Abstract
The majority of nucleotide binding domain leucine rich repeats-containing (NLR) family members has yet to be functionally characterized. Of the described NLRs, most are considered to be proinflammatory and facilitate IL-1β production. However, a newly defined sub-group of NLRs that function as negative regulators of inflammation have been identified based on their abilities to attenuate NF-κB signaling. NLRP12 (Monarch-1) is a prototypical member of this sub-group that negatively regulates both canonical and noncanonical NF-κB signaling in biochemical assays and in colitis and colon cancer models. The role of NLRP12 in infectious diseases has not been extensively studied. Here, we characterized the innate immune response of Nlrp12−/− mice following airway exposure to LPS, Klebsiella pneumoniae and Mycobacterium tuberculosis. In response to E. coli LPS, Nlrp12−/− mice showed a slight decrease in IL-1β and increase in IL-6 production, but these levels were not statistically significant. During K. pneumoniae infection, we observed subtle differences in cytokine levels and significantly reduced numbers of monocytes and lymphocytes in Nlrp12−/− mice. However, the physiological relevance of these findings is unclear as no overt differences in the development of lung disease were observed in the Nlrp12−/− mice. Likewise, Nlrp12−/− mice demonstrated pathologies similar to those observed in the wild type mice following M. tuberculosis infection. Together, these data suggest that NLRP12 does not significantly contribute to the in vivo host innate immune response to LPS stimulation, Klebsiella pneumonia infection or Mycobacterium tuberculosis.
Introduction
Initiation and termination of the host innate immune response to pathogenic bacteria infection is tightly regulated. The host response to respiratory bacteria is initially driven through the stimulation of TLRs. TLR activation signals through the NF-κB pathway to up-regulate the transcription of proinflammatory cytokines, including IL-1β, TNFα, and IL-6, which are essential for the recruitment of leukocytes to the lung and clearance of the infection. Mice deficient in these three proinflammatory cytokines have demonstrated significant increases in morbidity and mortality during respiratory infections with Klebsiella pneumoniae and Mycobacterium tuberculosis [1], [2].
While TLR recognition occurs on the plasma membrane and in endosomes, the nucleotide binding domain leucine rich repeats-containing family of proteins (NLRs) are a second family of pathogen recognition receptors (PRRs) that sense intracellular danger associated molecular patterns (DAMPs) and pathogen associated molecular patterns (PAMPs) in the cell cytoplasm [3], [4], [5], [6]. Thus far, over 23 distinct NLRs have been identified; however, the biological functions of the majority of these proteins have yet to be elucidated. The bulk of NLR publications have sought to characterize a sub-group of NLRs that function in the formation of a multi-protein complex termed the inflammasome, which includes a specific NLR, the adaptor protein PYCARD and Caspase-1. An inflammasome forms following the activation of a particular NLR by a specific danger signal, which ultimately results in the activation of caspase-1 and the subsequent post-translational cleavage of pro-IL-1β and pro-IL-18 into their active forms.
Unlike the majority of characterized proinflammatory NLRs, recent studies have identified a novel sub-group of NLRs that have the ability to attenuate inflammation [7], [8], [9], [10], [11], [12], [13]. The exact mechanism underlying this negative regulation is an area of active investigation; however, it appears that most of these NLRs function by inhibiting components of NF-κB signaling. The NLR, NLRP12 (formally known as MONARCH-1 or PYPAF7), is a prototypical member of this sub-group that functions as a negative regulator of the immune system through the attenuation of non-canonical NF-κB signaling and p52-dependent chemokine expression [12], [14]. This suppression is facilitated by the association of NLRP12 with the proteasome and the subsequent proteasome-dependent degradation of NF-κB inducing kinase (NIK) [12]. The interaction of NLRP12 with components of the NF-κB pathway ultimately results in the attenuated production of proinflammatory cytokines following PAMP stimulation and bacterial infections. In vivo, NLRP12 has been shown to dramatically influence the development of contact hypersensitivity, gastrointestinal inflammation and tumorigenesis [14], [15], [16].
Bacterial mediated infectious lung diseases are an important worldwide cause of morbidity and mortality. The gram negative bacterium Klebsiella pneumoniae is a leading cause of community- and hospital-acquired respiratory infection. The growing prevalence of antibiotic resistant strains constitutes a serious public health concern [17]. Pathogenic K. pneumoniae is capable of inducing severe bacterial pneumonia that is characterized by extensive lung inflammation, hemorrhage and necrotic lesion formation in the lungs, which can often progress to bacteremia and sepsis [18]. Similar to K. pneumoniae, the incidence of Mycobacterium tuberculosis (Mtb) is also a global threat based on the increasing prevalence of antibiotic resistant Mtb [19], [20]. One third of the world’s population is infected with Mtb and tuberculosis is associated with 1.4 million deaths per year [20]. The majority of patients infected with Mtb develop a latent tuberculosis infection. Individuals that are latently infected with Mtb will maintain a significant lifetime risk of disease reactivation despite being asymptomatic [20]. Disease reactivation is typically associated with some level of immune system compromise, such as HIV co-infection, or as a consequence of immunosuppressive drug therapy [21]. Thus, a deeper understanding of the underlying immune mechanisms that regulate active and latent Mtb infection is of immense clinical importance.
The innate immune response to K. pneumoniae and Mtb is influenced by NLR family members. Components of the NLRP3 inflammasome directly mediate the ex vivo and in vitro production of pro-inflammatory cytokines in response to K. pneumoniae infection in primary macrophages and monocytic cell lines [1]. Likewise, NLRP3 inflammasome components are essential for host defense against K. pneumoniae in vivo through the regulation of IL-1β production and necrosis in the lungs [1]. Components of the NLRP3 inflammasome are also essential for the production of active IL-1β in cultured human monocytes and primary mouse macrophages infected with Mtb. In addition, the NLR inflammasome adaptor protein PYCARD (ASC) exerts a novel NLRP3-independent role in granuloma formation and maintenance during in vivo Mtb infection [22]. Other NLRs, including NLRP12, have also been evaluated in the host immune response to gram negative bacteria and Mtb in vitro. Studies in the THP-1 human monocytic cell line found that Nlrp12 is highly expressed at baseline levels, and expression is down-regulated following stimulation with TNF-α, IFN-γ, or Mtb [10]. Downregulation of NLRP12 was suggested to be an important step in allowing the host to mount an effective inflammatory response [10]. Functionally, shRNA knockdown of NLRP12 results in increased levels of pro-inflammatory cytokines following LPS or Mtb challenge [10]. However, these studies were performed in vitro with human cell lines that express high basal levels of NLRP12; thus, validation in a more physiologically relevant setting is necessary. While several studies have assessed the contribution of proinflammatory NLRs in host defense, few studies have explored the in vivo role of the NLR family members that exert negative regulatory activities in host defense against pathogenic bacteria.
The identification and in vivo characterization of proteins that function as negative regulators of inflammation is an area of intense scientific and clinical importance for the development of clinical therapies. Previous studies have shown that NLRP12 functions as a negative regulator of inflammatory pathways in vitro; however, the role of NLRP12 in regulating host-pathogen interactions in vivo is not well defined. Here, we tested the hypothesis that mice lacking Nlrp12 would demonstrate increased morbidity, mortality and pathology following in vivo K. pneumoniae or Mtb infection. Based on the previous in vitro findings, we expected to observe accelerated disease progression, lung inflammation and cytokine production associated with an overzealous immune response in the Nlrp12−/− mice following K. pneumonia or Mtb bacterial infection.
Materials and Methods
Experimental Animals
All studies were conducted under the approval of the Institutional Care and Use Committee (IACUC) for The University of North Carolina at Chapel Hill and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Nlrp12−/− animals were kindly provided by Millennium Inc. and previously described [15]. All mice were maintained under specific pathogen free conditions and all experiments were performed with 6–12 week old age- and sex- matched mice. All mice were backcrossed onto C57Bl/6J for a minimum of 12 generations. Nlrp12−/− mice were compared to both purchased C57Bl/6J mice and littermate control mice from heterozygous breeding. All in vivo Mtb work was conducted under approved BSL-3/ABSL-3 protocols and conditions.
