Abstract
Objectives
Lipocalin-2 (Lcn2) is an innate immune protein expressed by a variety of cells and is highly upregulated during several pathological conditions including immune-complex (IC) mediated inflammatory/autoimmune disorders. However, the function of Lcn2 during IC-mediated inflammation is largely unknown. Therefore our objective was to investigate the role of Lcn2 in IC-mediated diseases.
Methods
The upregulation of Lcn2 was determined by ELISA in three different mouse models of IC-mediated autoimmune disease: systemic lupus erythematosus, collagen-induced arthritis and serum-induced arthritis. The in vivo role of Lcn2 during IC-mediated inflammation was investigated using Lcn2 knockout (Lcn2KO) mice and their wild type (WT) littermates.
Results
Lcn2 levels were significantly elevated in all the three autoimmune disease models. Further, in an acute skin inflammation model, Lcn2KO mice demonstrated a 50% reduction in inflammation with histopathological analysis revealing strikingly reduced immune cell infiltration compared to WT mice. Administration of recombinant Lcn2 to Lcn2KO mice restored inflammation to levels observed in WT mice. Neutralization of Lcn2 using a monoclonal antibody significantly reduced inflammation in WT mice. In contrast, Lcn2KO mice developed more severe serum-induced arthritis compared to WT mice. Histological analysis revealed extensive tissue and bone destruction with significantly reduced neutrophil infiltration but considerably more macrophage migration in Lcn2KO mice when compared to WT.
Conclusion
These results demonstrate that Lcn2 may regulate immune cell recruitment to the site of inflammation, a process essential for the controlled initiation, perpetuation and resolution of inflammatory processes. Thus, Lcn2 may present a promising target in the treatment of IC-mediated inflammatory/autoimmune diseases.
Key Indexing Terms: Lipocalin-2, immune-complexes, Fcγ receptors, autoimmune diseases
INTRODUCTION
Lipocalin 2 (Lcn2) belongs to a super family of small secreted proteins produced by a variety of cells including epithelia and neutrophils (1). Lcn2 and its human ortholog, neutrophil-gelatinase associated lipocalin (NGAL), are elevated by several orders of magnitude during inflammation/infection/injury (2, 3). Induction of Lcn2 during sterile inflammation suggests that it may have additional physiologic functions (4). The well-established functions of Lcn2 include antibacterial activity via sequestration of bacterial siderophores, iron homeostasis and cellular apoptosis (5–7). Lcn2 has been shown to be necessary for neutrophil recruitment in animal models of infection (8, 9) and a recent study confirms Lcn2 is indispensible for neutrophil migration, adhesion and function (10). Accordingly, Lcn2KO mice are susceptible to bacterial sepsis (3). Several investigations have shown the upregulation of Lcn2 in antibody-mediated inflammatory/autoimmune diseases such as systemic lupus erythromatous (SLE) in humans (11–13).
Autoimmune disease develops from an aberrant immune reaction against the host’s own antigens. Pathogenesis of antibody-mediated autoimmune disease involves both cellular and humoral immune responses. Unusual activation of autoantigen-specific T and B cells, viral infections and changes in the host’s cytokine profile are thought to drive development of antibody-mediated autoimmune disease (14). During IC-mediated autoimmune diseases such as arthritis, SLE and autoimmune vasculitis, autoantibodies bind to target host cells and initiate inflammation resulting in tissue injury (15, 16). Several in vivo and in vitro studies have shown that the interaction of the IC’s Fc domain with Fcγ receptors (FcγRs) expressed on inflammatory cells leads to the destruction of IC-bound target cells/tissues through antibody-dependent cytotoxicity (ADCC) and phagocytosis (17, 18). Therefore, it has been concluded that FcγRs plays a major role during the pathogenesis of several IC-mediated autoimmune diseases. Apart from FcγRs, ICs also interact with complement components and trigger the release of chemotactic peptide C5a, which also induces degranulation of mast cells (19, 20). Collectively, the interaction of ICs with FcγRs and complement components leads to the release of many chemokines and inflammatory mediators followed by destruction of autoantibody-coated target tissues during autoimmune disease.
More recently, upregulation of Lcn2, along with other inflammatory cytokines, has been reported in SLE patients (11–13, 21). However, the exact role of elevated Lcn2 in autoimmune disease (presumably sterile inflammation/aseptic disease) is largely unknown. Therefore, in this report, we have investigated the function of Lcn2 in an acute model of IC-mediated skin inflammation (reverse passive Arthus reaction) and a well-established serum-induced arthritis (SIA) model using genetically engineered Lcn2KO mice and their WT littermates. As arthritic symptoms in this SIA model persist for longer periods of time, it served as an excellent model to study Lcn2’s role during chronic inflammatory conditions. Our results demonstrate that Lcn2 levels are significantly elevated in three different models of autoimmune disease. Interestingly, in our acute model of IC-mediated skin inflammation, Lcn2 mice exhibited substantially reduced inflammation when compared to WT mice whereas, in SIA model, Lcn2KO mice develop severe arthritis as evidenced by paw swelling and histology. Our results demonstrate that Lcn2 is a host protective factor against systemic autoimmune disease.
MATERIALS AND METHODS
Reagents
Ovalbumin (OVA), Evan’s blue and Freund’s complete and incomplete adjuvant were purchased from Sigma (St. Louis, MO), rabbit anti-Ovalbumin IgG from Roche Molecular Biochemicals (Indianapolis, IN) and HRP-substrate and SDS-PAGE gels from BioRad (Hercules, CA). Iron-, siderophore- and endotoxin-free mouse recombinant Lcn2 (rec-Lcn2), Lcn2 neutralizing mAb (clone: 228418), rat IgG2a isotype control antibody (clone: 54447) and mouse Lcn2 Duoset ELISA kit are from R&D Systems (Minneapolis, MN), Micro BCA protein assay kit from Pierce (Rockford, IL) and bovine type II collagen from MD Biosciences, Inc (St. Paul, MN). Siemens Combostix reagent strips for urine analysis were procured from Emory University’s Hospital Pharmacy store (Atlanta, GA). Cell culture reagents were from Life Technologies (Gaithersburg, MD) and 2.4G2 (anti- mouse mAb CD16/32) mAb from BD biosciences (San Jose, CA). Rat anti-mouse neutrophil- (Ly-6G and Ly-6C: Clone NIMP-R14) and macrophage-specific (anti-F4/80: clone BM8) antibodies were purchased from Abcam, (Cambridge, MA). Affinity-purified polyclonal goat anti-rat antibody conjugated with Alexa-488 was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). KxB/N arthritic serum was a kind gift from Dr. Paul M Allen, Department of Pathology and Immunology, Washington University School of Medicine (St. Louis, MO).
Animal studies
Arthritis-susceptible DBA/J1 and SLE-prone NZB/WF1 (8–10 weeks old) female mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Lcn2KO (backcrossed to BL6 mice for more than 10 generations were obtained from Dr. Aderem (University of Washington) and were originally generated by Dr. Akira (Japan) and WT littermates on C57BL/6 background were bred and maintained at Emory University’s animal facility (Atlanta, GA). Genotyping of Lcn2KO was performed by Transnetyx, Inc (Cordova, TN). All animal experiments were conducted in accordance with Emory University IACUC.
