Abstract
The possible therapeutic benefits of B-cell depletion in combating tumoral immune escape have been debated. In support of this concept, metastasis of highly aggressive 4T1 breast cancer cells in mice can be abrogated by inactivation of tumor-evoked regulatory B cells (tBregs). Here we report the unexpected finding that B-cell depletion by CD20 antibody will greatly enhance cancer progression and metastasis. Both murine and human tBregs express low levels of CD20 and, as such, anti-CD20 mostly enriches for these cells. In the 4T1 model of murine breast cancer, this effect of enriching for tBregs suggests that B-cell depletion by anti-CD20 may not be beneficial at all in some cancers. In contrast, we show that in vivo targeted stimulation of B cells with CXCL13-coupled CpG-ODN can block cancer metastasis by inhibiting CD20Low tBregs. Mechanistic investigations suggested that CpG-ODN upregulates low surface levels of 4-1BBL on tBregs to elicit granzyme B-expressing cytolytic CD8+ T cells, offering some explanative power for the effect. These findings underscore the immunotherapeutic importance of tBreg inactivation as strategy to enhance cancer therapy by targeting both the regulatory and activating arms of the immune system in vivo.
Keywords: Rituximab, tBregs, metastasis, CpG therapy
INTRODUCTION
Cancer metastasis involves an active hijacking of regulatory immune cells in order to suppress antitumor effector immune responses. As a result, the increase in myeloid and myeloid-derived suppressor cells (MSC and MDSC) and regulatory T cells (Tregs) is often a sign of poor disease outcome in both mice and humans with cancer (1–3). However, the role of B cells, in particular regulatory B cells (Bregs), in this process remains poorly understood and is still debatable, although the existence of suppressive B cells has been known for more than 30 years. Protection from autoimmune diseases in mice and humans is shown to be mediated by unique subsets of Bregs (the definition first used by Mizoguchi (4)), such as murine IL-10 -producing B10 and B1b Bregs (4, 5); human IL-10 producing memory CD24hiCD27+ B-cells (6), CD25hi CD27hi CD86hi CD1dhi B cells (7), and CD19+CD24HighCD38High B cells (8). As such, the inactivation of B cells or Bregs exacerbates ulcerative colitis in patients with non-Hodgkin’s lymphoma, colitis, and Graves disease and increases the incidence of psoriasis in patients with psoriatic arthropathy (9–11). B cells also facilitate carcinogenesis of methylcholanthrene-induced (12, 13) and transplanted tumors (14). In humans with metastatic ovarian carcinoma, infiltration of CD19+ B cells was associated with worse disease outcome (15). Unless replenished with B220+ B cells, orthotopic tumors progress poorly in syngeneic mice deficient in B cells (16, 17). B cells promote a tumor suppressive milieu through the production of immunoglobulin or/and immunomodulatory factors and cytokines, or inducing the generation of regulatory T cells (18). For example, B-cell-expressed lymphotoxin α/β was linked with the androgen-independent growth of prostate cancer cells (19); and B cell expressed immunoglobulin was linked with inflammation in premalignant tissues and growth of HPV16-induced tumors (20). B cells can also mediate Th2 polarization and inhibition of antitumor cytotoxic activity of CD8+ T and NK cells by producing IL-10 (21) and TNFα (22).
To the best of our knowledge, only two clearly defined examples of cancer escape-promoting Bregs are reported. First, B10 cells reduce the therapeutic efficacy of anti-CD20 antibody against lymphoma by inhibiting monocyte activity and surface expression of FcγR in an IL-10-dependent fashion (23). Second, we recently found that 4T1 carcinoma cells actively convert normal B cells into TGFβ-producing Bregs, designated tumor-evoked Bregs (tBregs) in order to successfully metastasize (17). tBregs differ from the immune tolerance-inducing IL-10-producing Bregs and B cells (24–26). Phenotypically, they are poorly proliferative B2-like cells (IgDHigh) that express constitutively active Stat3 and surface markers CD25High B7-H1High CD81High CD86HighCCR6High and CD62LLowIgMInt/Low. tBregs facilitate lung metastasis by converting non-Treg CD4+ T cells into metastasis-promoting FoxP3+Tregs utilizing TGFβ (17), which in turn inactivate antitumor NK cells and protect metastasizing cancer cells (27). Since tBreg-like cells can be readily generated by treating normal human donor B cells ex vivo with conditioned media of various human cancer lines (17), human cancer metastasis may also utilize a similar mechanism. The clinical implication of tBregs is that, as long as cancer persists, it will induce tBregs and thereby initiate the chain of suppressive events. Thus, strategies that abrogate any step of this process are expected to inhibit cancer escape and metastasis. Indeed, the depletion of Tregs and tBregs with PC61 anti-CD25 antibody (Ab) successfully abrogates 4T1 breast cancer lung metastasis (17, 27). Despite these data, anti-CD20 Ab–mediated B-cell depletion instead increased the tumor burden in the lungs of mice intravenously injected with B16–F10 melanoma (28). Similarly, depletion of B cells with rituximab (anti-CD20 Ab for treatment of humans with B cell malignancies) did not provide clinical benefit in patients with renal cell carcinoma and melanoma (29), calling into question the importance of B cells or Bregs in cancer escape.
To reconcile this discrepancy, we hypothesized that the results could be explained if the anti-CD20 Ab treatment only depleted “normal functioning” or “immune stimulatory” CD20+ B cells that participate in induction of adaptive antitumor immune responses (30) promoting favorable disease outcome in some cancer patients (31). Indeed, our modeling study in mice with 4T1.2 breast cancer and analyses of B cells from B-CLL patients treated with rituximab indicate that anti-CD20 Ab enriches for 4-1BBLLow CD20Low CD19+ tBregs by depleting stimulatory B cells. As a result, this exacerbates both progression of primary tumor in the mammary gland and lung metastasis, suggesting that anti-CD20 Ab-mediated depletion of B cells may not provide therapeutic benefits for some cancers. To circumvent this problem, we devised a simple CXCL13–based strategy that blocks the generation of tBregs in vivo and activates B cells by delivering stimulatory CpG oligonucleotides (CpG-ODN) via their CXCR5 receptor. We found that CpG-ODN not only inhibits the activity of tBregs, but also enhances surface expression of 4-1BBL needed for the stimulation of T-cell responses.
MATERIALS AND METHODS
Female BALB/c, C57BL/6 mice and µMT mice with mature cell deficiency (B6.129P2-Igh-Jtm1Cgn/J) were from the Jackson Laboratory (Bar Harbor, ME). Jh KO mice were from Taconic Farms Inc. (Derwood, MD). The TCR transgenic pmel-1 (Vα1Vβ13 T cell receptor for H-2Db restricted mouse and human gp100 epitope) mice have been described previously (32). B16–F10 (CRL-6475), MDA-MB-231 (HTB-26), SW480 (CLL-228) and MCF7 (HTB-22) cells were purchased from the American Type Culture Collection. 4T1.2 cells, a subset of 4T1 cells, were a gift from Dr. Robin L. Anderson 14 (Peter McCallum Cancer Center, Australia). 938-mel was a gift from Dr. Ashani Weeraratna (Wistar Institute, PA). Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23, 1985). The experiments were performed using 4–8 week old female mice in a pathogen-free environment at the National Institute on Aging Animal Facility, Baltimore, MD. 4T1.2 cells (5×104–1×105). Mice were s.c. challenged into the fourth mammary gland of mice, and tumor progression and lung metastasis was assessed as previously described (27). B cells were depleted by 2–4 i.p. injections of anti-CD20 antibody (250 µg/mouse, clone 5D2, Genentech, Inc., San Francisco, CA), as indicated in the figure legends. Control IgG was from Sigma (St Louis, MO) and in-house purified IgG2a from murine A20 lymphoma.