Evaluation of Cytokine Levels in Primary Dendritic Cells
Wild type and Nlrp12−/− dendritic cells were isolated and cultured as previously described [14]. Once matured, the dendritic cells were stimulated with either 50 ng/ml of E. coli 0111:B4 LPS (Invivogen), 50 ng/ml of Klebsiella pneumoniae LPS (Sigma; source strain is ATCC 15380) or 50 µg/ml of trehalose-6,6-dibehenate (TDB), which is a synthetic analog of trehalose-6,6-dimycolate from Mycobacterium tuberculosis (Invivogen). Cell culture supernatants were collected following 4 hours of stimulation and TNF-α, IL-6 and IL-1β levels were assessed using ELISA (BD Biosciences). To evaluate IL-1β maturation and release, cells were treated with 2 mM ATP for 30 minutes prior to harvesting the supernatants. Pro- and cleaved IL-1β was detected by western blot in dendritic cell lysate and supernatant following 24 hours of LPS stimulation. Following the supernatant harvest, the remaining cells were lysed in 1% RIPA buffer. Immunoblots were probed with goat anti-mouse IL-1β primary antibody (R&D Biosystems), followed by rabbit anti-mouse HRP secondary antibody (Santa Cruz). Bands were visualized by Super Signal Chemiluminescence (Pierce).
Induction and Assessment of Acute Airway Inflammation
To assess lipopolysaccharide (LPS) induced acute airway inflammation, isoflurane inhalation was used to anesthetize the mice, and LPS (Sigma) isolated from Escherichia coli (sterile serotype 0111:B4) was instilled via intratracheal (i.t.) administration as previously described [23]. Control animals received an i.t. dose of saline. Mice were euthanized and airway inflammation was assessed 48 hours post LPS exposure.
Bacterial mediated acute airway inflammation was induced by K. pneumoniae lung challenge. K. pneumoniae 43816 (serotype 2) was obtained from the ATCC and cultured under suppliers recommended conditions. Prior to each experiment, bacteria density was estimated by measuring the absorbance at 600 nm and actual colony forming units (CFUs) were determined by plating an aliquot on LB agar plates. Mice were challenged via i.t. instillation with either 7.4×104 CFUs of K. pneumoniae in 50 µl of PBS or PBS alone (mock), as previously described [1]. To assess in vivo bacteria burden, mice were euthanized via i.p. injection with 2,2,2 tribromoethanol (avertin), bronchoalveolar lavage fluid (BALF) was collected as described below, and the cell free fluid was serially diluted and plated on LB agar plates. Additionally, the liver was removed, wet weight assessed, homogenized in 500 µl of HBSS with a Tissue Master 125 (Omni International) and centrifuged. The resulting supernatants were plated on LB agar plates.
Whole Body Aerosol Infection with Mycobacterium tuberculosis
Mycobacterium tuberculosis H37Rv strain was obtained from the American Type Culture Collection (ATCC). Bacteria were grown to log phase in Middlebrook 7H9 broth (Difco) with 0.2% glycerol, 1× albumin dextrose saline (ADS), and 0.05% Tween 80. Inoculum was assessed by plating infection media on Middlebrook 7H10 agar plates supplemented with glycerol and ADS. CFUs were counted after 21 days of incubation.
For in vivo airway infection, Nlrp12−/− and wild type mice were infected via aerosol as previously described [24]. Mice received 250–350 CFUs per lung, which was determined by sacrificing a subset of mice 1 day post infection as previously described [24]. Bacterial organ burden was quantified by plating serial dilutions of lung, liver, and spleen homogenates on 7H10 agar containing cycloheximide (1 µg/ml) and carbenicillin (50 µg/ml) to minimize contamination. CFUs were counted after 21 days of incubation and again after 35 days of incubation. All animal infections and organ harvests were carried out under BSL3/ABSL3 conditions.
Evaluation of Airway Inflammation
Upon completion of the LPS and K. pneumoniae models, mice were euthanized and serum was collected from animals by cardiac puncture. Bronchoalveolar lavage fluid (BALF) was collected to evaluate leukocytes and cytokine levels. The lungs were lavaged three times with 1 ml of HBSS. Following centrifugation, the cytokine levels in the cell free supernatants were evaluated by ELISA (BD Biosciences). Total BALF cellularity was determined from the resuspended cell pellet and evaluated using a hemocytometer. The composition of the BALF was determined by evaluating the morphology of ≥200 cells per sample following differential staining (Diff-Quik, Dade Behring) of cells that were cytospun onto slides.
At specific time points post-Mtb infection, mice were euthanized and the liver, spleen and right lung lobes were collected and homogenized for bacteria CFU analysis and cytokine profiles. Tissue homogenates were serial diluted and plated to determine CFUs. The remaining lung homogenates were centrifuged to remove cellular debris and the supernatant was double filter sterilized for cytokine analysis by ELISA.
For LPS and K. pneumoniae histopathology, whole lungs were fixed by inflation to 20-cm pressure and immersion in 10% buffered formalin. To evaluate Mtb histopathology, the left lung lobe was removed, manually inflated and immersed in buffered formalin. Inflammation was evaluated in sections (5 µm) of the left lung lobe, following hematoxylin and eosin (H&E) staining. Paraffin embedded sections were set and cut to reveal the maximum longitudinal visualization of the intrapulmonary main axial airway. Histopathology was evaluated and scored by blinded reviewers on a scale of 0 (absent) to 3 (severe), as previously described [1], [23], [25]. The parameters assessed included overall leukocyte infiltrations, perivascular and peribroncheolar cuffing, airway epithelial cell injury, extravasation, and the estimated percent of lung area involved with inflammation. Each individual parameter was scored and averaged to generate the histology score. Additional parameters were used to evaluate the granulomas in the Mtb histopathology as previously described [22]. Briefly, granuloma frequency was measured by counting the number of granulomas present in the total lung section, and granuloma size was evaluated using Image J software after the granuloma borders were defined. Ziehl-Neelsen (ZN) staining was utilized to evaluate acid fast bacterial localization within the lung and granuloma as previously described [22].
Statistical Analysis
We utilized GraphPad Prism 5 Statistical software to conduct Analysis Of Variance (ANOVA) followed by either Tukey-Kramer HSD or Newman-Keuls post-test to evaluate statistical significance for multiple comparisons. Single data point comparisons were evaluated by the Student’s two-tailed t-test. All data are presented as the mean +/− the standard error of the mean (SEM), and in all cases a p-value of less than 0.05 was considered statistically significant.