Reverse passive Arthus reaction (RPA)
RPA was carried out as previously described (22, 23). Briefly, a group of Lcn2KO and WT littermates (n=3) were injected with anti-Ovalbumin interadermally on the dorsal side (12.5 or 25 µg per site). Immediately, 500 µg of ovalbumin in 100 µl of PBS along with 1% Evan’s blue was injected into the tail vein. Both groups were sacrificed after 4h and the reverse side of the skin at the antibody injection site was collected and evaluated for extravasation of blue dye as dye intensity corresponds to severity of inflammation at the site of antibody injection. Photographs of the isolated skin samples were taken and intensity of the blue dye was estimated using Image J software (National Institute of Health, Bethesda) and KaleidaGraph (Synergy Software, Reading, PA). In some experiments recombinant Lcn2 (recLcn2; 10 µg/mouse) or Lcn2 neutralizing mAb (100 µg/mouse) were administered i.v. to Lcn2KO and WT mice, respectively, 1h before the initiation of RPA. Skin biopsies were subjected to histopathological analysis using H&E staining.
Collagen induced arthritis (CIA) and serum induced arthritis (SIA)
CIA was induced by immunizing the DBA/1J mice with bovine type II collagen as described (24). Briefly, bovine type II collagen (2 mg/ml) was emulsified thoroughly with an equal volume of complete Freund’s adjuvant with heat-killed mycobacterium (4 mg/ml) and 50 µl of the resulting emulsion (containing 100 µg of collagen) was injected intradermally at the base of the tail. After 21 days, a booster dose was given at the same concentration of collagen with incomplete Freund’s adjuvant. Serum prepared from the blood of arthritic K/BxN mice was used to induce SIA in Lcn2KO and WT littermates as described (25). Briefly, arthritis was induced by injecting 200 µl of K/BxN arthritic serum (i.p).
In both CIA and SIA models mice were monitored daily for the development of arthritis. Paw swelling was measured using a digital caliper. Blood was collected via retroorbital plexus and hemolysis-free serum was collected using serum separator tubes from BD Biosciences and stored at −70°C.
Development of SLE in NZB/F1 mice
SLE-prone NZB/WF1 mice of 8–10 weeks of age were purchased from Jackson Laboratories and maintained at Emory University’s animal facility until they developed autoimmune disease. Proteinuria was monitored once a week as an index of SLE development using commercially available Siemens Combostix reagent strips for urinalysis. Protein contents were expressed as mg of protein/ml of urine according to manufacturer protocol. Blood samples were collected at 8–12 weeks prior to the onset of disease and after 40 weeks once mice had fully developed disease. Serum samples were prepared and stored at −70°C.
Histological studies
Arthritic paws were removed and fixed in 4% buffered formalin, embedded in paraffin and stained with hematoxylin and eosin (Histoserve Inc, Germantown, MD). Severity of inflammation in the tissue sections was scored as follows: 0-no symptoms of inflammation (naïve paw), 1-mild infiltration of inflammatory cells, 2-infiltration of immune cells with muscle destruction, 3-immune cells with panus formation, 4-mild metacarpal bone erosion and 5-severe metacarpal and articular bone erosion. The average score by two individuals were used to plot the graphs. Immunohistochemistry of paraffin-embedded tissue was carried out by Emory University’s Immunohistochemistry Core Laboratory to detect neutrophils and macrophages using rat anti-mouse Ly-6 (for neutrophils) and anti-mouse F4/80 (for macrophages) as primary antibodies. Bound antibodies were detected using goat anti-rat antibody conjugated with Alexa-488. Histopathology photographs were taken using the Olympus BX53 microscope (x10 objective) and fluorescent images were captured using the x20 and x63 objectives on a Zeiss Axioscope (Carl Zeiss). DAPI (4',6-diamidino-2-phenylindole) stain was performed to detect the nucleus. Polymorphonuclear cells (neutrophils) were identified by nuclear structure followed by anti-Ly6 antibody.
ELISA
Hemolysis-free sera collected from the above animal models via retroorbital plexus were used to quantify circulating Lcn2 using mouse Lcn2 Duoset ELISA kit according to manufacturer instructions.
Statistical analysis
One-way analysis of variance (one-way ANOVA) and Student’s t-test (unpaired, two-tailed) were performed to compare the statistical significance between WT and Lcn2KO mice with p<0.05 (*) considered statically significant. GraphPad Prism 5 software was used to calculate statistical significance.
RESULTS
Upregulation of systemic Lcn2 in mouse models of autoimmune disease
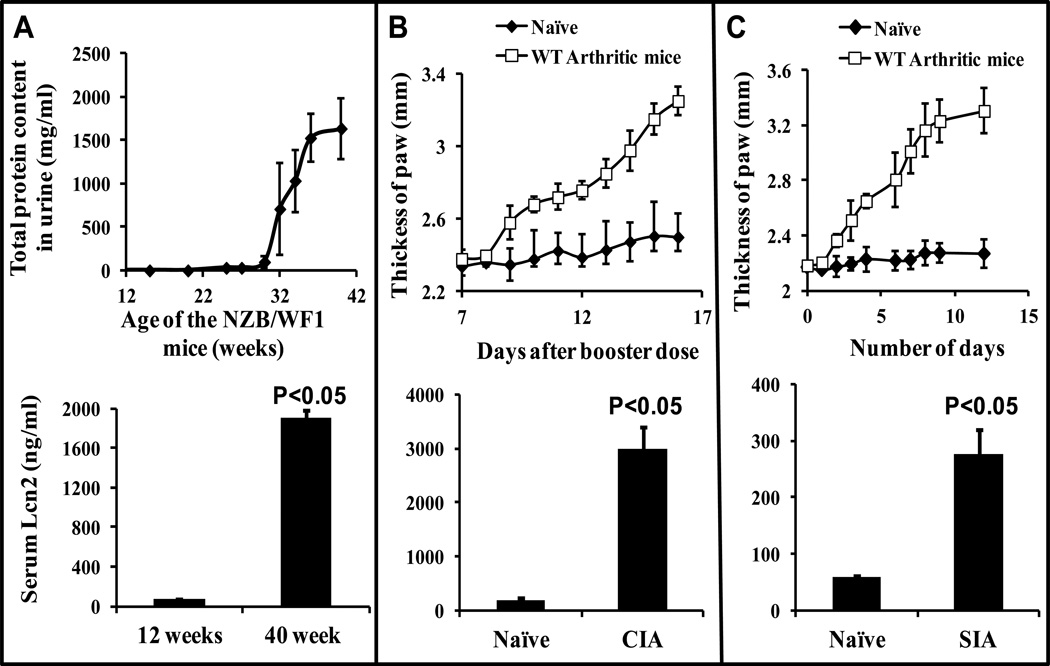
To study whether Lcn2 levels are elevated during the development of different IC-mediated autoimmune diseases, we quantitated systemic Lcn2 in mouse models of SLE and arthritis. Formation of ICs from autoantibodies against nuclear antigens has been implicated in the development of SLE (26, 27) . NZB/WF1 mice are susceptible to development of systemic autoimmune disease symptoms as they age and most die by 40–50 weeks as a result of multiple organ damage. Urine samples were collected once a week to monitor proteinuria as an index of SLE. Serum samples were collected once NZB/WF1 mice began secreting over 2 mg/ml of protein in their urine. Serum samples collected before (12 weeks old) and after (40 week old) the onset of lupus (Fig 1A, upper panel) was subjected to ELISA to investigate the upregulation of Lcn2. We observed a 29-fold increase in Lcn2 secretion after the onset of disease (at around 40 weeks of age) in SLE mice (Fig 1A, bottom panel) compared to the level of serum Lcn2 prior to onset of disease (at 12 weeks old).
