also have increased small bowel bacterial overgrowth, although no specific organism has been identified [6]. Previous studies, however, have been limited by the inability of conventional microbiological cultivation approaches to characterize human and mammalian gastrointestinal microbiota. In this regard, new advances in cultivation-independent approaches for microbiota analysis are now available for analysis of 16S rRNA profiles and taxon structure [7]. This approach provides a more complete picture of the composition of human intestinal microbiota, and may elucidate previously unrecognized bacterial patterns.
Lubiprostone is a bicyclic fatty acid of the prostone group derived from a metabolite of prostaglandin E1 [8], which is metabolized quickly and has low systemic bio-availability resulting in a local intestinal effect. In a number of studies, lubiprostone has been shown to stimulate Cl− secretion [9-14] resulting in accumulation of water in intestinal segments (enteropooling). This action is believed to be important for hydration and softening of stool, increased motility, and promotion of spontaneous bowel movements. Lubiprostone has been hypothesized to activate isoform 2 of the ClC channel family in addition to the CFTR Cl− channel [10-16]. As such, it has the potential to compensate for impaired Cl− secretion in CF patients. The activation of the alternate ClC-2 secretion pathway could potentially lead to improved fluid secretion, improved intestinal motility, and decreased mucus viscosity. This could ameliorate intestinal obstructive symptoms and pathogenic small bowel bacterial colonization in patients with CF. We hypothesized that lubiprostone can improve intestinal Cl− secretion in cystic fibrosis, and restore mucus properties and health-associated bacterial colonization. Using the ΔF508 mouse model of CF, we report that lubiprostone primarily induces a CFTR-independent anion secretion, which is associated with decreased thickness of the proximal and distal colonic mucus layer and altered structure of the enteric microbiota.
Methods
Mice
The ΔF508-mutation CFTR mouse line was obtained from Dr. Lane Clarke at the University of Missouri in Columbia [17]. This mouse has been used extensively in studies of ion transport abnormalities in cystic fibrosis (CF) because it carries the same CFTR mutation observed in the most common form of human CF. Both heterozygous (+/ΔF) and homozygous ΔF/ΔF mice were studied. All mice (including +/+) were obtained from the same breeding pairs to try to minimize variation in genetic background. Of note, this line of ΔF508 CF mouse was developed on a SvF129 × C57BL/6 background [17]. For our studies, the ΔF508 CF mice were outbred to Black Swiss mice (Taconic, Hudson, NY, USA) at intervals to reduce inbreeding depression and maintain viability of offspring. All mouse studies were approved by the University of Chicago Animal Care and Use Committee (IACUC protocol 71813). Mice were genotyped using tail snips taken at 3 weeks of age using CFM26, 27, and 28 primers for PCR as previously described [17]. Mice were maintained on standard mouse diet and Golytely (Braintree Labs, Braintree, MA, USA) was provided in the drinking water at one-half concentration for up to 2 weeks prior to experiments to prevent intestinal mucus obstruction. Autoclaved tap water was provided for all mice during the experiment to provide standard conditions. Experimental groups included (1) daily gavage of 50 μl volume with lubiprostone (10 mg/kg) in a medium chain triglyceride (MCT) solution vehicle (Miglyol 812 N), (2) daily gavage of 50 μl volume MCT solution vehicle, or (3) no treatment. The MCT vehicle was used for in vivo studies, as previously described [12]. Stool samples were then acquired 7 days after initiation of MCT or lubiprostone treatment. Mice were sacrificed and sections of all intestinal segments fixed for analysis of mucus thickness using histochemical analysis of stained sections. Mice used for these studies were between the ages of 8–16 weeks, weighing between 22 and 27 g. For in vivo treatment studies (inner mucus layer as well as microbiota), littermates were used.
Ion Transport Studies
Standard small volume Ussing chambers (Physiological Instruments, San Diego, CA, USA) were used to study the ex vivo effects of lubiprostone on intestinal ion transport. One-cm segments from jejunum, ileum, and proximal and distal colon were mounted ex vivo in the chambers for measurement of short circuit current (Isc) and transepithelial electrical resistance (TER). Tissues were placed into Ringer solution gassed with 95 %O2/5 % CO2 at 25 °C, as the intestinal strips contract in ice-cold saline. In addition, the muscularis propria was not dissected from the intestinal strips in order to preserve tissue integrity. Cyclooxygenase inhibitors and tetrodotoxin were not added to the tissue before placing in the Ussing chambers. Standard Krebs bicarbonate Ringer solution was used for the bathing media (composition in mmol/l: 127 NaCl, 5 KCl, 1.25 MgCl2, 1 CaCl2, NaHPO4, Na2HPO4, 25 NaHCO3) that was gassed with 5 % CO2/95 % O2 resulting in a pH of 7.4. To determine anion dependence of Isc responses, equimolar gluconate salts were used to replace [Cl−] in the buffer, keeping [HCO3−] constant. Lubiprostone [diluted in ethanol from a frozen stock (2 mM) in dimethyl sulfoxide] was added to either the mucosal or serosal chambers to examine its effects on stimulated Isc from a stock diluted in ethanol. In no case was there greater than 0.1 % v/v ethanol use in the Ussing chamber solutions. Inhibitor agents (dissolved in water or ethanol at 1,000 fold concentrations) were added to determine their effects on basal and lubiprostone-induced changes in Isc and TER. These agents included diphenylcarboxylic acid (DPC. mucosal), a general chloride channel blocker, CFTRinh172 (a specific CFTR channel blocker, mucosal and serosal), barium (to block basolateral K+ channels required for Cl− secretion, serosal), and bumetanide (a Na+–K+–2Cl− cotransport inhibitor whose action is also required for Cl− secretion, serosal). Voltage measurements were taken using agar/3 M KCl bridges connected to automatic voltage clamps.
