Abstract
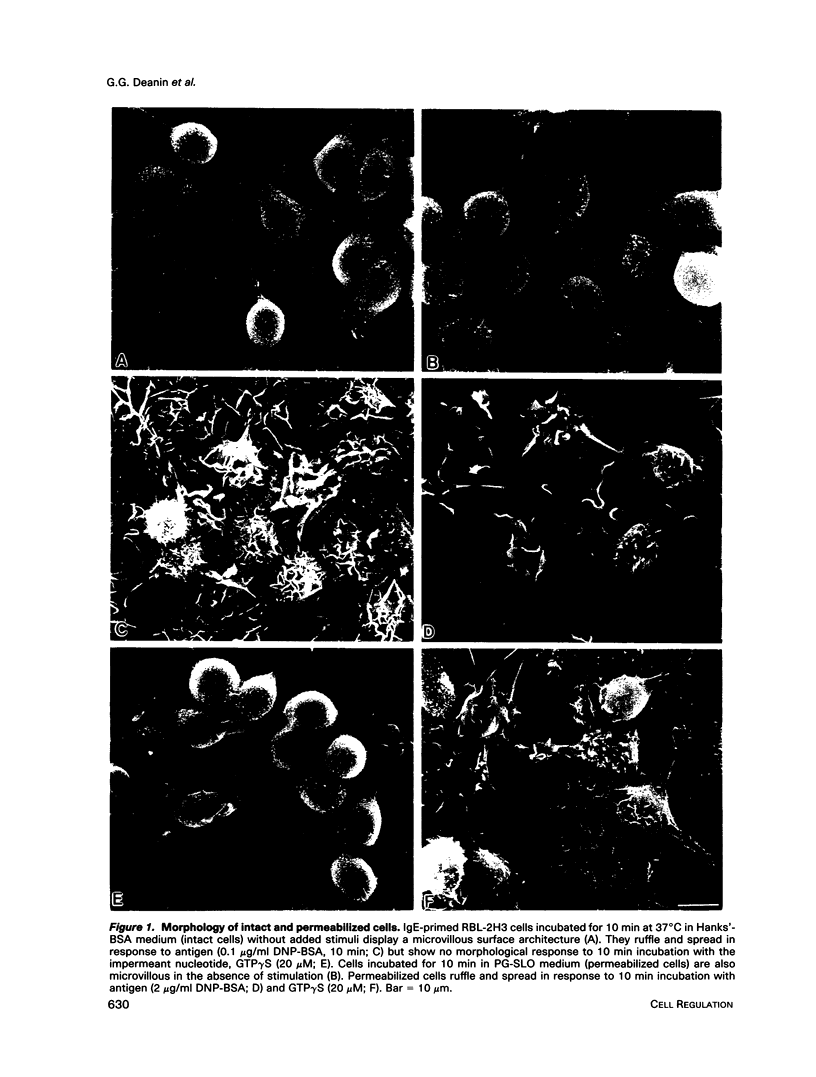
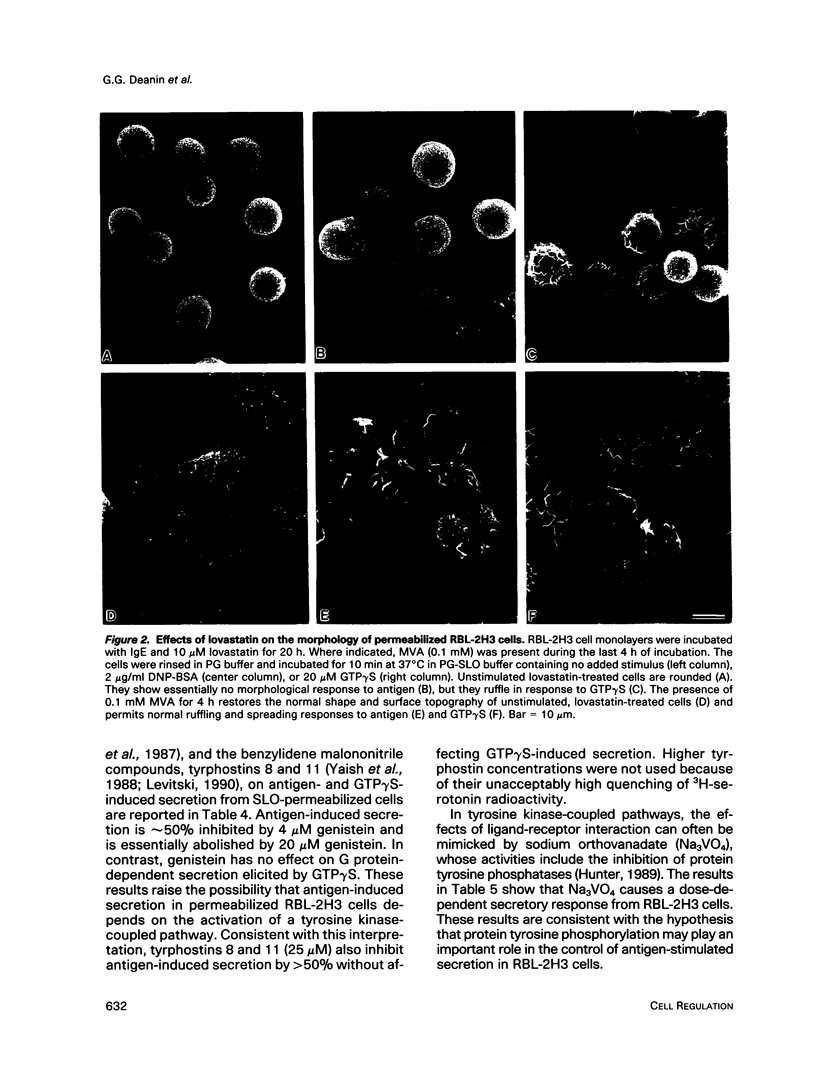
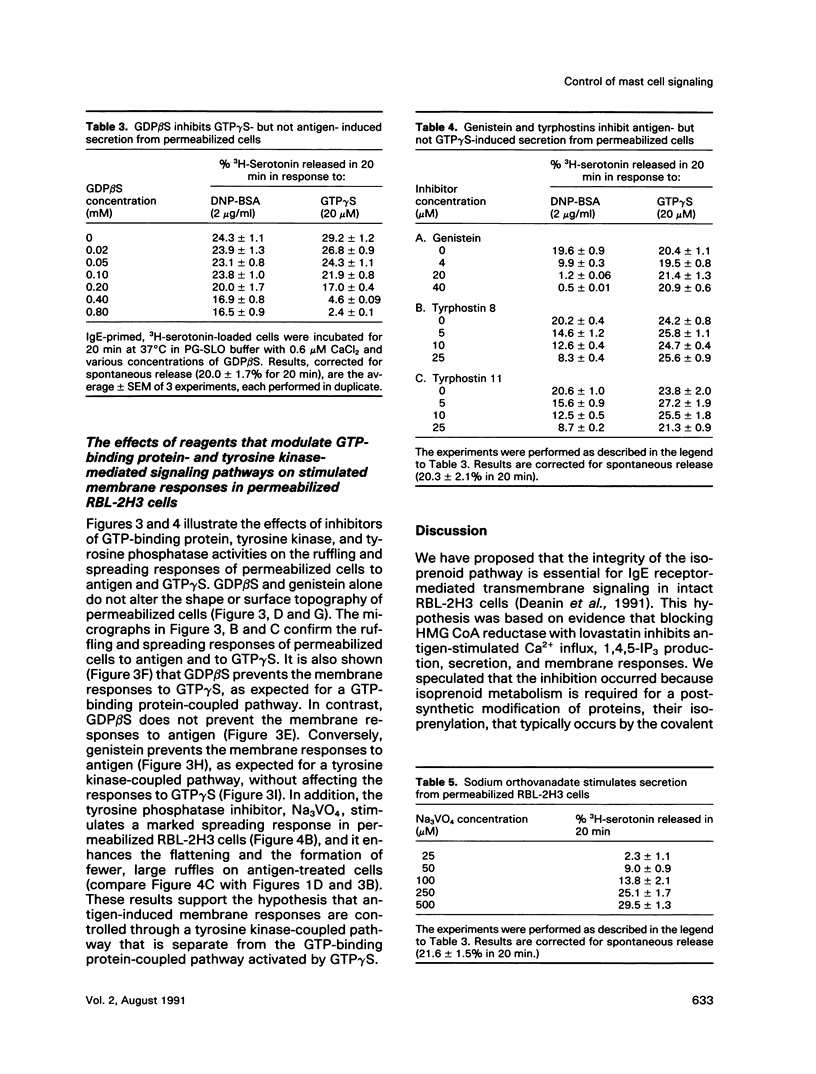
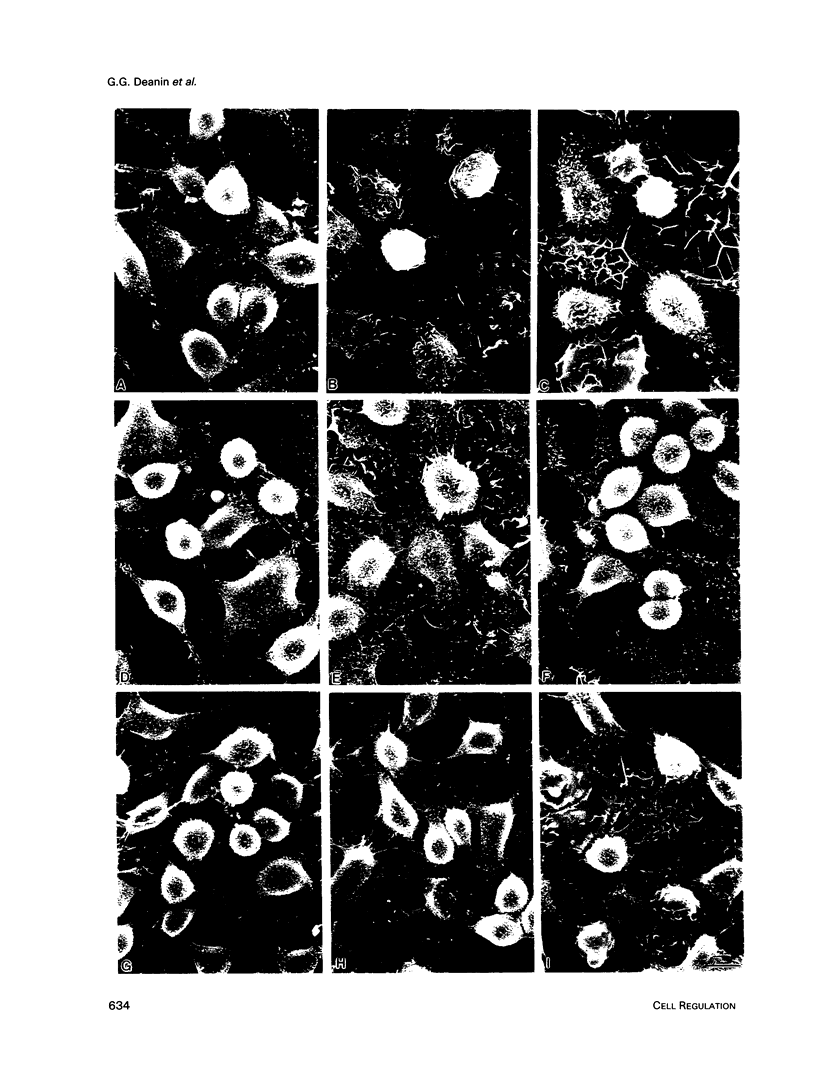
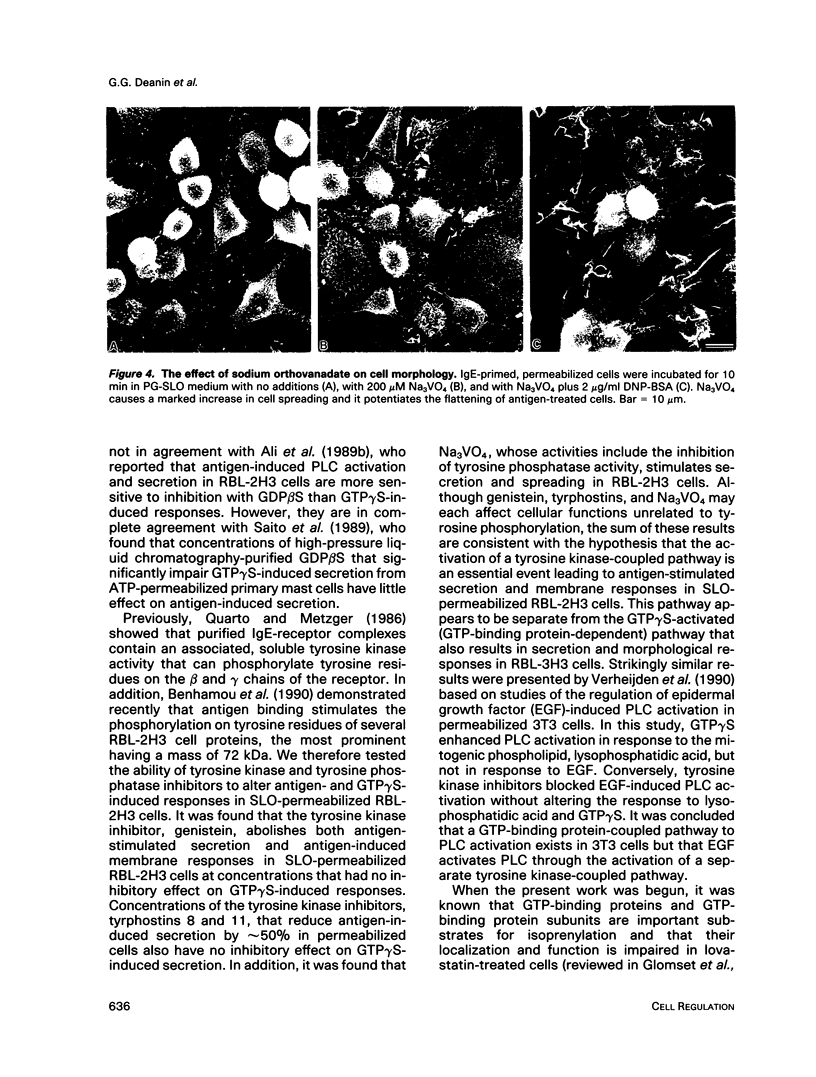
Previously, we reported that the isoprenoid pathway inhibitor, lovastatin, blocks the activation by IgE receptor cross-linking of 45Ca2+ influx, 1,4,5-inositol trisphosphate production, secretion, and membrane changes (ruffling, spreading) in intact RBL-2H3 rat basophilic leukemia cells. These results indicated that an isoprenoid pathway intermediate, very likely an isoprenylated protein, is importantly involved in the control of IgE receptor-mediated signal transduction. Here, we show that 20 h of pretreatment with lovastatin also inhibits antigen-induced secretion and membrane responses in streptolysin O-(SLO)-permeabilized cells. However, lovastatin does not inhibit secretion stimulated by the nonhydrolyzable GTP analog, GTP gamma S. Furthermore, the membrane responses to GTP gamma S persist, although in an attenuated form, in lovastatin-treated permeabilized cells. The relative insensitivity of GTP gamma S-induced responses to lovastatin was one of several indications that antigen and GTP gamma S may activate separate pathways leading to transmembrane responses in permeabilized cells. Further experiments showed that the beta-thio derivative of GDP, GDPBAS, inhibits the secretory and membrane responses to GTP gamma S, as expected for a GTP-binding protein-dependent signaling pathway, while having little effect on antigen-induced responses. Conversely, genistein blocks the secretory and membrane responses to antigen, as expected for a tyrosine kinase-dependent pathway, without altering the GTP gamma S-induced responses. From these results, and from additional data from cells treated with