Abstract
Objective
To estimate lifetime risk for HF by sex and race.
Background
Prior estimates of lifetime risk for developing heart failure (HF) range from 20% to 33% in predominantly white cohorts. Short-term risks for HF appear higher for blacks than whites, but only limited comparisons of lifetime risk for HF have been made.
Methods
Using public-release and internal datasets from NHLBI-sponsored cohorts, we estimated lifetime risks for developing HF to age 95, with death free of HF as the competing event, among participants in Chicago Heart Association Detection Project in Industry (CHA), Atherosclerosis Risk in Communities (ARIC), and Cardiovascular Health Study (CHS) cohorts.
Results
There were 39,578 participants (33,652 [85%] white; 5,926 [15%] black) followed for 716,976 person-years; 5,983 participants developed HF. At age 45 years, lifetime risks for HF through age 95 years in CHA and CHS were 30-42% in white men, 20-29% in black men, 32-39% in white women, and 24-46% in black women. Results for ARIC demonstrated similar lifetime risks for HF in blacks and whites through age 75 years (limit of follow-up). Lifetime risk for HF was higher with higher BP and BMI at all ages in both blacks and whites and did not diminish substantially with advancing index age.
Conclusions
These are among the first data to compare lifetime risks for HF between blacks and whites. Lifetime risks for HF are high and appear similar for black and white women, yet are somewhat lower for black compared with white men due to competing risks.
Keywords: lifetime risk, heart failure, epidemiology
BACKGROUND
HF is a growing public health crisis with increasing morbidity, mortality, and costs.(1) Previous declines in HF incidence over previous decades have flattened with increases in HF prevalence due to lower case fatality rates.(2,3) Indeed, HF prevalence increased by as much as 30% in Medicare beneficiaries from 1994 to 2003.(4) This increased prevalence of HF, however, may not be solely a result of patients surviving myocardial infarctions in the era of revascularization and aggressive medical therapy,(5) but more likely is associated with rising rates of obesity, hypertension, and diabetes, as well as improved survival among those with HF.(1-4,6-8)
Lifetime risk for developing HF has been estimated to range from 20% to 33% respectively, in the Framingham(9) and Rotterdam Studies,(10) two cohorts of almost exclusively white individuals of European ancestry. However, these studies used different criteria to define HF,(11,12) which may partially account for the differences in lifetime risk.
Whereas short-term risks for HF incidence appear to be significantly higher for blacks than whites in the US,(13,14) only limited comparisons of lifetime risk for HF have been made between white and black individuals.(15) Lifetime risk estimates account for the risk of incident HF as well as for the risk of death from competing causes, and overall and non-cardiovascular mortality risk is known to be higher among blacks than whites (especially in men). Therefore it is unknown whether lifetime risks for HF differ between blacks and whites. Further, knowledge of absolute lifetime risk estimates may be useful for policy-makers, patients, and physicians alike to estimate the current and future population burden of disease, as well as to estimate individual risks. A similar strategy used for breast cancer risk estimation has been cited as a contributor to increased breast cancer screening in the 1990s.(16,17)
We sought to define and compare the lifetime risks for HF by sex and race at selected ages in several diverse population samples by examining the results of prospective, observational studies with data on HF endpoints, namely the Chicago Heart Association Detection Project in Industry (CHA),(18) Atherosclerosis Risk In Communities Study (ARIC),(19) and Cardiovascular Health Study (CHS).(20)
METHODS
Cohorts
This analysis was undertaken as part of the Cardiovascular Lifetime Risk Pooling Project.(21) Using public-release and internal datasets from NHLBI-sponsored cohorts, namely the Chicago Heart Association Detection Project in Industry (CHA; internal dataset), Atherosclerosis Risk in Communities (ARIC; public-release dataset), and Cardiovascular Health Study (CHS; internal dataset), we estimated lifetime risk for developing overt HF.
The CHA study examined 37,572 participants in Chicago, Illinois between the ages of 18-74 years old (43% women, 10% black) from 1967 to 1973.(18) CHA participants were followed through 2003. Incident HF was defined by Medicare hospital discharge coding in the CHA cohort (ICD-9 codes 428.*, primary discharge diagnosis only). Since data were derived from Medicare hospitalization data, only HF hospitalizations from Medicare-eligible participants were captured beginning in 1984, the first year Medicare data were available for public use, through 2003. Follow-up for vital status was completed by direct mail, telephone, contact with employer, and matching of records with Social Security Administration files prior to 1979; from 1979 to 1994, follow-up was completed through the National Death Index.(13) Death certificates were obtained and coded for multiple causes by trained research staff according to the Eighth Revision of the International Classification of Diseases (ICD-8).(22) From 1995-1998, the National Death Index-plus service was used to obtain ICD Ninth Revision (ICD-9) cause of death coding and ICD Tenth Revision (ICD-10) coding from 1999 to 2003.(14,23) For this report, the underlying cause of death was used. HF mortality was defined as ICD-8 and ICD-9 code 428 and ICD-10 codes I50.1-I50.4.
