Abstract
To estimate the effects of ginger on apoptosis, proliferation, and differentiation in the normal-appearing colonic mucosa, we randomized 20 people at increased risk for colorectal cancer to 2.0 g of ginger or placebo daily for 28 days in a pilot trial. Overall expression and distributions of Bax, Bcl-2, p21, hTERT and MIB-1 (Ki-67) in colorectal crypts in rectal mucosa biopsies were measured using automated immunohistochemistry and quantitative image analysis. Relative to placebo, Bax expression in the ginger group decreased 15.6% (p = 0.78) in the whole crypts, 6.6% (p = 0.95) in the upper 40% (differentiation zone) of crypts, and 21.7% (p = 0.67) in the lower 60% (proliferative zone) of crypts; however, there was a 19% increase (p = 0.14) in Bax expression in the upper 40% relative to the whole crypt. While p21 and Bcl-2 expression remained relatively unchanged, hTERT expression in the whole crypts decreased by 41.2% (p = 0.05); the estimated treatment effect on hTERT expression was larger in the upper 40% of crypts (−47.9%; p = 0.04). In the ginger group, MIB-1 expression decreased in the whole crypts, upper 40% of crypts, and lower 60% of crypts by 16.9% (p = 0.39), 46.8% (p = 0.39), and 15.3% (p = 0.41), respectively. These pilot study results suggest that ginger may reduce proliferation in the normal-appearing colorectal epithelium and increase apoptosis and differentiation relative to proliferation—especially in the differentiation zone of the crypts, and support a larger study to further investigate these results.
Keywords: Differentiation (p21waf1/cip1), Apoptosis (Bax and Bcl-2), Proliferation (MIB-1/Ki-67 and hTERT), Colorectal Cancer, Ginger
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States (1). Reduced differentiation and deregulated cell cycle control provide the underlying platform for colon tumorigenesis; therefore, markers of cell cycle function, proliferation, and differentiation in the colorectal epithelium may serve as intermediate phenotypic biomarkers of risk for colorectal cancer and may be modifiable by dietary components.
Ginger root (Zingiber officinale) and its main phenolic constituents (gingerols, paradols, zingerone, and shogaols) have anti-oxidant (2, 3), anti-inflammatory (4, 5), and anti-carcinogenic properties (6, 7). Ginger root can also interfere with several cell-signaling pathways that are important in the early development of cancer (6). For example, ginger potentiated apoptotic indexes in vitro in different human cell lines through various signaling pathways (8–11). Also, several lines of evidence suggest that [6]-gingerol is effective in suppressing the transformation, hyperproliferation, and inflammatory processes that initiate and promote tumorigenesis (12–15). Despite its anticancer activity in vitro, the exact molecular mechanisms by which ginger may exert chemopreventive effects are not fully understood. While one randomized clinical trial found that ginger supplementation reduced PGE2 levels in healthy participants (16), there are no reported human in vivo investigations of the effects of ginger on apoptosis, proliferation, and differentiation markers in the normal colonic mucosa of people at increased risk for developing CRC.
The purpose of this study was to estimate the effects of 2.0 g of daily ginger extract supplementation on a marker of cell differentiation (p21waf1/cip1), two markers of 1 apoptosis (Bax and Bcl-2, which, respectively, promote and inhibit apoptosis), and two markers of cell proliferation (the MIB-1 epitope of Ki-67 and hTERT) in the normal-appearing colonic mucosa of people at increased risk for developing colorectal cancer in a pilot, randomized, double-blinded, placebo-controlled, clinical trial (Supplementary Figure S1). We hypothesized that ginger supplementation would increase differentiation (i.e., increase p21), decrease proliferation (i.e., decrease hTERT and MIB-1), and increase apoptosis (i.e., increase Bax and decrease Bcl-2).
METHODS
Participants
Participant recruitment and flow is depicted in Supplementary Figure S2. Participants were recruited from the surrounding community of Ann Arbor, MI through fliers posted around the University of Michigan, advertisements in local newspapers, and word-of-mouth between June 2009 and January 2010. Eligible participants were healthy male and female volunteers 18 years and older who were considered to be at increased risk for colorectal cancer. Increased CRC risk was defined as an individual who either had a first degree relative with colorectal cancer under the age of 60 at diagnosis or who had a previous adenomatous polyp or early (Dukes A, B or C) colon cancer resected. With the exception of curative surgery for small lesions, such as endoscopically removed cancers, eligible subjects were at least one year post-treatment for cancer. Exclusion criteria included lactose intolerance, a current diagnosis of peptic ulcer disease, gastrointestinal bleeding from gastric or duodenal ulcers, gastrin secreting tumors, a known allergy to ginger, supplement use/therapies that could obscure the ability to detect anti-inflammatory effects, and pregnant or lactating women. Individuals with hereditary non-polyposis colon cancer or familial adenomatous polyposis (HNPCC/FAP), inflammatory bowel disease, or coagulopathy/hereditary hemorrhagic disorders were also excluded. Over the 6-month recruitment period, 42 people were assessed for eligibility, of whom 21 (50%) met all eligibility criteria and were randomized (11 to placebo and 10 to 2.0 g ginger extract).
Participants were asked to avoid all foods containing ginger within 14 days prior to drug administration. This was confirmed by having participants complete a food checklist to verify that they were not consuming any ginger-rich foods. All participants were reimbursed for their time.
The study was approved by the University of Michigan Institutional Review Board. All study procedures were administered at the University of Michigan Clinical Research Unit (MCRU) after the participant gave written, informed consent.
Ginger Intervention
The ginger product used in this study was manufactured by Pure Encapsulations (Sudbury, MA USA). Pure Encapsulation’s ginger (Zingiber officinale radix) powder was processed using Good Manufacturing Procedures (GMP). Details on ginger and placebo content and quality control, randomization, toxicity assessment, and adherence were published previously (16). Briefly, the Investigational Drug Service of the University of Michigan (UMIDS) placed placebo and ginger extract powder into opaque red capsules. Participants were asked to ingest four 250 mg capsules twice daily for a total of eight capsules. Capsules were standardized to 5% gingerols and the placebo consisted of lactose powder. The dose was chosen based on the highest tolerated in healthy volunteers in a phase 1 study. The study biostatistician provided the UMIDS with randomization codes. Only the UMIDS staff was aware of participant treatment assignment. Toxicity was assessed weekly by telephone, email, or during clinic visit. Compliance was assessed by pill count. Participants were classified as adherent if the weekly monitoring suggested that 70% or more of the doses were taken as prescribed.