Antibodies used for FACS staining, such as anti-mouse and human FoxP3, anti-mouse CD20 were from eBioscience (San Diego, CA), while anti-mouse and human CD19, CD81, CD25, 4-1BBL, CD4, CD8, CD3, Ki67, IFNγ and Fc block were from Biolegend (San Diego, CA). Anti-IgM was from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA) TLR ligands from Invivogen (San Diego, CA) and phosphorothioated CpG oligonucleotides, such as ODN1826 and control ODN1826, were from Sigma Genosys (St Louis, MO).
BLC-arp (Biragyn et al., patent is pending), murine CXCL13 containing a single-stranded DNA/RNA –binding (RBD) portion of the capsid antigen of HBV encoding sequence, were constructed by replacing the PE38 fragment from chemotoxin constructs described elsewhere (33). BLC-arp was purified (>95% purity, verified by Coomassie Blue staining and western blotting) from yeast supernatant using SP-Sepharose™ Fast Flow and Heparin- HP trap columns (Invitrogen) on Fast performance liquid chromatography (Bio-Rad BioLogic Duoflow).
Human peripheral blood cell isolation
Human peripheral blood was collected by the Health Apheresis Unit and the Clinical Core Laboratory, the National Institute on Aging, under Human Subject Protocol # 2003054 and Tissue Procurement Protocol # 2003-071. PBMC’s were isolated using Ficoll-Paque (GE Healthcare, Waukesha, WI) density gradient separation according to the manufacturer’s instruction. B cells were isolated using B cell negative isolation (Miltenyi Biotec, Auburn, CA). CD3+ cells were isolated using the T cell enrichment columns from R&D Systems (Minneapolis, MN).
In vitro tBreg and T cell suppression assays
were performed as previously described (17). In brief, tBregs were generated from murine splenic B cells (>95% purity, isolated by negative selection using the RoboSep system, StemCell Technologies, Vancouver, Canada) or human peripheral blood B cells by incubating for two days in 50% conditioned medium of 4T1-PE cells (CM-PE), or MDA-MB-231, SW480, MCF7 or 938-mel cells in cRPMI (RPMI 1640 with 10% heat-inactivated fetal bovine serum, 10 mM HEPES, 1 mM sodium pyruvate, 0.01% 2-Mercaptoethanol, 2mM L-glutamine, 100U/ml penicillin and 100 µg/ml streptomycin) at a 37°C in humidified atmosphere with 5% CO2. Control B cells were treated with 100 ng/ml of recombinant mouse BAFF (R&D) in cRPMI. To assess in vivo-generated tBregs in tumor bearing mice, B cells were magnetically isolated from lymph nodes or spleens of tumor-bearing or naïve mice using anti-CD19-FITC Ab (Biolegend) and anti-FITC MicroBeads (Miltenyi Biotec). To test the suppressive activity of B cells, carboxyfluorescein succinimidyl ester (CFSE) or eFluor670 (eBioscience) –labeled splenic CD3+ T cells were with B cells for 5 days in the presence of 1.5–3 µg/ml of soluble anti-mouse CD3 Ab (BD Biosciences, San Jose, CA) or anti-CD3/28 coated beads (Invitrogen, Grand Island, NY). Decrease in dye expression within T cells correlates with their proliferation. The suppressive activity was also tested by determining the Ki67+ expression in target CD3+ T cells. For granzyme B induction in CD8 cells by CpG treated Bregs, we followed the same protocol as for the suppression assay.
To assess antigen-specific expansion of effector CD8+ cells in mice with B16–F10 melanoma, draining lymph node cells and splenocytes were stimulated ex vivo for 5–7 days with 5 µg melanoma gp10025–32 peptide and 20u/ml IL-2 and stained for CD8, Ki67 and GrzB.
In vivo manipulations
Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23, 1985). The experiments were performed using 4–8 weeks old female mice in a pathogen-free environment at the National Institute on Aging Animal Facility, Baltimore, MD. 4T1.2 cells (5×104–1×105) were s.c. challenged into the fourth mammary gland of BALB/c and Jh KO mice were, and tumor progression and lung metastasis was assessed as previously described (27). B cells were depleted by i.p. injections of anti-CD20 antibody (250 µg/mouse, two-four times). B16-F10 cells (1×105) were s.c. injected into C57BL/6, µMT or TCR transgenic pmel-1 mice and tumor progression was measured every other day as previously described (34). Ex vivo –generated tBregs or B cells (5×106) were injected i.v. into congenic mice one day before and 5 days after tumor challenge.
Statistical Analysis
The results are presented as the mean of triplicates ± SEM of at least three experiments. Differences were tested using Student’s t test and a 2 sided p-value less than 0.05 was considered statistically significant.
RESULTS
Cancer metastasis is enhanced by treatment with anti-CD20 Ab
Since tBregs actively facilitate lung metastasis by suppressing antitumor immune responses (17), the absence of tBregs is expected to hamper this process and inhibit cancer progression. Indeed, unlike WT BALB/c mice which had readily progressing 4T1.2 breast cancer cells in the mammary gland (primary site of challenge) and metastasis in the lungs (Fig.1A,B), congeneic Jh KO mice deficient in B cells (due to a deletion in the J segment of the immunoglobulin heavy chain locus) poorly supported primary tumor growth (Fig.1A) and lung metastasis (Fig.1B). These responses in Jh KO mice were completely reversed by adoptive transfer of tBregs from WT mice (Fig.1A,B), confirming the importance of tBregs in cancer escape (17). Thus, cancer progression and escape may also be therapeutically controlled by inactivation of B cells. To test this idea, we depleted B cells with anti-CD20 Ab in WT BALB/c mice before the challenge with 4T1.2 cancer cells. As shown in Fig.1C, B cell-depleted mice also abrogated lung metastasis of 4T1.2 cells, suggesting that this therapy successfully used in humans to combat B cell malignancies may also be utilized to treat metastasis in solid tumors. However, when we tested the therapeutic efficacy of anti-CD20 Ab in mice with established 4T1.2 cancer, all mice surprisingly succumbed to massive cancer growth and metastasis (Fig.1D,E). In fact, compared with control Ig-treated tumor bearing mice (Fig.1D,E), the anti-CD20 Ab treatment of mice with already established cancer further and significantly enhanced tumor growth (Fig.1D) and metastasis in the lungs (Fig.1C).
FIGURE 1. Depletion of CD20+ B cells promotes metastasis.