The Atherosclerosis Risk in Communities (ARIC) Study enrolled 15,721 participants ages 45-64 years at four sites (Minneapolis, Minnesota; Jackson, MS; Forsyth County, NC; Washington County, MD; 55% women, 27% black) from 1987 to 1989.(19) ARIC participants were followed through 2005. Individuals with prevalent HF were excluded using the Gothenburg criteria. Incident HF cases were ascertained by annual interviews of ARIC participants and review of local hospital discharge logs to find HF hospitalizations and by review of health department death certificates to find HF deaths. Incident HF was defined by Medicare hospital discharge coding for the ARIC cohort (ICD-9 codes 428.*; primary discharge diagnosis only) for hospitalization or by death certificate (ICD-9 codes 428.*; ICD-10 codes I50.*). Incident HF cases were adjudicated by a central committee of physician reviewers, as previously described.(13)
The Cardiovascular Health Study (CHS), a cohort of Medicare-eligible older Americans, enrolled 5,888 participants > 65 years from four sites (Sacramento County, CA; Allegheny County, PA; Forsyth County, NC; Washington County, MD; 58% women, 16% black) from 1989 to 1993.(20) CHS participants were followed through 2004. In this cohort, incident HF was defined by a central adjudication committee that relied upon results from semiannual contacts with participants and Medicare hospital discharge data (ICD-9 codes 428.*, primary discharge diagnosis only). Heart failure events were confirmed by physician review of identifying clinical evidence such as physician diagnosis, physical or x-ray findings or drug treatment with diuretics, digitalis, or vasodilators. The causes of death were adjudicated by the same end-points central adjudication committee.(20)
Statistical Analysis
All statistical analyses were performed using SAS statistical software (version 9.1; SAS Institute, Inc., Cary, NC). Lifetime risks were estimated using a modified life-table analysis using the Practical Incidence Estimator macro, in which each participant contributes information for each age attained during follow-up, as previously described.(24) For the calculation of lifetime risks, a modified Kaplan-Meier method was used which accounts for competing risk from death free of heart failure to avoid lifetime risk overestimation. In brief, the modified Kaplan-Meier analysis counts death free of heart failure as a competing risk rather than a withdrawal (as in the traditional Kaplan-Meier analysis) at the time of event (see Supplemental Appendix for additional details). All participants free from HF at selected index ages (45, 55, 65, and 75 years) were included. We estimated lifetime risk for HF to age 95, with death free of HF as the competing event, as described above. In order to demonstrate the difference between unadjusted Kaplan-Meier and adjusted lifetime risk estimates, we created cumulative risk curves using the same data. The difference between these curves represents the competing risks of death from causes other than heart failure.
Participants were also stratified by body mass index into three groups (BMI <25, 25-29, and >30 kg/m2) based on height and weight measured within two years of the index age in order to evaluate the association between obesity and lifetime risk for HF. We limited these analyses to a follow-up time of thirty years due to smaller sample sizes.
In order to investigate the association between blood pressure and lifetime risk for HF, we stratified participants by blood pressure level as measured within two (2) years of each index age. Again, we limited follow-up time to thirty years due to sample size. The blood pressure strata included: <120/<80 mmHg; 121-139 mmHg (systolic) or 81-89 mmHg (diastolic); 140-159 mmHg (systolic) or 90-99 mmHg (diastolic); and >160 mmHg (systolic), >100 mmHg (diastolic), or treated hypertension. Participants with treated hypertension were included in this final stratum due to the overall small size of this group, which was not adequately powered to address lifetime risks. It was also not known if such treated participants were treated to an optimal blood pressure target throughout each study. Inclusion of the treated participants would conservatively underestimate the lifetime risk for HF in this highest blood pressure stratum.
Finally, we performed analyses to examine the remaining lifetime risk for HF attributable to causes other than MI. In this analysis, we excluded participants with a history of recognized or unrecognized MI before or at the index examination and only considered those who developed HF without an intervening MI during follow-up.
Informed Consent
All cohorts included here have been approved by the institutional review board from each contributing institution, including Northwestern University. Participants provided informed consent at each examination.
RESULTS
Baseline characteristics for included participants in the CHA, ARIC, and CHS cohorts are shown in Table 1. After the index age of 45 years, CHA participants developed 3,972 HF events over 501,127 person-years of follow-up (7.9 per 1,000 person-years); after age 45, ARIC participants developed 1,010 HF events over 168,516 person-years of follow-up (6.0 per 1,000 person-years); and after the index age of 65 years, CHS participants developed 1,001 HF events over 47,333 person-years of follow-up (21.1 per 1,000 person-years).
Table 1.