Figure 1. Upregulation of systemic Lcn2 in animal models of autoimmune diseases.
(A) Serum samples from NZB/WF1 mice (n=5) at 12 and 40 weeks old (upper panel) were collected and subjected for ELISA (lower panel). (B upper panel) CIA was induced in DBA/J1 mice (n=5) using bovine collagen type-II. (C upper panel) SIA in C57BL/6J mice (n=3) by injecting K/BxN arthritic serum and paw thickness was measured as described in Materials and Methods. Serum samples were collected and assayed for Lcn2 by ELISA (B and C lower panel) as described under Materials and Methods. Figures are representative of three individual experiments. Data are mean ± SD of triplicates.
Next, arthritis was induced either by immunizing DBA/J1 mice with bovine type II collagen (CIA) or by injecting Kx/BN arthritic serum into C57BL/6 mice (SIA). After the onset of arthritis as determined by swelling of the paw (figure 1B and C; upper panel), serum samples were collected and subjected to ELISA in order to quantitate Lcn2. As shown in Fig.1B and C (bottom panel), systemic concentrations of Lcn2 were significantly increased in both the CIA (17 fold) and SIA (5 fold) arthritis models when compared with non-arthritic naïve mice. Taken together, these data suggest that Lcn2 levels are substantially elevated irrespective of the type of autoimmune disease.
Lcn2 deficient mice exhibit significantly reduced skin inflammation in RPA model
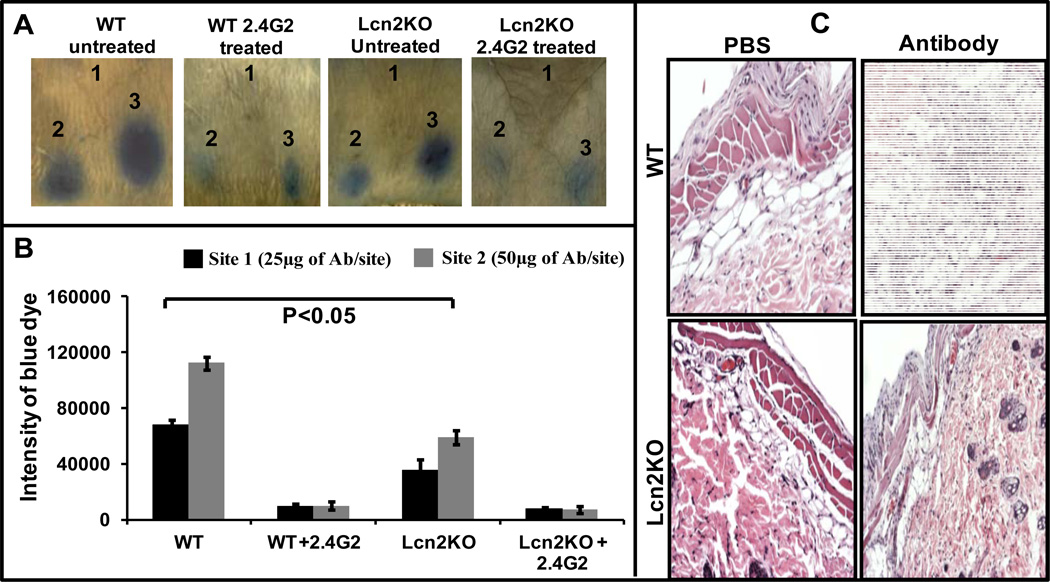
To investigate the role of Lcn2 during IC-mediated inflammation, we utilized an acute antibody-mediated inflammation model known as reverse-passive Arthus reaction (28). In this model, inflammation is initiated following antigen-antibody complex formation at the site of antibody injection. We induced inflammation by injecting rabbit anti- chicken ovalbumin (anti-OVA) interadermally on the dorsal side of the mice. The antigen, chicken ovalbumin (OVA), was injected intravenously along with 1% Evan’s blue dye. Infiltration of inflammatory cells and vascular leakage can be quantified by measuring extravasation of blue dye at the site of antibody injection (22, 23) as severity of inflammation is directly proportional to the intensity of the blue dye. As shown in Figure 2A, Lcn2KO mice exhibited reduced inflammation when compared to WT littermates. We observed an approximately 50% reduction in the intensity of antibody-mediated inflammation in Lcn2KO mice when compared to WT mice. The intensity of blue dye at the inflamed site was quantitated using KaleidaGraph software (Fig 2B). A group of mice (n=3) treated with a mAb (40 µg/mice, i.p) specific for mouse FcγRs (2.4G2) such as CD16A and CD32B completely inhibited IC-mediated inflammation (Fig 2A and B) and served as a specificity control. Since 2.4G2 does not block FcγRI and FcγRIV, IC-mediated RPA may be mediated solely by FcγRIII. Histopathological analysis of the inflamed sites (Fig 2C; section is from site 2, Fig 2A) showed a striking reduction in immune cell infiltration in Lcn2KO mice when compared to WT mice.
Figure 2. Lcn2 is required to potentiate IC-mediated inflammation in RPA model.
(A) RPA was initiated as described under Materials and Methods. After 3h mice were euthanized and the dorsal side of the skin photographed for analysis. The photographs are representative of three individual mice. (B) Blue dye intensity was quantified using ImageJ and KaleidaGraph software. Data are expressed as ± SD. (C) Panel-1: Skin biopsy from PBS injection site of WT untreated mouse with RPA (from Figure 2A, site 1). No specific pathologic changes are identified. Panel-2: Skin biopsy from anti-Ova antibody injection site of WT untreated mouse with RPA (from Figure 2A, site 3). The epidermis and dermis are essentially unexceptional. Subdermal fat is edematous and shows infiltration of inflammatory cells at the site of inflammation. Panel-3: Skin biopsy from PBS injection site of Lcn2KO untreated mouse with RPA (from Figure 2A, site 2). No specific pathologic difference between untreated WT and Lcn2KO mice. Panel-4: Skin biopsy from anti-Ova antibody injection site of Lcn2KO mouse (from Figure 2A, site 3). Though the pathology of Lcn2KO is indistinguishable, there is a substantial decrease in the infiltration of inflammatory cells than the WT mice.