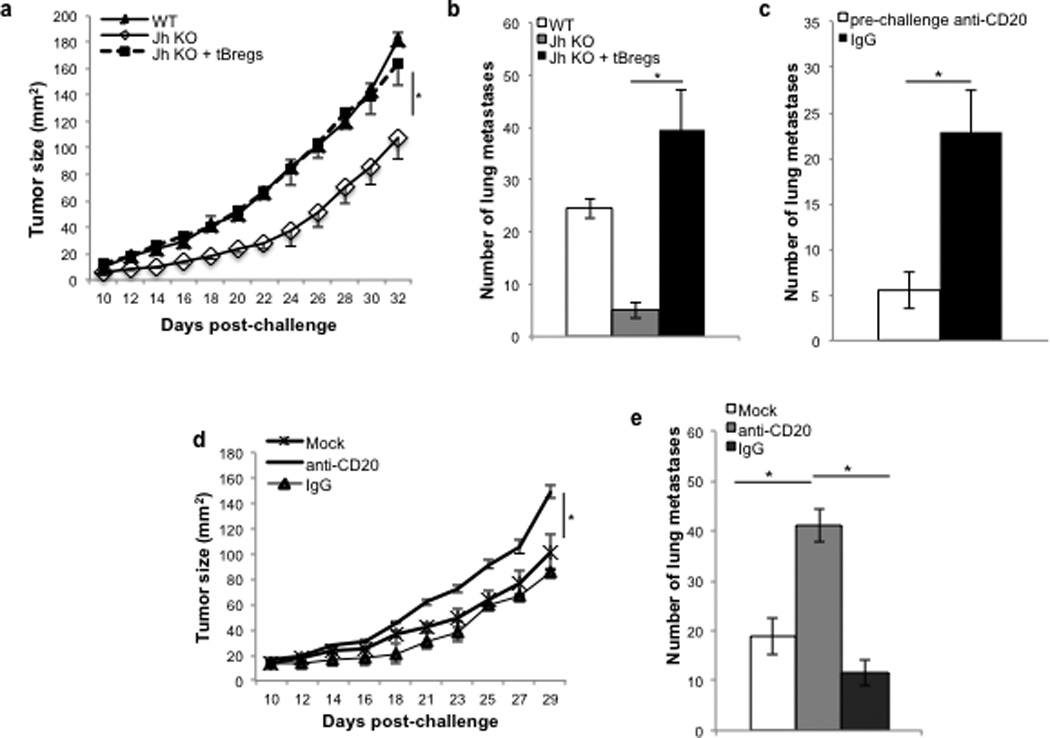
4T1.2 cells (5×104) were subcutaneously injected in the fourth mammary gland of Jh KO (A,B) and BALB/c (A–E) mice to assess tumor progression (A,D) and number of metastatic foci (at day 32 post challenge, B,C,E). To evaluate the role of tBregs (A,B) or anti-CD20 Ab –mediated B cell depletion (C–E), 4T1.2 tumor-bearing Jh KO mice were adoptively transferred with tBregs (A,B) or BALB/c mice were intraperitoneally injected with 250 µg/mouse anti-CD20 Ab or control isotype-matched antibody (IgG, mock) at 5, 10 and 15 days post tumor challenge (D–E) or at -10, -6 and -2 before tumor challenge (C). Y-axis shows tumor size (A,D), or number of metastatic foci (B,C,E) ± SEM of four-eight mice per group experiments reproduced at least three times. From here on, * P<0.05 of indicated differences between groups are considered significant.
This surprising result was not due to inability or poor efficiency of B-cell depletion, as every mouse treated with anti-CD20 Ab had drastically reduced numbers of CD19+ B cells in peripheral blood (PB), secondary lymphoid organs and lungs of tumor-bearing mice (>80%, Fig.2A). However, and importantly, the remaining B cells expressed enhanced levels of surface markers characteristic of tBregs (CD25+ CD81High within CD19+ cells, Fig.2B and sup.Fig.1A), suggesting that they could be enriched for tBregs. To confirm this possibility, we isolated CD19+ B cells from tumor-bearing mice treated with anti-CD20 Ab or control IgG2a to test their ability to suppress the activity of T cells ex vivo. As we reported that 4T1.2 cancer induces the generation of tBregs in vivo (17, 27), B cells from IgG2a-treated tumor-bearing mice significantly suppressed the activity of CD4+ and CD8+ T cells as compared with B cells from naïve mice (Fig.2C). However, their suppressive activity was drastically enhanced if B cells were isolated from tumor-bearing mice treated with anti-CD20 Ab (Fig.2C). Moreover, these enriched B cells (Fig.2D) as well as ex vivo-generated tBregs (by treating normal B cells with cancer conditioned media (CM) (17), sup. Fig.1B) expressed only low levels of CD20. Taken together, these results suggest that, although cancer progression and metastasis require B cells, the anti-CD20 Ab-mediated depletion of CD20+ B cells instead worsens the disease by enriching for CD20Low tBregs.
FIGURE 2. Depletion of CD20+ B cells after tumor challenge enriches for tBregs.
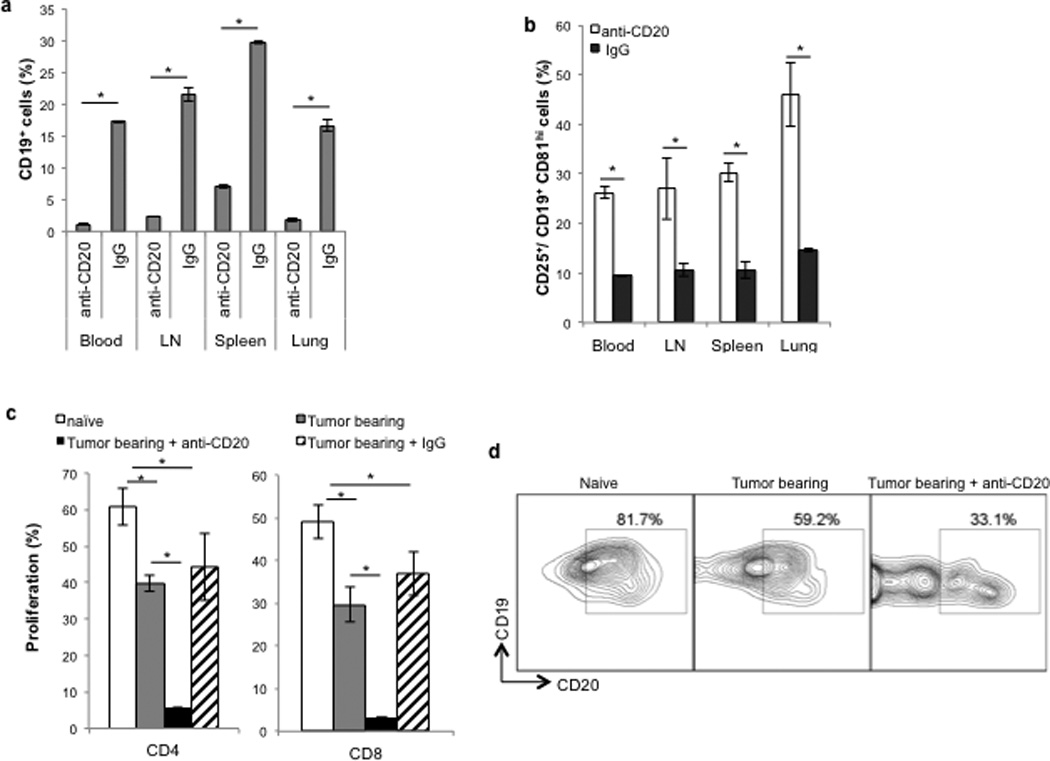
Anti-CD20 Ab efficiently depletes CD19+ cells in various organs (A) of 4T1.2 tumor-bearing BALB/c mice used in Figure 1D,E, but enriches for tBregs (CD81hiCD25+ within CD19+ gated cells, B). CD19+ cells isolated from lymph nodes of tumor-bearing mice suppressed proliferation of syngeneic CD4+ and CD8+ T cells (C). In particular, B cells from anti-CD20 Ab –treated mice were much more suppressive for both CD4+ and CD8+ T cells (C). Proliferation was assessed using CFSE dilution assay of T cells stimulated with anti-CD3 Ab and incubated with equal numbers of B cells for 5 days. CD19+ cells from the spleen of tumor-bearing mice -treated with anti-CD20 Ab expressed low levels of surface CD20 as comaperd with naïve mice (Naïve, D) and non-treated tumor-bearing mice (Tumor-bearing, D). A representative FACS staining dot plots of CD20+ cells within CD19+ gated cells (D). Y-axis shows % of CD19+ cells (A), tBregs (B), or proliferated CD4+ and CD8+ T cells (C) ± SEM of four-eight mice per group experiments reproduced at least three times.