For all short circuit current studies, individual tissues were stimulated with one concentration of lubiprostone rather than sequential addition of increasing concentrations to the same tissue. This experimental paradigm was employed as there appeared to be tachyphylaxis to this agent. In these studies, pretreatment of wild-type (WT) mouse ileum with lubiprostone decreased the short circuit current response to forskolin [13], similar to results observed in human colonic T84 cells [14]. For some studies, a low (30 nM) and high concentration (1,000 nm) were used since lubiprostone appears to have a concentration-dependent effect on duodenal HCO3− and Cl− transport [12]. Lubiprostone-stimulated duodenal bicarbonate secretion was stimulated in a dose-dependent manner starting at low 10 nM and increasing to 1 μM, whereas net Cl− output and net water output were only increased at concentrations above 0.1 μM, suggesting two stimulated transport pathways. Additional data from Xenopus A6 cells suggested that concentrations of lubiprostone less than 100 nM stimulate the ClC-2 Cl− channel, whereas concentrations greater than 100 nM activate the CFTR Cl− channel [16].
Mucus Thickness Measurements
For measurements of mucus thickness, chloroform-based Carnoy’s fixative was used to better preserve mucus integrity as formaldehyde fixation has been noted to dehydrate mucus. Tissues were immediately placed into Carnoy’s fixative (chloroform:ethanol: acetic acid 60:30:10 by vol.) for 4 h, transferred to 100 % ethanol and immediately processed. Paraffin sections (4 μm) were cut and stained with both periodic acid Schiff as well as Alcian blue (Sigma Chemical, St. Louis, MO, USA). Slides were imaged on a Leica DM2500 microscope and analyzed using NIH Image J software. Three sections were used for each analysis and, on each section, 20 random locations were used for mucus layer thickness analysis.
DNA Extraction, 16S rRNA Gene PCR, and Clone Library Sequence Analysis
Fecal samples were first homogenized in 1 ml extraction buffer [50 mM Tris (pH 7.4), 100 mM EDTA (pH 8.0), 400 mM NaCl, 0.5 % SDS] containing 20 μl proteinase K (20 mg/ml). A 500-μl slurry of 0.1-mm-diameter zirconia/silica beads (BioSpec Products, Bartlesville, OK, USA) was added into the extraction tubes and lysed with a Mini-Beadbeater-8k Cell Disrupter (BioSpec Products) set for 5 min. After overnight incubation at 55 °C, standard DNA extraction with phenol:chloroform:isoamyl alcohol, and precipitation with ethanol was performed. Isolated DNA was dissolved in TE buffer and stored at −80 °C.
Bacterial populations in the samples were analyzed by 16S rRNA gene sequence-based analysis as described previously [18]. Briefly, 16S rRNA gene sequences were amplified from DNA templates using broad-range primers 8F (5′-AGAGTT TGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). PCR products were separated by agarose gel electrophoresis and products were purified by QIAquick gel extraction kit (Qiagen, Valencia, CA, USA) and cloned into pCR-2.1-TOPO® vectors (Invitrogen, Carlsbad, CA, USA) using the TOPO-TA Cloning Kit according to the manufacturer’s instructions. From each library, 100 colonies were picked randomly and processed for DNA sequencing using 8F as the primer.
Sequence Alignment and Phylogenetic Analysis
The 16S rRNA gene sequences were analyzed as described previously [18]. Briefly, raw sequence data were processed and aligned by using the RDP pipeline server at the Ribosomal Database Project II (RDP-II) website (http://rdp.cme.msu.edu/pipeline) [19]. The program DOTUR with the furthest neighbor algorithm was used to group sequences into operational taxonomical units (OTUs) or phylotypes which represented the number of 16S rRNA sequence similarity groupings. A 97 % cutoff for similarity was used. For principal component analysis (PCA), all 16S rRNA gene sequences were imported into the ARB software package [20] and aligned into a phylogenetic tree which was used to perform clustering analysis using online UniFrac without abundance weighting [21]. The p test in the UniFrac was performed to determine whether each sample was significantly different from others. All sequences have been deposited in GenBank: JQ694723–JQ695800.
