Abstract
We developed atomic force microscope (AFM) based protocols that enable isolation and characterization of antibody based reagents that selectively bind target protein variants using low nanogram amounts or less of unpurified starting material. We isolated single chain antibody fragments (scFvs) that specifically recognize an oligomeric amyloid-beta (Aβ) species correlated with Alzheimer’s disease (AD) using only a few nanograms of an enriched but not purified sample obtained from human AD brain tissue. We employed several subtractive panning steps to remove all phage binding non-desired antigens and then employed a single positive panning step using minimal antigen. We also used AFM to characterize the specificity of the isolated clones, again using minimal material, selecting the C6 scFv based on expression levels. We show that C6 selectively binds cell and brain derived oligomeric Aβ. The protocols described are readily adapted to isolating antibody based reagents against other antigenic targets with limited availability.
Keywords: biopanning; single chain antibody fragments, antibody libraries; beta-amyloid; oligomeric aggregates; nanotechnology; atomic force microscopy
INTRODUCTION
Antibodies or antibody fragments have been developed toward a wide array of different target antigens for a variety of different diagnostic applications using either conventional immunization protocols or through various surface display techniques 1-3. In addition, monoclonal antibodies represent a rapidly growing and very promising therapeutic approach for treating a variety of diseases including cancer, rheumatoid arthritis, Crohn’s disease and multiple sclerosis 4 with over 30 different antibodies approved for therapeutic use. Despite the enormous success in generating antibodies against desired antigens, there are many important biological targets that have proven to be difficult to generate antibodies against, especially antigen targets that are unstable, difficult to purify, or available in only limited quantities. One general class of such biological targets includes various antigen targets involved in protein misfolding diseases. Over 30 human health diseases have been connected to misfolding or misprocessing of proteins including cancer (p53), diabetes (islet amyloid polypeptide), Alzheimer’s (beta-amyloid and tau), Parkinson’s (alpha-synuclein), and Huntington’s diseases (huntingtin), Amyotrophic lateral sclerosis (superoxide dismutase) and prion based diseases. In many of these diseases, specific misfolded protein variants such as small soluble oligomeric forms of the amyloid-beta protein 5 or a misfolded form of the prion protein 6 have been associated with cell dysfunction and disease progression. The role of specific protein aggregate species in disease onset and progression has been greatly hindered by a lack of suitably selective reagents to identify the presence of the various different aggregate species involved in these critically important diseases. Methods are needed that would facilitate isolation of antibody based reagents that can selectively recognize different protein variants in these diseases given that many of these targets are present in small amounts and may be unstable and difficult to purify.
Towards this end we developed a biopanning technique combining the diversity of phage display antibody libraries with the imaging capabilities of atomic force microscopy (AFM) that enables us to isolate antibody fragments against specific protein morphologies 7. Using this technology, we isolated scFvs against oligomeric protein species implicated in Alzheimer’s (AD) 8, 9 and Parkinson’s diseases 7, 10, 11. Here we have further refined this technology to enable us to isolate and characterize antibody fragments that selectively recognize a target antigen that is available in only limited amounts (low nanogram) and that is not purified.
The target we selected here is a brain derived oligomeric Aβ species which has been shown to be important in the onset and progression of AD 12, 13. Aggregation and deposition of Aβ has long been correlated with AD, however there has been considerable confusion regarding the role of Aβ in AD since numerous different aggregate forms of Aβ have been identified and characterized including fibrils, proto-fibrils, annular structures, globular structures, amorphous aggregates and various soluble oligomers 14-17. Numerous studies indicate that small oligomeric morphologies of Aβ are the primary toxic species in AD 16, and antibody fragments generated against these synthetic oligomeric Aβ species recognize naturally occurring Aβ aggregates in human brain tissue 8, 9. However additional studies have also indicated that an SDS-stable naturally occurring low-n oligomeric Aβ species inhibits long term potentiation in mammalian hippocampus 18, correlates well with dementia in AD patients 19, causes short term memory loss in rats 20, and affects dendritic morphology in neuronal cells resulting in synaptic losses 21. Therefore these naturally derived SDS-stable Aβ oligomers represent an important biological target different from other aggregate species of in vitro derived Aβ oligomers. Unfortunately, these SDS-stable brain derived oligomeric Aβ aggregates are available in very limited amounts and are difficult targets to generate antibodies against. Therefore they represent an ideal target for our AFM based biopanning protocols.
To generate an antibody fragment that specifically recognizes the target brain derived oligomeric Aβ species, but that do not also cross-react with monomeric, fibrillar or synthetic oligomeric Aβ species, we modified our panning protocol to account for the very limited availability of unpurified starting material available. By incorporating a series of “subtractive panning” steps, we eliminated essentially 100% of phage binding to off-target antigens including Aβ monomers and other brain derived proteins; and subsequently, employing only a single round of positive biopanning using only a few nanograms of the target antigen, we were able to isolate a pool of antibody clones where virtually all the clones selectively bound the desired target. We selected higher affinity clones and verified binding specificity by AFM, again using only a few nanograms of the unpurified target. This nanoscale method should be applicable to and facilitate isolation of antibody based reagents to many biologically relevant targets that are currently very difficult to generate antibodies against.