Results
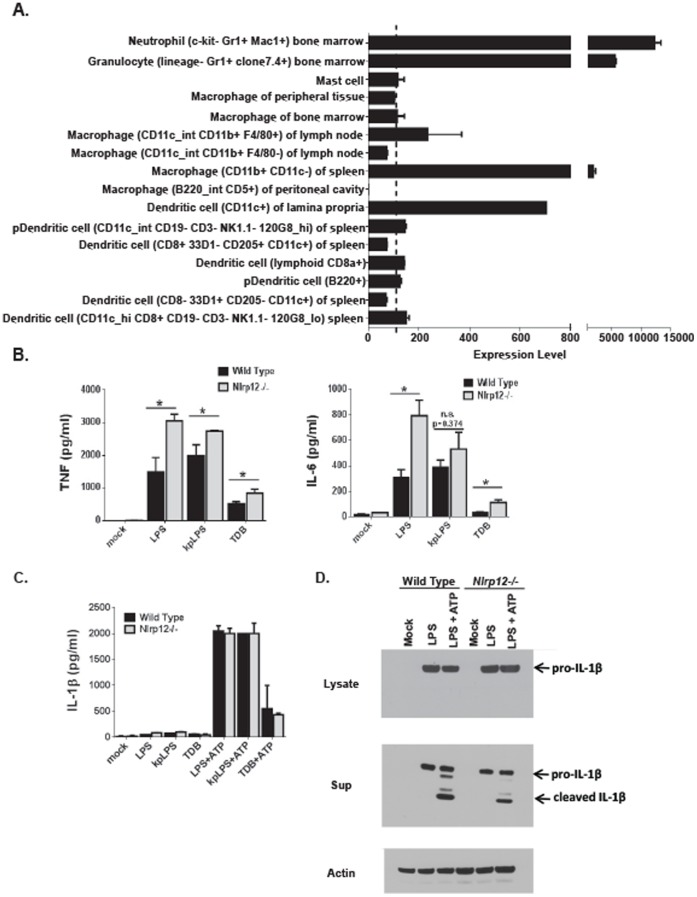
NLRP12 Attenuates the Release of TNFα and IL-6 in Bone Marrow Derived Dendritic Cells, but does not Contribute to IL-1β Maturation or Release
Previous studies characterizing NLRP12 in human monocyte cell lines revealed that NLRP12 functions as a negative regulator of NF-κB signaling following stimulation with TLR agonist, TNFα and infection with M. tuberculosis [10], [12]. To expand upon these previous findings, we sought to evaluate the contribution of NLRP12 in mediating the host immune response in mice. Initially, we utilized bioinformatics to evaluate Nlrp12 expression patterns in specific cell subpopulations. Nlrp12 expression in mouse cells associated with the host immune response was compiled using a publically accessible microarray meta-analysis search engine (Nextbio website. Available: http://www.nextbio.com/b/search/ba.nb. Accessed 2013 March 11), as previously described [14]. This analysis revealed highly variable levels in the expression of Nlrp12 in select cell populations relevant to the host immune response. Nlrp12 was found to be highly expressed in neutrophils and consistently expressed in dendritic cell populations ( Figure 1A ). However, macrophage Nlrp12 expression was highly variable. For example, cell populations from the spleen expressed high levels of Nlrp12, whereas populations from the peritoneal cavity did not have any detectable levels ( Figure 1A ). To evaluate cytokine production in response to pathogen stimulation, dendritic cells were isolated from the bone marrow, cultured and matured as previously described [14]. These primary cells were stimulated with pathogen associated molecular patterns (PAMPs) associated with E. coli, K. pneumoniae and M. tuberculosis infection. TNFα levels were significantly increased in Nlrp12−/− dendritic cell cultures following stimulation with E. coli derived LPS, K. pneumoniae derived LPS and the M. tuberculosis PAMP TDB ( Figure 1B ). Similarly, IL-6 levels were also significantly increased in Nlrp12−/− isolated cells stimulated with E. coli LPS and TDP ( Figure 1B ). Together, these data are consistent with previously published findings that suggest NLRP12 functions as a negative regulator of inflammatory signaling [10], [12], [14].
Figure 1. Dendritic cell stimulation with PAMPs associated with E. coli, K. pneumoniae and M. tuberculosis.
A) Nlrp12 expression in relevant immune system cell types was compiled using a publically accessible microarray meta-analysis search engine (Nextbio website. Available: http://www.nextbio.com/b/search/ba.nb. Accessed 2013 March 11). B) Primary bone marrow derived dendritic cells were isolated, cultured and stimulated with either E. coli LPS (LPS)(50 ng/ml), K. pneumoniae LPS (kpLPS)(50 ng/ml) or trehalose-6,6-dibehenate (TDB; a synthetic analog of TDM from M. tuberculosis)(50 µg/ml). The levels of TNFα and IL-6 were evaluated in the supernatants following 4 hours of stimulation. Experiments were repeated at least 3 separate times with dendritic cells harvested from 3 individual mice for each genotype and treatment in each replicate. C) IL-1β was evaluated in the supernatants 4 hours after stimulation, following an additional treatment with 2 mM ATP 30 minutes prior to harvesting the supernatants. Experiments were repeated at least 3 separate times with dendritic cells harvested from 3 individual mice for each genotype and treatment in each replicate. D) Pro- and cleaved IL-1β was detected by western blot in dendritic cell lysate and supernatant following LPS stimulation. Experiments were repeated at least 3 separate times with dendritic cells harvested and pooled from 3 individual mice from each genotype and treatment in each replicate.
NLRP12 has been suggested to be capable of inflammasome formation and facilitate IL-1β maturation [26], [27]. Thus, in addition to evaluating TNFα and IL-6, we also evaluated IL-1β production and maturation using ELISA and western blot. We detected a significant increase in IL-1β release into the supernatant by ELISA following stimulation with all three PAMPs and ATP, in both Nlrp12−/− and wild type cells ( Figure 1C ). However, no significant differences were detected in IL-1β levels between the wild type and Nlrp12−/− cells ( Figure 1C ). Moreover, western blot analysis revealed that a significant amount of pro-IL-1β was present in the lysate from both wild type and Nlrp12−/− cells treated with LPS ( Figure 1D ). Likewise, both pro- and cleaved-IL-1β was consistently detected in the supernatant from wild type and Nlrp12−/− dendritic cells. Thus, these findings indicate that NLRP12 does not function in IL-1β maturation in mouse bone marrow derived dendritic cells following stimulation with the PAMPs tested. These data are consistent with other published findings that failed to support a role for NLRP12 in inflammasome formation under similar experimental conditions as those tested here [15].
NLRP12 does not Play a Role in LPS Mediated Acute Lung Inflammation
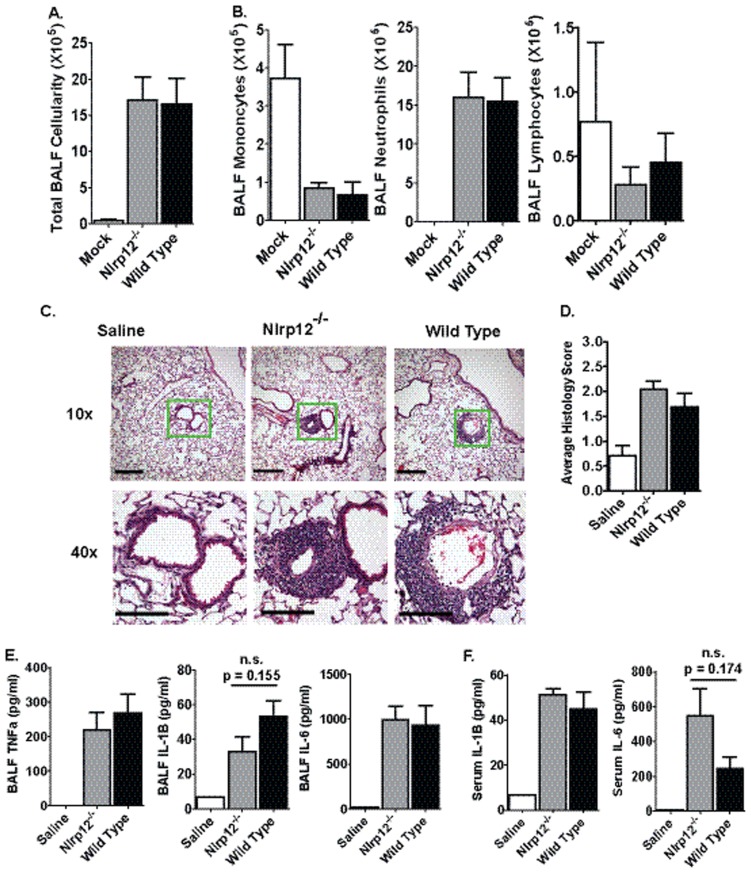
Lipopolysaccharide (LPS) is a component of the cell wall of gram-negative bacteria and is ubiquitous in the environment. Inhalation of LPS has been shown to exacerbate airway reactivity in human asthmatics and activates the host innate immune system by acting as a PAMP to stimulate TLR4 receptors [28]. NLRP12 has previously been shown, in vitro, to down-regulate LPS induced NF-κB activation in human monocytes. This occurs through a mechanism that involves the inhibition of TLR/IL-1 receptor signaling proteins (MyD88, IRAK-1 and TRAF6) and the hyperphosphorylation of IRAK-1 [10]. These data suggest that NLRP12 may also function to down regulate the host innate immune response to LPS in a model of acute airway inflammation. Nlrp12−/− and wild type mice were challenged with 50 µg of LPS through intratracheal (i.t.) administration and harvested 16–20 hrs post-administration. The LPS challenge resulted in a significant increase in total bronchoalveolar lavage fluid (BALF) cellularity, which was consistent with a significant increase in airway neutrophilia following LPS treatment ( Figure 2A ). However, no significant differences were observed between Nlrp12−/− and wild type mice in either the total number or composition of the leukocyte populations present in the BALF ( Figure 2B ). This suggests that airway inflammation occurs independent of NLRP12 following LPS treatment.