Results
Effects of Lubiprostone on Short Circuit Current (Isc)
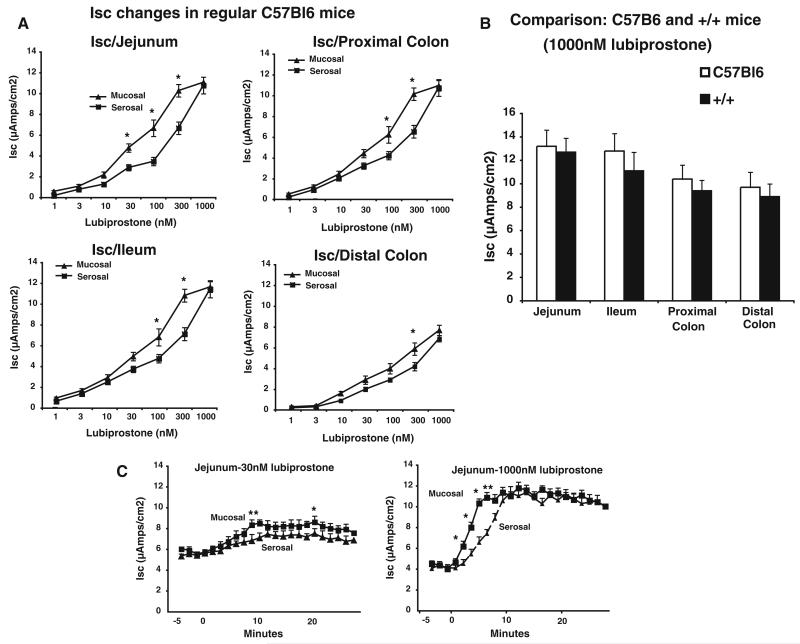
The effects of lubiprostone were tested in C57BL/6J mice as well as in mice or mixed backgrounds that were heterozygous or homozygous for the ΔF508 allele CF or wt CF gene (respectively +/ΔF, ΔF/ΔF, and +/+). As the genetic background might influence the response to lubiprostone, the same breeding pairs of mice outbred to Black Swiss mice were used to minimize genetic variation. We also initially compared C57BL/6J mice to the +/+ mice to determine that if the effects of lubiprostone were comparable in those of mixed background mouse lines. All transport studies were performed ex vivo in Ussing chambers. Consistent with previous reports, we found that lubiprostone increased Isc in all gut segments when added to either the mucosal or serosal sides of mouse intestine of C57BL/6J mice (Fig. 1a). A maximal concentration of 1,000 nM lubiprostone was used to compare maximal Isc changes in mice with CF+/+ alleles of mixed and C57BL/6J backgrounds. No significant differences were noted in any intestinal segment (Fig. 1b). In C57BL/6J mice, we determined the effects of lubiprostone were slightly more rapid when applied mucosally (Fig. 1b) in all segments of the mouse intestine (data not shown). As a control for the subsequent in vivo studies, 50 μl of medium chain triglyceride solution without lubiprostone was added to determine its effects on Isc. In both +/+ and +/ΔF mice, no effects on Isc were observed (data not shown).
Fig. 1.
Lubiprostone stimulates short circuit current in jejunum and distal colon of mouse intestine. a Segments of the intestine along the longitudinal length of C57BL/6J mice were mounted in Ussing chambers and the concentration dependence of mucosal or serosal addition of lubiprostone-stimulated changes in short circuit determined. Maximal Isc changes were recorded. *p < 0.05 comparing mucosal versus serosal addition at same concentration by paired Student’s t test. b Comparison of Isc changes in C57BL/6J and +/+ mice obtained by breeding +/ΔF508 mice in intestinal segments. No statistical differences were noted in any segment by paired Student’s t test. c For the jejunum, time courses of mucosal versus serosal addition of 30 or 1,000 nM lubiprostone are presented. Data are mean ± SEM for 6 mice for each set of experiments. *p < 0.05 comparing mucosal versus serosal addition at same time point for mucosal versus serosal addition by paired Student’s t test
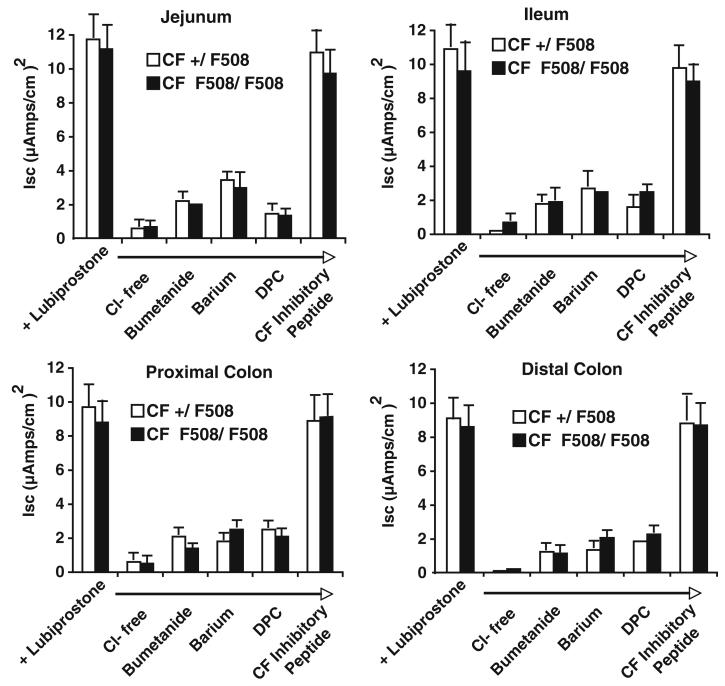