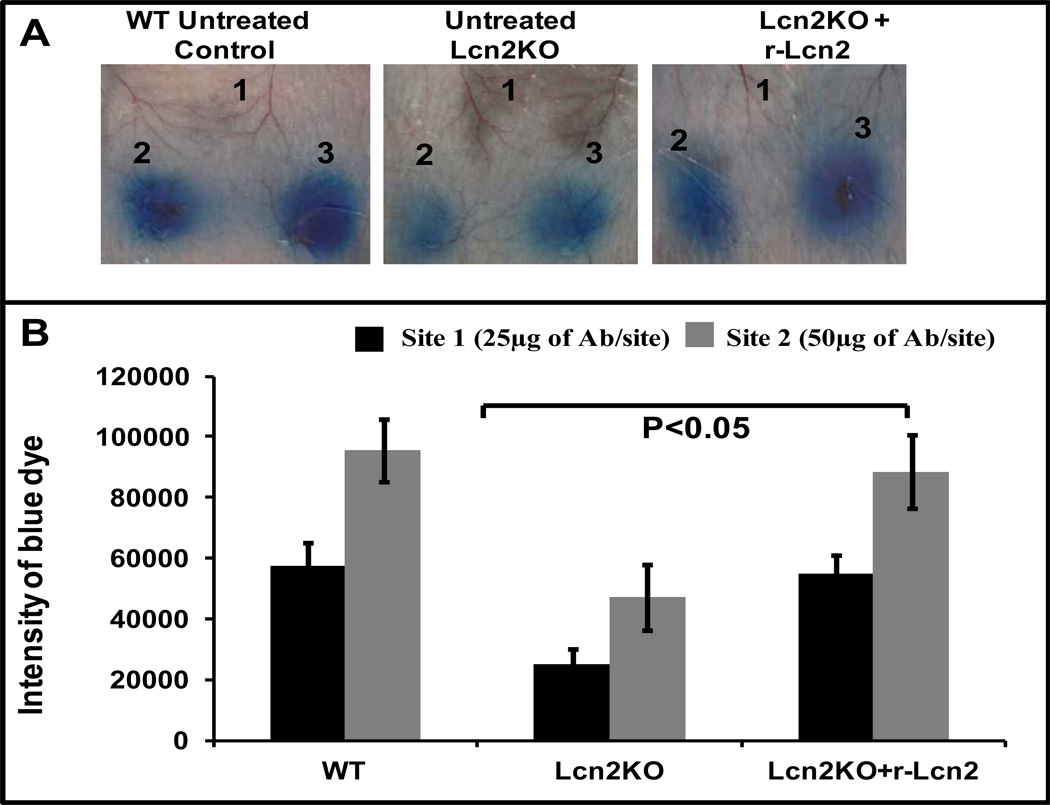
Administration of recombinant Lcn2 (rec-Lcn2) to Lcn2KO mice resulted in similar degree of IC-induced inflammation as in WT mice
To further substantiate our results, Lcn2KO mice were administered commercially available iron-, siderophore- and endotoxin-free rec-Lcn2 (10 µg/mouse) 1h prior to RPA. We used a dose of rec-Lcn2 that produced systemic Lcn2 levels 5-fold higher than systemic basal levels (100 ng/ml of blood) in Lcn2KO mice (data not shown). As shown in Fig 3A and B, Lcn2KO mice administered rec-Lcn2 exhibited inflammation similar to that found in WT mice in response to ICs as evidenced by blue dye extravasation. These data suggest that Lcn2 is necessary for immune cell infiltration and, thus, the potentiation of antibody-mediated skin inflammation.
Figure 3. Rec-Lcn2 administration to Lcn2 deficient mice induces similar levels of IC-induced Evan’s blue extravasation in WT mice.
(A) A group of Lcn2KO mice (n=3) were injected with rec-Lcn2 intravenously (100 µg/mouse). After 1h, these mice were injected intradermally with PBS (site 1) or anti-Ova (12.5 µg at site 2 and 25 µg at site 3). RPA was initiated as described above in three separate groups of mice (n=3): WT untreated control, Lcn2KO untreated control and rec-Lcn2 treated Lcn2KO. After 3h the mice were euthanized and the dorsal side of the skin was photographed for analysis. The figure shows three representative mice. (B) The blue dye seen in the photographs was quantified using ImageJ and KaleidaGraph softwares for groups with or without rec-Lcn2 treatment. Data are presented as the mean ± SD from three experiments.
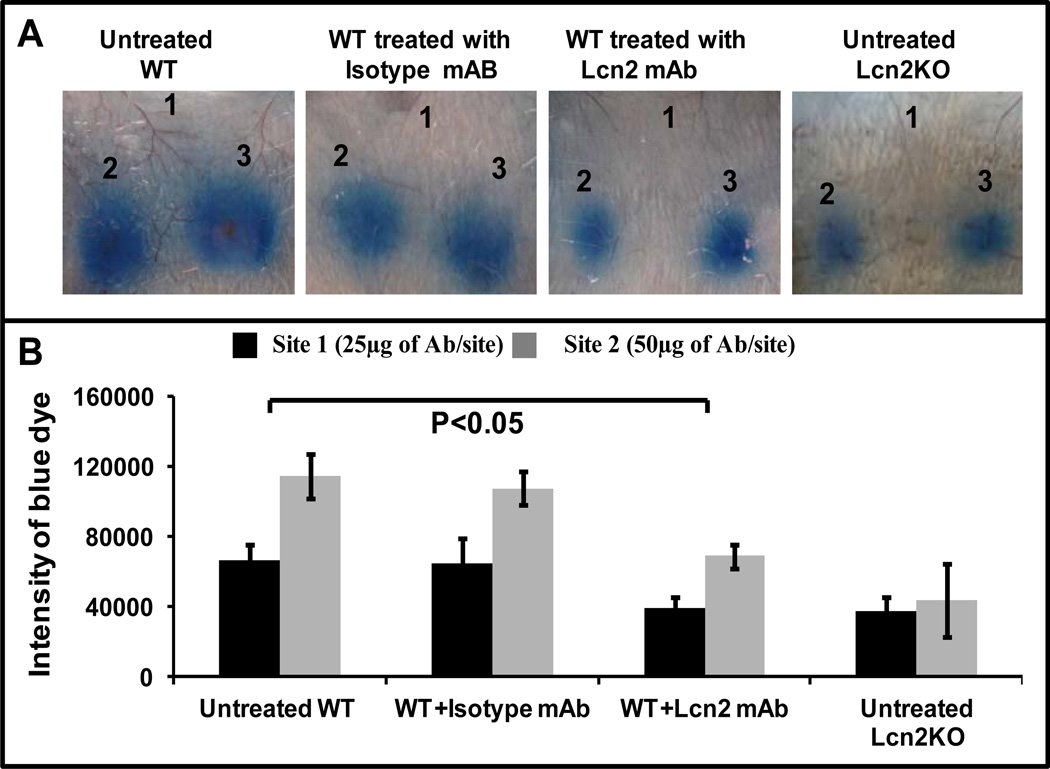
Neutralization of Lcn2 in WT mice alleviates IC-mediated inflammation
Next, to confirm the role of Lcn2 in RPA, endogenous Lcn2 in WT mice was neutralized using an established (29) mAb against murine Lcn2. Lcn2-specific antibody (10 µg/mouse) was administered 1h prior to initiation of RPA. We observed that neutralization of Lcn2 significantly reduced Evan’s blue dye leakage in WT mice (Fig 4A and B) to levels similar to those exhibited by Lcn2KO mice. Collectively, these results suggest that Lcn2 is required for inflammatory cell migration during IC-mediated inflammation. To exclude the possibility that the dose of rec-Lcn2 we used may exceed biological levels leading to difficulty in interpreting our findings we utilized an alternate approach involving the development of IC-mediated arthritis to confirm our results.