Baseline characteristics of participants free from heart failure from Chicago Heart Association Detection in Industry Project (CHA), Atherosclerosis Risk in Communities (ARIC), and Cardiovascular Health Study (CHS) cohorts. Sample sizes are lower than total sample sizes for these cohorts since these measures reflect data collected only at pre-specified index ages of 45 and 65 years. (SBP=systolic blood pressure; DBP=diastolic blood pressure; BMI=body mass
| Chicago Heart Association Detection in Industry Project (CHA) |
Atherosclerosi s Risk in Communities (ARIC) |
Cardiovascular Health Study (CHS) |
|
|---|---|---|---|
| N=19,391 | N=15,732 | N=4,455 | |
| Index age, years | 45 | 45 | 65 |
| Follow-up (person-years) | 501,127 | 168,516 | 47,333 |
| Total HF events | 3,972 | 1,010 | 1,001 |
| WHITE MEN, No. (%) | 10,017 (51.7) | 5,426 (34.5) | 1,492 (33.5) |
| SBP, mmHg (SD) | 142.9 (20.3) | 120.1 (16.1) | 136.1 (21.1) |
| DBP, mmHg (SD) | 84.3 (11.7) | 73.2 (9.8) | 72.3 (11.3) |
| BMI, kg/m2 (SD) | 27.2 (3.6) | 27.4 (4.0) | 26.4 (3.6) |
| BP medication, % | 6.8 | 20.3 | 34.5 |
| History of diabetes, % | 4.1 | 7.8 | 8.0 |
| History of myocardial infarction, % | 2.7 | 1.8 | 9.5 |
| BLACK MEN, No. (%) | 545 (2.8) | 1,629 (10.4) | 248 (5.6) |
| SBP, mmHg (SD) | 146.3 (24.1) | 130.5 (21.9) | 138.1 (20.9) |
| DBP, mmHg (SD) | 87.9 (14.3) | 82.5 (13.0) | 76.6 (11.6) |
| BMI, kg/m2 (SD) | 27.3 (4.2) | 27.6 (4.9) | 26.7 (3.9) |
| BP medication, % | 6.3 | 34.4 | 44.8 |
| History of diabetes, % | 3.7 | 16.2 | 11.4 |
| History of myocardial infarction, % | 1.3 | 1.9 | 4.4 |
| WHITE WOMEN, No. (%) | 8,379 (43.2) | 6,050 (38.5) | 2,288 (51.0) |
| SBP (mm Hg) | 138.3 (20.8) | 117.0 (17.6) | 135.1 (21.1) |