The effects of lubiprostone on current in +/ΔF and ΔF/ΔF mice were similar to those of +/+ (comparison of Isc changes in Fig. 1a and control values in Fig. 2). To determine if the changes in Isc were due to active Cl− secretion, a Cl−-free buffer was used. Additionally, pharmacologic inhibition of transporters required for Cl− secretion were tested in the +/ΔF and ΔF/ΔF mice (Fig. 2). In both +/ΔF and ΔF/ΔF mice, omission of Cl− from the bathing medium abolished the Isc response in all segments of mouse intestine (Fig. 2). Lubiprostone-stimulated changes in Isc were also blocked by serosal addition of bumetanide, a Na+-K+-2Cl− cotransport inhibitor, serosal addition of barium, a K+ channel blocker, and luminal addition of DPC (diphenylcarboxylic acid, a general Cl− channel inhibitor (Fig. 2). In contrast, changes in lubiprostone-stimulated Isc were not blocked by luminal addition of the CFTR channel inhibitor-172 (Fig. 2).
Fig. 2.
Lubiprostone-stimulated short circuit current changes represent non-CFTR mediated chloride secretion. Lubiprostone-stimulated current is a Cl− secretory current and occurs in heterozygous and homozygous ΔF508 mice. Segments from jejunum, ileum, and proximal and distal colon of +/ΔF and ΔF/ΔF mice were mounted in Ussing chambers, allowed to equilibrate for 15 min, pretreated with inhibitors for at least 10 min to the serosal side (bumetanide, 10 uM; barium, 3 mM) or mucosal side (diphenylcarboxylic acid, DPC, 1 mM; CF172 inhibitor peptide, 30 uM), and then stimulated with 1,000 nM lubiprostone. Maximal changes in Isc were recorded. Data are mean ± SEM for 6 mice
Lubiprostone Decreases Thickness of the Inner Mucus Layer
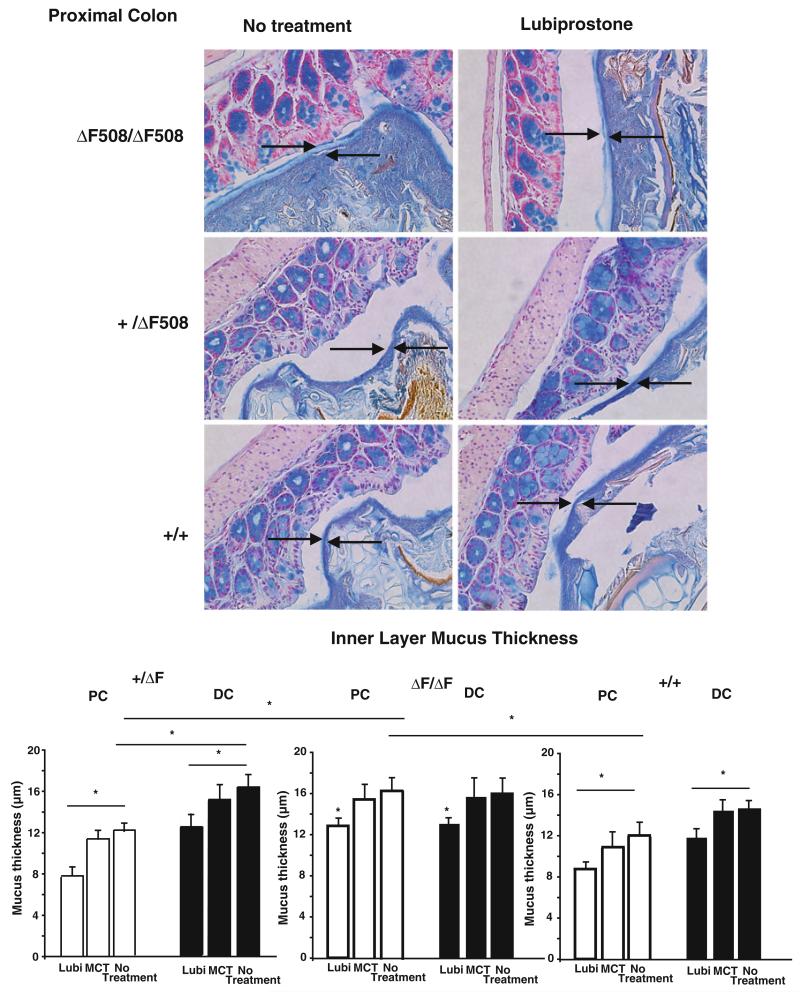
For the analysis of mucus, thickness of the mucus layer overlying the mucosal surface was measured in Alcian blue-stained, Carnoy-fixed specimens. Intestinal segments were carefully and rapidly removed and placed into Carnoy’s fixative. No attempts were made to remove luminal contents in order to preserve the integrity of the mucus. Since the mucus layer was negligible in the small intestine of all mice, measurements were only performed on mucus of the proximal and distal colon (Fig. 3). As inner layer mucus thickness demonstrates variability within the same segment of the intestine, three separate sections were examined for each mouse, and for each slide, 20 different locations were selected in each section. In the proximal colon, the inner mucus layer thickness was statistically greater in the ΔF/ΔF compared with the +/ΔF or +/+ mice by analysis of variance using a Bonferroni correction (Fig. 3). Distal colonic inner layer mucus was similar in all mice. Lubiprostone decreased the inner mucus layer thickness in both proximal and distal colon in both +/ΔF and ΔF/ΔF mice (Fig. 3, arrows demonstrate representative regions where mucus thickness was measured). Treatment with the MCT vehicle did not significantly alter the thickness of the inner mucus layer in either segment.