Flexible sigmoidoscopy and tissue collection
Participants underwent two flexible sigmoidoscopies, one at baseline and the second within 24 hours of the last ginger/placebo dose on day 28. Participants did not have to be fasting for their visits and did not take a bowel cleansing preparation or enema. Participants were placed in a left lateral decubitus position and a flexible sigmoidoscope was passed at least 15 cm above the anal sphincter. Four tissue samples were taken by opening and pressing the biopsy forceps perpendicular to the mucosal surface with mild pressure. Each biopsy specimen was taken 2 cm or more from other biopsy sites in the distal sigmoid colonic mucosa.
Tissue handling and disposition
Tissues were handled according to previously reported methods (17, 18). The biopsies were immediately placed in phosphate buffered saline, then reoriented under a dissecting microscope and transferred to 10% normal buffered formalin within 15 minutes, and then transferred to 70% ethanol 24 hours after initial placement in formalin. The paraffin blocks, each containing four biopsies, were then cut into 3-μm-thick sections, with levels 40 μm apart. Five slides with three biopsy levels each were processed and stained within 7 days of being cut, yielding a total of 15 biopsy levels per participant.
Immunohistochemistry protocol
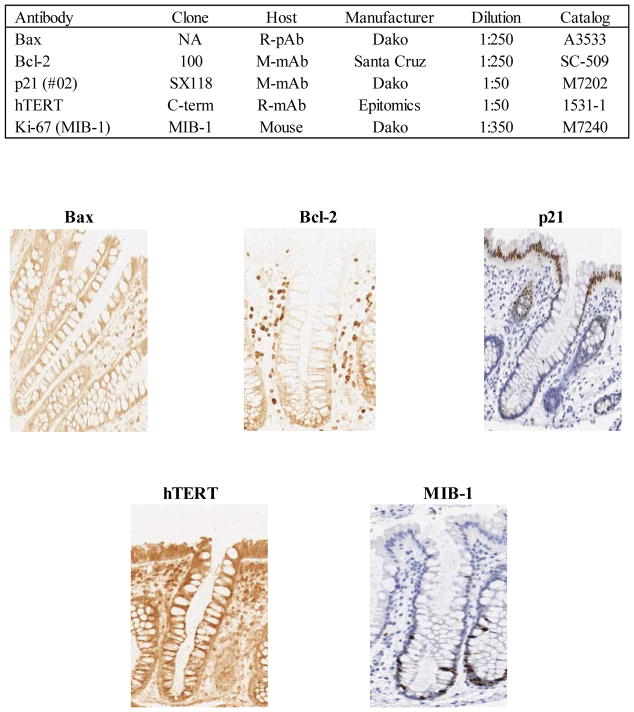
Slides were immunohistochemically processed using a labeled streptavidin-biotin method for Bax, Bcl-2, p21, hTERT, and MIB-1 (epitope of Ki-67) as summarized in Figure 1. Slides were not counterstained. After staining, the slides were coverslipped with a Leica CV5000 Coverslipper (Leica Microsystems, Inc., IL). Baseline and follow-up visit biopsy slides were included in the same staining batch, and each staining batch included both positive and negative control slides.
Figure 1.
Summary of biomarker immunohistochemical protocols and images (at 200x magnification) of colon crypts immunohistochemically processed for: Bax, apoptotic marker; Bcl-2, anti-apoptotic marker; p21, differentiation marker; MIB-1, proliferation marker (short term); hTERT, proliferation marker (long term)
A quantitative image analysis method (“scoring”) was used to evaluate the expression of the biomarkers in the colon crypts, as described previously (17, 18). Briefly, the basic scoring method (Figure 2) was an image analysis scoring procedure for antigens that were labeled with a wide range of intensities in gradient distributions along the crypt axis. A “scorable” crypt was defined as an intact hemicrypt that extended from the muscularis mucosa to the colon lumen. “Hemicrypts” (one half of a crypt bisected from crypt base to colon lumen) were manually traced by a trained technician and divided by the software into a number of segments corresponding to the average width of colonocytes. Overall hemicrypt- and segment-specific biomarker-labeling optical densities were then calculated by the software and stored into a Microsoft Access database. The goal was to score at least 16 hemicrypts per each biopsy visit, for a total of 32 hemicrypts.
Figure 2.
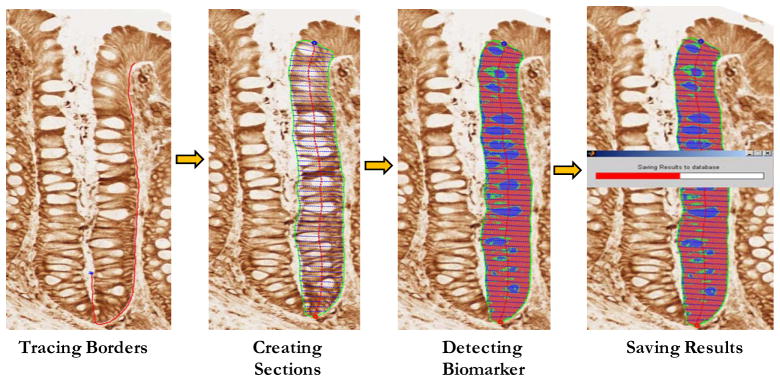
Quantitative image analysis using Aperio Scanscope and CellularEyes software
Blinded subsets of previously analyzed slides were re-analyzed by the technician during the study to assess intra-reader reliability, which was > 0.90 for all biomarkers.
Statistical methods and sample size
Balance between treatment groups on baseline characteristics was tested using independent sample t-tests for continuous variables and Pearson’s chi square and Fisher’s exact tests, as appropriate, for categorical variables. Slide “scoring” reliability was analyzed using intra-class correlation coefficients.
The mean optical density of Bax, Bcl-2, MIB-1, hTERT, and p21 labeling in normal colon crypts was calculated for each patient at baseline and 28-day follow-up by summing all the densities from all analyzed crypts from the biopsy specimens and dividing by the number of crypts analyzed. The crypt differentiation compartment was defined a priori as the upper 40% of the crypts (differentiation zone), and the crypt proliferation compartment was defined as the bottom 60% of the crypts (proliferation zone). Measures of the within-crypt distributions of the biomarkers were calculated for each patient by taking the mean of the biomarker densities in the upper 40% of crypts and dividing it by the biomarker densities in the whole crypts (Φh).