Figure 2. LPS mediated acute airway inflammation in Nlrp12−/− mice.
Mice were challenged with 1 mg/kg of E. coli LPS (serotype 0111:B4) i.t. and airway inflammation was assessed 48 hours post-challenge. A) LPS induced a significant increase in BALF cellularity. B) No significant differences were detected in the cellular composition of the BALF between Nlrp12−/− and wild type mice. C) Airway challenge with LPS resulted in a significant influx of neutrophils to the airway. Histopathology revealed a significant amount of perivascular and peribroncholar cuffing and some slight alveolar occlusion following lung LPS exposure. The magnification bar at 10× = 10 µM and 40× = 5 µM. D) Histopathology analysis and histology scoring revealed no significant differences between the Nlrp12−/− and wild type mice. E–F) LPS induced a significant increase in local (BALF) and systemic (serum) levels of proinflammatory cytokines. No significant differences were observed between Nlrp12−/− and wild type mice. Saline, n = 3; LPS-treated Nlrp12−/−, n = 6; LPS-treated Wild Type, n = 6. Experiments were repeated 3 separate times with the same numbers of mice in each replicate.
Airway LPS exposure results in neutrophil infiltration of the lung parenchyma. Previous ex vivo data demonstrated that primary dendritic cells and neutrophils isolated from Nlrp12−/− mice displayed significantly attenuated cell migration in a model of skin hypersensitivity [15]. While BALF cellularity is a widely accepted and accurate surrogate marker for airway inflammation, it does not always fully reflect the extent of inflammation in the lung parenchyma. To further evaluate inflammation, lung histopathology was evaluated from the main bronchi of the left lobe and representative sections were examined ( Figure 2C ). Lung histopathology assessments revealed that LPS induced a significant increase in infiltrating neutrophils in the areas around the lung vasculature (perivascular), the lung parenchyma, and the large and small airways (peribronchiolar) ( Figure 2C ). However, no significant differences were observed in the lung histopathology between LPS challenged Nlrp12−/− and wild type mice ( Figure 2D ). Together, the BALF cellularity assessments and the histopathology findings show that NLRP12 does not play a significant role in LPS-induced acute lung inflammation.
Previous in vitro studies have demonstrated that NLRP12 functions as a negative regulator of TNFα signaling and the LPS-induced production of IL-6 and IL-1β in human monocyte cell lines [10]. However, the in vitro role of NLRP12 appears to be cell type and species specific. Studies utilizing ex vivo primary cells from Nlrp12−/− mice indicate that NLRP12 does not appear to influence the production of TNFα, IL-6 or IL-1β in mouse bone marrow derived dendritic cells [14], [15]. Thus, we sought to evaluate the in vivo contribution of NLRP12 in the local (BALF) and systemic (serum) cytokine response following airway administration of LPS ( Figure 2E–F ). We observed a significant increase in TNFα, IL-1β and IL-6 in the BALF following LPS challenge in both Nlrp12−/− and wild type mice ( Figure 2E ). Likewise, LPS challenge increased the serum levels of IL-1β and IL-6 ( Figure 2F ). TNFα was not detected in the serum following LPS challenge (data not shown). NLRP12 has been shown to associate with the inflammasome adaptor protein ASC/PYCARD, which suggests that NLRP12 may be capable of inflammasome formation under certain conditions [26], [27]. As seen in Figure 2E and F , BALF IL-1β levels were consistently attenuated and serum IL-6 levels were consistently increased in the Nlrp12−/− mice following LPS administration. However, these results did not achieve statistical significance. No other significant differences were observed in cytokine levels between the Nlrp12−/− and wild type mice ( Figure 2E–F ).
Mice Lacking Nlrp12 Demonstrate Normal Immune Responses following Klebsiella pneumoniae Airway Infection
The use of bacterial LPS to induce acute lung inflammation is a well established and highly advantageous model that is commonly employed to assess the effects of Gram-negative bacteria. However, live bacterial pathogens activate the host innate immune system through a diverse range of PAMPs, effector proteins and antimicrobial peptides. Thus, one of the often cited limitations of LPS based models is that endotoxin exposure provides a limited view of the actual events that occur during a live bacteria infection in the lungs [29]. Several NLR proteins play integral roles in mediating the host innate immune response to a variety of lung pathogens. For example, NLRP3 is critical to the host immune response following airway infections with Klebsiella pneumoniae and Chlamydia pneumoniae [1], [30]. NLRC4 plays a vital role in regulating IL-1β and IL-18 production during lung mucosal defense following infections with Pseudomonas aeruginosa and Burkholderia pseudomallei [31], [32]. Likewise, the non-inflammasome forming NLR, NLRC2/NOD2, plays a role in neutrophil recruitment and bacterial killing during E. coli-mediated pneumonia in mice [33]. Thus, we next sought to determine if the host innate immune response is significantly altered in Nlrp12−/− mice during a live bacterial lung infection with K. pneumoniae.
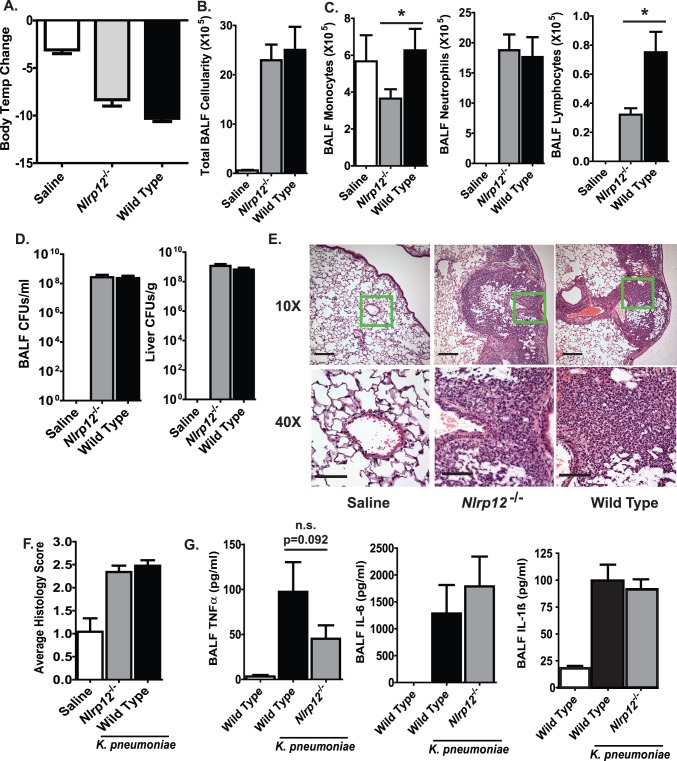
The local administration of K. pneumoniae to the lungs results in significant morbidity, mortality, pneumonia and systemic manifestations of sepsis [1]. Nlrp12−/− mice were challenged via i.t. installation with 7.4×104 CFUs of K. pneumoniae. Previous studies have shown that disease progression peaks at 48 hrs post-infection in wild type C57Bl/6 mice in this K. pneumoniae model [1]. To assess the clinical manifestations of disease progression, body temperature was utilized as a highly sensitive surrogate marker for disease progression. As shown in Figure 3A , 48 hrs after K. pneumoniae exposure, infected animals demonstrated a significant decrease in body temperature. However, no significant differences were detected between Nlrp12−/− and wild type animals ( Figure 3A ). Airway inflammation was assessed by BALF cellularity, which revealed a significant increase in total cellularity following K. pneumoniae infection ( Figure 3B ). Additional morphological analysis of the BALF cellularity revealed that the increase in cellularity was associated with a significant influx of neutrophils ( Figure 3C ). Notably, Nlrp12−/− mice exhibited significantly reduced levels of monocytes and lymphocytes compared to wild type mice. However, the overall significance of the reduced numbers of these specific cell populations in the K. pneumoniae model is unknown as global disease progression was unaffected. Local and systemic bacteria burdens can dramatically influence the host innate immune response and typically reflect disease progression. To measure the bacteria load, BALF was collected and livers were harvested from mice 48 hrs after K. pneumoniae infection. Similar amounts of bacteria were observed in the lungs and livers of Nlrp12−/− and wild type mice, and no significant differences in bacteria burden were observed between Nlrp12−/− and wild type animals ( Figure 3D ). This indicates that NLRP12 does not influence K. pneumoniae clearance or dissemination in this acute lung infection model.