tyrphostins and sodium orthovanadate, we propose that IgE receptor-mediated secretion from permeabilized RBL-2H3 cells occurs by a tyrosine kinase-dependent pathway that requires isoprenoid pathway activity for function.We propose further that RBL-2H3 cells contain a separate GTP-binding protein-mediated signaling pathway whose direct activation by GTP gamma S is either independent of isoprenoid pathway activity or depends on the activity of an isoprenylated protein that is not significantly depleted after 20 h of lovastatin treatment.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Mach W., Föhr K. J., Gratzl M. Poration by alpha-toxin and streptolysin O: an approach to analyze intracellular processes. Methods Cell Biol. 1989;31:63–90. doi: 10.1016/s0091-679x(08)61602-7. [DOI] [PubMed] [Google Scholar]
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Ali H., Collado-Escobar D. M., Beaven M. A. The rise in concentration of free Ca2+ and of pH provides sequential, synergistic signals for secretion in antigen-stimulated rat basophilic leukemia (RBL-2H3) cells. J Immunol. 1989 Oct 15;143(8):2626–2633. [PubMed] [Google Scholar]
- Ali H., Cunha-Melo J. R., Beaven M. A. Receptor-mediated release of inositol 1,4,5-trisphosphate and inositol 1,4-bisphosphate in rat basophilic leukemia RBL-2H3 cells permeabilized with streptolysin O. Biochim Biophys Acta. 1989 Jan 17;1010(1):88–99. doi: 10.1016/0167-4889(89)90188-2. [DOI] [PubMed] [Google Scholar]
- Anderson D., Koch C. A., Grey L., Ellis C., Moran M. F., Pawson T. Binding of SH2 domains of phospholipase C gamma 1, GAP, and Src to activated growth factor receptors. Science. 1990 Nov 16;250(4983):979–982. doi: 10.1126/science.2173144. [DOI] [PubMed] [Google Scholar]