Rituximab enriches for human tBregs expressing low levels of CD20
We reported that human cancers also induce the generation of tBreg-like cells (17), which may function similarly by regulating immune responses at the sites of tumor progression and metastasis. In concordance, we detected clusters of B cells residing in the lungs as well as in primary tumors and surrounding stromal tissues of humans with metastatic breast cancer (5/5 cases, sup. Fig.1C). Thus, human tBregs may also be CD20Low and, as such, may escape anti-CD20 antibody-mediated depletion. Since the lack of access to fresh samples from breast cancer patients hampers their characterization, we first tested this possibility by evaluating expression of CD20 on the surface of ex vivo-generated tBregs by treating human peripheral blood B cells with CM of MDA-MB-231 breast cancer cells or SW480 colon cancer cells (17). Unlike control B cells (mock-treated or cultured with CM of breast cancer MCF7 cells or 938 melanoma cells, Fig.3A and sup.Fig.1D), tBregs not only efficiently suppressed the activity of human T cells (B-MDA, Fig.3A; and B-MDA and BSW480, sup.Fig.1D), but also expressed significantly reduced surface CD20 (Fig.3B and sup.Fig.1E). The regulatory activity of human tBregs was mostly retained in CD20Low B cells when they were segregated using a FACS-sorter (purity >95%, Fig.3C and sup.Fig.2A). Similarly, CD20Low B cells remaining after in vitro anti-CD20 Ab-mediated depletion of CD20High cells also efficiently suppressed T cell activity (sup.Fig.2B,C).
FIGURE 3. Human tBregs also express low levels of CD20.
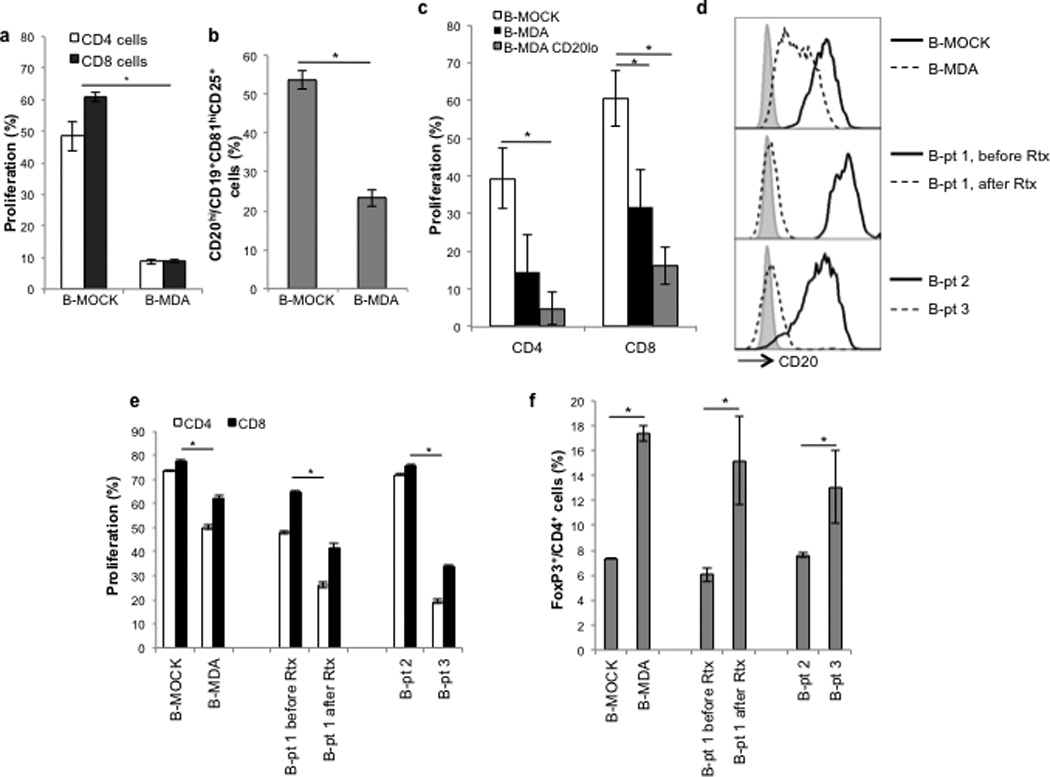
Ex vivo generated human tBregs (from healthy donor B cells -treated with CM of MDA-MB-231 cells (17)) not only suppressed proliferation of human CD4+ and CD8+ T cells stimulated with anti-CD3 Ab (B-MDA, A), but also reduced cells expressing high levels of CD20 within CD81hiCD25+ CD19+ cells (B). The suppressive activity (for CD4+ and CD8+ T cells) of human tBregs was retained within CD20Low B cells (C), as shown by FACS sorting for CD20Low B cells (enrichment >95%). Y-axis shows (%) Ki67+ CD4+ and CD8+ T cells ± SEM (C) of triplicate experiments. (D–F) Rituximab enriches for CD20Low tBregs in patients with B-CLL, as shown by the increased presence of B cells expressing CD20Low (broken line, D). In particular, compared with B cells before the treatment (continuous line, middle panel, D), the majority of B cells of patient 1 were CD20Low after rituximab treatment (broken line, D). These CD20Low cells efficiently suppressed proliferation of allogeneic CD4+ and CD8+ T cells (E) and induced FoxP3+ Treg conversion when mixed with CD25− CD4+ T cells (% of FoxP3+ within CD4+, F). All data shown here were from triplicate experiments reproduced at least three times.
Next, to confirm the enrichment of CD20Low tBregs after anti-CD20 Ab treatment in humans, we tested B cells from 13 patients with B-CLL and found 9 of them clearly containing CD20Low B cells in the peripheral blood (Table 1 and Fig.3D), compared with untreated patients (patient 2, Fig.3D and Table 1) or healthy subjects (Fig.3B, D). Importantly, these CD20Low B cells functioned like tBregs, i.e. efficiently suppressed activity of T cells (Fig.3E) and induced FoxP3+ Treg conversion from CD25− CD4+ non-regulatory T cells (Fig.3F). Since all 6 out of these 9 samples with CD20Low B cells were from people treated with rituximab (Table 1), it is tempting to speculate that they were enriched by anti-CD20 Ab-induced B-cell depletion as in mice with 4T1.2 cancer. For example, while B cells from the patient 1 before the treatment did not reveal the presence of suppressive CD20Low B cells (Fig.3D–F), the majority of its B cells after rituximab treatment became CD20Low (Fig.3D) and readily suppressed T-cell activity (Fig.3E) and converted Tregs (Fig.3F).
Table 1.
CD20loCD19+ B cells from B-CLL patients suppress T cells
| Patients | Compared to ND | Rituximab | ||
|---|---|---|---|---|
| CD20 decrease * | Suppression | 4-1BBL | ||
| 1 | + | +++ | Low | + |
| − | − | Low | − | |
| 2 | − | − | ND | − |
| − | − | ND | − | |
| 3 | + | +++ | Low | + |
| 4 | + | ++ | Low | − |
| 5 | + | ++ | Low | − |
| 6 | + | ++ | Equal | + |
| 7 | + | + | ND | + |
| 8 | + | + | High | − |
| 9 | − | − | Equal | − |
| 10 | − | − | High | − |
| 11 | − | − | Equal | − |
| 12 | − | + | Low | + |
| 13 | + | ++ | low | + |
CD19+ B cells from B-CLL patients were phenotyped for CD20 and 4-1BBL expression and tested for T cell suppression
+ corresponds to > 40% decrease compared to ND
Activation of TLR9 blocks the generation and function of tBregs
Considering the failure of anti-CD20 therapy to deplete tBregs, we decided to seek a different strategy to neutralize tBregs. When we screened various TLR ligands for their ability to inactivate tBregs, the majority of them failed to do so (Fig.4A,B and sup.Fig.3A). For example, LPS did not inhibit tBregs (Fig.4A,B and sup.Fig.3A) nor convert B cells into tBregs (17), despite expression of TLR4 (data not shown). In contrast, only TLR7–9 ligands (single stranded RNA and unmethylated CpG-ODN) reversed the phenotype (upregulated CD20 and downregulated CD25+CD81High, Fig.4A and sup.Fig.3A,B) and blocked the regulatory activity of murine and human tBregs (Fig.4B and sup.Fig.3A–C). Hence, CpG-ODN can efficiently inhibit tBregs and, as such, it may also provide therapeutic benefit in tumor-bearing mice blocking tBreg-mediated metastasis.