Figure 4. Neutralization of Lcn2 in vivo in WT mice reduces severity of IC-induced inflammation.
(A) A group of WT mice (n=3) were injected with Lcn2 neutralizing mAb intravenously (100 µg/mouse). After 1h, these mice were injected intradermally with PBS (site 1) or anti-Ova (12.5 µg in site 2 and 25 µg in site 3). RPA was initiated as described above in four separate groups of mice (n=3): WT untreated control, WT treated with Lcn2 mAb, WT treated with isotype control mAb and Lcn2KO untreated control. After 3h the mice were euthanized and the dorsal side of the skin was photographed for analysis. The figure shows three representative mice. (B) Intensity of dermal lesions, seen blue in the photographs, was quantified using ImageJ and KaleidaGraph software. Data are presented as the mean ± SD from three experiments.
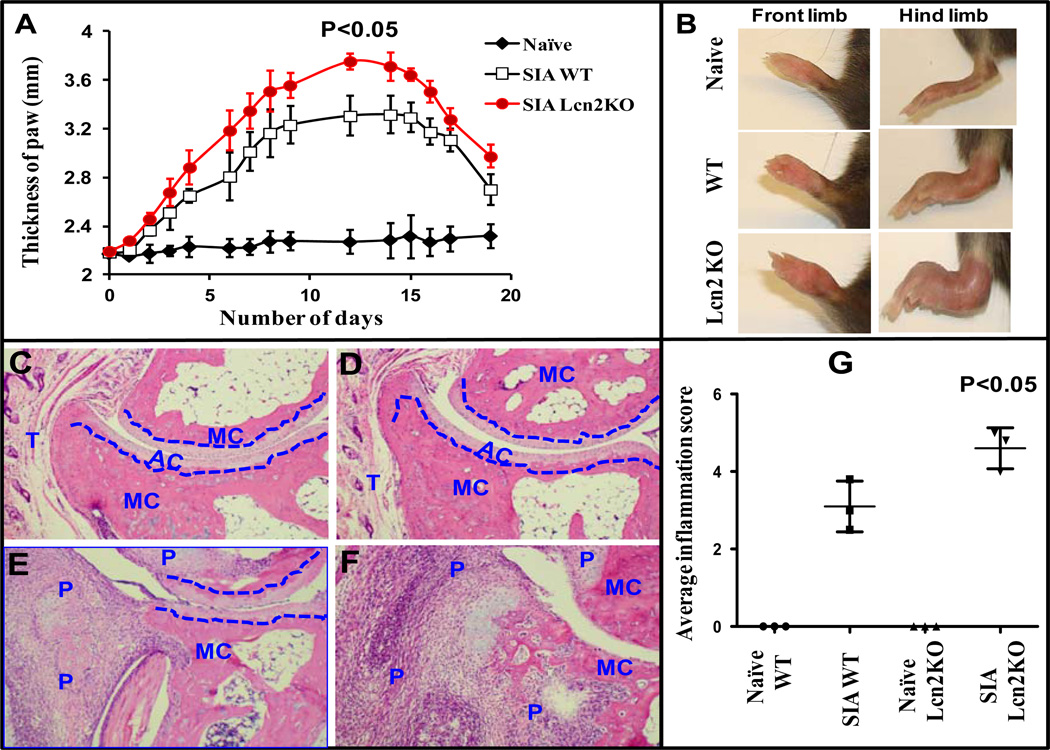
Lcn2 deficiency aggravates disease severity in SIA model
It has been shown that C57BL/6 mice are resistant to arthritis development in CIA (30, 31). Therefore, we utilized a well-established SIA model to investigate whether Lcn2 deficiency confers a similar degree of protection during the development of arthritis. We induced SIA in age- and gender-matched Lcn2KO and their WT littermates by injecting K/BxN arthritic serum (0.2 ml) intraperitoneally and monitored disease severity over a period of three weeks. SIA developed in WT and Lcn2KO mice in a time-dependent manner and reached disease severity by day 12 (Fig 5A). We observed that there was significantly more severe disease (P<0.05) as indicated by paw thickness in Lcn2KO mice compared to WT mice (Fig 5A). By day 12, Lcn2KO mice suffered greater disease when compared to WT littermates and were rendered nearly immobile as a result of paw swelling (Fig 5B). Consistent with clinical severity, synovial inflammation was significantly (p<0.05) elevated in Lcn2KO mice (Fig 5G) as evidenced by histopathological observation 12 days after K/BxN serum transfer. As a specificity control, unarthritic paws from naïve Ln2KO and WT mice were compared. The paws of unarthritic mice showed intact metacarpal bone, articular cartilage structure and tendon muscle without any detectable immune cells (Fig 5C and D). As shown in figure 5F, there was marked destruction of soft tissue, metacarpal bone and articular cartilage with extensive panus formation in Lcn2KO mice when compared to their WT littermates (Fig 5E). Taken together, these results suggest that Lcn2 plays a protective role during chronic IC-mediated inflammation and may also play an indispensible role during tissue remodeling and in preserving bone homeostasis.
Figure 5. Lcn2 deficient mice develop severe arthritis when compared to WT in SIA model.
SIA was induced as described under Materials and Methods. The mice were monitored for the development of arthritis by ankle thickness (A) and photographs were taken on day 12 (B). Histological section of ankle joints stained with haematoxylin and eosin (original magnification 10x). Tissue sections of hind limbs from naïve WT (C) and Lcn2KO mice (D) show remarkably intact bone structure. Tissue sections of arthritic hind limbs exhibit greater infiltration of immune cells, bone erosion and cartilage destruction in Lcn2KO (F) compared to WT mice (E). Histological scoring and assessment of bone erosion in arthritic paws of Lcn2KO and WT mice were carried out as described under Materials and Methods and represented graphically (G). The blue dotted line differentiates the metacarpal (MC) bone from articular cartilage (AR). P and T stand for panus formation and tendon muscle, respectively. A group of mice (n=3) treated with PBS (naïve) served as specificity control. Photographs are representative of three individual mice. Data are expressed as ± SEM.