| DBP (mmHg) | 80.7 (11.5) | 69.5 (9.8) | 69.2 (10.8) |
| BMI (kg/m2) | 25.2 (1.4) | 26.6 (5.5) | 26.2 (4.9) |
| BP medication, % | 9.3 | 19.5 | 37.7 |
| History of diabetes, % | 2.6 | 6.6 | 6.5 |
| History of myocardial infarction, % | 2.2 | 0.8 | 4.7 |
| BLACK WOMEN, No. (%) | 450 (2.3) | 2,627 (16.7) | 427 (9.6) |
| SBP (mm Hg) | 140.1 (24.4) | 128.2 (21.5) | 143.1 (23.3) |
| DBP (mmHg) | 84.3 (14.4) | 78.2 (11.7) | 74.7 (11.1) |
| BMI (kg/m2) | 26.5 (4.9) | 30.8 (6.5) | 29.4 (5.9) |
| BP medication, % | 13.3 | 44.6 | 62.3 |
| History of diabetes, % | 3.9 | 18.7 | 9.0 |
| History of myocardial infarction, % index) |
0.9 | 1.3 | 4.2 |
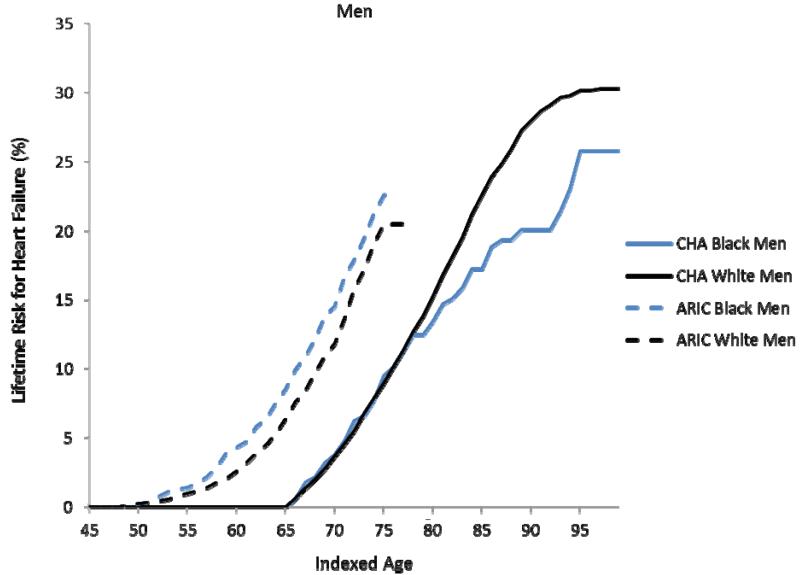
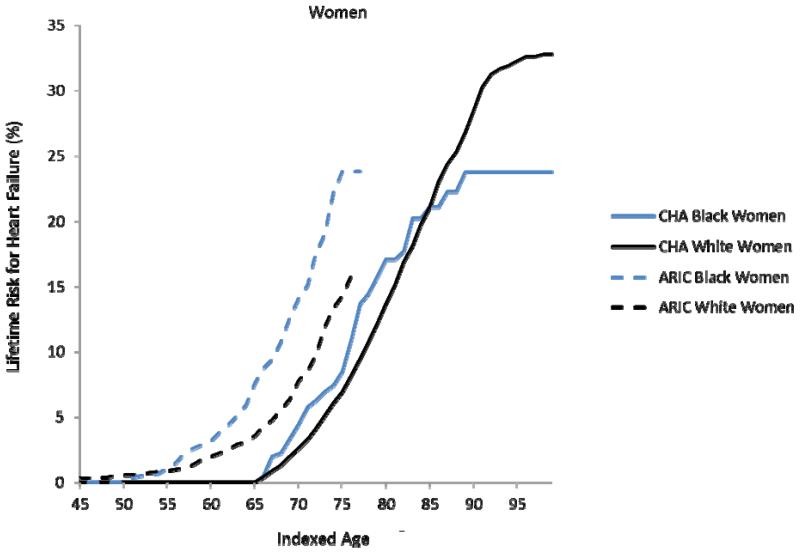
In the CHA cohort, the remaining lifetime risks for HF at age 45 through 95 were 30.2% (95%CI 28.9, 31.5) for white men, 20.1% (15.8, 24.4) for black men (through 90 years), 32.3% (30.8, 33.8) for white women, and 23.7% (17.8, 29.8) for black women (through 90 years) (Figures 1a-b). The overlap of these estimates in women through index ages 75 years and 85 years indicates that lifetime risks for HF were similar between black and white women, whereas lifetime risks for HF were generally lower among black men than white men in CHA. With advancing index ages, lifetime risks for HF did not decrease, despite an increase in the competing risk of HF-free death in all groups. For example, the lifetime risks for HF in white men in CHA at ages 45 and 55 were 30.2% (95%CI 28.9, 31.5) and 28.5% (27.1, 29.9), respectively.
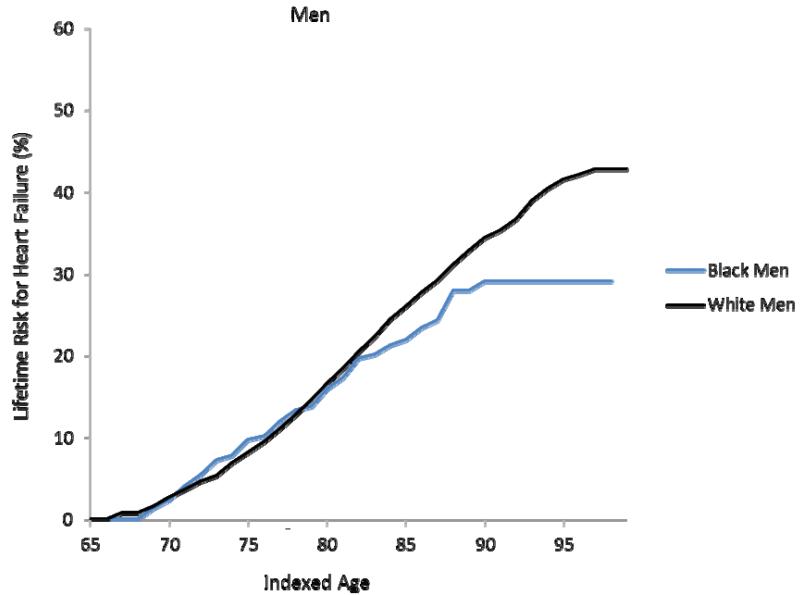
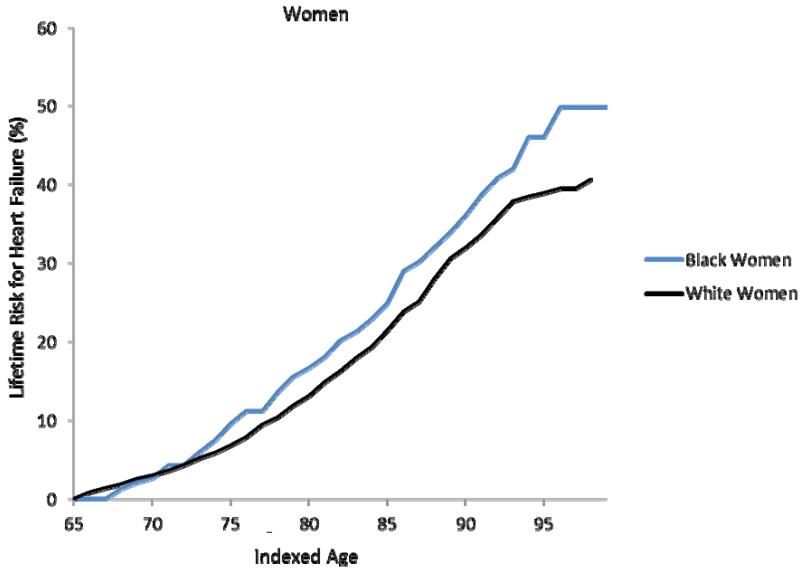
Figure 1a-d. Lifetime risks for heart failure in white and black Americans.