MATERIALS AND METHODS
Phage Display scFv Library
The Sheets phage display scFv library 22 was provided by Dr Yu (Eunice) Zhou, Department of Anesthesia, University of San Francisco. Production of phage was performed essentially as described 23.
Brain Derived Antigens
The brain derived antigens including Aβ aggregate samples were a generous gift from Dr. Dennis Selkoe, (Harvard Medical School, Boston). A 40ng aliquot of enriched brain derived samples containing SDS-stable Aβ oligomers or Aβ monomers were obtained as lyophilized powder. The brain derived Aβ oligomers were prepared as described previously 18. Prior to the biopanning experiments, the samples were re-suspended in TBS buffer to a final Aβ concentration of 5 nM, aliquoted and stored at −20 °C. Brain samples from which Aβ had been depleted by immunoprecipitation were also used for subtractive panning and as controls.
Preparation of Synthetic Aβ
Aβ40 was synthesized in the Proteomics and Protein Chemistry Laboratory at Arizona State University, purified by HPLC, lyophilized and stored as its Trifluoroacetate salt Aβ40 at −20°C. Samples were prepared as previously described 9. Briefly, Aβ40 was solubilized in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) at a concentration of 1 mg/mL to avoid aggregates. Aliquots of 250 μL were air dried and stored at −20 °C. Prior to use, the aliquots of monomeric Aβ were re-suspended in dimethyl-sulfoxide (DMSO) and diluted to final concentration in Tris-HCl buffer (25 mM Tris, 150 mM NaCl, pH 7.5).
Atomic Force Microscope (AFM) Imaging
AFM analysis was performed as described previously 24. Samples were deposited on mica, dried and imaged in air using a MultiMode AFM NanoScope IIIA system (Veeco/Digital Instruments, Santa Barbara, CA) operating in tapping mode using silicon probes (Model: OTESPA, Veeco, Santa Barbara, CA) 24.
Biopanning against Natural Brain Derived Antigen
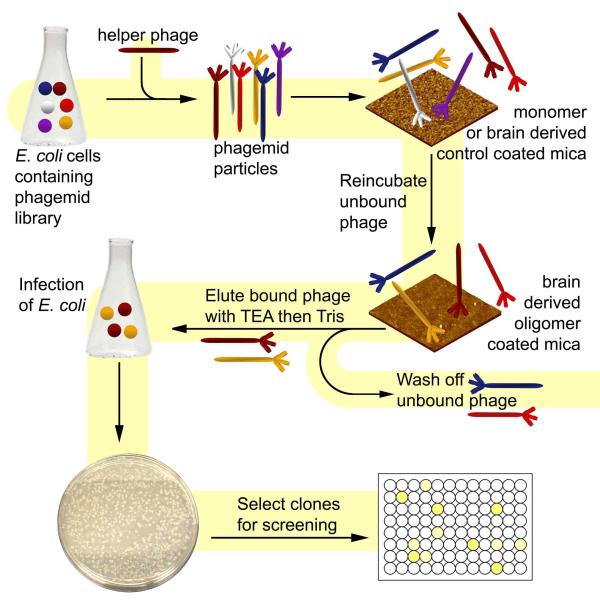
The biopanning process was divided into two stages. The first stage, referred to as “Subtractive panning”, was used to eliminate phage that bind to non-desired antigens. The second stage, “Positive panning”, was used to isolate phage that bind to brain derived oligomeric Aβ (Fig. 1).
FIGURE 1. Biopanning Schematic.
Panning protocol showing different steps involved in isolating antibody based reagents against low concentrations of natural brain derived Aβ oligomers.
Subtractive panning
Subtractive panning was performed to eliminate phage binding to non-desired antigens. A 1ng aliquot of brain derived control proteins, 1ng of brain derived monomers and 1μg of synthetic monomers were separately deposited on multiple pieces of mica and air dried. A 100μL aliquot of amplified phage containing 1012 pfu was serially added first to the brain derived control, next to brain derived monomeric Aβ, and finally to multiple pieces containing synthetic monomeric Aβ. The phage was allowed to incubate with each piece of antigen coated mica for 10 min before removal and addition to the next sample. The subtractive panning steps were performed in duplicate with one set used for panning and the second set used for AFM imaging to monitor the panning process. Serial addition of phage to mica substrates containing the synthetic Aβ monomer samples was continued until no phage was observed binding to antigen by AFM analysis. Aliquots of 1xPBS were periodically added to restore phage solution volume lost due to evaporation from mica surface.