FIGURE 4. CXCR5-targeted delivery of CpG-ODN blocks tBregs and activates B cells eliciting cytolytic CD8+ T cells.
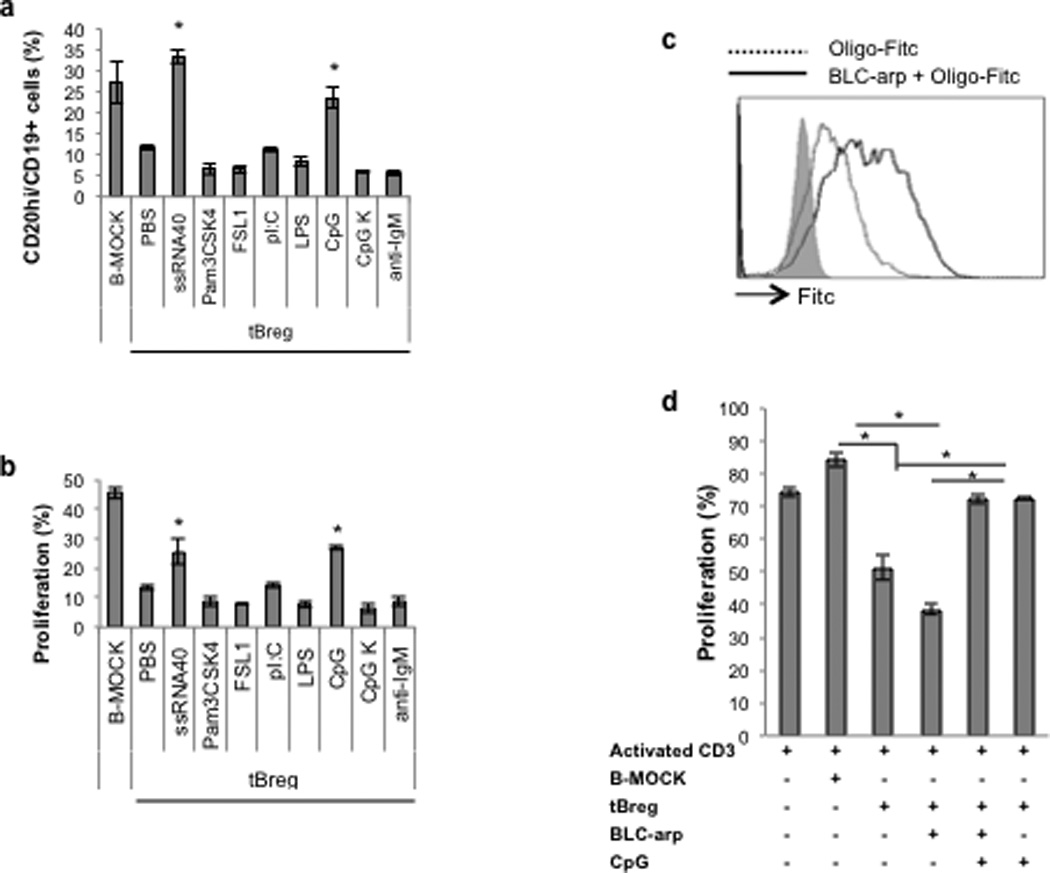
(A,B) Ex vivo generation of murine tBregs was done in the absence or presence of various TLR ligands (5 µg/ml of ssRNA40; 10 µg/ml of Pam3CSK4 or FSL-1; 5 µg/ml of LPS; 1 µg/ml ODN1826 PS (CpG) and control CpG K) or 10 µg/ml anti-IgM. After 48h, cells were stained for CD20 expression (A) and evaluated for the ability to suppress proliferation of CD3+ T cells stimulated with anti-CD3 Ab (B). FITC- labeled CpG is better uptaken by tBregs when coupled with BLC-arp (continuous line, C), as compared with free CpG (broken line, C). Shaded area is for untreated control cells (C). BLC-arp/CpG (3 µg/ml ODN1826 PS) blocks activity of murine tBregs in vitro, at the same extent as free CpG, as shown by the inability to inhibit proliferation of CFSE-labeled CD3+ T cells (D). Controls were B cells treated with BAFF (B-Mock) and untreated tBregs.
Targeted delivery of CpG-ODN to CXCR5-expressing cells abrogates lung metastasis by inhibiting tBregs and activating B cells to promote antigen-specific CD8+ T cells
Since systemic injections of free CpG-ODN can promote lymphoid follicle destruction and immunosuppression (35), to circumvent this potential problem we devised a CXCL13-based strategy (BLC-arp, sup.Fig.3D) to deliver CpG-ODN to CXCR5-expressing B cells in vivo. As we previously demonstrated that an RNA-binding domain (RBD)-containing chemokines bind and transduce cells with oligonucleotides via chemokine receptors (Biragyn et al., MS submitted, 2012), CpG-ODN efficiently transduced (Fig.4C) and inactivated tBregs (Fig.4D) inducing B-cell activity even at suboptimal doses (50 ng/ml, sup.Fig.3E,F), if coupled with BLC-arp (BLC-arp/CpG).
To test the clinical relevance of tBreg inactivation, 4T1.2 tumor–bearing mice were treated 5 times intravenously with BLC-arp coupled with 5 µg/ml CpG or control CpG oligonucleotides. While all control mice succumbed to massive lung metastasis, treatment with BLC-arp/CpG abrogated lung metastasis (Fig.5A). Of note: Control “non-stimulatory” CpG also reduced (at significantly lesser extent, if compared with CpG-ODN, p<0.05) lung metastasis (p<0.05, BLC-arp/ CpG K vs. Mock, Fig.5A), suggesting that any unmethylated DNA oligonucleotide may similarly function, if delivered with the help of BLC-arp. Importantly, when CD19+ B cells were isolated from these tumor-bearing mice, B cells from BLC-arp/CpG-treated mice were no longer CD20Low (Fig.5B) and could not suppress the activity of CD4+ and CD8+ T cells (Fig.5C). Instead, B cells from these mice activated T cell responses, as they induced proliferation of T cells (Fig.5C and sup.Fig.4A) and expanded GrzB-expressing CD8+ T cells (sup.Fig.4B,C) that readily and significantly lysed 4T1.2 cancer cells (Fig.5D). Hence, an inefficient induction of cytolytic CD8+ T cell responses in these cancer model (compare naïve vs tumor, Fig.5D), can be efficiently reversed using BLC-arp/CpG by inactivating tBregs and rendering B cells stimulatory.
FIGURE 5. In vivo-targeted delivery of CpG-ODN blocks lung metastasis.