Fig. 3.
Lubiprostone decreases inner layer mucus thickness in proximal and distal colon. Mice were treated with lubiprostone (10 mg/kg) once daily for 7 days and intestinal tissue removed, fixed in Carnoy’s fixative, and sections stained for mucus with Alcian blue and counterstained with nuclear fast red. a Images shown are representative of those of 5 or 6 animals in each group in the proximal colon. Arrows indicate mucus thickness that was measured using NIH Image J software. b Data are mean ± SEM for 5 mice (MCT) or 6 mice (no treatment or lubiprostone (Lubi) groups). *p < 0.05 by analysis of variance using a Bonferrroni correction
Effects of Lubiprostone on Colonic Bacterial Composition
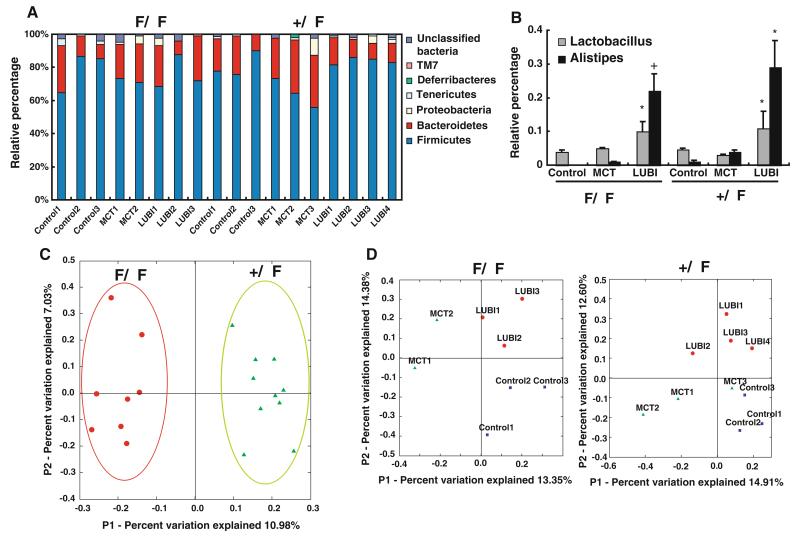
To gain insight into the specific gut bacterial phylotypes associated with altered mucus, 16S rRNA gene clone libraries were established and sequenced. A total of 1,519 partial 16S rRNA gene sequences (average sequence length of 650 nucleotides) were obtained from 18 mice (10 +/ΔF and 8 ΔF/ΔF: the 8 ΔF/ΔF mice came from 8 different litters from 6 different mothers because of low breeding numbers; all 10 +/ΔF were from the same litters). 16S rRNA gene sequences were assigned into operational taxonomic units (OTUs) or phylotypes at a similarity cutoff value of 97 (sequences which were more than 97 % similar were considered the same). The RDP Classifier (Ribosomal Database Project, Michigan State University, http://rdp.cme.msu.edu) tool to assign taxonomy to each 16S rRNA gene sequence,in both +/ΔF and ΔF/ΔF mice the majority of sequences were classified into two phyla, Firmicutes and Bacteroidetes. Proteobacteria, Deferribacteres, Tenericutes, TM7, and a small portion of unclassified bacteria were represented at lower levels in most of the samples (Fig. 4a). After the lubiprostone treatment, no phyla shifts were observed (Fig. 4a). However, at the genus level, two genera, Alistipes and Lactobacillus, both Firmicutes, were increased by lubiprostone treatment compared to both control and MCT vehicle (Fig. 4b).
Fig. 4.