Primary analyses were based on assigned treatment at the time of randomization, regardless of adherence status (intent-to-treat analysis). Treatment effects were evaluated by assessing the differences in biomarker concentration from baseline to the 28-day follow-up visit between the ginger and placebo group by a repeated-measures linear mixed effects model. The model included the intercept, indicators for treatment group and visit (baseline and follow-up), and a treatment by visit interaction term. Since optical density is measured in arbitrary units, to provide perspective on the magnitude of the treatment effects we also calculated relative effects, defined as: [treatment group follow-up mean/treatment group baseline mean]/[placebo group follow-up mean/placebo group baseline mean]. The relative effect provides a conservative estimate of the proportional change in the treatment group relative to that in the placebo group, and its interpretation is somewhat analogous to that of an odds ratio (e.g., a relative effect of 2.0 would mean that the proportional change in the treatment group was two times that in the placebo group). Since the treatment groups were balanced on risk factors at baseline, no adjustment was made for other covariates.
To assess the effects of ginger supplementation on cellular functioning, two cell cycle summary scores were created; summary scores included Bax, Bcl-2, p21, and one marker of proliferation (either hTERT or MIB-1). Scores for each biomarker were based on the percent change in biomarker expression over the treatment period ([participant biomarker at follow-up – participant biomarker at baseline]/participant biomarker at baseline). Percent changes were divided into seven equal interval categories, which were determined a priori, (≤ −75%, −75% to −45.1%, −45% to −15.1%, −15% to 15%, 15.1% to 45%, 45.1% to 75% and ≥75%) and corresponded to a score ranging from −3 to 3. The combined cell cycle score was created for each participant by summing the interval scores of each individual biomarker (Bcl-2, hTERT and MIB-1 were included with a negative sign as increases in these biomarkers are generally thought to be associated with greater risk for tumorigenesis). As such, positive scores reflect higher levels of apoptosis and/or differentiation relative to proliferation while negative scores reflect the opposite balance.
A post-hoc power analysis to determine the sample size needed in a full scale study to detect at p ≤ 0.05 the treatment effects estimated in this pilot study was conducted assuming using a 2-sample t test to compare mean changes in biomarker labeling optical densities and statistical power of 0.8.
Statistical analyses were performed using SAS 9.2 statistical software (SAS Institute Inc., Cary, NC, USA). A P value ≤ 0.05 (two-sided) was considered statistically significant. In addition to analyzing cell cycle scores and overall mean changes in biomarker expression, ratios comparing changes within and between each of the biomarkers were tested. Given that a total of 42 tests were conducted, at least two would be expected to be significant by chance alone at the 0.05 level of significance.
RESULTS
Characteristics of study participants
Treatment groups did not differ significantly on characteristics measured at baseline (Table 1). The mean age of participants was 51 years, 35% were male, 75% were white, and 50% had a first degree relative with colorectal cancer under the age 60 at the time of diagnosis. One participant was removed from the study after randomization as they were found to not to be at increased risk for CRC. Nine (90%) participants in the ginger group reported an adverse event over the course of the study compared to four participants (40%) in the placebo group (p = 0.06; Supplementary Table S1). No toxicities greater than NCI Common Toxicity Criteria (v. 4.0) Grade I were reported. GI symptoms, which included bloating, gas, nausea, and heartburn, were the most commonly reported adverse event, occurring in 70% of individuals in the ginger group and 30% of individuals in the placebo group (p = 0.18).
Table 1.
Selected baseline characteristics of participants
| Characteristics | Ginger n=10 | Placebo n=10 | P† |
|---|---|---|---|
| Sex, No. (%) | |||
| Men | 4 (40) | 3 (30) | 0.64 |
| Women | 6 (60) | 7 (70) | |
| Race, No. (%) | |||
| White | 8 (80) | 7 (70) | 0.38 |
| Other* | 2 (20) | 3 (30) | |
| Age, mean (SD), years | 51.1 (11.7) | 50.8 (14.6) | 0.95 |
| Reason for being high risk for CRC‡, No. (%) | |||
| First degree relative | 4 (40) | 6 (60) | 0.47 |
| Previous adenoma | 6 (60) | 6 (60) | |
| Previous CRC | 1 (10) | 0 (0) | |
African American, Asian, Pacific Islander, American Indian/Alaskan Native
Independent sample t-test or Pearson’s Chi-square, as appropriate
CRC=Colorectal cancer; 1st degree relative must have had a diagnosis of colorectal cancer before the age of 60; Prior colorectal cancer must have been fully excised and either Duke’s A or B; Values add up to >100% due to participants having several reasons for being at high risk for colorectal cancer
Over the four-week trial period, participants in the ginger and placebo groups took 98% and 95%, respectively, of the pills administered at trial onset.
Effects of ginger on the separate and relative expressions of Bax, Bcl-2, p21, hTERT and MIB-1 in normal colorectal crypts
At baseline, there were no statistically significant differences between treatment groups in Bax, Bcl-2, p21, hTERT, or MIB-1 levels.
Apoptosis Markers
There were no statistically significant changes in the whole crypt expression of Bax or Bcl-2 after four weeks of treatment, although there was a suggestion that the mean biomarker levels decreased slightly (Table 2). Although Bax expression in the ginger group relative to the placebo group decreased by 15.6% (p = 0.78) in the whole crypts, it increased by 19% (p = 0.14) in the Φh of crypts. There were no statistically significant treatment effects on the expression of Bcl-2 in the whole crypts or in the Φh of crypts. After four weeks of treatment, the Bax/Bcl-2 ratio decreased 26.6% (p = 0.62) in the whole crypts and increased 16.7% (p = 0.58) in the Φh of crypts, relative to the placebo group.
Table 2.