Figure 3. The host innate immune response to Klebsiella pneumoniae infection in Nlrp12−/− mice. A).
Body temperature was monitored daily to assess morbidity. K.pneumoniae infection induced a significant decrease in body temperature in both Nlrp12−/− and wild type mice. B) The lungs were lavaged with HBSS and total BALF cellularity was determined. C) No significant differences in were observed in the BALF neutrophil populations between Nlrp12−/− and wild type mice. However, a significant decrease in the number of monocytes (p = 0.042) and lymphocytes (p = 0.032) were observed in the BALF from the Nlrp12−/− mice compared to the wild type. *P<0.05. D) No differences in bacterial burden were found between the BALF and livers of Nlrp12−/− and wild type animals. E) Histopathology revealed a significant amount of alveolar occlusion following K. pneumoniae infection in both Nlrp12−/− and wild type mice. The magnification bar at 10× = 10 µM and 40× = 5 µM. F) Histology scoring confirmed that no significant differences were identified between the Nlrp12−/− and wild type mice. G) A significant increase in TNFα, IL-6 and IL-1β was observed in the BALF from all K. pneumoniae challenged animals. Saline, n = 3; Nlrp12−/−, n = 13; Wild Type, n = 15. Experiments were repeated 3 separate times with a minimum of 7 K. pneumoniae infected Nlrp12−/− and 7 wild type mice in each group. n.s. = not significant; p = 0.0917.
K. pneumoniae infection is characterized by the development of severe pneumonia and parenchymal disease. Thus, to determine if histopathology was significantly altered in Nlrp12−/− mice, lungs were harvested 48 hrs post-infection and histopathology was evaluated. As expected, all of the mice challenged with K. pneumoniae showed significantly increased inflammatory cell infiltration and extensive alveolar space occlusion ( Figure 3E ). Subsequent histological scoring revealed that there were no significant differences in lung histopathology between Nlrp12−/− and wild type animals ( Figure 3F ). Together, these data suggest that NLRP12 deficiency does not alter the clinical progression of K. pneumoniae infection in mice.
In addition to inducing robust airway inflammation, K. pneumoniae infection also induces the local and systemic production of several cytokines that have been previously associated with NLRP12 function [1]. As shown in Figure 3G , airway challenges with K. pneumoniae induced a significant increase in BALF TNFα, IL-6 and IL-1β levels. In the case of TNFα, we consistently observed a trend towards attenuated levels in the Nlrp12−/− mice; however, the levels of TNFα were never found to be significantly different between the Nlrp12−/− and wild type animals ( Figure 3G ). Likewise, no statistically significant differences were detected in BALF IL-6 or IL-1β between the wild type and Nlrp12−/− mice ( Figure 3G ). Together, these data suggest that NLRP12 does not play a substantial role in regulating the production of several critical cytokines in response to K. pneumoniae airway infection.
NLRP12 does not Play a Role in the in vivo Host Immune Response to Mycobacterium tuberculosis Infection
In contrast to the acute lung infection caused by K. pneumoniae, Mycobacterium tuberculosis (Mtb) is a chronic lung pathogen. Previous data obtained from an Mtb infected human macrophage line indicates that proinflammatory cytokine levels are increased in the absence of NLRP12 [10]. These data demonstrate that NLRP12 is important for dampening the host immune response during Mtb infection. Thus, we tested if the absence of NLRP12 would cause an overzealous host immune response leading to increased morbidity, mortality and lung inflammation during in vivo Mtb infection.
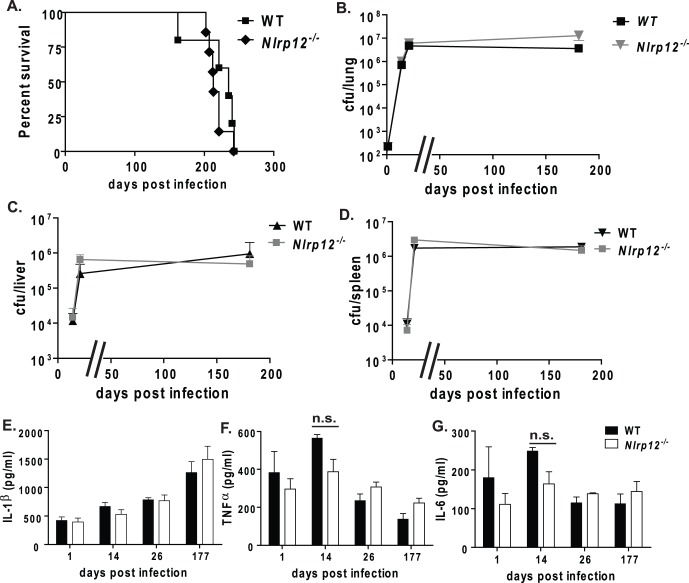
Nlrp12−/− and wild type mice were infected with Mtb via aerosol infection. We found that the mean survival was 217 days post-infection for Nlrp12−/− mice and 220 days for wild type mice ( Figure 4A ). We did not observe any differences in morbidity or mortality between the Nlrp12−/− and wild type animals, indicating that NLRP12 does not play a prominent role in host protection during Mtb infection. In addition to survival, we also sought to determine if NLRP12 has an effect on bacterial growth during in vivo infection by measuring the bacterial burden within the lungs at various time points post-infection. The lungs of Nlrp12−/− and wild type mice were harvested at 1, 14, 26 and 177 days post-infection to evaluate bacterial burden and cytokines following Mtb infection. We did not observe any differences in the amount of Mtb present within the lungs of Nlrp12−/− or wild type mice at any time point post-infection ( Figure 4B ). In addition to the local bacteria burden, we also measured the ability of Mtb to disseminate to secondary sites of infection. Bacterial dissemination to the liver and spleen occurs by 14 days post-infection in wild type C57Bl/6 mice [22]. We found no differences between the bacterial burden of the liver and spleen when we compared Nlrp12−/− and wild type animals ( Figure 4C–D ). These results indicate that NLRP12 does not influence Mtb growth or dissemination at either the primary or secondary sites of infection.
Figure 4. NLRP12 is not protective during Mtb infection.