Bacterial population analysis for +/ΔF and ΔF/ΔF mice using 16S rRNA clone libraries. DNA was extracted from stool, 16S rRNA amplified by PCR, cloned into libraries, and clones sequenced and analyzed as described in “Methods”. a Phylum distribution of control, MCT vehicle, and lubiprostone (Lubi) mice for +/ΔF and ΔF/ΔF mice, b changes in Lactobacillus and Alistipes in +/ΔF and ΔF/ΔF mice treated with MCT or lubiprostone (LUBI), c 16S rRNA gene-based principal coordinate analysis (PCA) of bacterial populations including all +/ΔF and ΔF/ΔF mice, and d PCA within groups of +/ΔF and ΔF/ΔF mice demonstrates that the CFTR genotype has the larger affect shaping gut microbiota. Clustering of treatment only occurs when +/ΔF and ΔF/ΔF groups are analyzed individually. Individual mice correspond to mice (as columns) in (a). *p < 0.05; +p < 0.01 compared with untreated by paired Student’s t test
Unsupervised analysis of the clone library 16S rRNA data from all groups showed clustering by mouse genotype (Fig. 4c). In contrast, no clustering was observed that distinguished treatment, suggesting that genotype plays a greater role in microbiota assemblage in this situation. In contrast, when the data from the two mouse genotypes were analyzed separately (+/ΔF and ΔF/ΔF) by PCA analysis, clustering was observed in each group (+/ΔF and ΔF/ΔF) that had undergone different treatments (control, MCT, and lubiprostone) (Fig. 4d).
Discussion
Cystic fibrosis is a devastating disease caused by a number of functional disturbances arising from mutations of the cystic fibrosis transmembrane receptor, among them defective Cl− secretion that results in complications that involve the respiratory and GI tract. Measures to compensate for this latter defect, by promoting alternative pathways for Cl− and concomitant fluid transport, could potentially improve outcomes. Lubiprostone, an agent with local intestinal effects that stimulates Cl− transport, is currently being used for treatment of constipation-predominant irritable bowel syndrome [22]. As its action is via a pathway not solely dependent on CFTR, the present study was undertaken to determine whether lubiprostone is capable of modifying intestinal mucus and affecting colonic bacterial composition in a CF model.
Our study demonstrates that lubiprostone stimulates active chloride secretion in both the murine small and large intestine. This effect was negated in Cl−-free solutions and decreased by inhibitors known to block NKCC1, K+ channels, and Cl− channels that mediate Cl− secretion. Lubiprostone did stimulate active Cl− secretion in mice homozygous for the ΔF508 CF mutation, an effect that was not blocked by the CFTR Cl− channel inhibitor CFinh172. These data would therefore implicate the activation of a non-CFTR Cl− channel by lubiprostone. Our data are consistent with data from guinea pig intestine where a non-CFTR Cl− channel also appeared to be involved [11]. In these studies, a number of prostaglandin receptor blockers were ineffective in blocking the Cl−secretory response stimulated by lubiprostone, suggesting an independent and unique mechanism of action for this agent involving direct activation of the ClC-2 Cl− channel. However, our results differ from those of Bijvelds et al. [13], who found that lubiprostone stimulated active anion secretion that was CFTR-dependent and inhibited by the CFTR inhibitor 172. Furthermore, our mice demonstrated a maximal Isc increase of approximately 12 μamp/cm2 (in jejunum where the largest increase was observed) compared with over 100 μamp/cm2 in the ΔF/ΔF mice of Bijvelds. Studies of Mizumori et al. [12] suggested that lubiprostone activates Cl− secretion by stimulation of both ClC-2 and CFTR. We believe that these seemingly contradictory findings could be dose-related. At concentrations below 1 μM, as used in the present study, lubiprostone may activate ClC-2, while at higher concentrations it may activate the EP4 prosta-glandin receptor. The subsequent generation of cAMP by this receptor would activate CFTR [13, 14]. In support of this, CF mice express ClC2 in lung and exhibit net Cl− secretion in response to lubiprostone. In support of the activation of non-CFTR-dependent Cl− transport, it should be noted that lubiprostone stimulates Cl− secretion in respiratory epithelium of cystic fibrosis mice [23] where ClC-2 is expressed. It is also possible that differences in mouse genetic background could contribute to the differences observed. It should be noted in the studies of Bijvelds that, in the ΔF/ΔF mice, some Cl− secretion stimulated by lubiprostone was observed in these mice, albeit less than that observed in wild-type mouse intestine. It should also be noted that the actions of the CFTRinh172 may be incomplete [24]. Additionally, in our Ussing chamber studies, tissues are not stripped of muscularis propria which limits penetration of the drug from the basolateral side; however, the CFTRinh was added to both mucosal and serosal sides of the tissue.
We next investigated the effect of altered Cl− transport on intestinal mucus. Fixation artifact can make measurements of intestinal mucus difficult. In vivo measurements, such as those described [25-28] are more physiological, but require special expertise and equipment. In vitro measurements of mucus thickness are attended with potential fixation artifacts, but may be useful for comparative purposes. The inner mucus layer thickness measured in the present studies was of the order of 10–15 μm, less than the 40μ50 μm measured using the above-cited in vivo studies and could be caused in part by dehydration. Other causes such as inner mucus layer pulling away from the mucosa during fixation can be confounding. Within each section, the variability in mucus thickness is also large as observed by the large standard errors of the data.