- Becker E. L. The relationship of the chemotactic behavior of the complement-derived factors, C3a, C5a, and C567, and a bacterial chemotactic factor to their ability to activate the proesterase 1 of rabbit polymorphonuclear leukocytes. J Exp Med. 1972 Feb 1;135(2):376–387. doi: 10.1084/jem.135.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou M., Gutkind J. S., Robbins K. C., Siraganian R. P. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985 Jan;47(1):52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank U., Ra C., Miller L., White K., Metzger H., Kinet J. P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989 Jan 12;337(6203):187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Deanin G. G., Cutts J. L., Pfeiffer J. R., Oliver J. M. Role of isoprenoid metabolism in IgE receptor-mediated signal transduction. J Immunol. 1991 May 15;146(10):3528–3535. [PubMed] [Google Scholar]
- Endo A., Kuroda M., Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976 Dec 31;72(2):323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- Glomset J. A., Gelb M. H., Farnsworth C. C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990 Apr;15(4):139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Law D. A., DeFranco A. L. Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature. 1990 Jun 28;345(6278):810–813. doi: 10.1038/345810a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hart M. J., Polakis P. G., Evans T., Cerione R. A. The identification and characterization of an epidermal growth factor-stimulated phosphorylation of a specific low molecular weight GTP-binding protein in a reconstituted phospholipid vesicle system. J Biol Chem. 1990 Apr 15;265(11):5990–6001. [PubMed] [Google Scholar]