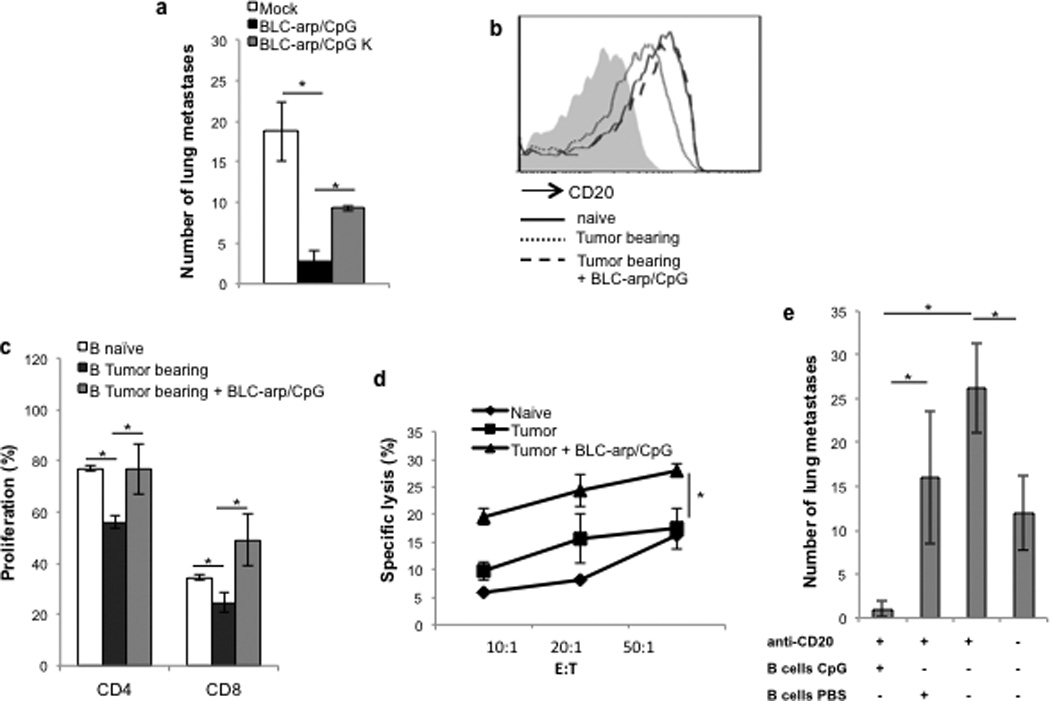
(A,E). BALB/c mice with established 4T1.2 cancer (as in Fig.1) were i.v. injected with BLC-Arp (30 µg) coupled with 5 µg ODN1826 PS (CpG) control CpG (CpG K) on days 3, 7, 12, 16 and 21 post tumor challenge to test for metastasis suppression (A). CD20 expression within CD19+ B cells in blood of BLC-arp/CpG-treated tumor-bearing mice (broken line, B) compared with the untreated tumor-bearing mice (mock, A, and dotted line, B). BLC-arp/CpG retarded lung metastasis (black bar, A) and increased CD20 expression on B cells (broken line, B). Expression of CD20 in B cells of naïve mice is in continuous line (B). Importantly, draining lymph node B cells from BLC-arp/CpG –treated tumor-bearing mice did not suppress proliferation of CD4+ and CD8+ T cells (C), instead becoming stimulatory for CD8+ T cells (p<0.05, naïve B cells vs. BLC-arp/CpG –treated tBregs, C). The inability of 4T1.2 cancer to induce cytolytic CD8+ T cells (compare tumor vs naïve, D) is reversed in 4T1.2 cancer-bearing mice treated with BLC-arp/CpG (D). Y-axis shows % of specific lysis (Cr51 release) ± SEM of 4T1.2 cells incubated with purified CD8+ T cells at indicated E:T ratio. To test the stimulatory activity of BLC-arp/CpG and to show that the effect is due to direct B cell stimulation 4T1.2 tumor –bearing mice were first depleted of CD20+ B cells by treating with anti-CD20 Ab on days 4 and 6 post tumor challenge (E). Then, mice were separated into two random groups and adoptively transferred with syngeneic B cells pretreated ex-vivo for 3h with 10 µg/ml CpG (B cells CpG) or PBS (B cells PBS). The cells were thoroughly washed with PBS to remove free CpG before i.v. injections of 5×106 B cells at days 10 and 15. Y-axis shows number of lung metastatic foci ± SEM in the lungs of 4–6 mice per group at day 32. All data shown here were reproduced at least three times.
Next, to confirm that the observed results were due to B cell activation, we first depleted B cells in tumor-bearing mice by injecting anti-CD20 Ab at days 3 and 5 post tumor challenge and, then at days 10 and 15, the mice were adoptively transferred with mock or CpG-ODN-pretreated B cells from syngeneic naïve mice. The enhanced metastasis of 4T1.2 cancer-bearing mice after anti-CD20 Ab treatment (Fig.5E and also see Fig.1E) was not only reversed, but metastasis was almost completely lost if the mice were adoptively transferred with CpG-treated B cells (Fig.5E) or CpG-treated Bregs (data not shown). These results were in striking contrast with the transfer of mock-treated B cells, which only slightly reduced the enhanced lung metastasis to the levels of control untreated mice (presumably indicating the restoration of B cells responses, Fig.5E), suggesting a dominant effect of adoptively transferred CpG-treated B cells/tBregs over in-situ tBregs. This is not an artifact of the cancer model or mouse strain we used, as a different tumor, B16 melanoma in congenic B-cell–deficient µMT mice also progressed poorly unless replenished with tBregs from WT C57BL/6 mice (sup.Fig.5A) (17). The transfer of tBregs inhibited melanoma peptide gp10025–32-specific IFNγ-expressing CD8+ T cells in µMT mice (sup.Fig.5B,C) and in B-cell-competent pmel mice (transgenic for CD8+ T cells specific for gp10025–32 (32), sup.Fig.5D,E), which was almost completely lost if tBregs were treated with CpG (sup.Fig.5A–E).
CpG–mediated T-cell activation requires upregulation of 4-1BBL on tBregs
tBregs as well as LPS–stimulated B cells (B-LPS) up regulated CD40, B7.1 and B7.2 (17), excluding their direct involvement in suppression of T cells. However, we found that, compared with B-LPS or even with normal B cells, expression of 4-1BBL, an immunostimulatory receptor that activates CD8+ T cells and NK cells via 4-1BB (36, 37), was significantly reduced on murine and human tBregs (Fig.6 and suppl.Fig.6A,B). Compared with naïve mouse B cells, the expression of 4-1BBL was reduced in CD25+ B cells homing to the primary tumor, the secondary lymphoid organs and lungs of 4T1.2 cancer–bearing mice (Fig.6B), which was further decreased in tBregs from mice treated with anti-CD20 Ab (Suppl.Fig.6B). However, BLC-arp/CpG treatment reversed the reduced expression of 4-1BBL on murine and human tBregs (Fig.6C) as well as in vivo in tumor-bearing mice (Fig.6D). Importantly, B cells from tumor-bearing mice, which were protected from lung metastasis as a result of BLC-arp/CpG-induced inactivation of tBregs and induction of cytolytic CD8+ T cells (Fig.5A–E), also expressed significantly up regulated 4-1BBL (Fig.6D). Thus, CpG could render tBregs stimulatory by reversing the insufficient signaling of the 4-1BBL/4-1BB axis. Indeed, tBregs could not suppress and instead activated T cells, if the missing signaling was substituted with the agonistic anti-4-1BB Ab (anti-CD137 Ab, Fig.6E).
FIGURE 6. CpG renders tBregs stimulatory by up regulating 4-1BBL on tBregs.