As discussed in a number of publications [2, 27-29], the organization of intestinal mucus is complex, and mucus resides both as an inner mucus layer and as a layer mixed with luminal contents. Our measurements may be different since our manner of preparation differs slightly and likely results in removal of some mucus. Care was taken to minimize the manipulation of the tissue. Small sections were cut with fresh razor blades and the tissues were not washed or rinsed in saline as this may disturb the mucus and cause it to denude.
For all mice (+/+, +/ΔF, and ΔF/ΔF), the thickness of the inner mucus layer in the distal colon was greater and did not differ between the CF genotypes. Lubiprostone reduced the inner layer mucus thickness in both the proximal as well as the distal colon of all three types of mice, an effect potentially mediated through induction of Cl− movement. This effect most likely resulted from compositional changes in the mucus caused by lubiprostone, possibly through promotion of non-CFTR Cl secretion. We speculate that changes in the properties of the inner mucus layer could affect the assemblage of the gut microbiota. While taxonomical annotation of DNA sequences revealed no major differences at the phylum level between any of the samples, and no new phyla were observed after treatment with MCT or lubiprostone, differences between fecal microbiota for the three experimental groups were clearly observed by principal component analysis. Lubiprostone increased Lactobacillus (Firmicutes) and Alistipes (Bacteroidetes). Changes in microbial 16S rRNA profiles unfortunately provide minimal information on the functional consequences that might impact host responses. The generation of metabolic substrates such as short chain fatty acids by microbes like Lactobacillus could potentially have a beneficial effect on mucosal and systemic processes. Lactobacilli can also have anti-inflammatory activities [30, 31] and anti-neoplastic effects [32, 33]. Less is known about Alistipes and its potential contribution to regulation of intestinal functions [34-36]. Different conditions, including other bacteria in the population and metabolic substrates, must be considered in the potential for bacteria to be beneficial or pathogenic. The results of the lubiprostone-induced changes must be interpreted with the knowledge that it was provided via the MCT vehicle also had effects on the microbiome.
In summary, we report that lubiprostone stimulates active anion secretion in the small and large intestine of mice through a non-CFTR-dependent mechanism. We presume this is mediated through ClC-2, although our studies could not confirm with certainty whether the transporter is apical or basolateral. We hypothesize that the enhanced intestinal secretion observed in wild-type/ΔF508 hetetozygous (+/ΔF) and ΔF508 homozygous (ΔF/ΔF) mice accounts for the changes in mucus thickness. It also appears that lubiprostone affects the composition of the colonic microbiota of F508 homozygous (ΔF/ΔF) mice, an effect that is independent of the MCT vehicle used. These changes in microbiota are likely to be related to the alterations in mucus composition and physical properties caused by lubiprostone that may have implications for treatment of intestinal symptoms in patients with cystic fibrosis.
Acknowledgments
Supported by a grant from Takeda Pharma-ceuticals North America, Inc., NIH Digestive Disease Research Center grant DK-42086 (E.B.C.), NIH grant R37 DK47722 (E.B.C.), the University of Chicago Cancer Research Center (CA-01459), a fellowship grant from the Crohn’s and Colitis Foundation of America (Y.W.), NIH grant HD059123 (E.C.C.).
Footnotes
Conflict of interest The authors disclose no other potential conflicts of interests or financial relationship with the organization that sponsored the research.
Contributor Information
Mark W. Musch, Division of Biological Sciences, Department of Medicine, The University of Chicago, 900 E. 57th St., Chicago, IL 60637, USA
Yunwei Wang, Division of Biological Sciences, Department of Medicine, The University of Chicago, 900 E. 57th St., Chicago, IL 60637, USA.