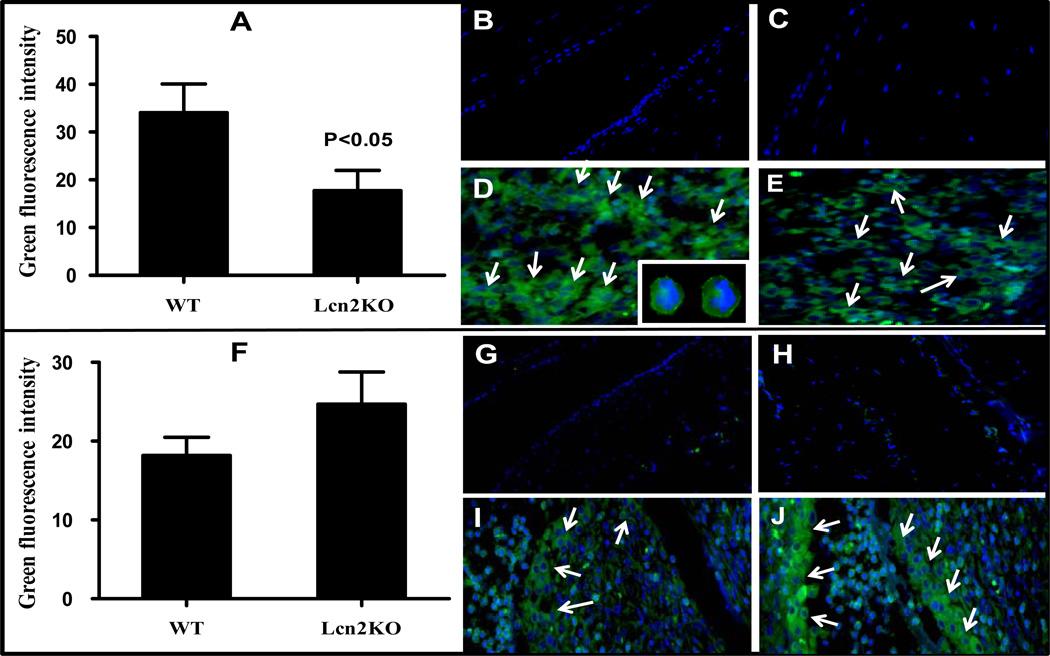
Lcn2 is essential for neutrophil extravasation but may not be required for macrophage migration to the site of inflammation
It has been shown that neutrophils and macrophages are necessary for initiation and progression of inflammation and play major roles during the development of SIA (25, 32). Therefore, to confirm the type of immune cell responsible for causing the severe arthritic damage observed in Lcn2KO mice, immunohistochemistry was performed. Consistent with a recent report (10), we have also observed a significant (Fig 6A) reduction (about 50–60%) in neutrophil infiltration in Lcn2KO (Fig 6E) compared to WT mice (Fig 6D) whereas neutrophils were undetectable in tissue sections from naïve mice (Fig 6B and C). The presence of neutrophils was confirmed based on polynuclear structures (DAPI staining) and Ly-6 surface marker (Fig 6D, insets). These results suggest that Lcn2 is required for the migration of neutrophils to the site of inflammation. To investigate whether macrophages are associated with severe pathogenesis of SIA in Lcn2KO mice, we performed macrophage staining using F4/80 antibody. Interestingly, we observed considerably more macrophage accumulation in arthritic Lcn2KO than in WT mice (Fig 6F), with macrophages concentrated in the panus region near the metacarpal bone (Fig 6I and J). Macrophages were not detected in unarthritic Lcn2KO and WT mice near metacarpal bone or tendon muscle region (Fig 6G and H). At present, the cause of such elevated macrophage accumulation in Lcn2KO mice is unclear (Fig 6F) although we have made similar observations in a dextran sulfate sodium (DSS)-induced inflammatory bowel disease (colitis) model (unpublished data). Therefore, it is possible that macrophages may be responsible for causing severe SIA pathology in Lcn2KO mice.
Figure 6. Lcn2 is a prerequisite for neutrophil, but not macrophage, migration to the site of inflammation during the pathogenesis of SIA.
Histological sections of Lcn2KO and WT mice were stained to detect neutrophils/macrophages as described under Materials and Methods. Unarthritic paws of WT (B) and Lcn2KO (C) mice did not exhibit neutrophils whereas neutrophil infiltration was significantly reduced in arthritic Lcn2KO (E) when compared to WT (D) mice. Inset: Neutrophils are identified based on nuclear structure (DAPI) followed by surface marker (Ly-6) staining. These pictures were taken at 63x original magnification. Unarthritic paws of WT (G) and Lcn2KO (H) mice did not exhibit macrophage accumulation near metacarpal bone or tendon muscle whereas considerably more macrophages accumulated in arthritic Lcn2KO (J) when compared to WT (I) mice. Green fluorescence intensity of each section was calculated using Image J software and graphically represented using GraphPad Prism 5 software (A and F). Pictures were taken at 20x original magnification and the regions of neutrophil/macrophage accumulation are presented in the figure for the purpose of clarity. Arrows indicates the presence of neutrophils/macrophages. Data are representative of three individual mice (n=3) and are expressed as ± SD.
DISCUSSION
In humans, systemic upregulation of Lcn2 significantly correlates with the development of many inflammatory disorders such as peritonitis, atherosclerosis (33), vasculitis/Kawasaki disease (34), inflammatory bowel diseases (35) and SLE (11–13, 21). Apart from these studies, the upregulation of Lcn2 has also been observed in the sputum of asthma and chronic obstructive pulmonary disease (COPD) patients and synovial fluid of rheumatoid arthritis patients (36). Recently, Lcn2 has been identified as an inducer of chemoattractants that mediate cell migration during CNS injury (37). Though the bactericidal activity of Lcn2 has been well characterized (3, 8, 38) and is known to be upregulated during various inflammatory conditions (4, 39), its biological role during IC-mediated inflammation is still obscure. In this report, we have shown that Lcn2 plays a protective role in the development of IC-mediated inflammatory diseases such as arthritis and SLE. We observed a several fold increase in circulating Lcn2 during IC-mediated autoimmune disease such as SLE and arthritis. To delineate the function of Lcn2 during IC-mediated inflammation, we utilized an acute inflammation model known as RPA. We found that extravasation of inflammatory cells at the site of inflammation was reduced in Lcn2KO mice when compared to WT. Supplementing rec-Lcn2 to Lcn2KO mice facilitated inflammation such that treated Lcn2KO were indistinguishable from WT mice whereas neutralizing circulating endogenous Lcn2 using mAb in WT mice reduced inflammation to levels comparable to Lcn2KO mice. These data support earlier studies (8, 37) indicating that Lcn2 is required for the migration of inflammatory cells to the site of inflammation in RPA. Studies with Fcγ and FcγR knockout mouse have shown that RPA is mediated largely by FcγRs (40–42). To investigate whether the interaction of FcγRs with ICs leads to the secretion of Lcn2 from inflammatory cells such as macrophages, we incubated ICs with P388D1 cells (mouse macrophage cell line) for 24 hr at 37°C and used culture supernatant to detect secretion of Lcn2 by P388D1 cells. Our results demonstrate a 5–6 fold increase in Lcn2 secretion when compared to untreated cells (data not shown). Hence, it is possible that the interaction of ICs with FcγRs expressed on inflammatory cells residing at the site of inflammation causes upregulation of Lcn2, which, in turn, may act as an inducer of chemoattractants. Therefore, we hypothesize that there might be crosstalk between FcγRs and Lcn2 upregulation during IC-mediated inflammation. It has been shown that the complement pathway also plays a vital role during the IC-mediated inflammation and, while it is possible this may also be a contributing factor, we have previously shown that the complement pathway plays little or no role during RPA in C57BL/6 mice (22).