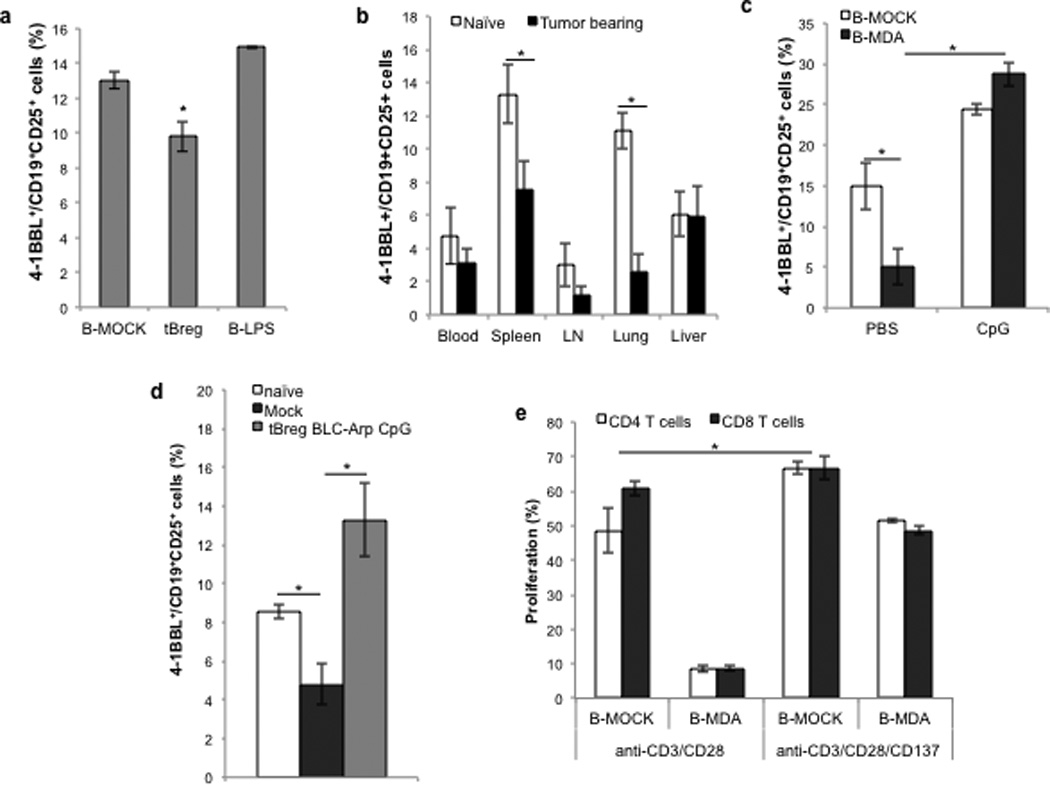
Compared with LPS-stimulated B cells (B-LPS), ex vivo-generated murine tBregs reduce surface expression of 4-1BBL (A). Shown is % ± SEM of 4-1BBL+ within CD25+ CD19+ B cells (B) of a triplicate experiment. (B) Compared with naïve mice, 4T1.2 cancer-bearing BALB/c mice have less B cells expressing 4-1BBL. Shown is % ± SEM (4 mice/group) of 4-1BBL+ within CD25+ CD19+ B cells in blood, spleen, draining lymph nodes (LN), lungs and liver. The low levels of 4-1BBL on ex vivo generated human (B-MDA, C) and murine tBregs from mice with 4T1.2 cancer (D) were drastically reversed by in vitro (C) and in vivo (D) BLC-arp/CpG treatment, respectively. The suppressive activity of tBregs was blocked in the presence of agonistic anti-4-1BB (CD137) Ab that presumably supplemented the missing 4-1BBL signaling to T cells (E). All data shown here were from triplicate experiments reproduced at least three times.
DISCUSSION
Here, we provide a new mechanistic insight to reconcile the debate of the last 30 years (12–14) on the role of B-cell inactivation in cancer therapy. The hypothesis was that if B cells promote carcinogenesis in mice (12–14, 19, 20) and their genetic dysfunction usually retards tumor progression (16, 17), anti-CD20 treatments should provide therapeutic benefit in mice bearing highly metastatic 4T1.2 breast cancer. However, although 4T1.2 cancer loses its ability to metastasize in B-cell deficient mice, the outcome of anti-CD20 Ab treatment in immune competent mice differed depending on the time of use. While 4T1.2 cancer metastasis was abrogated in mice treated with anti-CD20 Ab before tumor challenge, surprisingly its metastasis was instead significantly enhanced if B cells were depleted with anti-CD20 Ab in mice with already established tumor. Our data indicate that this is due to anti-CD20 Ab-induced enrichment of CD20Low tBregs after the depletion of “immunostimulatory” CD20High B cells, which are presumably de novo generated from normal B cells in response to 4T1.2 cancer (17). We also confirmed similar enrichment of suppressive CD20Low tBregs in modeling studies with B cells treated with cancer cell CM in vitro and, importantly, in 6/13 human patients with B-CLL after treatment with rituximab. In one patient, while B cells prior the treatment did not reveal signs of tBregs, the majority of the remaining B cells after rituximab depletion were CD20Low tBregs. Since these CD20Low tBregs readily suppressed T cell activity and induced FoxP3+ Tregs from non-Treg CD4+ T cells, a function we previously associated with murine breast cancer-induced tBregs (17), it is tempting to speculate that rituximab (which mostly depletes mature and memory CD20+ B cells, but not long-lived plasma cells (38)) may also promote immunosuppression in some cancer patients. For example, it remains to be seen whether recent failure of rituximab to benefit patients with renal cell carcinoma and melanoma (29) may also be governed by a similar mechanism. However, CD20+ B cells are required for optimal anticancer immunity, and their presence in metastatic lymph nodes is a sign of favorable disease outcome in patients with head and neck cancer (31). In support, anti-CD20 Ab–induced progression of B16 melanoma in C57BL/6 mice is thought to be a result of the depletion of CD20+ B cells (28) that induce anticancer effector immune responses (39). Thus, in cancers where tBregs facilitate escape and metastasis, the therapeutic emphasis should be to avoid further worsening the disease outcome by depleting also the beneficial antitumor CD20+ B cells. For this purpose, we created an alternative strategy that inactivates tBregs and at the same time activates antitumor B cells. Although administration of free CpG-ODN can induce lymphoid follicle destruction and immunosuppression (35), here we show that relatively low doses of CpG-ODN (<10 µg) can neutralize the metastasis-promoting activity of tBregs if delivered in vivo into CXCR5-expressing B cells using modified CXCL13 chemokine (BLC-arp). BLC-arp/CpG efficiently abrogated lung metastasis in mice bearing an aggressive 4T1.2 breast cancer by activating B cells to elicit cytolytic antitumor CD8+ T cells.
Interestingly, besides being CD20Low, tBregs also expressed low levels of 4-1BBL, an important co-stimulatory molecule involved in activation and amplification of Th1-polarized T-cell responses, CD8+ T cells and NK cells by signaling through 4-1BB (CD137) (36, 37). Although the biological relevance of this pathway remains poorly understood and is a subject of a different study, we can speculate that the expression of 4-1BBL is low to provide insufficient co-stimulatory signaling to T cells. Confirming this prediction, when we circumvented the missing signal to 4-1BB on T cells, such as by treating with agonistic anti-CD137 Ab or activating with CpG-ODN, the suppressive activity of tBregs was abrogated. Alternatively, by being 4-1BBLLow, tBregs may avoid a reverse signaling required for their inactivation, as 4-1BBL signaling induces inflammatory responses from epithelial cells in renal ischemia-reperfusion injury (40). Thus, our findings raise an interesting possibility that similar tBreg-like cells may also control autoimmune responses, if we consider that cancer escape is utilizing an existing regulatory machinery. Moreover, the opposing results observed after depletion CD20+ B cells with rituximab in humans with autoimmune diseases (such as impairment of T-cell responses (41) and amelioration of RA, MS and T1D (18) and exacerbation of ulcerative colitis and psoriatic arthropathy in patients with Graves disease and non-Hodgkin lymphoma (9–11)) may also be due to the rituximab-induced misbalance of CD20+ B cells and tBreg-like cells. Similarly CD25+ B cells that induce anergy of activated T cells by competing for IL-2 upon treatment with S. aureus Cowan 1 antigen were recently reported (42), suggesting that tBregs may be induced by parasite infection. Unlike them, tBregs efficiently inhibit both resting and activated T cells, including CD4+ and CD8+ T cells without induction of cell death or use of IL-2. To date, we found that tBregs (17, 27) phenotypically and functionally differ from other Bregs involved in autoimmune responses (5, 43, 44) and LPS- or BCR-activated suppressive B cells (45, 46).