Lifetime risks (LR) for heart failure at index age 45 years for black and white men (1a) and women (1b) in the Chicago Heart Association Detection in Industry Project (CHA) and Atherosclerosis Risk in Communities (ARIC) cohorts and LR for heart failure at index age 65 years for black and white men (1c) and women (1d) in the Cardiovascular Health Study (CHS).
In the ARIC cohort, the remaining lifetime risk for HF through age 75, which was near the limit of follow-up, was 19.1% (17.0, 21.2) for white men and 21.3% (17.5, 25.1) for black men (Figure 1a). In women, the lifetime risk for HF through age 75 was 13.4% (11.3, 15.4) in white women compared to 23.9% (20.1, 27.6) in black women (Figure 1b). At the index ages of 45 and 55 years, remaining lifetime risks for HF through age 75, the limit of follow-up in the ARIC cohort) appeared higher for whites and blacks in ARIC compared to whites and blacks in CHA.
In the CHS cohort, the remaining lifetime risk for HF at age 65 through 95 was 41.6% (38.0, 45.1) for white men and 29.1% (21.1, 37.1) for black men (Figure 1c). Remaining lifetime risks for HF were 38.5% (35.2, 41.9) for white women and 46.1% (38.0, 54.1) for black women (Figure 1d). As seen in the CHA cohort, the lifetime risk for HF in CHS participants also did not substantially decrease with advancing index age.
Lifetime Risks for HF by BMI Strata
When we stratified participants by BMI, lower BMI strata were associated with lower adjusted cumulative risks for HF in all sex-race groups through age 75 years. Table 2 demonstrates that remaining lifetime risk for HF tended to increase with higher BMI at all index ages for all sex and race groups. For example, lifetime risk for HF among black women at index age of 45 years ranged from 4.4% (0.2, 8.6) in women with BMI < 25 kg/m2 to 21.8% (7.5, 36.1) in women with BMI ≥ 30 kg/m2. Similar trends were also seen in the ARIC and CHS cohorts (data not shown).
Table 2.
Thirty-year cumulative risk (95% CI) for HF, adjusted for competing risk of death, at index age 45 by sex-race group in the CHA by BMI strata. (BMI=body mass index; HF=heart failure)
| BMI Group | White Men | Black Men | White Women | Black Women |
|---|---|---|---|---|
| Chicago Heart Association Detection in Industry Project (CHA): Index age 45; Follow-up age 75 | ||||
| <25 kg/m2 | 9.1 (7.2, 10.9) |
12.8 (4.5, 21.1) |
7.7 (6.3, 9.0) |
4.4 (0.2, 8.6) |
| 25-29 kg/m2 | 11.8 (10.3, 13.3) |
6.0 (1.4, 10.7) |
11.5 (9.1, 13.9) |
11.4 (4.0, 18.8) |
| ≥30 kg/m2 | 19.3 (16.1, 22.5) |
21.3 (7.3, 35.4) |
16.6 (12.0, 21.2) |
21.8 (7.5, 36.1) |
Lifetime Risks for HF by Blood Pressure Strata
When participants were stratified by blood pressure level, lower lifetime risks were seen in participants with optimal blood pressure compared to higher lifetime risks with stage II/treated hypertension. Table 3 demonstrates that remaining cumulative risk for HF, adjusted for the competing risk of death through age 75 years, tended to increase with higher BP for all sex and race groups in CHA at an index age of 45 years. For example, adjusted cumulative risks in white men ranged from 9.8% (5.6, 13.9) with blood pressure ≤120/≤80mmHg to 16.3% (13.5, 19.2) with systolic blood pressure ≥ 160, diastolic blood pressure ≥ 100 mmHg or treated hypertension. A similar pattern of results was observed at all index ages for all sex/race groups in CHA and the other cohorts (data not shown).
Table 3.
Thirty-year cumulative risk (95% CI) for HF, adjusted for competing risk of death, at index age 45 by sex-race group in CHA by blood pressure strata.