Since RPA is an acute IC-mediated inflammation model, we extended our studies to investigate the function of Lcn2 in the K/BxN arthritis model. In contrast to our acute RPA model, we observed that Lcn2KO mice developed severe arthritis when compared to WT mice. The reason for contrasting results between the RPA and SIA models is not clear; however, it may be due to the fact that RPA is an acute model featuring only transient tissue damage that may not require all possible tissue repair mechanisms for recovery of the inflamed region. It is interesting to note that Lcn2 is highly elevated during tissue involution/remodeling (43). Therefore, it is possible that, since SIA persists for a longer period of time, the absence of Lcn2-mediated tissue repair may result in the increased severity of arthritis observed in Lcn2KO when compared to WT mice. Histopathological analysis of Lcn2KO and WT arthritic paws revealed significant metacarpal bone erosion and articular cartilage damage with extensive panus formation in Lcn2KO, but not WT mice, suggesting that Lcn2 may play a vital role in preserving bone architecture. It has been shown that neutrophils and macrophages are predominantly responsible for initiation and progression of SIA (25, 32). Similar to Schroll et al (10), we observed that neutrophil infiltration is severely reduced in Lcn2KO mice during development of SIA, suggesting that Lcn2 is prerequisite for neutrophil infiltration and that, perhaps, SIA is mediated by cells other than neutrophils in Lcn2 deficient mice. Consistent with that reasoning, we observed greater macrophage accumulation in Lcn2KO mice during development of SIA. While the mechanism of such increased macrophage accumulation is unknown, it is possible that production of macrophage-specific chemokines may have increased over time in Lcn2KO mice as a compensatory mechanism to mediate inflammation in the absence of a timely neutrophil response. Therefore, we hypothesize that macrophages may be responsible for causing the severe arthritic pathology observed in Lcn2KO mice. Various in vivo studies have shown that the inflammation and tissue repair mechanisms occur in parallel during inflammatory conditions in order to limit host tissue damage and that Lcn2 plays a significant role in tissue remodeling (44–46). Therefore, in continuation of this view, our data suggests that SIA-induced Lcn2KO mice experience continuous inflammation on a background of impaired tissue remodeling capacity and, thereby, suffer exacerbated arthritis. Such damage in Lcn2KO mice may be primarily mediated by macrophages when compared to WT mice. Nonetheless, other cell types including mast cells and platelets may also be playing a role as has been observed during the pathogenesis of SIA (47, 48).
Further, apart from the innate immune cellular contribution to the pathogenesis of SIA, it is possible that Lcn2 deficient mice exhibit elevated intracellular labile iron that may participate in Fenton’s reaction, generating reactive oxygen species and thus increasing oxidative stress. Upregulation of Lcn2 during inflammatory conditions may aid in scavenging reactive oxygen species indirectly by chelating iron. We have also found that Lcn2 offers significant protection against endotoxin-induced sepsis in mice whereby Lcn2KO mice exhibit significantly elevated oxidative stress markers and LPS-induced toxicity (49).
In conclusion, the data presented in this report suggest that engagement of FcγRs expressed on local inflammatory cells by ICs leads to the induction of Lcn2. Such IC-induced Lcn2 expression may be necessary for the migration of inflammatory cells to the site of not only infection (8, 9, 50) but also inflammation during acute IC-mediated inflammatory events whereas, in chronic IC-mediated disorders, Lcn2 may play a protective role and prevent severe tissue damage by facilitating tissue remodeling. Therefore, our results show that Lcn2 may play a dual role (initiation and resolution) during the pathogenesis of IC-mediated inflammation. Taken together, these studies suggest that Lcn2 plays a vital role during the pathogenesis of IC-mediated inflammatory/autoimmune disease conditions by modulating the inflammatory response of immune cells.
Acknowledgments
Funding: This work was supported by American Heart Association award (11SDG5710004) to R.S and NIH K01 (DK083275) award to M.V-K. DK072564, DK079392 to C.A.P
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
REFERENCES
- 1.Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000;1482(1–2):9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 2.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 3.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432(7019):917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 4.Jiang W, Constante M, Santos MM. Anemia upregulates lipocalin 2 in the liver and serum. Blood cells, molecules & diseases. 2008;41(2):169–174. doi: 10.1016/j.bcmd.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123(7):1293–1305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 6.Devireddy LR, Hart DO, Goetz DH, Green MR. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 2010;141(6):1006–1017. doi: 10.1016/j.cell.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao G, Clifton M, Hoette TM, Mori K, Deng SX, Qiu A, et al. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat Chem Biol. 2010;6(8):602–609. doi: 10.1038/nchembio.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachman MA, Miller VL, Weiser JN. Mucosal lipocalin 2 has pro-inflammatory and iron-sequestering effects in response to bacterial enterobactin. PLoS Pathog. 2009;5(10):e1000622. doi: 10.1371/journal.ppat.1000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu H, Santoni-Rugiu E, Ralfkiaer E, Porse BT, Moser C, Hoiby N, et al. Lipocalin 2 is protective against E. coli pneumonia. Respir Res. 2010;11:96. doi: 10.1186/1465-9921-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroll A, Eller K, Feistritzer C, Nairz M, Sonnweber T, Moser PA, et al. Lipocalin 2 ameliorates granulocyte functionality. Eur J Immunol. 2012 Sep 11; doi: 10.1002/eji.201142351. [DOI] [PubMed] [Google Scholar]
- 11.Pitashny M, Schwartz N, Qing X, Hojaili B, Aranow C, Mackay M, et al. Urinary lipocalin-2 is associated with renal disease activity in human lupus nephritis. Arthritis Rheum. 2007;56(6):1894–1903. doi: 10.1002/art.22594. [DOI] [PubMed] [Google Scholar]
- 12.Rubinstein T, Pitashny M, Putterman C. The novel role of neutrophil gelatinase-B associated lipocalin (NGAL)/Lipocalin-2 as a biomarker for lupus nephritis. Autoimmun Rev. 2008;7(3):229–234. doi: 10.1016/j.autrev.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Brunner HI, Mueller M, Rutherford C, Passo MH, Witte D, Grom A, et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2577–2584. doi: 10.1002/art.22008. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz RS. Autoimmunity and autoimmune diseases. New York: Raven Press Ltd; 1993. [Google Scholar]