Positive Panning
A 1ng aliquot of natural brain derived SDS-stable oligomers was deposited onto mica and air dried. Following subtractive panning, a 20 μL aliquot of remaining phage was added to the mica surface, incubated for 10 min and then sequentially washed with 2 mL PBS-0.1% Tween-20, 2 mL PBS and 2 mL water and then air dried. This positive panning step was performed in duplicate, with one set used for the actual panning process and the second set used for AFM imaging to monitor the panning process. Bound phage particles were eluted and recovered as previously described 7, 10, 11.
Selection of High Affinity Clones
Approximately 400 clones were recovered from the single round of positive panning and each clone was individually grown in 96 well plates. Phage production from the individual clones was induced by addition of helper phage as previously described 23. Serial dilutions of the natural brain derived Aβ oligomer sample (1:10 – 1:10,000) were deposited on mica. Phage from all 400 clones were pooled and added to each of the mica samples. Unbound phage were washed off with 2 mL PBS-0.1% Tween-20 and water and remaining phage particles were visualized by AFM. Phage were eluted from mica containing the different antigen dilutions as described above and used to infect E. coli TG1 and plated onto LB agar plates containing 100ug/ml ampicillin. Single clones were picked from the plate corresponding to the lowest concentration of oligomeric Aβ, plasmid DNA was isolated and checked by sequence analysis to verify sequence of the isolated scFvs.
Dot Blot Assay to Screen for Expression Levels
To check expression levels, plasmid DNA from the positive clones identified above were transformed into the non-suppressor bacterial strain E. coli HB2151 for production of soluble scFv. Individually selected clones were grown and scFv production was induced by addition of 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) as described earlier 23. A 5 μl aliquot of the supernatant and lysate fractions from the different clones were deposited onto a gridded nitrocellulose membrane. The membrane was blocked with 5% Milk-PBS (5g Carnation nonfat dry milk in 100ml PBS buffer) for at least one hour at room temperature followed by incubation overnight with a 1:1000 dilution of the primary anti-myc tag antibody 9E10 (Sigma). Immunoreactivity was detected after a 1-h incubation at room temperature using a 1:1000 dilution of the secondary anti-mouse IgG HRP antibody (Sigma). The membrane was stained with 3,3′-Diaminobenzidine Tetrahydrochloride (DAB) solution (Sigma). The C6 scFv was selected for further study based on expression levels.
Binding Specificity by Phage Assay
Binding specificity to brain derived oligomers of the C6 scFv as well as the A4 and E1 scFvs isolated previously against synthetic Aβ oligomers 8, 9 were assayed by AFM. Phage was produced from E. coli TG1 essentially as described 7, 10, 11. Purified phage was added to naturally derived and synthetically derived Aβ oligomers on the mica surface and phage binding to the antigen was visualized by AFM.
Production and Purification of Soluble ScFv
To express and purify soluble protein, the C6 gene was cloned into a pIT2 E. coli expression vector, and transformed into E. coli HB2151. The supernatant and cell lysate from a 1L culture were combined and concentrated in a tangential flow filter (Millipore) using a 10 kDa filter membrane (Millipore). The concentrated supernatant/lysate was used for dot blot and Western blot assays.
For scFv purification, the 6xHis tagged scFv was purified by mixing with 1 ml Nickel NTA sepharose beads (Qiagen, CA) for 2 hours, followed by elution with an imidazole gradient. Fractions containing scFvs were pooled and dialyzed into 1X PBS. Protein expression and purity was checked by SDS-PAGE and Western blot. A bicinchoninic acid (BCA) protein assay (Sigma) was used to determine scFv concentration as previously described 9.
Culture of Human APP Over-expressing Cells
A Chinese hamster ovary (CHO) cell line stably transfected with cDNA encoding mutant human APP751, 7PA2 was a kind gift from Dr. Dennis Selkoe (Harvard Medical School, Boston). Cells were grown in Dulbecco Modified Eagle medium (DMEM) containing 10% fetal bovine serum, 1% L-glutamine and 1% penicillin/streptomycin (Gibco). Selection for mutant APP expressing cells was performed using 1 mg/ml G-418 (Calbiochem), an amyloglycoside antibiotic. Once the cells reach 95% confluence, 7PA2 cells were plated onto 6 well plates and used for further studies.
Detection of Aβ Expressed by Human APP Over-expressing Cells
The 7PA2 cells were grown for 2 days after which the cell culture media was removed and concentrated. Total protein concentration was determined by BCA and cell culture media containing 25 μg of protein was separated on a 10% Tris/Tricine gel and transferred onto a 0.2 μm nitro-cellulose membrane (Bio-Rad). Cell media from untransfected CHO cells was used as a control. The membrane was probed for 24 hours with the concentrated supernatant containing C6 scFv. The membrane was then incubated overnight with a 1:1000 dilution of the primary anti-myc tag antibody. Immunoreactivity was detected following 1 h incubation with a 1:1000 dilution of a HRP conjugated goat anti-mouse IgG as secondary antibody. The membrane was stained with DAB solution as described above (Sigma).