Erika C. Claud, Division of Biological Sciences, Department of Medicine, The University of Chicago, 900 E. 57th St., Chicago, IL 60637, USA; Division of Biological Sciences, Department of Pediatrics, The University of Chicago, 900 E. 57th St., Chicago, IL 60637, USA
Eugene B. Chang, Division of Biological Sciences, Department of Medicine, The University of Chicago, 900 E. 57th St., Chicago, IL 60637, USA; Knapp Center for Biomedical Discovery, The University of Chicago, 900 E. 57th St., Chicago, IL 60637, USA
References
- 1.Rowe SM. Cystic fibrosis. New Eng J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Gouyer V, Gottrand F, Desseyn JL. The extraordinarily complex but highly structured organization of intestinal mucus-gel unveiled in multicolor images. PLOS One. 2011;6:e18761. doi: 10.1371/journal.pone.0018761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilger MA. Cystic fibrosis: nutritional and intestinal disorders. Pediatr Pulmonol. 1995;20:56–57. [Google Scholar]
- 4.Werlin SL, Benuri-Silbiger I, Kerem E, et al. Evidence of intestinal inflammation in patients with cystic fibrosis. J Pediatr Gastroenterol Nut. 2010;51:304–308. doi: 10.1097/MPG.0b013e3181d1b013. [DOI] [PubMed] [Google Scholar]
- 5.Saiman L, Siegel J. Infection control in cystic fibrosis. Clin Microbiol Rev. 2004;17:57–71. doi: 10.1128/CMR.17.1.57-71.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridge JL, Conrad C, Gerson L, et al. Risk factors for small bowel bacterial overgrowth in cystic fibrosis. J Pediatr Gastroenterol Nut. 2007;44:212–218. doi: 10.1097/MPG.0b013e31802c0ceb. [DOI] [PubMed] [Google Scholar]
- 7.Eckburg PB, Bil EM, Bernstein CN, et al. Divesity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacy BE, Levy LC. Lubiprostone: a chloride channel activator. J Clin Gastroenterol. 2007;41:345–351. doi: 10.1097/01.mcg.0000225665.68920.df. [DOI] [PubMed] [Google Scholar]
- 9.Ueno R, Osama H, Habe T. Oral SPI-0211 increases intestinal fluid secretion and chloride concentration without altering serum electrolyte levels. Gastroenterology. 2004;126:A298. [Google Scholar]
- 10.Cuppoletti J, Malinowska DH, Tewari KP, et al. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol. 2004;287:C1173–C1183. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 11.Fei G, Wang YZ, Liu S, et al. Stimulation of mucosal secretion by lubiprostone (SPI-0211) in Guinea pig small intestine and colon. Am J Physiol Gastrointest Liver Physiol. 2009;296:823–832. doi: 10.1152/ajpgi.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizumori M, Akiba Y, Kaunitz JD. Lubiprostone stimulates duodenal bicarbonate secretion in rats. Dig Dis Sci. 2009;54:2063–2069. doi: 10.1007/s10620-009-0907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bijvelds MJ, Bot AG, Escher JC, De Jonge HR. Activation of intestinal Cl− secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology. 2009;137:976–985. doi: 10.1053/j.gastro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Ao M, Venkatasubramanian J, Boonkaewwan C, Benya R, Rao M. Lubiprostone activates Cl− secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci. 2011;56:339–351. doi: 10.1007/s10620-010-1495-8. [DOI] [PubMed] [Google Scholar]
- 15.MacVinish LJ, Cope G, Ropenga A, Cuthbert AW. Chloride transporting capability of Calu-3 epithelia following persistent knockdown of the cystic fibrosis transmembrane conductance regulator, CFTR. Br J Pharmacol. 2007;150:1055–1065. doi: 10.1038/sj.bjp.0707175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G234–G251. doi: 10.1152/ajpgi.00366.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeiher BG, Eichwald E, Zabner J, et al. A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest. 1995;96:2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Hoenig JD, Malin KJ, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3:944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole JR, Chai B, Marsh TL, et al. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acid Res. 2003;31:442–443. doi: 10.1093/nar/gkg039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludwig W, Strunk O, Westram R, et al. ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32:1363–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozupone C, Hamady M, Knight R. UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics. 2006;7:371. doi: 10.1186/1471-2105-7-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drossman DA, Chey WD, Johanson JR, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329–341. doi: 10.1111/j.1365-2036.2008.03881.x. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald KD, McKenzie KR, Henderson MJ, et al. Lubiprostone activated non-CFTR dependent respiratory epithlelial chloride secretion in cystic fibrosis mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L933–L940. doi: 10.1152/ajplung.90221.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang XF, Reddy MM, Quinton PM. Effects of a new cystic fibrosis transmembrane conductance regulator inhibitor on Cl− conductance in human sweat ducts. Exp Physiol. 2004;89:417–425. doi: 10.1113/expphysiol.2003.027003. [DOI] [PubMed] [Google Scholar]
- 25.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 26.Gustafsson JK, Ermund A, Johansson MEV, Schutte A, Hansson GC, Sjovall H. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol. 2012;302:G430–G438. doi: 10.1152/ajpgi.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;205:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson MEV, Holmen Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 2011;108:4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swidsinski A, Loening-Baucke V, Theissig T, et al. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56:343–350. doi: 10.1136/gut.2006.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paolillo R, RomanoCarratelli C, Sorrentino S, Mazzola N, Rizzo A. Immunomodulatory effects of Lactobacillus plantarum on human colon cancer cells. Int Immunopharmacol. 2009;9:1265–1271. doi: 10.1016/j.intimp.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Tao Y, Drabik KA, Waypa TS, et al. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–C1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 32.Kazaie K, Zadeh M, Khan MW, et al. Ablating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci USA. 2012;109:10462–10467. doi: 10.1073/pnas.1207230109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asha GD, Devaraja TN. Lactobacillus sp. As probiotics for human health with special emphasis on colorectal cancer. Ind J Sci Technol. 2011;4:1008–1014. [Google Scholar]
- 34.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–108. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Guraner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;36:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 36.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]