Abstract
Objectives
Antiretroviral (ARV) resistance is of concern. Opioid agonist treatment ( i.e., methadone or buprenorphine) is effective and decreases HIV transmission risk behaviors and HIV seroconversion. Despite prevention efforts, injection drug use (IDU) and risky sexual behaviors remain prevalent in patients receiving opioid agonist treatment. The purpose of this study is to determine in HIV-infected patients receiving opioid agonist treatment, the prevalence of HIV transmission risk behaviors, the prevalence of ARV resistance, and the prevalence of ARV resistance among those with risk behaviors.
Methods
The design was a cross-sectional, study of patients recruited from opioid treatment programs and outpatient practices. We measured demographic, drug treatment, and HIV clinical information (including ARV adherence), self-reported HIV risk behaviors and drug use, urine toxicologies, and genotype testing for ARV resistance (with both standard assays and Ultradeep sequencing). Data analysis included descriptive statistics.
Results
59 subjects enrolled. 64% were male, 24% were white, and mean age was 46 years. 53% were receiving methadone and 47% buprenorphine. 80% were on opioid agonist treatment for 12 weeks or more. 14% reported unprotected sex, 7% reported sharing needles or works, and 60% had positive urine toxicology for illicit drug use. 15% had evidence of HIV resistance by standard genotyping, 7% with single class resistance, 3% with double class resistance, and 5% with triple class resistance. Ultradeep sequencing found additional class resistance in 5 subjects. 22% of subjects with evidence of transmission risk behaviors vs. 14% of subjects without risk behaviors had evidence of ARV resistance.
Conclusions
Improved prevention and treatment efforts may be needed for HIV-infected, opioid dependent individuals receiving opioid agonist treatment to decrease transmission of ARV resistant virus, especially in resource limited settings.
Keywords: HIV, methadone, buprenorphine, risk taking, drug resistance, viral
Introduction
HIV-infected opioid dependent patients can transmit HIV by sharing injection paraphernalia (needles and/or works) and through unprotected sex. Opioid agonist treatment (i.e., methadone or buprenorphine) is an effective treatment for opioid dependence that can decrease the frequency of injection-related sharing and HIV seroconversion.(D. S. Metzger et al., 1993; David S. Metzger, Woody, & O'Brien; Sullivan et al., 2008) HIV-infected opioid dependent patients receiving these medications, however, may still continue to use injection drugs and share injection paraphernalia.(Gowing, Farrell, Bornemann, Sullivan, & Ali, 2011) In addition, opioid agonist treatment does not address sexual risk behavior in these patients.(Gowing, et al., 2011; Sullivan, et al., 2008)
The advent of combination antiretroviral (ARV) treatment has revolutionized the care of HIV-infected patients. ARV resistance, which is the natural response of most viruses facing drug pressure, occurs in 10–15% of newly infected individuals and 30–50% of untreated injection drug users (IDUs).(Kozal, 2009; Kozal et al., 2005; Kozal et al., 2004) It results from partial HIV treatment (i.e., absence of combined ARV regimens), poor adherence to ARV, or acquisition of a resistant viral strain from sexual activity or injection drug use (IDU). Additionally, ARV resistance leads to poor HIV outcomes.(Kozal et al., 2007) ARV resistance is a concern, especially in resource-limited settings where second-line ARVs are scarce or nonexistent. A variety of strategies have been implemented to minimize ARV resistance including resistance testing prior to initiation of ARVs, the use of high potency therapies, promotion of medication adherence, development of new viral drug classes, and risk behavior counseling.(Fisher, Cornman, Norton, & Fisher, 2006; Fisher et al., 2006; Kozal, 2009; Panel on Antiretroviral Guidelines for Adults and Adolescents, January 10, 2011) Currently, six ARV classes exist and resistance can occur with every ARV agent available. Certain mutations may render resistance to an entire class of ARV medication, and therefore markedly limit the medication options available to certain patients.(Korthuis et al., 2011)
The prevalence of paraphernalia sharing, unprotected sex, and antiretroviral resistance in HIV-infected patients receiving opioid agonist treatment is unknown. The purpose of this investigation was to determine, in HIV-infected patients receiving opioid agonist treatment, the prevalence of the prevalence of risk behaviors (paraphernalia sharing and unprotected sex), the prevalence of ARV resistance, and the prevalence of ARV resistance among those with risk behaviors.