Protein Dot Blot Assays
A 2 μl aliquot of an in vitro generated monomeric, oligomeric and fibrillar Aβ or alpha-synuclein sample was removed at selected time points during aggregation and deposited onto a gridded nitrocellulose membrane as described 11, 25. The membrane was blocked with 5% Milk-PBS (5g Carnation nonfat dry milk in 100 ml PBS buffer) for at least one hour at room temperature. The membrane was then incubated overnight with concentrated supernatants containing the C6 nanobody at 4 °C, followed by labeling with a 1:1000 dilution of the primary anti-myc tag antibody 9E10 (Sigma) for 2 hours. Immunoreactivity was detected as described above.
Aβ Aggregation assay
A sample of 50 μM Aβ prepared in Tris-HCl buffer was incubated alone and with 5μM C6 at 37°C. Aliquots were removed at selected time intervals and analyzed by Western blot. Aliquots were collected and separated on a 10% Tris/Tricine gel and transferred onto a 0.2 μm nitro-cellulose membrane (Bio-Rad). The membrane was probed for 24 hours with a 1:1000 dilution mouse monoclonal antibody 6E10 (Calbiochem, USA) which recognizes the N-terminus of the Aβ peptide and immunoreactivity was detected following a 1-hour incubation with a 1:1000 dilution of a HRP conjugated goat anti-mouse IgG as secondary antibody to identify monomeric and oligomeric Aβ aggregates.
Brain Dot Blot Assays
Unfixed frozen mouse brain tissue from 10-week old 3xTg 26 and wild-type mice obtained from Dr. Jon Valla. Brains were chilled in 4 °C PBS, mounted in a plexiglas frame, and coronally sectioned at 1mm. Sections were frozen and stored at −80°C until processing. The brain tissue was weighed on ice and 4 volumes of homogenization buffer (50 mM Tris-HCL, 10 mM EDTA pH: 7.5) with 1% SDS was added followed by sonication of the samples. A 1:100 dilution of the protease inhibitor cocktail (Halt Protease inhibitor, Pierce) was added and the samples were centrifuged at 13,000 rpm long enough to remove any insoluble material (typically 15-45min at 4°C). A 5 ul aliquot of the resulting extracts were added to a 0.2μm nitrocellulose membrane. The membrane was blocked with 5 % PBS-Milk and probed with C6 phage (1010 pfu) in 10 ml of 2% milk, for 2 h at room temperature. Bound phage were detected after a 1-hour incubation using a 1/1000 dilution of HRP-conjugated mouse anti-M13 antibody.
RESULTS
Biopanning against Naturally Derived Aβ oligomers
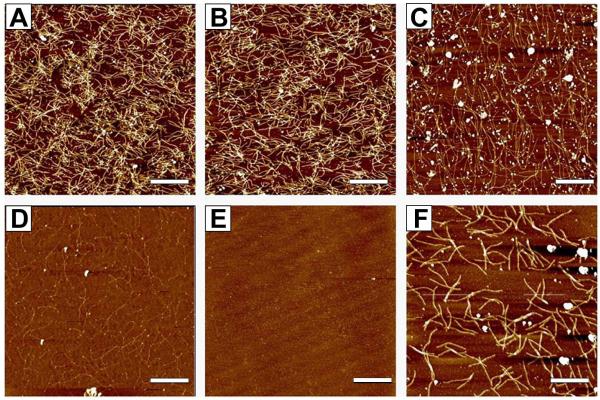
Serial subtractive panning performed against brain derived proteins from which Aβ has been depleted (Fig 2A), brain derived monomer (Fig. 2B) and synthetic Aβ monomers (Fig 2C-E) resulted in elimination of virtually all phage binding to these off-target antigens as monitored by AFM. The aliquot of phage remaining after the subtractive panning steps was added to mica coated with the brain derived SDS-stable Aβ oligomers (Fig 2F) for recovery of phage binding the target antigen. Approximately 400 single clones were recovered from the single positive panning step.
FIGURE 2. Biopanning process monitored by AFM.
Subtractive panning protocols were used against: A) 1ng brain derived proteins from which Aβ has been depleted; B) 1ng brain derived Aβ monomer; and C-E) 1μg synthetic Aβ monomers. The subtractive panning process was monitored by AFM imaging and repeated until no phage binding to the synthetic Aβ sample was observed. C) 1st synthetic monomer sample; D) 3rd synthetic monomer sample; E) 5th synthetic monomer sample. F) Recovered phage from last monomer mica was added to mica containing 1ng dimer sample. Scale bar represents 1μm.