Changes in biomarkers of apoptosis, proliferation, and differentiation in colorectal crypts
| Biomarker Expression in colorectal crypts a | n | Baseline | 4-week Follow-up | Absolute Treatment Effect b | Relative Treatment Effect c | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | P d | |||
| Bax | |||||||||
| Placebo | 10 | ||||||||
| Whole crypt | 658 | 333 | 520 | 391 | N/A | N/A | N/A | N/A | |
| Differentiation zone | 240 | 129 | 187 | 158 | N/A | N/A | N/A | N/A | |
| Proliferation zone | 391 | 211 | 310 | 224 | N/A | N/A | N/A | N/A | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 0.37 | 0.07 | 0.35 | 0.05 | N/A | N/A | N/A | N/A | |
| Ginger | 9 | ||||||||
| Whole crypt | 585 | 232 | 390 | 164 | −56 | 194 | 0.78 | 0.84 | |
| Differentiation zone | 213 | 109 | 155 | 62 | −5 | 79 | 0.95 | 0.93 | |
| Proliferation zone | 348 | 138 | 216 | 106 | −50 | 117 | 0.67 | 0.78 | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 0.36 | 0.08 | 0.40 | 0.06 | 0.06 | 0.04 | 0.14 | 1.19 | |
| Bcl-2 | |||||||||
| Placebo | 10 | ||||||||
| Whole crypt | 539 | 278 | 501 | 255 | N/A | N/A | N/A | N/A | |
| Differentiation zone | 67 | 80 | 59 | 53 | N/A | N/A | N/A | N/A | |
| Proliferation zone | 498 | 219 | 439 | 212 | N/A | N/A | N/A | N/A | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 0.11 | 0.06 | 0.11 | 0.05 | N/A | N/A | N/A | N/A | |
| Ginger | 10 | ||||||||
| Whole crypt | 648 | 236 | 570 | 185 | −40 | 152 | 0.80 | 0.95 | |
| Differentiation zone | 54 | 27 | 48 | 21 | 2 | 32 | 0.95 | 1.01 | |
| Proliferation zone | 592 | 223 | 520 | 173 | −43 | 131 | 0.75 | 1.00 | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 0.09 | 0.03 | 0.09 | 0.03 | 0.50 | 0.03 | 0.87 | 1.03 | |
| p21 | |||||||||
| Placebo | 10 | ||||||||
| Whole crypt | 355 | 141 | 388 | 148 | N/A | N/A | N/A | N/A | |
| Differentiation zone | 318 | 117 | 360 | 146 | N/A | N/A | N/A | N/A | |
| Proliferation zone | 37 | 50 | 28 | 22 | N/A | N/A | N/A | N/A | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 0.91 | 0.10 | 0.93 | 0.06 | N/A | N/A | N/A | N/A | |
| Ginger | 10 | ||||||||
| Whole crypt | 311 | 124 | 278 | 103 | −66 | 82 | 0.43 | 0.82 | |
| Differentiation zone | 286 | 121 | 269 | 100 | −59 | 77 | 0.45 | 0.83 | |
| Proliferation zone | 25 | 30 | 9 | 6 | −7 | 20 | 0.73 | 0.47 | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 0.90 | 0.11 | 0.97 | 0.02 | 0.05 | 0.05 | 0.33 | 1.06 | |
| hTERT | |||||||||
| Placebo | 10 | ||||||||
| Whole crypt | 2,054 | 769 | 2,715 | 1,114 | N/A | N/A | N/A | N/A | |
| Differentiation zone | 661 | 286 | 896 | 454 | N/A | N/A | N/A | N/A | |
| Proliferation zone | 1,347 | 467 | 1,760 | 630 | N/A | N/A | N/A | N/A | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 0.32 | 0.04 | 0.32 | 0.04 | N/A | N/A | N/A | N/A | |
| Ginger | 10 | ||||||||
| Whole crypt | 2,651 | 1,084 | 2,059 | 683 | −1,253 | 589 | 0.05 | 0.59 | |
| Differentiation zone | 911 | 405 | 643 | 208 | −502 | 223 | 0.04 | 0.52 | |
| Proliferation zone | 1,668 | 667 | 1,369 | 478 | −713 | 359 | 0.06 | 0.63 | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 0.34 | 0.04 | 0.32 | 0.06 | −0.02 | 0.03 | 0.57 | 0.95 | |
| MIB-1 | |||||||||
| Placebo | 10 | ||||||||
| Whole crypt | 1,388 | 452 | 1,571 | 598 | N/A | N/A | N/A | N/A | |
| Differentiation zone | 45 | 51 | 72 | 92 | N/A | N/A | N/A | N/A | |
| Proliferation zone | 1,343 | 420 | 1,498 | 514 | N/A | N/A | N/A | N/A | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 0.03 | 0.03 | 0.04 | 0.03 | N/A | N/A | N/A | N/A | |
| Ginger | 10 | ||||||||
| Whole crypt | 1,432 | 536 | 1,346 | 284 | −268 | 305 | 0.39 | 0.83 | |
| Differentiation zone | 68 | 68 | 58 | 43 | −37 | 42 | 0.39 | 0.53 | |
| Proliferation zone | 1,363 | 496 | 1,287 | 263 | −231 | 275 | 0.41 | 0.85 | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 0.04 | 0.04 | 0.04 | 0.03 | −0.01 | 0.02 | 0.60 | 0.65 | |
| Bax/Bcl-2 Ratio | |||||||||
| Placebo | 10 | ||||||||
| Whole crypt | 1.40 | 0.74 | 1.38 | 1.19 | N/A | N/A | N/A | N/A | |
| Differentiation zone | 5.32 | 2.82 | 4.88 | 3.91 | N/A | N/A | N/A | N/A | |
| Proliferation zone | 0.96 | 0.60 | 0.97 | 0.91 | N/A | N/A | N/A | N/A | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 4.16 | 2.01 | 3.93 | 1.74 | N/A | N/A | N/A | N/A | |
| Ginger | 9 | ||||||||
| Whole crypt | 0.99 | 0.47 | 0.72 | 0.36 | −0.26 | 0.51 | 0.62 | 0.73 | |
| Differentiation zone | 4.82 | 3.12 | 3.47 | 1.56 | −0.92 | 1.95 | 0.64 | 0.78 | |
| Proliferation zone | 0.64 | 0.27 | 0.44 | 0.25 | −0.21 | 0.38 | 0.60 | 0.68 | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 4.64 | 1.84 | 5.12 | 2.00 | 0.71 | 1.23 | 0.58 | 1.17 | |
| Bax/hTERT Ratio | |||||||||
| Placebo | 10 | ||||||||
| Whole crypt | 0.37 | 0.25 | 0.22 | 0.19 | N/A | N/A | N/A | N/A | |
| Differentiation zone | 0.43 | 0.29 | 0.25 | 0.25 | N/A | N/A | N/A | N/A | |
| Proliferation zone | 0.33 | 0.23 | 0.20 | 0.17 | N/A | N/A | N/A | N/A | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 1.16 | 0.16 | 1.11 | 0.21 | N/A | N/A | N/A | N/A | |
| Ginger | 9 | ||||||||
| Whole crypt | 0.23 | 0.08 | 0.25 | 0.25 | 0.17 | 0.13 | 0.22 | 1.82 | |
| Differentiation zone | 0.25 | 0.11 | 0.29 | 0.24 | 0.22 | 0.15 | 0.17 | 2.00 | |