- Holowka D., Metzger H. Further characterization of the beta-component of the receptor for immunoglobulin E. Mol Immunol. 1982 Feb;19(2):219–227. doi: 10.1016/0161-5890(82)90334-0. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein-tyrosine phosphatases: the other side of the coin. Cell. 1989 Sep 22;58(6):1013–1016. doi: 10.1016/0092-8674(89)90496-0. [DOI] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Samelson L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990 Mar 1;144(5):1591–1599. [PubMed] [Google Scholar]
- Kaneko I., Hazama-Shimada Y., Endo A. Inhibitory effects on lipid metabolism in cultured cells of ML-236B, a potent inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme-A reductase. Eur J Biochem. 1978 Jun 15;87(2):313–321. doi: 10.1111/j.1432-1033.1978.tb12380.x. [DOI] [PubMed] [Google Scholar]
- Kita T., Brown M. S., Goldstein J. L. Feedback regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in livers of mice treated with mevinolin, a competitive inhibitor of the reductase. J Clin Invest. 1980 Nov;66(5):1094–1100. doi: 10.1172/JCI109938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P. J., Ledbetter J. A., McConnell F. M., Draves K., Deans J., Schieven G. L., Clark E. A. The role of tyrosine phosphorylation in signal transduction through surface Ig in human B cells. Inhibition of tyrosine phosphorylation prevents intracellular calcium release. J Immunol. 1991 Jan 15;146(2):715–722. [PubMed] [Google Scholar]
- Levitzki A. Tyrphostins--potential antiproliferative agents and novel molecular tools. Biochem Pharmacol. 1990 Sep 1;40(5):913–918. doi: 10.1016/0006-2952(90)90474-y. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- Ludowyke R. I., Peleg I., Beaven M. A., Adelstein R. S. Antigen-induced secretion of histamine and the phosphorylation of myosin by protein kinase C in rat basophilic leukemia cells. J Biol Chem. 1989 Jul 25;264(21):12492–12501. [PubMed] [Google Scholar]