Phage Recovered from Panning Binds Specifically to Brain Derived Oligomers
We verified that phage recovered from the positive panning steps specifically bound SDS-stable oligomeric Aβ and not other off-target antigens. Pooled phage from the approximately 400 recovered clones showed abundant binding to the brain derived oligomeric Aβ sample, but not to monomeric Aβ or other brain derived proteins (Fig. 3).
FIGURE 3. Phage from 400 recovered clones specifically binds to brain derived Aβ oligomers.

Phage produced from 400 clones recovered from the biopanning process were added to: A) 1ng Aβ depleted brain sample; B) 1ng brain derived Aβ monomer; C) 1μg synthetic Aβ monomer; D) 1ng brain derived Aβ oligomers. Scale bar represents 1μm.
Selection for High Affinity Phage
To select phage with the highest affinity for oligomeric Aβ we added an aliquot of the pooled phage from the 400 recovered clones to serial dilutions of brain derived Aβ oligomers. At low antigen concentrations, high affinity clones should preferentially bind over low affinity variants. Phage recovered from the mica samples containing 10pg of brain derived Aβ oligomers were recovered for further analyses.
Production of Soluble ScFv
DNA sequence analysis indicated that there were 18 distinct clones from the 30 clones recovered from the mica sample containing 10pg of brain derived Aβ. Expression levels of the 18 clones were analyzed and clone C6 was selected for further studies based on expression level (Fig. 4).
FIGURE 4. Dot-blot to determine expression levels of isolated clones.

Phage binding to the 10pg brain derived dimers were eluted and transformed into E. coli HB2151 competent cells. Single clones were picked and tested for levels of soluble protein expression by dot blot analysis.
The clone with the highest expression (*) C6, was selected for further study.
C6 Phage Specifically Recognizes Brain Derived Oligomers
We verified that C6 phage specifically binds brain derived Aβ oligomers and not other brain derived proteins or synthetic Aβ oligomers. C6 phage specifically bound natural brain derived oligomers but not synthetic Aβ aggregates pre-incubated for either 1 or 3 days (Fig. 5A-C). In contrast the previously isolated scFvs that specifically recognized synthetic oligomeric forms of Aβ, E1 and A4, bound the 1 day (Fig. 5D-F) and 3 day (Fig. 5G-I) Aβ aggregates respectively but not the brain derived Aβ oligomers 8, 9.
FIGURE 5. Comparison of C6, E1 and A4 phage binding to different Aβ oligomers.
C6 phage binds A) natural brain derived Aβ oligomers but not to the B) 1 day synthetic Aβ aggregates, or the C) 3 day synthetic Aβ aggregates. Phage from E1 does not bind the D) Natural brain derived oligomers, but recognizes synthetic oligomers at E) 1 day and F) 3 days of aggregation. A4 phage does not bind to G) natural brain derived Aβ oligomers or the H) 1 day synthetic Aβ aggregates, but recognizes the F) 3 day synthetic Aβ aggregates. Scale bar represents 1μm.
Purification of C6 ScFv
Soluble C6 protein was expressed and purified by metal ion chromatography. Purified protein showed the expected 29kDa band corresponding to a full length scFv (data not shown).
C6 Selectively Recognizes a Cell derived Oligomeric Aβ Species
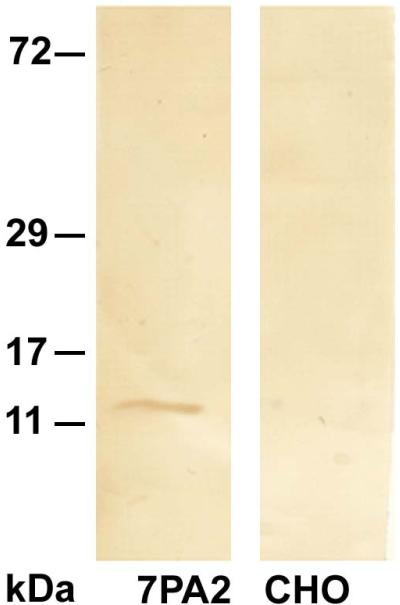
When cell supernatant from hAPP over-expressing 7PA2 cells was analyzed by Western blot probed with C6 scFv, an approximately 11 kDa band was detected, likely corresponding to either an SDS-stable dimeric or trimeric aggregate of Aβ (Fig. 6, lane 1) consistent with previous reports21. No band was observed when the untransfected CHO cell medium was probed with C6 scFv (Fig. 6, lane 2). Dot blots containing in vitro generated monomeric, oligomeric and fibrillar Aβ species did not show reactivity with C6 (data not shown) nor did a similar blot containing different forms of a-synuclein (data not shown).
FIGURE 6. C6 scFv detects SDS stable oligomeric Aβ secreted by 7PA2 cells.
A 25μg aliquot of cell media of cell media was separated on a 10% Tris/Tricine gel, transferred onto a nitrocellulose membrane for Western blot analysis and probed with C6 scFv. C6 shows reactivity with a 12-16 kDa band corresponding to trimeric or tetrameric Aβ (Lane 1). Lane 2- control showing media from untransfected CHO cells.