| Proliferation zone | 0.22 | 0.07 | 0.22 | 0.25 | 0.14 | 0.13 | 0.27 | 1.73 | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 1.04 | 0.15 | 1.25 | 0.23 | 0.26 | 0.12 | 0.05 | 1.26 | |
| Bax/MIB-1 Ratio | |||||||||
| Placebo | 10 | ||||||||
| Whole crypt | 0.48 | 0.23 | 0.32 | 0.20 | N/A | N/A | N/A | N/A | |
| Differentiation zone | 81.19 | 156.40 | 15.44 | 14.50 | N/A | N/A | N/A | N/A | |
| Proliferation zone | 0.49 | 0.24 | 0.33 | 0.22 | N/A | N/A | N/A | N/A | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 71.55 | 143.30 | 20.03 | 19.60 | N/A | N/A | N/A | N/A | |
| Ginger | 9 | ||||||||
| Whole crypt | 0.46 | 0.24 | 0.29 | 0.11 | −0.01 | 0.13 | 0.96 | 0.96 | |
| Differentiation zone | 42.25 | 66.75 | 18.26 | 29.01 | 41.76 | 57.31 | 0.48 | 2.27 | |
| Proliferation zone | 0.49 | 0.26 | 0.31 | 0.12 | −0.02 | 0.14 | 0.89 | 0.94 | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 24.60 | 31.16 | 18.43 | 19.24 | 45.35 | 49.71 | 0.37 | 2.68 | |
| p21/hTERT Ratio | |||||||||
| Placebo | 10 | ||||||||
| Whole crypt | 0.18 | 0.07 | 0.16 | 0.07 | N/A | N/A | N/A | N/A | |
| Differentiation zone | 0.53 | 0.21 | 0.47 | 0.23 | N/A | N/A | N/A | N/A | |
| Proliferation zone | 0.03 | 0.05 | 0.02 | 0.02 | N/A | N/A | N/A | N/A | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 2.92 | 0.54 | 2.92 | 0.32 | N/A | N/A | N/A | N/A | |
| Ginger | 10 | ||||||||
| Whole crypt | 0.13 | 0.08 | 0.15 | 0.08 | 0.05 | 0.05 | 0.34 | 1.35 | |
| Differentiation zone | 0.37 | 0.26 | 0.45 | 0.18 | 0.14 | 0.14 | 0.32 | 1.39 | |
| Proliferation zone | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.02 | 0.86 | 0.73 | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 2.69 | 0.36 | 3.07 | 0.50 | 0.38 | 0.28 | 0.18 | 1.14 | |
| p21/MIB-1 Ratio | |||||||||
| Placebo | 10 | ||||||||
| Whole crypt | 0.28 | 0.12 | 0.26 | 0.11 | N/A | N/A | N/A | N/A | |
| Differentiation zone | 57.13 | 129.18 | 15.52 | 17.56 | N/A | N/A | N/A | N/A | |
| Proliferation zone | 0.03 | 0.03 | 0.02 | 0.02 | N/A | N/A | N/A | N/A | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 192.44 | 422.80 | 53.55 | 53.07 | N/A | N/A | N/A | N/A | |
| Ginger | 10 | ||||||||
| Whole crypt | 0.25 | 0.16 | 0.21 | 0.21 | −0.03 | 0.08 | 0.69 | 0.87 | |
| Differentiation zone | 26.76 | 51.35 | 12.62 | 13.91 | 27.46 | 44.53 | 0.55 | 1.74 | |
| Proliferation zone | 0.02 | 0.02 | 0.01 | 0.00 | 0.00 | 0.01 | 0.80 | 0.54 | |
| Ratio of Differentiation Zone/Whole Crypt (Φh) | 73.76 | 89.08 | 59.90 | 65.26 | 125.05 | 139.20 | 0.38 | 2.92 | |
Differentiation zone: the upper 40% of the crypts; Proliferation zone: the bottom 60% of the crypts
Absolute Treatment Effect is the absolute change from baseline to follow-up in the ginger group minus the absolute change from baseline to follow-up in the placebo group from mixed model
Relative Treatment Effect is defined as: (ginger group follow-up/ginger group baseline)/(placebo follow-up/placebo baseline)
P values for difference between each the ginger group and placebo group from mixed model
P values for differences between follow-up visit and baseline visit from mixed model
Differentiation Marker
Although p21 expression in the ginger group relative to the placebo group decreased by 18.2% (p = 0.43) in the whole crypts, 16.9% (p = 0.45) in the differentiation zone, and 53.2% (p = 0.73) in the proliferation zone, the p21 labeling index Φh increased by 5.7% (p = 0.33).
Proliferation Markers
The estimated relative treatment effects on MIB-1 expression in the whole crypts, the differentiation zone, and the proliferation zone were decreases of 16.9% (p = 0.39), 46.8% (p = 0.39), and 15.3% (p = 0.41), respectively. Also, MIB-1 expression in the Φh of crypts decreased by 35.5% (p = 0.60) in the ginger group relative to the placebo group.
In the ginger group, hTERT expression in the whole crypts statistically significantly decreased by 41.2% (p = 0.05) relative to the placebo group. The relative change in hTERT expression was slightly more pronounced in the differentiation zone of crypts (−47.9%; p = 0.04).
Differentiation Relative to Proliferation
Following four weeks of treatment, in the ginger group relative to placebo, the p21/hTERT ratio increased 34.6% (p = 0.34) in the whole crypts, 39% (p = 0.32) in the differentiation zone of crypts, and 14.2% (p = 0.18) in the Φh of crypts. The p21/MIB-1 ratio decreased 23.3% (p = 0.69) in the whole crypts, 73.6% (p = 0.55) in the differentiation zone of crypts, and 192% (p = 0.38) in the Φh of crypts.
Apoptosis Relative to Proliferation
In the ginger group relative to the placebo group, the Bax/hTERT ratio increased 82% (p = 0.22) in the whole crypts and 25.6% (p = 0.05) in the Φh of crypts, whereas the Bax/MIB-1 ratio remained relatively unchanged in the whole crypts (−3.9%; p = 0.96), but increased 127% (p = 0.48) in the differentiation zone of crypts and 168% (p = 0.37) in the Φh of crypts.
Effects of ginger on the cell cycle score
The effects of ginger supplementation on the “cell cycle scores” are summarized in Table 3. There was no evidence for a treatment effect on the cell cycle score that included MIB-1 as the proliferation marker. In contrast, the estimated treatment effect on the cell cycle score that included the long-term proliferation marker, hTERT, was a 74% increase in the ginger group relative to the placebo group ([−0.33 vs. −1.30]; p = 0.35).
Table 3.