A) Nlrp12 −/− mice infected with Mtb had no difference in survival compared to wild type mice (median survival was 217 days and 220 days, respectively). B–D) Bacterial burden in the lungs, liver, and spleen was comparable between Nlrp12−/− and wild type mice at all time points assessed. E) Similar amounts of IL-1β were observed in whole lung homogenates from Nlrp12 −/− mice compared to wild-type lungs during both early and chronic phases of Mtb infection. F–G. Cytokine measurements of TNFα (F) and IL-6 (G) showed that Nlrp12 −/− and wild-type lungs had similar amounts of cytokines at one day post-infection and during chronic Mtb infection. At 14 days post-infection, Nlrp12 −/− lungs had reduced cytokines compared to wild-type lungs that neared significance (p = 0.056 and p = 0.06, respectively). Bacteria burden and cytokine measurements at each time point were taken from 3 mice per genotype per time point. Nlrp12−/−, n = 19; Wild type, n = 17; Experiments were repeated 2 separate times with a minimum of 12 Mtb infected Nlrp12−/− and wild type mice in each group. At least 3 mice per strain per time-point were used for CFU and cytokine assessments.
To assess the host inflammatory response to Mtb, the proinflammatory cytokines IL-1β, TNFα, and IL-6 were measured from lung homogenates of Nlrp12−/− and wild type mice ( Figure 4E–G ). The amount of lung IL-1β increased in both Nlrp12−/− and wild type mice as Mtb infection progressed. TNFα and IL-6 levels were also comparable between the lungs of Nlrp12−/− and wild type mice at one day postinfection and during the chronic phase of the infection. At day 14, we found that Nlrp12−/− lungs had reduced TNFα and IL-6 compared to wild type mice, which approached significance (p = 0.056 and p = 0.06, respectively). However, the physiological relevance of these findings is currently unclear and is in contrast to the anti-inflammatory role previously described for NLRP12 in human monocytic cell lines. This finding is reminiscent of NLRP3, which profoundly influences IL-1β production in cultured macrophages in response to Mtb, but does not significantly influence the in vivo model [22], [34]. Together, these data suggest that NLRP12 does not significantly affect lung cytokine levels, bacterial burden or mouse survival in an in vivo model of Mtb infection. Thus, we conclude that NLRP12 does not play a significant role in attenuating or exacerbating Mtb infection.
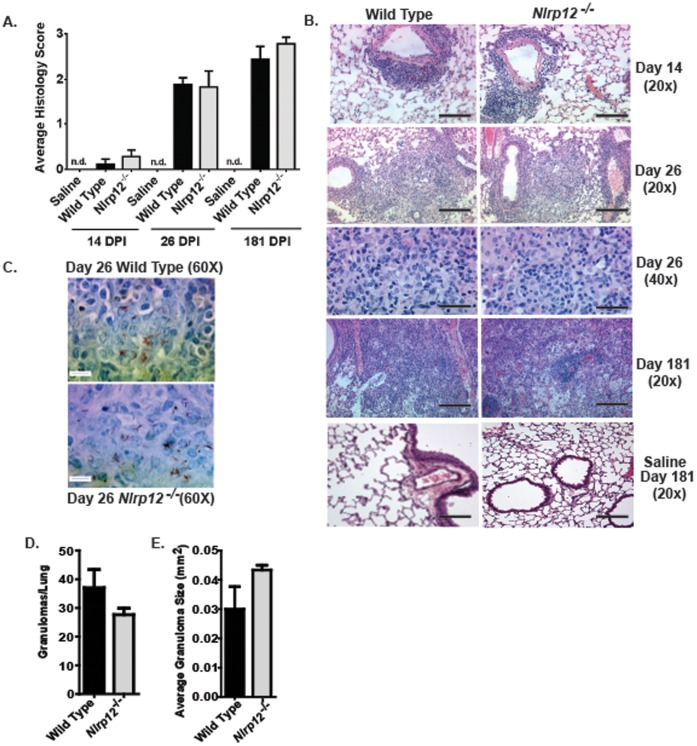
Chronic Mtb infection results in a significant increase in lung inflammation and the formation of highly structured granulomas. A diverse array of cytokines has been shown to influence the formulation and maintenance of Mtb granulomas, including TNFα and IL-6 [35], [36]. These cytokines are important for the innate immune response during the initial exposure of the host to Mtb, but they are also important for regulating the host immune response during chronic Mtb infection. We observed a trending decrease in lung TNFα and IL-6 levels in the Nlrp12−/− mice. Thus, we next sought to evaluate lung histopathology over the time course of Mtb infection. Lungs were harvested at 14, 26 and 181 days post-infection, which represents the early growth phase, the entry to the persistence phase and the chronic phase of Mtb infection in C57Bl/6 mice, respectively [24]. Lung sections at each time point were evaluated and histopathological features were scored. As seen in Figure 5A , we observed significant changes in lung histopathology over the time course of the infection. During the early stages of the infection (Day 14), neutrophilic infiltration was concentrated around the vasculature ( Figure 5B ). However, as the disease progressed, we observed increased areas of concentrated immune cells throughout the lung parenchyma, which is indicative of granuloma lesions ( Figure 5B ). No significant differences in lung inflammation were observed between Nlrp12−/− and wild type animals. In addition to assessments of inflammation, paraffin embedded lung sections were stained with Ziehl-Neelsen acid fast stain to identify Mtb in situ. Our earlier data suggested that the overall lung bacterial burden was unaffected by the loss of NLRP12 ( Figure 4B ). Consistent with these earlier findings, we did not detect any differences in the bacteria burden or bacterial localization in the Nlrp12−/− and wild type mice, shown by acid-fast staining for Mtb bacilli ( Figure 5C ). In all cases, the majority of the bacteria were associated with the granulomas. We also characterized the granulomas in each lung section as previously described [22]. As shown in Figure 5D–E , the number of granulomas observed per lung and the average size of the granulomas were not significantly different between the Nlrp12−/− and wild type mice. Together, these data demonstrate that the host response to Mtb, including granuloma formation and maintenance, are unaffected by the loss of NLRP12.
Figure 5. Lung histopathology and Mtb granuloma formation is unaltered in Nlrp12 deficient mice.
Histopathological analyses of lung sections were conducted at 14, 26 and 181 days post infection (DPI) with aerosol Mtb. A) Semiquantative histology scoring revealed a similar increase in lung inflammation over the course of the Mtb infection between Nlrp12−/− and wild type mice. B) Histopathological progression of leukocyte infiltration at 14, 26 and 181 DPI. Mild to moderate perivascular cuffing was observed around the vasculature of the lungs by 14 DPI. Extensive leukocyte infiltration around the vasculature, airways and throughout the lung parachema was observed by 26 DPI. Large, well formed granulomas were observed at 181 days post infection, which affected extensive regions of the lungs. No signs of abnormal inflammation or granuloma formation were detected in the saline treated mice at any time point evaluated. The magnification bar at 20× = 10 µM and 40× = 5 µM. C) Images from acid fast stained lung sections revealed that the leukocyte infiltration was associated with concentrated areas of bacteria infiltration and was consistent with early stage granuloma formation. The magnification bar at 60× = 5 µM. D–E) The number of granulomas per lung and the size of each granuloma was determined and no significant differences in granuloma formation was observed between either Nlrp12−/− or wild type mice. Experiments were repeated 2 separate times with a minimum of 12 Mtb infected Nlrp12−/− and 12 wild type mice in each group. At least 3 mice per strain per time-point were used for analysis.
Discussion
Several NLRs have been shown to negatively regulate NF-κB signaling [7], [8], [9], [10], [11], [12], [13]. Of these NLRs, NLRP12 is the prototypical member and has been shown to negatively regulate components of both canonical and non-canonical NF-κB pathways in vitro and more recently found to display this role in vivo in colitis and colon cancer models [14], [16]. The canonical NF-κB signaling pathway is a critical modulator of innate immunity and is essential for host protection against pulmonary K. pneumoniae and Mtb infection [31], [37], [38]. While components of the non-canonical NF-κB and NIK signaling pathways have not been directly evaluated in the context of K. pneumoniae or Mtb infection, LPS from Salmonella enterica has been shown to activate both canonical and non-canonical NF-κB signaling pathways [39]. Likewise, NIK deficient aly/aly mice have demonstrated protection in LPS models of bone resorption and osteoclastogenesis [40]. Thus, elements of the non-canonical pathway likely influence host innate immunity to several gram negative bacteria species in vivo.