C6 Recognizes Aβ Aggregates in Brain Tissue
To determine whether C6 could also recognize small oligomeric Aβ aggregates in brain tissue, we probed homogenized 10-week old mouse brain tissue from wild type and triple transgenic (3xTg) mice developed by LaFerla et al 26 with C6. Strong reactivity was observed with the 10 week old 3xTg samples while little or no binding was observed with the 10 week wild-type mouse tissue (Fig 7).
FIGURE 7. C6 recognizes Aβ oligomeric aggregates in transgenic AD mouse brain tissue.

Brain extracts from 10-week old 3xTg AD transgenic mice 26 and wild type mice were homogenized, blotted onto a nitrocellulose membrane and probed with C6 phage. Values are normalized to control BSA sample. Image shows increased staining of brain extract from transgenic mouse brain compared to wild type mice brain extract.
C6 Stabilizes Formation of SDS-Stable Aβ aggregates
Aliquots of 2-4 day aggregated Aβ generated in vitro without C6 show only a single strong monomeric Aβ band when separated on a denaturing SDS gel and probed with the anti-Aβ antibody 6E10 (Fig 8). In contrast, the Aβ samples co-incubated with C6 show a reduction in monomeric Aβ levels after 2-4 days (Fig 8A) and the presence of numerous larger low intensity bands corresponding to different size Aβ aggregates (Fig. 8B) indicating the specific interaction between C6 and aggregated Aβ.
FIGURE 8. C6 stabilizes SDS-stable oligomeric Aβ.
A 50 μM Aβ solution was incubated alone or in the presence of 5μM C6 nanobody. Aliquots were taken at different incubation times, separated on an SDS-gel and probed with 6E10 monoclonal antibody. A) Monomeric Aβ bands present at of 0 hours (Lane 1) and pooled aliquots from 2-4days (Lane 2). B) Oligomeric Aβ bands present in pooled aliquots of Aβ samples incubated alone (Lane 1) or co-incubated with C6 (Lane 2) for 2-4 days.
DISCUSSION
We previously developed a biopanning technique combining atomic force microscopy and phage display technology 7 that enabled us to isolate antibody fragments against specific morphologies of target proteins that we could generate in vitro, including oligomeric forms of Aβ8, 9 and α-synuclein 10, 11. Combining the nano-imaging capabilities of AFM with the binding diversity of phage display antibody libraries allowed us to monitor the panning process, even allowing us to recover bound phage from a single target molecule 27. Here we have expanded these capabilities so that we can isolate and characterize scFvs against selected biologically important protein variants derived from human tissue. In this case our target was a toxic low-n SDS-stable Aβ oligomeric species which is selectively found in human AD brain tissue 18. Availability of the Aβ aggregate species was very limited as we were able to obtain only 40 nanograms of enriched but not purified Aβ aggregates isolated from human AD brain tissue from Dr. Selkoe (Harvard Medical School). Since only very limited amounts of the enriched antigen target were available for isolation and characterization of the scFvs, we modified the panning protocol to both facilitate isolation of scFvs to the target using minimal antigen, and to also minimize the amount of antigen needed to characterize binding specificity of the isolated clones (Fig. 1). We included several subtractive panning steps to eliminate phage binding to likely off-target antigens including brain derived proteins and monomeric Aβ, and synthetic Aβ. The subtractive panning steps enabled us to eliminate virtually all phage binding to off-target antigens, and allowed for isolation of phage that bind specifically to the brain derived oligomeric Aβ aggregates using minimal target sample (Fig. 2). Since we monitored the subtractive panning steps by AFM to ensure that all phage binding to off-target antigen were removed, we also ensured that virtually all of the clones recovered from the positive panning step would selectively bind our antigen target. This process ensured that we would not only need very little antigen to isolate scFvs against our target antigen, but we would also need very little antigen to characterize the binding specificity. After a single round of positive panning, we isolated approximately 400 clones from a starting phage library of around 1012 clones. Phage from each of these 400 clones was independently amplified to ensure that phage from all the clones were amplified to the same extent. The 400 phage stocks were pooled and assayed to verify specificity for the target antigen, showing abundant binding to the target brain derived Aβ oligomer samples but essentially no binding to the control antigens (Fig. 3).
Since availability of antigen was limited, we modified the screening protocol to facilitate isolation of the higher affinity variants from the 400 clones. At low antigen concentrations, phage will compete for antigen sites and high affinity phage will preferentially bind over low affinity clones. The high affinity screen resulted in identification of 18 clones with unique sequences. Further selection of these 18 clones was based on protein expression levels where C6 had the highest expression and was selected for further studies (Fig. 4).