| Blood Pressure Group | White Men | Black Men | White Women | Black Women |
|---|---|---|---|---|
| Chicago Heart Association Detection in Industry Project (CHA) Index age 45; Follow-up age 75 years | ||||
| ≤120/≤80 mmHg | 9.8 (5.6, 13.9) |
--* | 9.9 (6.9, 12.8) |
6.9 (0, 16.1) |
| 121-139 or 81-89 mmHg | 10.9 (9.1, 12.7) |
7.5 (1.2, 13.8) |
8.6 (7.0, 10.4) |
7.9 (1.3, 14.5) |
| 140-159 or 90-99 mmHg | 12.1 (10.3, 14.0) |
13.9 (5.9, 22.0) |
9.2 (7.0, 11.4) |
6.7 (0, 14.0) |
| ≥160 or ≥100 mmHg or treated hypertension |
16.3 (13.5, 19.2) |
12.7 (3.2, 22.2) |
13.3 (9.7, 16.9) |
16.0 (6.4, 25.5) |
Insufficient number of events to provide stable estimate. (HF=heart failure)
Lifetime Risks for in the Absence of Antecedent Myocardial Infarction
We performed sex- and race-specific estimates of lifetime risk for HF after accounting for the competing risk of antecedent myocardial infarction (MI) (Table 4). Lifetime risks were lower in participants without prior MI in nearly all sex-race groups but remained high, ranging from 18.6% (14.4, 22.9) in black men to 28.7% (27.3, 30.2) in white women at index age of 45 years in CHA and from 22.1% (15.0, 29.2) in black men to 36.3% (28.8, 43.8) in black women at index age of 65 years in CHS. Similar trends were seen for ARIC, despite the limited follow-up to age 75 years.
Table 4.
Cumulative risk (95% CI) for HF, adjusted for competing risk of death and antecedent myocardial infarction, at selected index ages through given follow-up ages by sex-race groups.
| White Men | Black Men | White Women | Black Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up age |
75
years |
85
years |
95
years |
75
years |
85
years |
95
years |
75
years |
85
years |
95
years |
75
years |
85
years |
95
years |
| Chicago Heart Association Detection in Industry Project (CHA) | ||||||||||||
| Index age 45 |
7.8 (7.2, 8.3) |
19.5 (18.6, 20.4) |
26.1 (24.9, 27.3) |
8.6 (6.2, 10.9) |
15.8 (12.2, 19.4) |
18.6* (14.4, 22.9) |
6.2 (5.7, 6.7) |
18.9 (17.9, 19.9) |
28.7 (27.3, 30.2) |
8.3 (5.7, 10.9) |
20.0 (15.1, 24.9) |
22.7* (16.7, 28.6) |
| Index age 55 |
11.4 (10.6, 12.3) |
18.0 (17.0, 19.1) |
24.5 (23.2, 25.9) |
7.7 (4.3, 11.0) |
15.4 (10.9, 19.9) |
18.1* (13.1, 23.0) |
4.5 (4.0, 5.1) |
18.4 (17.3, 19.5) |
28.3 (26.8, 29.9) |
6.7 (2.7, 10.7) |
19.3 (12.8, 25.7) |
22.0* (14.7, 29.2) |
| Atherosclerosis Risk in Communities (ARIC) | ||||||||||||
| Index age 45 |
14.2 (12.4, 16.0) |
-- | -- |
15.9 (12.6, 19.1) |
-- | -- |
10.6 (8.8, 12.4) |
-- | -- |
19.9 (16.4, 23.4) |
-- | -- |
| Index age 55 |
13.9 (12.1, 15.7) |
-- | -- |
16.1 (12.7, 19.6) |
-- | -- |
10.4 (8.6, 12.2) |
-- | -- |
20.1 (16.5, 23.8) |
-- | -- |
| Cardiovascular Health Study (CHS) | ||||||||||||
| Index age 65 |
5.5 (3.2, 7.8) |
16.1 (13.4, 18.7) |
26.4 (23.2, 29.7) |
8.0 (3.1, 12.8) |
17.6 (11.5, 23.7 |
22.1* (15.0, 29.2) |
4.9 (3.3, 6.5) |
14.5 (12.5, 16.5) |
29.9 (26.8, 33.0) |
8.9 (4.6, 13.1) |
20.3 (15.1, 25.4) |
36.3 (28.8, 43.8) |
| Index age 75 | -- |
13.1 (11.0, 15.2) |
26.0 (22.6, 29.3) |
-- |
14.2 (8.5, 19.9) |
20.7* (13.1, 28.3) |
-- |
10.7 (9.2, 12.2 |
28.1 (24.9, 31.3) |
-- |
12.5 (8.4, 16.6) |
30.5 (22.7, 38.4) |
Limited to 90-year follow-up. (HF=heart failure)
Unadjusted Risk for HF Compared to Lifetime Risk
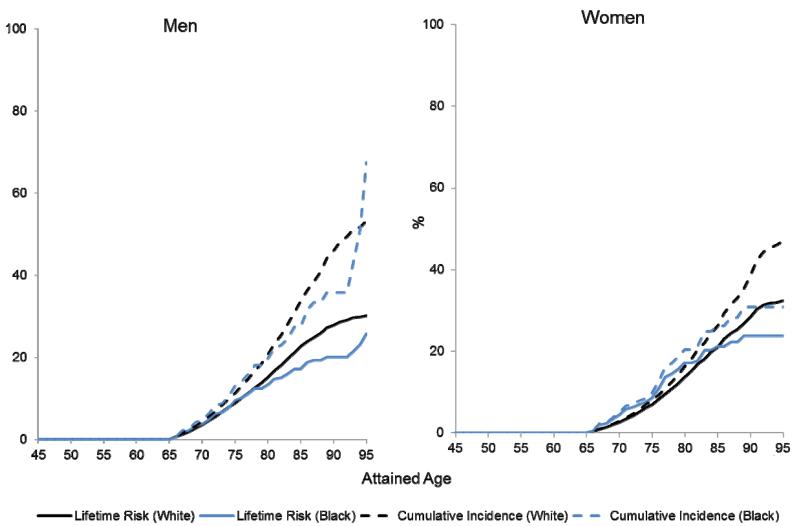
Figures 2a (men) and 2b (women) compare unadjusted risks for HF (Kaplan-Meier cumulative incidence) with the lifetime risks for HF in the CHA cohort at the index age of 45 years old. Since the unadjusted Kaplan-Meier cumulative incidence does not account for the risk of HF-free death, the competing event, the Kaplan-Meier estimates for cumulative incidence of HF are substantially higher than the adjusted cumulative lifetime risks. The differences between the unadjusted and adjusted cumulative incidence curves represent the burden of competing risk from death free of HF. For example, the unadjusted risk of non-cardiovascular disease death rates in the CHA cohort are shown in Supplemental Figure 1, separately for black and white men and women at an index age of 45. After 20 years of follow-up, the incidence of non-CVD death in black men was 11.8%, compared with 7.6% in white men. Therefore, the greater burden of non-CVD mortality among black men particularly appears to limit lifetime risks for HF in this group.
Figure 2a (men) and 2b (women). Lifetime and unadjusted risks for heart failure.