- Maeyama K., Hohman R. J., Ali H., Cunha-Melo J. R., Beaven M. A. Assessment of IgE-receptor function through measurement of hydrolysis of membrane inositol phospholipids. New insights on the phenomena of biphasic antigen concentration-response curves and desensitization. J Immunol. 1988 Jun 1;140(11):3919–3927. [PubMed] [Google Scholar]
- Maltese W. A. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J. 1990 Dec;4(15):3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- Maltese W. A., Sheridan K. M. Isoprenoid modification of G25K (Gp), a low molecular mass GTP-binding protein distinct from p21ras. J Biol Chem. 1990 Oct 15;265(29):17883–17890. [PubMed] [Google Scholar]
- McCloskey M. A. Cholera toxin potentiates IgE-coupled inositol phospholipid hydrolysis and mediator secretion by RBL-2H3 cells. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7260–7264. doi: 10.1073/pnas.85.19.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Isakov N., Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990 Mar 30;247(4950):1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- Narasimhan V., Holowka D., Fewtrell C., Baird B. Cholera toxin increases the rate of antigen-stimulated calcium influx in rat basophilic leukemia cells. J Biol Chem. 1988 Dec 25;263(36):19626–19632. [PubMed] [Google Scholar]
- Oliver J. M., Seagrave J., Stump R. F., Pfeiffer J. R., Deanin G. G. Signal transduction and cellular response in RBL-2H3 mast cells. Prog Allergy. 1988;42:185–245. [PubMed] [Google Scholar]
- Pfeiffer J. R., Seagrave J. C., Davis B. H., Deanin G. G., Oliver J. M. Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J Cell Biol. 1985 Dec;101(6):2145–2155. doi: 10.1083/jcb.101.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarto R., Metzger H. The receptor for immunoglobulin E: examination for kinase activity and as a substrate for kinases. Mol Immunol. 1986 Nov;23(11):1215–1223. doi: 10.1016/0161-5890(86)90154-9. [DOI] [PubMed] [Google Scholar]