Although earlier studies from others emphasized the role of tumor-induced Gr1+ CD11b+ MDSCs in metastasis of 4T1 cancer cells (47–49), the data presented here further confirms our previous reports underscoring the importance of tBregs and tBreg- induced Tregs in facilitation of lung metastasis (17, 27). The process can be inhibited and lung metastasis can be successfully abrogated by depleting tBregs with anti-B220 Ab (17) or by treatment with anti-CD25 Ab that depletes both tBregs and Tregs (17, 27). Further supporting this idea, we also observed that, although B-cell deficient mice did not support lung metastasis of 4T1.2 cells, the expansion of MDSCs (associated by others with lung metastasis (50)) was comparable to that in tumor-bearing WT mice (data not shown). Taken together, our data not only suggest that anti-CD20 Ab/rituximab may not provide benefits in solid tumors, as it enriches for CD20Low 4-1BBLLow tBregs and thereby further enhances lung metastasis by exacerbating tBreg-mediated immunosuppression. Instead, we propose an alternative and simple strategy to control cancer escape by targeting both the regulatory and effector arms of the immune system with a modified CXCL13 (BLC-arp). We show that BLC-arp-coupled CpG-ODN can efficiently abrogate lung metastasis by inactivating CD20Low 4-1BBLLow tBregs and activating immunostimulatory B cells. Although 4T1 cancer poorly elicits CD8+ T cell responses, BLC-arp/CpG induced expansion of antigen-specific IFNγ-expressing effector and cytolytic CD8+ T cells due to the blockage of tBregs and activation of B cells.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Dr. Dan Longo (NIA/NIH) and Ana Lustig (NIA/NIH) for helpful comments and suggestions; Karen Madara (NIA/NIH) for providing human blood samples. This research was supported by the Intramural Research Program of the National Institute on Aging, NIH.
The entire work was funded and performed at the National Institute on Aging
Footnotes
Conflict of Interest: The authors do not have any conflict of interest.
References
- 1.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 2.Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 3.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 4.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. The Journal of experimental medicine. 1997;186:1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessel A, Haj T, Peri R, Snir A, Melamed D, Sabo E, et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev. 2012;11:670–677. doi: 10.1016/j.autrev.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Mielke F, Schneider-Obermeyer J, Dorner T. Onset of psoriasis with psoriatic arthropathy during rituximab treatment of non-Hodgkin lymphoma. Ann Rheum Dis. 2008;67:1056–1057. doi: 10.1136/ard.2007.080929. [DOI] [PubMed] [Google Scholar]
- 10.Dass S, Vital EM, Emery P. Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum. 2007;56:2715–2718. doi: 10.1002/art.22811. [DOI] [PubMed] [Google Scholar]
- 11.Goetz M, Atreya R, Ghalibafian M, Galle PR, Neurath MF. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm Bowel Dis. 2007;13:1365–1368. doi: 10.1002/ibd.20215. [DOI] [PubMed] [Google Scholar]
- 12.Brodt P, Gordon J. Anti-tumor immunity in B lymphocyte-deprived mice. I. Immunity to a chemically induced tumor. Journal of immunology. 1978;121:359–362. [PubMed] [Google Scholar]
- 13.Brodt P, Gordon J. Natural resistance mechanisms may play a role in protection against chemical carcinogenesis. Cancer immunology, immunotherapy : CII. 1982;13:125–127. doi: 10.1007/BF00205312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monach PA, Schreiber H, Rowley DA. CD4+ and B lymphocytes in transplantation immunity. II. Augmented rejection of tumor allografts by mice lacking B cells. Transplantation. 1993;55:1356–1361. [PubMed] [Google Scholar]
- 15.Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG, et al. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol. 2006;125:451–458. [PubMed] [Google Scholar]
- 16.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nature Medicine. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 17.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4 T cells to T-regulatory cells. Cancer research. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev. 2010;237:264–283. doi: 10.1111/j.1600-065X.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- 19.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer research. 2006;66:7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 22.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, et al. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. The Journal of clinical investigation. 2011;121:4268–4280. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. The Journal of clinical investigation. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrne SN, Halliday GM. B cells activated in lymph nodes in response to ultraviolet irradiation or by interleukin-10 inhibit dendritic cell induction of immunity. J Invest Dermatol. 2005;124:570–578. doi: 10.1111/j.0022-202X.2005.23615.x. [DOI] [PubMed] [Google Scholar]
- 26.Sun JB, Flach CF, Czerkinsky C, Holmgren J. B lymphocytes promote expansion of regulatory T cells in oral tolerance: powerful induction by antigen coupled to cholera toxin B subunit. Journal of immunology. 2008;181:8278–8287. doi: 10.4049/jimmunol.181.12.8278. [DOI] [PubMed] [Google Scholar]
- 27.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorrentino R, Morello S, Forte G, Montinaro A, De Vita G, Luciano A, et al. B cells contribute to the antitumor activity of CpG-oligodeoxynucleotide in a mouse model of metastatic lung carcinoma. Am J Respir Crit Care Med. 2011;183:1369–1379. doi: 10.1164/rccm.201010-1738OC. [DOI] [PubMed] [Google Scholar]
- 29.Aklilu M, Stadler WM, Markiewicz M, Vogelzang NJ, Mahowald M, Johnson M, et al. Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2004;15:1109–1114. doi: 10.1093/annonc/mdh280. [DOI] [PubMed] [Google Scholar]
- 30.Candolfi M, Curtin JF, Yagiz K, Assi H, Wibowo MK, Alzadeh GE, et al. B cells are critical to T-cell-mediated antitumor immunity induced by a combined immune-stimulatory/conditionally cytotoxic therapy for glioblastoma. Neoplasia. 2011;13:947–960. doi: 10.1593/neo.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baatar D, Olkhanud P, Newton D, Sumitomo K, Biragyn A. CCR4-expressing T cell tumors can be specifically controlled via delivery of toxins to chemokine receptors. J Immunol. 2007;179:1996–2004. doi: 10.4049/jimmunol.179.3.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiavo R, Baatar D, Olkhanud P, Indig FE, Restifo N, Taub D, et al. Chemokine receptor targeting efficiently directs antigens to MHC class I pathways and elicits antigen-specific CD8+ T-cell responses. Blood. 2006;107:4597–4605. doi: 10.1182/blood-2005-08-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nature Medicine. 2004;10:187–192. doi: 10.1038/nm987. [DOI] [PubMed] [Google Scholar]
- 36.Watts TH, DeBenedette MA. T cell co-stimulatory molecules other than CD28. Curr Opin Immunol. 1999;11:286–293. doi: 10.1016/s0952-7915(99)80046-6. [DOI] [PubMed] [Google Scholar]
- 37.Vinay DS, Kwon BS. 4-1BB signaling beyond T cells. Cell Mol Immunol. 2011;8:281–284. doi: 10.1038/cmi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4802–4807. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, Hirano N, Anderson KS, Butler MO, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–3325. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 40.Kim HJ, Lee JS, Kim JD, Cha HJ, Kim A, Lee SK, et al. Reverse signaling through the costimulatory ligand CD137L in epithelial cells is essential for natural killer cell-mediated acute tissue inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E13–E22. doi: 10.1073/pnas.1112256109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tretter T, Venigalla RK, Eckstein V, Saffrich R, Sertel S, Ho AD, et al. Induction of CD4+ T-cell anergy and apoptosis by activated human B cells. Blood. 2008;112:4555–4564. doi: 10.1182/blood-2008-02-140087. [DOI] [PubMed] [Google Scholar]
- 43.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 45.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 46.Hussain S, Delovitch TL. Intravenous transfusion of BCR-activated B cells protects NOD mice from type 1 diabetes in an IL-10-dependent manner. J Immunol. 2007;179:7225–7232. doi: 10.4049/jimmunol.179.11.7225. [DOI] [PubMed] [Google Scholar]
- 47.DuPre SA, Hunter KW., Jr Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: association with tumor-derived growth factors. Exp Mol Pathol. 2007;82:12–24. doi: 10.1016/j.yexmp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–2211. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 49.Serafini P, De SC, Marigo I, Cingarlini S, Dolcetti L, Gallina G, et al. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