Changes in cell cycle score
| n | Treatment Effect | ||
|---|---|---|---|
| Mean | SD | ||
| Cell Cycle Score (w/ MIB-1) | |||
| Placebo | 10 | −0.70 | 2.63 |
| Ginger | 9 | −0.78 | 3.31 |
| Cell Cycle Score (w/hTERT) | |||
| Placebo | 10 | −1.30 | 2.87 |
| Ginger | 9 | −0.33 | 1.22 |
P-value is based on an independent samples t-test
DISCUSSION
Our results suggest that ginger supplementation may reduce proliferation in the crypts of the normal-appearing colorectal epithelium and increase apoptosis and differentiation relative to proliferation—especially in the differentiation zone of crypts of individuals at increased risk for CRC. These findings are consistent with previous studies that suggested that the chemopreventive properties of ginger may lie in its ability to regulate cell function and viability (8–11). In vitro and animal studies also suggest that ginger and its constituents may act as chemopreventive agents by reducing COX-2 expression (5, 13, 19), increasing immune function (20, 21), lowering the activity of microbial enzymes (β-glucuronidase and mucinase) (22), and blocking angiogenic signals that supply blood to tumor cells (14, 23).
Our results suggest that expression of proliferation markers may decrease in response to ginger supplementation, a finding that is consistent with our hypothesis as well as results from animal models (19, 20) and in vitro (21–23) studies. Our strongest indication of a treatment effect on proliferation was the estimated effect on hTERT expression. A decrease in hTERT expression is consistent with previous reports, which found that ginger inhibited hTERT and c-Myc expression in human non-small lung cancer cells (24). While the estimated treatment effect on our other marker of proliferation MIB-1 was not as strong, the estimated effect was more pronounced in the upper sections of the colorectal crypts, suggesting that ginger may decrease proliferation in the parts of the colorectal crypts most exposed to bowel lumen carcinogens.
Although we hypothesized that ginger supplementation would increase the apoptosis promotion marker Bax and the differentiation marker p21, our results suggested that ginger reduced Bax and p21 expression. These findings are not consistent with previous in vitro (8–11, 25) and animal studies (26, 27), although similar null results were reported in several rodent models (28, 29) and in vitro studies of HT-29 and Caco-2 colon tumor cell lines (25, 30). A possible explanation is that the biomarkers used may not have been the best measures of cellular differentiation and apoptosis in normal colorectal crypts. However, p21 is a potent inducer of differentiation in intestinal colonocytes, and its expression is known to be down-regulated during the early stages of colon tumorigenesis (31). Abnormalities in p21 expression have been linked to carcinogenesis (32) and p21 loss is observed in 79% of colon cancer tumors (33, 34). As such, p21 is a viable marker of differentiation and its expression may be considered an intermediate biomarker of risk for colorectal cancer. Additionally, there is substantial evidence that markers of apoptosis, including Bax and Bcl-2 expression, are plausible candidates for treatable biomarkers of risk for colorectal neoplasms (35, 36).
If cell differentiation and apoptotic markers are valid intermediate biomarkers of risk for colorectal cancer, interpretation of both marker expression and influence of ginger supplementation on marker expression may differ based upon stage of carcinogenesis progression. Chemopreventive agents whose influences are confined to a specific stage of colorectal tumorigenesis could have been missed using the current study population. For example, ginger may influence p21 expression in individuals with a family history of CRC (and those without prior adenomas or CRC), but this finding would be obscured in the current study population, which also includes individuals with a previously resected adenomatous polyp or CRC. Additionally, the relatively short length of the study may have been insufficient for ginger to produce any measurable changes within the colonic mucosa.
There are other possible explanations for our finding, including chance—especially considering the pilot study’s small sample size—and non-transferability of in vitro and animal model results to human models; all previous studies were either in vitro or animal experimental studies and the chemopreventive effects of ginger observed in these studies may not necessarily translate to decreased CRC risk in humans. Additionally, previous studies focused on the effects of ginger on tumor rather than normal cells. As such, ginger’s anti-carcinogenic effects may be confined to active cancer cell lines or may be expressed differently in normal human cells.
While both the Bax/Bcl-2 ratio and p21 expression decreased in the treatment group, relative to the placebo group, our results suggest that ginger “normalized” the distribution of Bax and p21 expression in the crypts. Animal studies and preliminary evidence in humans suggest that an upward shift in the proliferative zone of normal colonic mucosa is a precursor for colon neoplasia (37, 38). Furthermore, patients with a history of sporadic adenoma (39–41) and those with a family history of colorectal cancer (40, 42, 43) were found to have an increased proliferation rate and an upward (luminal) extension of the proliferation zone. Thus, “normalization” of the distributions of cell cycle activities in the crypt may be an integral mechanism by which ginger may suppress the initiation of colorectal carcinogenesis in individuals at increased risk for CRC.
Apoptosis and proliferation occur through tightly regulated processes. As such, decreased apoptosis may simply reflect decreased proliferation. Our results indicated a positive correlation between Bax and both hTERT and MIB-1. The positive correlation between apoptosis and proliferation indices suggests that apoptosis may reflect not only cell death, but also proliferation activity. This finding, which is in agreement with previous studies using rodent models (44, 45), suggests that a link exists between the two pathways and may, in part, be explained by cell cycle sequence: apoptosis primarily occurs in the late G1 and G2 phases, but not in the G0 and M phases (46).
This trial highlights the importance of studying all major cell cycle functions when assessing the impact of certain chemopreventive agents. The processes of apoptosis, differentiation, and proliferation are intricately correlated; research focusing strictly on one or two phenotypic cell markers will most likely not provide enough information to accurately describe the status of key systems within colonocytes whose malfunction can lead to the development of colorectal cancer.
This study had several limitations and strengths. Proliferation, differentiation, and apoptosis markers are not proven biomarkers of risk for colon cancer; however, substantial basic science literature supports an important role for cell cycle functioning in colon carcinogenesis. Given that the endpoints investigated were intermediate markers of risk, this study cannot prove that ginger-mediated reductions in proliferation will translate to actual reductions in colon cancer risk. The study design also provided neither estimates of the rapidity of a response to ginger nor whether an effect would be apparent with prolonged use. Furthermore, given the small sample size of the pilot, randomized, controlled trial, the study had limited statistical power; thus, our findings may have been due to chance. A post-hoc sample size analysis using markers that would be suitable for a future trial indicated that a sample size ranging from 16 participants (hTERT) to 432 participants (Bcl-2) would be required to detect a statistically significant difference in biomarker levels between treatment groups. Also, the small size did not allow us to conduct additional subgroup analyses by risk type (e.g., family history, previous adenoma, previous CRC). Also, this study would need to be further replicated in larger studies and among several independent groups. On the other hand, this study, to the best of our knowledge, is the first randomized, double-blinded, placebo-controlled trial to have assessed the effects of ginger on apoptosis, proliferation, and differentiation markers in the normal-appearing colorectal epithelium. Also, there was high protocol adherence by study participants and the study was strengthened by the use of automated immunohistochemical staining and novel quantitative image analysis procedures, and high biopsy analysis reliability.