- Saito H., Ishizaka K., Ishizaka T. Effect of nonhydrolyzable guanosine phosphate on IgE-mediated activation of phospholipase C and histamine release from rodent mast cells. J Immunol. 1989 Jul 1;143(1):250–258. [PubMed] [Google Scholar]
- Samelson L. E., Phillips A. F., Luong E. T., Klausner R. D. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4358–4362. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bolen J. B., Bookman M. A. Alterations in tyrosine protein phosphorylation induced by antibody-mediated cross-linking of the CD4 receptor of T lymphocytes. Mol Cell Biol. 1989 Oct;9(10):4441–4446. doi: 10.1128/mcb.9.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijden G. F., Verlaan I., Schlessinger J., Moolenaar W. H. Epidermal growth factor-induced phosphoinositide hydrolysis in permeabilized 3T3 cells: lack of guanosine triphosphate dependence and inhibition by tyrosine-containing peptides. Cell Regul. 1990 Aug;1(9):615–620. doi: 10.1091/mbc.1.9.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. S., Deanin G. G., Oliver J. M. Regulation of IgE receptor-mediated secretion from RBL-2H3 mast cells by GTP binding-proteins and calcium. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1064–1069. doi: 10.1016/0006-291x(91)91528-k. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Deanin G. G., Standefer J. C., Vanderjagt D., Oliver J. M. Depletion of guanine nucleotides with mycophenolic acid suppresses IgE receptor-mediated degranulation in rat basophilic leukemia cells. J Immunol. 1989 Jul 1;143(1):259–265. [PubMed] [Google Scholar]
- Yaish P., Gazit A., Gilon C., Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988 Nov 11;242(4880):933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]