Specificity of the C6 phage for the SDS-stable brain derived Aβ oligomers, but not to synthetic Aβ oligomers or other off target antigens, was verified by AFM (Fig. 5). We previously identified two scFvs, A4 and E1 that selectively recognize two different in vitro generated oligomeric Aβ species 8, 9. Here we show that C6 recognizes an SDS-stable Aβ species isolated from brain tissue which is a conformationally distinct Aβ aggregate species from the small soluble Aβ aggregate species recognized by either A4 or E1. Since all of these aggregate species are found in human AD brain tissue 8, 9, 18 a variety of different low-n Aβ aggregate species can be naturally generated in human tissue, potentially with a variety of toxic mechanisms.
The C6 scFv recognizes an approximately 11 kDa Aβ oligomeric species produced by 7PA2 cells, a CHO cell line that over-expresses hAPP (Fig. 6), indicating that C6 recognizes an SDS-stable dimeric or trimeric Aβ species consistent with previous results21. Height distribution analysis of the brain derived oligomeric species bound by C6 phage (Fig. 5A) indicate that C6 binds Aβ aggregates with an average height of 2.1 nm which, based on similar height distribution studies using photo-cross-linked Aβ species, corresponds to tetrameric Aβ 28. These results indicate that C6 binds a naturally occurring tetrameric Aβ species. C6 preferentially recognized oligomeric Aβ in the brain tissue of 10-week old 3xTg mouse models of AD, but not in age matched controls (Fig 7), indicating that the Aβ species recognized by C6 does occur in vivo. Since the negative panning protocols removed all phage containing antibody fragments against off target items including other brain proteins and brain derived and synthetic monomeric Aβ, we were able to isolate an scFv that selectively binds a brain derived oligomeric Aβ species, even though the enriched brain derived sample we utilized to isolate the scFvs may have contained other brain proteins and monomeric Aβ. C6 binds a major component of the brain derived Aβ sample (Fig 5A) further demonstrating that C6 binds oligomeric Aβ and not a minor component the co-immunoprecipates with Aβ.
The results obtained here indicate reagents such as C6 can be very useful tools to more accurately diagnose neurodegenerative diseases and may help to monitor progression and treatment of these diseases. Despite promising results in animal models, several recent clinical trials to treat AD, including passive immunization protocols to lower Aβ levels have met with limited success (reviewed in 29, 30), although better results were obtained with patients who received treatment at an earlier stage of the disease 31. These studies indicate the need for better early diagnostic biomarkers of AD when treatment may have more benefit. Reagents that selectively recognize small oligomeric forms of Aβ have great promise as early biomarkers for AD 32, 33. Here we demonstrate that a novel biopanning protocol can be effectively used to isolate antibody based reagents against specific protein morphologies even when the target antigen is available in trace amounts and cannot be purified. Such highly selective morphology specific reagents can be powerful tools for diagnosis of various neurodegenerative diseases.
Acknowledgements
We thank Lalitha Venkataraman for help with the synuclein studies. This work was supported by grants from the Arizona Department of Health Services for the Arizona Alzheimer’s Consortium. We are grateful to the Banner/Sun Health Research Institute Brain Donation Program of Sun City, Arizona for the provision of human brain tissue. The Brain Donation Program is supported by the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Prescott Family Initiative of the Michael J. Fox Foundation for Parkinson’s Research.
REFERENCES
- 1.Hudson PJ, Souriau C. Engineered antibodies. Nat Med. Jan. 2003;9:129–134. doi: 10.1038/nm0103-129. [DOI] [PubMed] [Google Scholar]
- 2.Jain M, Kamal N, Batra SK. Engineering antibodies for clinical applications. Trends Biotechnol. Jul. 2007;25:307–316. doi: 10.1016/j.tibtech.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Demarest SJ, Glaser SM. Antibody therapeutics, antibody engineering, and the merits of protein stability. Curr Opin Drug Discov Devel. 2008 Sep;11:675–687. [PubMed] [Google Scholar]
- 4.ElBakri A, Nelson PN, Abu Odeh RO. The state of antibody therapy. Human Immunology. 71:1243. doi: 10.1016/j.humimm.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008 Sep 1;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobb NJ, Surewicz WK. Prion diseases and their biochemical mechanisms. Biochemistry. 2009 Mar 31;48:2574–2585. doi: 10.1021/bi900108v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkhordarian H, Emadi S, Schulz P, Sierks MR. Isolating recombinant antibodies against specific protein morphologies using atomic force microscopy and phage display technologies. Protein Eng Des Sel. 2006 Nov;19:497–502. doi: 10.1093/protein/gzl036. [DOI] [PubMed] [Google Scholar]