In summary, these preliminary results suggest that 2.0 g ginger extract taken daily may reduce proliferation in the crypts of normal-appearing colorectal epithelium and increase apoptosis and differentiation relative to proliferation–especially in the differentiation zone of crypts, and support a full-scale clinical trial to further investigate these results.
Supplementary Material
Acknowledgments
GRANT SUPPORT
This publication was made possible in part by Grant Number P30 CA047904, P30 CA 48592, CA130810 (GI SPORE) and K07CA102592, K24CA80846 from the National Cancer Institute (NCI) and University of Michigan Clinical Research Center UL1RR024986, and the Kutsche Family Memorial Endowment. The ginger extract was generously donated by Pure Encapsulations ® (Sudbury, MA).
Footnotes
Trial registration ID: NCT01344538
None of the authors had a conflict of interest.
References
- 1.Society AC. Cancer Facts and Figures. Atlanta, Ga: American Cancer Society; 2011. [Google Scholar]
- 2.Ahmed RS, Seth V, Banerjee BD. Influence of dietary ginger (Zingiber officinales Rosc) on antioxidant defense system in rat: comparison with ascorbic acid. Indian J Exp Biol. 2000;38(6):604–6. [PubMed] [Google Scholar]
- 3.Sekiwa Y, Kubota K, Kobayashi A. Isolation of novel glucosides related to gingerdiol from ginger and their antioxidative activities. J Agric Food Chem. 2000;48(2):373–7. doi: 10.1021/jf990674x. [DOI] [PubMed] [Google Scholar]
- 4.Grzanna R, Lindmark L, Frondoza CG. Ginger--an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8(2):125–32. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- 5.Lantz RC, Chen GJ, Sarihan M, Solyom AM, Jolad SD, Timmermann BN. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine. 2007;14(2–3):123–8. doi: 10.1016/j.phymed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45(5):683–90. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Manju V, Nalini N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1,2 dimethylhydrazine-induced colon cancer. Clin Chim Acta. 2005;358(1–2):60–7. doi: 10.1016/j.cccn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Keum YS, Kim J, Lee KH, Park KK, Surh YJ, Lee JM, et al. Induction of apoptosis and caspase-3 activation by chemopreventive [6]-paradol and structurally related compounds in KB cells. Cancer Lett. 2002;177(1):41–7. doi: 10.1016/s0304-3835(01)00781-9. [DOI] [PubMed] [Google Scholar]
- 9.Miyoshi N, Nakamura Y, Ueda Y, Abe M, Ozawa Y, Uchida K, et al. Dietary ginger constituents, galanals A and B, are potent apoptosis inducers in Human T lymphoma Jurkat cells. Cancer Lett. 2003;199(2):113–9. doi: 10.1016/s0304-3835(03)00381-1. [DOI] [PubMed] [Google Scholar]
- 10.Lee E, Surh YJ. Induction of apoptosis in HL-60 cells by pungent vanilloids, [6]-gingerol and [6]-paradol. Cancer Lett. 1998;134(2):163–8. doi: 10.1016/s0304-3835(98)00253-5. [DOI] [PubMed] [Google Scholar]
- 11.Pan MH, Hsieh MC, Kuo JM, Lai CS, Wu H, Sang S, et al. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol Nutr Food Res. 2008;52(5):527–37. doi: 10.1002/mnfr.200700157. [DOI] [PubMed] [Google Scholar]
- 12.Bode AM, Ma WY, Surh YJ, Dong Z. Inhibition of epidermal growth factor-induced cell transformation and activator protein 1 activation by [6]-gingerol. Cancer Res. 2001;61(3):850–3. [PubMed] [Google Scholar]
- 13.Kim SO, Kundu JK, Shin YK, Park JH, Cho MH, Kim TY, et al. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene. 2005;24(15):2558–67. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- 14.Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, Han S, et al. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2005;335(2):300–8. doi: 10.1016/j.bbrc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 15.Lee HS, Seo EY, Kang NE, Kim WK. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J Nutr Biochem. 2008;19(5):313–9. doi: 10.1016/j.jnutbio.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Zick SM, Turgeon DK, Vareed SK, Ruffin MT, Litzinger AJ, Wright BD, et al. Phase II study of the effects of ginger root extract on eicosanoids in colon mucosa in people at normal risk for colorectal cancer. Cancer Prev Res (Phila) 2011;4(11):1929–37. doi: 10.1158/1940-6207.CAPR-11-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedirko V, Bostick RM, Long Q, Flanders WD, McCullough ML, Sidelnikov E, et al. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2010;19(1):280–91. doi: 10.1158/1055-9965.EPI-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedirko V, Bostick RM, Flanders WD, Long Q, Shaukat A, Rutherford RE, et al. Effects of vitamin D and calcium supplementation on markers of apoptosis in normal colon mucosa: a randomized, double-blind, placebo-controlled clinical trial. Cancer Prev Res (Phila) 2009;2(3):213–23. doi: 10.1158/1940-6207.CAPR-08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khater DS. The influence of ginger as a chemopreventive agent on proliferation and apoptosis in chemically induced oral carcinogenesis. Nature and Science. 2010;8(11):44–51. [Google Scholar]
- 20.Lu J, Guan S, Shen X, Qian W, Huang G, Deng X, et al. Immunosuppressive activity of 8-gingerol on immune responses in mice. Molecules. 2011;16(3):2636–45. doi: 10.3390/molecules16032636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown AC, Shah C, Liu J, Pham JT, Zhang JG, Jadus MR. Ginger’s (Zingiber officinale Roscoe) inhibition of rat colonic adenocarcinoma cells proliferation and angiogenesis in vitro. Phytother Res. 2009;23(5):640–5. doi: 10.1002/ptr.2677. [DOI] [PubMed] [Google Scholar]
- 22.Yagihashi S, Miura Y, Yagasaki K. Inhibitory effect of gingerol on the proliferation and invasion of hepatoma cells in culture. Cytotechnology. 2008;57(2):129–36. doi: 10.1007/s10616-008-9121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo EYLH, Kim WK. Effect of [6]-Gingerol on Inhibition of Cell Proliferation in MDA-MB-231 Human Breast Cancer Cells. Korean J Nutr. 2005;38(8):656–662. [Google Scholar]