Lifetime risks (LR) are compared with unadjusted risks for heart failure at index age 45 by sex-race group in the Chicago Heart Association Detection in Industry Project (CHA) Cohort. The difference between lifetime risk estimate and unadjusted cumulative incidence (Kaplan-Meier estimate) is an estimate of the competing risk for each sex/race group.
DISCUSSION
These are among the first data to explore the lifetime risk for HF in different race/ethnic groups. Overall, remaining lifetime risks for HF are high (20-45%) in all groups and appear similar for black and white women and somewhat lower for black men compared to white men. Despite differences in birth cohort, age, and diagnostic criteria, we find remarkably similar results between blacks and whites. Risk factors for increasing lifetime risks for HF in whites— such as obesity, blood pressure, and non-fatal myocardial infarction—appear to increase the lifetime risks for HF in blacks in a similar fashion. The rising obesity prevalence in the US makes this consistent association particularly relevant to the future HF risk. Also, lifetime risk for HF did not diminish with advancing age since it is more common in older individuals, despite the increased risk of HF-free death, and hence shorter remaining lifespan during which HF can occur.
The unadjusted risk for HF in black men was highest among all sex-race groups, as has been described in a population of younger adults.(25) However, the lower lifetime risks for HF appears to be a product of higher overall risks for HF among African-Americans that were counter-balanced by greater competing risks for death from non-cardiovascular causes, particularly among African-American men, due to causes such as homicide, renal failure, and HIV.(26)
The lifetime risks for developing HF in the present cohorts are higher than previously reported in the Framingham and Rotterdam cohorts. These differences may be related, in part, to how incident HF was defined in each cohort. The Framingham Heart Study, in which the lifetime risks for HF were estimated to be approximately 20% at all index ages, has a more stringent definition of HF as compared to other cohorts.(27)
Nevertheless, hospital discharge data, which were used by the cohorts in the present study, demonstrate an increased number of hospitalizations for HF and rising costs associated with these hospitalizations.(1) Our use of primary hospital discharge diagnosis may even underestimate admissions with associated HF diagnoses. Unlike coronary artery disease and stroke, where individuals might “escape” some of their lifetime risk at older index ages,(28) lifetime risks for developing new onset HF continue to remain as high as 40% at age 75, despite greater competing risks and substantially shorter remaining lifespan at such an age. If risk factors for HF are not treated more aggressively in both whites and blacks, HF incidence and costs will likely increase further, particularly as the US population ages.
Limitations
Our paper has several limitations. First, we evaluated cohorts that defined HF differently, which likely affected lifetime risk estimates across different populations. However, these cohorts defined HF through hospital discharge coding, which represents a clinically relevant entity. Second, different cohorts had different entry criteria and different index ages ranging from <45 to ≥65, which also limits our analysis of these cohorts across a wider age range. Third, our analysis of ARIC was limited to public-release dataset, which likely explains our lower estimates compared with the internal dataset with longer follow-up through 85 years.(15) However, our estimates through age 75 years were similar to those recently reported. Fourth, data were collected from participants in different age ranges at different time points, which could lead to birth cohort effects. The differences in lifetime risks for HF between CHA and ARIC may be partially explained by the increased prevalence of obesity that occurred from 1967-73 (CHA enrollment) to 1987-1989 (ARIC enrollment). On the other hand, since CHA HF events were collected through Medicare discharge data, HF events that occurred before age 65 years or the onset of hemodialysis or disability would have been missed, leading to an underestimation of lifetime risks. The prevalence of HF is less than 2% in US adults <60 years,(1) suggesting that few incident cases would have been missed, but data from ARIC indicate that more cases would be missed among black men than other sex/race groups. Similar trends between white and black participants at younger ages as seen in ARIC also suggests that these lifetime risk estimates are likely stable.