NLRP12 has been previously shown in vitro to function as a negative regulator of the host innate immune response to both LPS and MTb in human THP-1 monocytic cell lines [10], [12]. This function was shown to be associated with the ability of NLRP12 to interfere with both TLR2 and TLR4 pathways and antagonize signaling events downstream of MyD88, IRAK-1, TRAF2, TRAF6 and RIP1. The mechanism associated with this attenuation was attributed to the inhibition of IRAK-1 hyper-phosphyorylation [10]. While these initial studies were essential for the characterization of NLRP12, the physiological relevance of this NLR in infectious diseases is unclear. The previous studies were all conducted in vitro and utilized cultured human cell lines [10], [11], [12], [26]. Recent studies have begun to examine the in vivo role of NLRP12 utilizing Nlrp12−/− mice. In studies evaluating the development of allergic airway inflammation, no significant differences were observed between Nlrp12−/− mice and wild type animals [41]. However, NLRP12 was shown to influence the development of contact hypersensitivity and colitis associated cancer, which are both disease models with a strong innate immune system component. In the contact hypersensitivity models, dendritic cells and neutrophils from Nlrp12−/− mice failed to respond to select chemokines, which resulted in reduced migration and attenuated disease progression [15]. However, in models of experimental colitis and tumorigenesis, Nlrp12−/− mice demonstrated significantly increased inflammation and cancer progression associated with reduced inhibition of NF-κB signaling [14], [16]. Thus, it appears that NLRP12 displays distinct tissue- and disease-specific functions in vivo. Together, the previous in vitro findings and the in vivo data associated with the innate immune response suggested that the Nlrp12−/− mice would serve as an appropriate model to evaluate disease pathogenesis during bacterial pneumonia and Mtb infection. However, as our data shows, we were unable to identify a functional role for NLRP12 in either disease.
Together, these data demonstrate the importance of validating findings from cell culture/cell lines to mouse models. This is especially evident in the Mtb findings, which showed a convincingly strong in vitro phenotype for NLRP12 that was not apparent in our in vivo experiments. Likewise, previous in vitro data suggested that we would observe a significant in vivo phenotype in the Nlrp12−/− mice following LPS stimulation and K. pneumoniae infection, which we did not observe in our current study. Similar disconnects have been observed between the in vitro data and in vivo data for several NLRs and inflammasome adaptor proteins. For example, recent reports utilizing human cell lines and primary cells from knockout mice reported that components of the NLRP3 inflammasome were necessary for the host immune response against MTb infection [22], [42], [43]. However, follow-up studies utilizing Nlrp3−/− and Caspase-1−/− mice revealed that these inflammasome components did not directly affect the host immune response to in vivo Mtb infection [22], [34], [44]. Thus, many NLRs, including NLRP12, likely function through unpredictable mechanisms in the complex in vivo environment.
The most likely explanation for the discrepancy between the previous in vitro findings for NLRP12 and the data presented here, which suggest that NLRP12 does not significantly contribute to the in vivo host innate immune response to the pathogens evaluated, is that the studies are fundamentally different (i.e. in vivo vs. in vitro; human vs. mouse). However, it is possible that NLRP12 does contribute to the host innate immune response in a temporal, cell type-, tissue-, dose- or PAMP-specific manner and the models utilized here were too broad to discern its function. This hypothesis is supported by other findings that have shown differential and transient expression of NLRP12 in human and rodent cells [10], [15], [45]. Of particular relevance to the current work, NLRP12 was shown to be significantly elevated in cells collected from the BALF of rats that were challenged in vivo with either silica particles or LPS [45]. However, this up-regulation was shown to be transient, temporal and PAMP/DAMP specific [45]. The in vivo bacteria models utilized in the current study are associated with robust immune responses, which may obscure the contribution of indirect or subtle regulators of innate immunity. Thus, it is possible that the in vivo contribution of NLRP12 may have been obscured by the high levels of inflammation, which was not a factor in the previous contact hypersensitivity study [15]. In an attempt to circumvent this issue, we utilized LPS as a less robust, bacteria-free model to assess the innate immune response. However, similar to the bacteria studies, we also failed to detect a significant role for NLRP12. It is certainly possible that other in vivo models, which combine lower levels of inflammation with higher resolution analyses, may reveal a role for NLRP12 in mediating the innate immune response to pathogens. For example, a recent study showed a role for NLRP12 in inflammasome formation and IL-1β/IL-18 maturation in response to a subcutaneous infection with a genetically attenuated strain of Yersinia, but not other activators that engage the NLRP3 inflammasome [27]. Thus, it appears that the inflammasome function of NLRP12 is spatial and/or pathogen specific.
A second possible explanation for the discordance between the in vitro and in vivo findings is that other genes may be compensating for the loss of NLRP12 in vivo. Several recent papers have identified other NLRs that function as negative regulators of canonical and noncanonical NF-κB signaling, many of which may be capable of compensating for the loss of NLRP12. For example, NLRC5 has been shown to attenuate NF-κB signaling following in vitro PAMP stimulation [46], [47]. NLRC5 has also been suggested to inhibit NF-κB signaling through its interaction with IKKα and IKKβ [47], [48], although controversy exists regarding its negative regulatory function [49]. In addition to members of the NLR family, the NF-κB pathway is highly regulated, both positively and negatively, by a large assortment of other proteins. Thus, it is possible that some additional negative regulators may function, either up-stream or down-stream, to compensate for the loss of NLRP12 activity during lung inflammation.
Our data suggests that NLRP12 does not contribute to host defense in response to a limited selection of PAMPs and pathogens. However, it should be noted that future in vivo assessments in the lung or in other tissues using other model systems may discern novel functions for NLRP12 in mediating innate immunity. Here, we assessed the role of NLRP12 in acute and chronic models of bacterial pneumonia and Mtb infection. Each of these diseases impact a growing number of humans world wide and it is essential that we expand our understanding of the underlying disease mechanisms associated with the host immune response in each of these cases. The lack of a role for NLRP12 in the disease processes assessed here is encouraging from the aspect of pharmacological inhibitors that target other inflammatory disorders, such as contact hypersensitivity or inflammatory bowel disease, where NLRP12 was found to play a role in a disease model.
Acknowledgments
We would like to thank Dr. Fayyaz S. Sutterwala (University of Iowa), Dr. Beverly H. Koller (UNC) and Dr. John Bertin (GSK) for their technical support and supplying the initial breeder mice used to establish our colony. We would also like to thank Drs. Denis Gris, Monika Schneider, Kelly E. Roney, Brian P. O’Connor and Chris B. Moore for their technical assistance.