- 24.Tuntiwechapikul W, Taka T, Songsomboon C, Kaewtunjai N, Imsumran A, Makonkawkeyoon L, et al. Ginger extract inhibits human telomerase reverse transcriptase and c-Myc expression in A549 lung cancer cells. J Med Food. 2010;13(6):1347–54. doi: 10.1089/jmf.2010.1191. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Cekanova M, Baek SJ. Multiple mechanisms are involved in 6-gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells. Mol Carcinog. 2008;47(3):197–208. doi: 10.1002/mc.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong CH, Bode AM, Pugliese A, Cho YY, Kim HG, Shim JH, et al. [6]-Gingerol suppresses colon cancer growth by targeting leukotriene A4 hydrolase. Cancer Res. 2009;69(13):5584–91. doi: 10.1158/0008-5472.CAN-09-0491. [DOI] [PubMed] [Google Scholar]
- 27.Lee E, Park KK, Lee JM, Chun KS, Kang JY, Lee SS, et al. Suppression of mouse skin tumor promotion and induction of apoptosis in HL-60 cells by Alpinia oxyphylla Miquel (Zingiberaceae) Carcinogenesis. 1998;19(8):1377–81. doi: 10.1093/carcin/19.8.1377. [DOI] [PubMed] [Google Scholar]
- 28.Bidinotto LT, Spinardi-Barbisan AL, Rocha NS, Salvadori DM, Barbisan LF. Effects of ginger (Zingiber officinale Roscoe) on DNA damage and development of urothelial tumors in a mouse bladder carcinogenesis model. Environ Mol Mutagen. 2006;47(8):624–30. doi: 10.1002/em.20248. [DOI] [PubMed] [Google Scholar]
- 29.Dias MC, Spinardi-Barbisan AL, Rodrigues MA, de Camargo JL, Teran E, Barbisan LF. Lack of chemopreventive effects of ginger on colon carcinogenesis induced by 1,2-dimethylhydrazine in rats. Food Chem Toxicol. 2006;44(6):877–84. doi: 10.1016/j.fct.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Yodkeeree S, Sung B, Limtrakul P, Aggarwal BB. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: Evidence for an essential role of reactive oxygen species. Cancer Res. 2009;69(16):6581–9. doi: 10.1158/0008-5472.CAN-09-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14(2):159–69. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Nosho K, Yamamoto H, Adachi Y, Endo T, Hinoda Y, Imai K. Gene expression profiling of colorectal adenomas and early invasive carcinomas by cDNA array analysis. Br J Cancer. 2005;92(7):1193–200. doi: 10.1038/sj.bjc.6602442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogino S, Nosho K, Shima K, Baba Y, Irahara N, Kirkner GJ, et al. p21 expression in colon cancer and modifying effects of patient age and body mass index on prognosis. Cancer Epidemiol Biomarkers Prev. 2009;18(9):2513–21. doi: 10.1158/1055-9965.EPI-09-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366(6456):701–4. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 35.Lu QL, Abel P, Foster CS, Lalani EN. bcl-2: role in epithelial differentiation and oncogenesis. Hum Pathol. 1996;27(2):102–10. doi: 10.1016/s0046-8177(96)90362-7. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Nomura S, Sakai T, Nariya S. Expression of bcl-2 oncoprotein in gastrointestinal and uterine carcinomas and their premalignant lesions. Hum Pathol. 1997;28(3):309–15. doi: 10.1016/s0046-8177(97)90129-5. [DOI] [PubMed] [Google Scholar]
- 37.Wargovich MJ, Baer AR. Basic and clinical investigations of dietary calcium in the prevention of colorectal cancer. Prev Med. 1989;18(5):672–9. doi: 10.1016/0091-7435(89)90038-8. [DOI] [PubMed] [Google Scholar]
- 38.Bostick RM, Fosdick L, Wood JR, Grambsch P, Grandits GA, Lillemoe TJ, et al. Calcium and colorectal epithelial cell proliferation in sporadic adenoma patients: a randomized, double-blinded, placebo-controlled clinical trial. J Natl Cancer Inst. 1995;87(17):1307–15. doi: 10.1093/jnci/87.17.1307. [DOI] [PubMed] [Google Scholar]
- 39.Paganelli GM, Biasco G, Santucci R, Brandi G, Lalli AA, Miglioli M, et al. Rectal cell proliferation and colorectal cancer risk level in patients with nonfamilial adenomatous polyps of the large bowel. Cancer. 1991;68(11):2451–4. doi: 10.1002/1097-0142(19911201)68:11<2451::aid-cncr2820681121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Lipkin M, Uehara K, Winawer S, Sanchez A, Bauer C, Phillips R, et al. Seventh-Day Adventist vegetarians have a quiescent proliferative activity in colonic mucosa. Cancer Lett. 1985;26(2):139–44. doi: 10.1016/0304-3835(85)90019-9. [DOI] [PubMed] [Google Scholar]
- 41.Stadler J, Yeung KS, Furrer R, Marcon N, Himal HS, Bruce WR. Proliferative activity of rectal mucosa and soluble fecal bile acids in patients with normal colons and in patients with colonic polyps or cancer. Cancer Lett. 1988;38(3):315–20. doi: 10.1016/0304-3835(88)90023-7. [DOI] [PubMed] [Google Scholar]
- 42.Lipkin M, Blattner WE, Fraumeni JF, Jr, Lynch HT, Deschner E, Winawer S. Tritiated thymidine (phi p, phi h) labeling distribution as a marker for hereditary predisposition to colon cancer. Cancer Res. 1983;43(4):1899–904. [PubMed] [Google Scholar]
- 43.Gerdes H, Gillin JS, Zimbalist E, Urmacher C, Lipkin M, Winawer SJ. Expansion of the epithelial cell proliferative compartment and frequency of adenomatous polyps in the colon correlate with the strength of family history of colorectal cancer. Cancer Res. 1993;53(2):279–82. [PubMed] [Google Scholar]
- 44.Reid S, Ritchie A, Boring L, Gosling J, Cooper S, Hangoc G, et al. Enhanced myeloid progenitor cell cycling and apoptosis in mice lacking the chemokine receptor, CCR2. Blood. 1999;93(5):1524–33. [PubMed] [Google Scholar]
- 45.Traver D, Akashi K, Weissman IL, Lagasse E. Mice defective in two apoptosis pathways in the myeloid lineage develop acute myeloblastic leukemia. Immunity. 1998;9(1):47–57. doi: 10.1016/s1074-7613(00)80587-7. [DOI] [PubMed] [Google Scholar]
- 46.Scopa CD, Tsamandas AC, Zolota V, Kalofonos HP, Batistatou A, Vagianos C. Potential role of bcl-2 and ki-67 expression and apoptosis in colorectal carcinoma: a clinicopathologic study. Dig Dis Sci. 2003;48(10):1990–7. doi: 10.1023/a:1026178506348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.