Conclusions
Lifetime risks for HF are very high for both black and white men and women, underscoring the importance of population-wide preventive efforts to curb the growing burden of HF. These data should help clinicians, researchers, and policy-makers more clearly understand how great of a problem HF currently is and will continue to be unless preventive measures are broadly implemented.
Acknowledgments
We thank the investigators of all the cohort studies included in this analysis for their hard work and dedication in collecting the underlying data, and especially the study participants, whose dedication and commitment have formed the basis of profound observations regarding health and disease that have contributed to improved health, longevity, and quality of life for millions of persons.
Funding Support
Role of the Funding Source (NHLBI)
Supported by NHLBI to Dr. Lloyd-Jones for the Lifetime Risk Pooling Project (R21 HL085375).
The most recent follow-up of the Chicago Heart Association Detection in Industry Project was supported by the NHLBI (5R01HL081141-04).
The Atherosclerosis Risk in Communities Study is conducted and supported by the NHLBI in collaboration with the study investigators. This study was conducted with the use of a limited-access dataset obtained by the National Heart, Lung, and Blood Institute (NHLBI) and does not necessarily reflect the opinions or views of the study investigators or the NHLBI.
The research reported in this article was supported by contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Abbreviations
- ARIC
Atherosclerosis Risk In Communities Study
- BMI
body mass index
- BP
blood pressure
- CHA
Chicago Heart Association Detection Project in Industry
- CHS
Cardiovascular Health Study
- CVD
cardiovascular disease
- HF
heart failure
- ICD
International Classification of Diseases
- MI
myocardial infarction
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 4.Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994-2003. Arch Intern Med. 2008;168:418–24. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 5.Baker DW. Prevention of heart failure. J Card Fail. 2002;8:333–46. doi: 10.1054/jcaf.2002.0805333. [DOI] [PubMed] [Google Scholar]
- 6.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 7.Lee DS, Massaro JM, Wang TJ, et al. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension. 2007;50:869–76. doi: 10.1161/HYPERTENSIONAHA.107.095380. [DOI] [PubMed] [Google Scholar]
- 8.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–37. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 10.Bleumink GS, Knetsch AM, Sturkenboom MC, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25:1614–9. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 12.Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527–60. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- 13.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–22. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 14.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–45. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avery CL, Loehr LR, Baggett C, et al. The ARIC (Atherosclerosis Risk in Communities) Study The Population Burden of Heart Failure Attributable to Modifiable Risk Factors. J Am Coll Cardiol. 2012;60:1640–6. doi: 10.1016/j.jacc.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackman DK, Bennett EM, Miller DS. Trends in self-reported use of mammograms (1989-1997) and Papanicolaou tests (1991-1997)--Behavioral Risk Factor Surveillance System. MMWR CDC Surveill Summ. 1999;48:1–22. [PubMed] [Google Scholar]
- 17.Visser LE, Bleumink GS, Trienekens PH, Vulto AG, Hofman A, Stricker BH. The risk of overanticoagulation in patients with heart failure on coumarin anticoagulants. Br J Haematol. 2004;127:85–9. doi: 10.1111/j.1365-2141.2004.05162.x. [DOI] [PubMed] [Google Scholar]
- 18.Stamler J, Dyer AR, Shekelle RB, Neaton J, Stamler R. Relationship of baseline major risk factors to coronary and all-cause mortality, and to longevity: findings from long-term follow-up of Chicago cohorts. Cardiology. 1993;82:191–222. doi: 10.1159/000175868. [DOI] [PubMed] [Google Scholar]
- 19.The Atherosclerosis Risk in Communities (ARIC) Study. The ARIC investigators design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 20.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 21.Berry JDDA, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM. Lifetime risks for cardiovascular disease. New England Journal of Medicine. 2012;366:312–9. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics NCfH . Health, United States, 1995. Public Health Service; Hyattsville, Md: 1996. [Google Scholar]
- 23.Campbell VA, Crews JE, Moriarty DG, Zack MM, Blackman DK. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults--United States, 1993-1997. MMWR CDC Surveill Summ. 1999;48:131–56. [PubMed] [Google Scholar]
- 24.Beiser A, D’Agostino RB, Sr., Seshadri S, Sullivan LM, Wolf PA, The Practical Incidence Estimators (PIE) macro Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. Stat Med. 2000;19:1495–522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–90. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. [PubMed] [Google Scholar]
- 27.McKee PA, Castelli WP, McNamara PM, Kannel WB, the Framingham study The natural history of congestive heart failure. N Engl J Med. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 28.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–8. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]