- 8.Kasturirangan S, Lin L, Emadi S, Boddapati S, Schulz P, Sierks MR. Nanobody specific for oligomeric beta-amyloid stabilizes non-toxic form. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.09.020. In press. [DOI] [PubMed] [Google Scholar]
- 9.Zameer A, Kasturirangan S, Emadi S, Nimmagadda SV, Sierks MR. Anti-oligomeric Abeta single-chain variable domain antibody blocks Abeta-induced toxicity against human neuroblastoma cells. J Mol Biol. 2008 Dec 26;384:917–928. doi: 10.1016/j.jmb.2008.09.068. [DOI] [PubMed] [Google Scholar]
- 10.Emadi S, Barkhordarian H, Wang MS, Schulz P, Sierks MR. Isolation of a human single chain antibody fragment against oligomeric alpha-synuclein that inhibits aggregation and prevents alpha-synuclein-induced toxicity. J Mol Biol. 2007 May 11;368:1132–1144. doi: 10.1016/j.jmb.2007.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emadi S, Kasturirangan S, Wang MS, Schulz P, Sierks MR. Detecting morphologically distinct oligomeric forms of alpha-synuclein. J Biol Chem. 2009 Apr 24;284:11048–11058. doi: 10.1074/jbc.M806559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 13.Walsh DM, Klyubin I, Shankar GM, et al. The role of cell-derived oligomers of Abeta in Alzheimer’s disease and avenues for therapeutic intervention. Biochem Soc Trans. 2005 Nov;33(Pt 5):1087–1090. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- 14.Caughey B, Lansbury PT., Jr Protofibrils, Pores, Fibrils, and Neurodegeneration: Separating the Responsible Protein Aggregates from the Innocent Bystanders. Annu Rev Neurosci. 2003;9:9. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 15.Harper JD, Wong SS, Lieber CM, Lansbury PTJ. Observation of metastable Aβ amyloid protofibrils by atomic force microscopy. Chemistry & Biology. 1997;4:119–125. doi: 10.1016/s1074-5521(97)90255-6. [DOI] [PubMed] [Google Scholar]
- 16.Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yankner BA. Mechanisms of Neuronal Degeneration in Alzheimer’s Disease. Neuron. 1996;16:921–923. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 18.Shankar GM, Li S, Mehta TH, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008 Aug;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mc Donald JM, Savva GM, Brayne C, et al. The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain. 2010 May;133(Pt 5):1328–1341. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleary JP, Walsh DM, Hofmeister JJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005 Jan;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 21.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007 Mar 14;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheets MD, Amersdorfer P, Finnern R, et al. Efficient construction of a large nonimmune phage antibody library: the production of high-affinity human single-chain antibodies to protein antigens [published erratum appears in Proc Natl Acad Sci U S A 1999 Jan 19;96(2):795] Proc Natl Acad Sci U S A. 1998;95:6157–6162. doi: 10.1073/pnas.95.11.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G. By-passing Immunization Human Antibodies from V-gene Libraries Displayed on Phage. J. Mol. Biol. 1991;222:581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- 24.Wang MS, Zameer A, Emadi S, Sierks MR. Characterizing antibody specificity to different protein morphologies by AFM. Langmuir. 2009 Jan 20;25:912–918. doi: 10.1021/la8025914. [DOI] [PubMed] [Google Scholar]
- 25.Kasturirangan S, Lin L, Emadi S, Boddapati S, Schulz P, Sierks MR. Nanobody specific for oligomeric beta-amyloid stabilizes non-toxic form. Neurobiol Aging. 2012;33:1320–1328. doi: 10.1016/j.neurobiolaging.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003 Jul 31;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 27.Shlyakhtenko LS, Yuan B, Emadi S, Lyubchenko YL, Sierks MR. Single-molecule selection and recovery of structure-specific antibodies using atomic force microscopy. Nanomedicine. 2007 Sep;3:192–197. doi: 10.1016/j.nano.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Ono K, Condron MM, Teplow DB. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc Natl Acad Sci U S A. 2009 Sep 1;106:14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. Feb;6:108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panza F, Frisardi V, Solfrizzi V, et al. Immunotherapy for Alzheimer’s disease: from anti-beta-amyloid to tau-based immunization strategies. Immunotherapy. Feb;4:213–238. doi: 10.2217/imt.11.170. [DOI] [PubMed] [Google Scholar]
- 31.Callaway E. Alzheimer’s drugs take a new tack. Nature. Sep 6;489:13–14. doi: 10.1038/489013a. [DOI] [PubMed] [Google Scholar]
- 32.Santos AN, Ewers M, Minthon L, et al. Amyloid-beta oligomers in cerebrospinal fluid are associated with cognitive decline in patients with Alzheimer’s disease. J Alzheimers Dis. 29:171–176. doi: 10.3233/JAD-2012-111361. [DOI] [PubMed] [Google Scholar]
- 33.Sierks MR, Chatterjee G, McGraw C, Kasturirangan S, Schulz P, Prasad S. CSF Levels of oligomeric alpha-synuclein and beta-amyloid as biomarkers for neurodegenerative disease. Integrative Biology. 2011 doi: 10.1039/c1ib00018g. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]