- 15.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura A, Takai T. A role of FcgammaRIIB in the development of collagen-induced arthritis. Biomed Pharmacother. 2004;58(5):292–298. doi: 10.1016/j.biopha.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Hogarth PM. Fc receptors are major mediators of antibody based inflammation in autoimmunity. Curr Opin Immunol. 2002;14(6):798–802. doi: 10.1016/s0952-7915(02)00409-0. [DOI] [PubMed] [Google Scholar]
- 18.Takai T. Fc receptors and their role in immune regulation and autoimmunity. J Clin Immunol. 2005;25(1):1–18. doi: 10.1007/s10875-005-0353-8. [DOI] [PubMed] [Google Scholar]
- 19.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Ramos BF, Jakschik BA. Augmentation of reverse arthus reaction by mast cells in mice. J Clin Invest. 1991;88(3):841–846. doi: 10.1172/JCI115385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz N, Michaelson JS, Putterman C. Lipocalin-2, TWEAK, and other cytokines as urinary biomarkers for lupus nephritis. Ann N Y Acad Sci. 2007;1109:265–274. doi: 10.1196/annals.1398.032. [DOI] [PubMed] [Google Scholar]
- 22.Shashidharamurthy R, Hennigar RA, Fuchs S, Palaniswami P, Sherman M, Selvaraj P. Extravasations and emigration of neutrophils to the inflammatory site depend on the interaction of immune-complex with Fcgamma receptors and can be effectively blocked by decoy Fcgamma receptors. Blood. 2008;111(2):894–904. doi: 10.1182/blood-2007-04-085944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shashidharamurthy R, Machiah D, Bozeman EN, Srivatsan S, Patel J, Cho A, et al. Hydrodynamic delivery of plasmid DNA encoding human FcgammaR-Ig dimers blocks immune-complex mediated inflammation in mice. Gene Ther. 2012;19(9):877–885. doi: 10.1038/gt.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinau S, Martinsson P, Gustavsson S, Heyman B. Importance of CD23 for collagen-induced arthritis: delayed onset and reduced severity in CD23-deficient mice. J Immunol. 1999;162(7):4266–4270. [PubMed] [Google Scholar]
- 25.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167(3):1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 26.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279(5353):1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 27.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56(7):481–490. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arthus M. Injections répétées de serum de cheval chez le lapin. C R Soc Biol. 1903;55:817–825. [Google Scholar]
- 29.Nairz M, Theurl I, Schroll A, Theurl M, Fritsche G, Lindner E, et al. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood. 2009;114(17):3642–3651. doi: 10.1182/blood-2009-05-223354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan M, Kang I, Craft J, Yin Z. Resistance to development of collagen-induced arthritis in C57BL/6 mice is due to a defect in secondary, but not in primary, immune response. J Clin Immunol. 2004;24(5):481–491. doi: 10.1023/B:JOCI.0000040919.16739.44. [DOI] [PubMed] [Google Scholar]
- 31.Yang HT, Jirholt J, Svensson L, Sundvall M, Jansson L, Pettersson U, et al. Identification of genes controlling collagen-induced arthritis in mice: striking homology with susceptibility loci previously identified in the rat. J Immunol. 1999;163(5):2916–2921. [PubMed] [Google Scholar]
- 32.Solomon S, Rajasekaran N, Jeisy-Walder E, Snapper SB, Illges H. A crucial role for macrophages in the pathology of K/B×N serum-induced arthritis. Eur J Immunol. 2005;35(10):3064–3073. doi: 10.1002/eji.200526167. [DOI] [PubMed] [Google Scholar]
- 33.Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, et al. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26(1):136–142. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- 34.Biezeveld MH, van Mierlo G, Lutter R, Kuipers IM, Dekker T, Hack CE, et al. Sustained activation of neutrophils in the course of Kawasaki disease: an association with matrix metalloproteinases. Clin Exp Immunol. 2005;141(1):183–188. doi: 10.1111/j.1365-2249.2005.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38(3):414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu S, Venge P. Lipocalins as biochemical markers of disease. Biochim Biophys Acta. 2000;1482(1–2):298–307. doi: 10.1016/s0167-4838(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Kim JH, Seo JW, Han HS, Lee WH, Mori K, et al. Lipocalin-2 is a chemokine inducer in the central nervous system: Role of CXCL10 in lipocalin-2-induced cell migration. J Biol Chem. 2011;286(51):43855–43870. doi: 10.1074/jbc.M111.299248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10(5):1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 39.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17(2):216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76(3):519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 41.Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5(2):181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 42.Ravetch JV, Clynes RA. Divergent roles for Fc receptors and complement in vivo. Annu Rev Immunol. 1998;16:421–432. doi: 10.1146/annurev.immunol.16.1.421. [DOI] [PubMed] [Google Scholar]
- 43.Ryon J, Bendickson L, Nilsen-Hamilton M. High expression in involuting reproductive tissues of uterocalin/24p3, a lipocalin and acute phase protein. The Biochemical journal. 2002;367(Pt 1):271–277. doi: 10.1042/BJ20020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391(Pt 2):441–448. doi: 10.1042/BJ20051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sola A, Weigert A, Jung M, Vinuesa E, Brecht K, Weis N, et al. Sphingosine-1-phosphate signalling induces the production of Lcn-2 by macrophages to promote kidney regeneration. J Pathol. 2012;225(4):597–608. doi: 10.1002/path.2982. [DOI] [PubMed] [Google Scholar]
- 46.Vinuesa E, Sola A, Jung M, Alfaro V, Hotter G. Lipocalin-2-induced renal regeneration depends on cytokines. Am J Physiol Renal Physiol. 2008;295(5):F1554–F1562. doi: 10.1152/ajprenal.90250.2008. [DOI] [PubMed] [Google Scholar]
- 47.Boilard E, Blanco P, Nigrovic PA. Platelets: active players in the pathogenesis of arthritis and SLE. Nat Rev Rheumatol. 2012;8(9):534–542. doi: 10.1038/nrrheum.2012.118. [DOI] [PubMed] [Google Scholar]
- 48.Nigrovic PA, Lee DM. Synovial mast cells: role in acute and chronic arthritis. Immunol Rev. 2007;217:19–37. doi: 10.1111/j.1600-065X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 49.Srinivasan G, Aitken JD, Zhang B, Carvalho FA, Chassaing B, Shashidharamurthy R, et al. Lipocalin 2 deficiency dysregulates iron homeostasis and exacerbates endotoxin-induced sepsis. J Immunol. 2012;189(4):1911–1919. doi: 10.4049/jimmunol.1200892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saiga H, Nishimura J, Kuwata H, Okuyama M, Matsumoto S, Sato S, et al. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J Immunol. 2008;181(12):8521–8527. doi: 10.4049/jimmunol.181.12.8521. [DOI] [PubMed] [Google Scholar]