Funding Statement
This work was supported by the National Institutes of Health U19-AI067798 and U19-AI077437(JPYT); F32-AI-082895-01, T32-AR007416 and T32-CA009156 (ICA). This work was also supported by a National Institutes of Health National Research Service Trainee Award (JCA) and the American Cancer Society (JDL and ICA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, et al. (2009) NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and independent pathways. J Immunol 183: 2008–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bean AG, Roach DR, Briscoe H, France MP, Korner H, et al. (1999) Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol 162: 3504–3511. [PubMed] [Google Scholar]
- 3. Harton JA, Linhoff MW, Zhang J, Ting JP (2002) Cutting edge: CATERPILLER: a large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine rich repeat domains. J Immunol 169: 4088–4093. [DOI] [PubMed] [Google Scholar]
- 4. Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124: 783–801. [DOI] [PubMed] [Google Scholar]
- 5. Meylan E, Tschopp J, Karin M (2006) Intracellular pattern recognition receptors in the host response. Nature 442: 39–44. [DOI] [PubMed] [Google Scholar]
- 6.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, et al.. (2008) The NLR gene family: a standard nomenclature. Immunity 28, 285–287. [DOI] [PMC free article] [PubMed]
- 7. Schneider M, Zimmermann AG, Roberts R, Zhang L, Swanson KV, et al. (2012) NLRC3 Attenuates TLR Signaling via Modification of TRAF6 and NF-κB. Nature Immunology. Sep 13(9): 823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, et al. (2011) NLRX1 Protein Attenuates Inflammatory Responses to Infection by Interfering with the RIG-I-MAVS and TRAF6-NF-κB Signaling Pathways. Immunity. Jun 24 34(6): 854–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conti BJ, Davis BK, Zhang J, O'Connor W, Williams KL, et al. (2005) CATERPILLER 16.2 (CLR16.2), a novel NBD/LRR family member that negatively regulates T cell function. J Biol Chem 280: 18375–18385. [DOI] [PubMed] [Google Scholar]
- 10. Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, et al. (2005) The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor alpha-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. J Biol Chem 280: 39914–39924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams KL, Taxman DJ, Linhoff MW, Reed W, Ting JP (2003) Cutting edge: Monarch-1: a pyrin/nucleotide-binding domain/leucine-rich repeat protein that controls classical and nonclassical MHC class I genes. J Immunol 170: 5354–5358. [DOI] [PubMed] [Google Scholar]
- 12. Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, et al. (2007) Monarch-1 suppresses non-canonical NF-kappaB activation and p52 dependent chemokine expression in monocytes. J Immunol 178: 1256–1260. [DOI] [PubMed] [Google Scholar]
- 13. Cui J, Zhu L, Xia X, Wang HY, Legras X, et al. (2010) NLRC5 negatively regulates the NF kappaB and type I interferon signaling pathways. Cell. 141: 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allen IC, Wilson JE, Schneider M, Lich JD, Authur JC, et al. (2012) NLRP12 Functions as a Negative Regulator of Noncanonical NF-κB Signaling and Tumorigenesis during Colitis Associated Cancer. Immunity. May 25 36(5): 742–54. [Google Scholar]
- 15. Arthur JC, Lich JD, Ye Z, Allen IC, Gris D, et al. (2010) Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J Immunol 185: 4515–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, et al. (2011) The NOD like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. Nov 15 20(5): 649–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sikarwar AS, Batra HV (2011) Prevalence of Antimicrobial Drug Resistance of Klebisella pneumonia in India. International Journal of Bioscience, Biochemistry and Bioinformatics. Sept 1(3): 211–215. [Google Scholar]
- 18. Rammaert B, Goyet S, Beauté J, Hem S, Te V, et al. (2012) Klebsiella pneumoniae related community-acquired acute lower respiratory infections in Cambodia: clinical characteristics and treatment. BMC Infect Dis. Jan 10 12: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao Y, Xu S, Wang L, Chin DP, Wang S, et al. (2012) National survey of drug-resistant tuberculosis in China. N Engl J Med. Jun 7 366(23): 2161–70. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. (2011) WHO Tuberculosis facts sheet. Geneva. Available at: http://www.who.int/tb/publications/factsheets/en/index.html. Accessed 2013 March 11.
- 21. Brassard P, Kezouh A, Suissa S (2006) Antirheumatic drugs and the risk of tuberculosis. Clin Infect Dis. Sep 15 43(6): 717–22. [DOI] [PubMed] [Google Scholar]
- 22. McElvania Tekippe E, Allen IC, Hulseberg PD, Sullivan JT, McCann JR, et al. (2010) Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS One 5: e12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allen IC, Pace AJ, Jania LA, Ledford JG, Latour AM, et al. (2006) Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am J Physiol Lung Cell Mol Physiol 291: L1005–1017. [DOI] [PubMed] [Google Scholar]
- 24. Kurtz S, McKinnon KP, Runge MS, Ting JP, Braunstein M (2006) The SecA2 secretion factor of Mycobacterium tuberculosis promotes growth in macrophages and inhibits the host immune response. Infect Immun 74: 6855–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, et al. (2009) The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30: 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, et al. (2002) PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem 277: 29874–29880. [DOI] [PubMed] [Google Scholar]
- 27. Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, et al. (2012) The NLRP12 Inflammasome recognizes Yersinia pestis. Immunity 37(1): 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simpson A, Martinez FD (2010) The role of lipopolysaccharide in the development of atopy in humans. Clin Exp Allergy. Feb 40(2): 209–23. [DOI] [PubMed] [Google Scholar]
- 29. Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, et al. (2010) Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol 184: 5743–5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, et al. (2007) Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. Dec 24 204(13): 3235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F (2011) Inflammasome dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1β is deleterious. PLoS Pathog. Dec 7(12): e1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Theivanthiran B, Batra S, Balamayooran G, Cai S, Kobayashi K, et al. (2012) NOD2 Signaling Contributes to Host Defense in the Lungs against Escherichia coli Infection. Infect Immun. Jul 80(7): 2558–69. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, et al. (2010) Caspase 1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. Apr 1 184(7): 3326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P (1989) The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56: 731–740. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Broser M, Rom WN (1994) Activation of the interleukin 6 gene by Mycobacterium tuberculosis or lipopolysaccharide is mediated by nuclear factors NF-IL6 and NF-kappa B. Proc Natl Acad Sci U S A 91, 2225–2229. [DOI] [PMC free article] [PubMed]
- 37. Yamada H, Mizuno S, Reza-Gholizadeh M, Sugawara I (2001) Relative importance of NF-kappaB p50 in mycobacterial infection. Infect Immun. Nov 69(11): 7100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S (2009) Both TRIF- and MyD88 dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol. Nov 15;1 83(10): 6629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soysa NS, Alles N, Takahashi M, Aoki K, Ohya K (2011) Defective nuclear factor-κB inducing kinase in aly/aly mice prevents bone resorption induced by local injection of lipopolysaccharide. J Periodontal Res. Apr 46(2): 280–4. [DOI] [PubMed] [Google Scholar]
- 41. Allen IC, Lich JD, Arthur JC, Jania CM, Roberts RA, et al. (2012) Characterization of NLRP12 during the Development of Allergic Airway Disease in Mice. PLoS One. Jan 7(1): e30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carlsson F, Kim J, Dumitru C, Barck KH, Carano RA, et al. (2010) Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog 6: e1000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, et al.. (2010) Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol 12, 1046–1063. [DOI] [PubMed]
- 44. Dorhoi A, Nouailles G, Jörg S, Hagens K, Heinemann E, et al. (2012) Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur J Immunol. 2012 Feb 42(2): 374–84. [DOI] [PubMed] [Google Scholar]
- 45. Rao KM, Meighan T (2006) Exposure in vivo to silica or lipopolysaccharide produces transient or sustained upregulation, respectively, of PYPAF7 and MEFV genes in bronchoalveolar lavage cells in rats. J Toxicol Environ Health A 69: 481–490. [DOI] [PubMed] [Google Scholar]
- 46. Benko S, Magalhaes JG, Philpott DJ, Girardin SE (2010) NLRC5 limits the activation of inflammatory pathways. J Immunol 185: 1681–1691. [DOI] [PubMed] [Google Scholar]
- 47. Cui J, Zhu L, Xia X, Wang HY, Legras X, et al. (2010) NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell 141: 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tong Y, Cui J, Li Q, Zou J, Wang HY, Wang RF (2012) Enhanced TLR-induced NF κB signaling and type I interferon responses in NLRC5 deficient mice. Cell Res. May 22(5): 822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kumar H, Pandey S, Zou J, Kumagai Y, Takahashi K, et al. (2011) NLRC5 deficiency does not influence cytokine induction by virus and bacteria infections. J Immunol. Jan 15 186(2): 994–1000. [DOI] [PubMed] [Google Scholar]