Methods
Study Design
For this cross-sectional study, patients were recruited from opioid treatment programs and office-based practices in New Haven and Waterbury, CT. Patients were eligible for study inclusion if they were HIV-infected, at least 18 years of age, receiving methadone or buprenorphine for at least one month, and English or Spanish speaking. The study protocol was approved by the Human Investigation Committee (HIC) for the Yale University School of Medicine. Patients were compensated with a $20 gift card upon study completion.
Data collection
We collected demographic and HIV clinical information via patient interview and chart review. ARV adherence was assessed using a self-report instrument assessing medication use for the prior 7 days.(Terry Beirn Community Programs for Clinical Research on AIDS, 2004) IDU paraphernelia sharing and unprotected sexual behaviors were assessed for the prior 90 days using the AIDS/HIV Risk Inventory and the Addiction Severity Index.(McLellan, Luborsky, Woody, & O'Brien; Sullivan et al.) Subjects were asked to quantify number of partners and events occurring for each behavior and to report the HIV-serostatus (HIV-infected or HIV-uninfected or status unknown) of their partners. We also reviewed urine toxicology testing from 6-months prior (when available).
Standard DNA sequencing was used to detect HIV genotypic resistance using consensus population sequencing of the HIV-1 pol gene.(Kozal et al.) Mutations were considered resistant if they met criteria for ARV resistance by Stanford University HIV Database (HIVdb) 2009 algorithm.(Stanford University, 2009) We used the Stanford HIVdb resistance algorithm as it is a validated genotypic drug-resistance test interpretation algorithm which is open access and has a transparent mutation genotypic susceptibility scoring that is considered the standard in the HIV drug resistance field. (Rhee et al., 2009) Secondary mutations or polymorphisms listed for reverse transcriptase or protease inhibitors were not included. We also collected prior genotypic drug resistance patterns through chart review and included these prior patterns in the assessment of ARV resistance.
Ultra-deep sequencing
HIV infections in patients exist as viral quasi-species, a collection of genetically diverse viral variants.(Li et al., 2011; Panel on Antiretroviral Guidelines for Adults and Adolescents, January 10, 2011) Not all the viral variants that make up the collection in a person are detected by standard resistance assays. Therefore, we used ultra-deep sequencing techniques (Kozal MJ, Chiarella J, & St. John EP, 2011; Simen et al., 2009) to identify and accurately quantify minor resistant variants present at very low (<1%) levels in patient samples. (Kozal MJ, et al., 2011; Lataillade et al., 2010; Simen, et al., 2009) Samples with adequate HIV viral loads (typically >10,000 copies/mL) were further evaluated for low-level resistant variants to 0.4% of the circulating viral quasi-species (Kozal MJ, et al., 2011; Lataillade, et al., 2010) that may have been missed by standard genotyping methods.(Kozal MJ, et al., 2011; Lataillade, et al., 2010; Li, et al., 2011; Simen, et al., 2009) Ultra-deep sequencing was still performed on samples with HIV viral loads bellow 10,000 copies/mL, however, the levels of mutations identified in these samples represent the proportion of sequenced PCR amplicons containing the mutation and may or may not represent the actual proportion in the plasma sample.(Kozal MJ, et al., 2011; Lataillade, et al., 2010)
Sample size and data analysis
A formal sample size calculation was not conducted for this cross-sectional analysis. We anticipated a prevalence of HIV drug resistance of 10–15% in HIV treatment-naïve patients and 30–50% in those receiving cART. (Kozal, et al., 2005; Kozal, et al., 2004; Novak et al., 2005) and established a recruitment goal of 90 patients.
Using descriptive analyses, we explored the prevalence and frequencies of risk behaviors including IDU focusing on sharing paraphernalia (needles and/or works) and unprotected sex stratified by HIV-serostatus of partners (HIV-infected vs. HIV-uninfected or status unknown); prevalence of ARV resistance; and the prevalence of standard ARV resistance among risk behavior groups. We also report the prevalence of minor resistant variants based on ultra-deep sequencing. Data analysis was performed using SAS version 9.2.
Results
A total of 59 subjects were enrolled in the study. 64% of the sample were male, 32% were white, 53% were receiving methadone and 47% were receiving buprenorphine. 80% of the sample was on opioid agonist therapy for at least 12 weeks. Median duration of HIV disease was 19 years and 89% were on antiretroviral medication. 32% of the sample had a detectable viral load (Table 1).
Table 1.
Patient characteristics, N=59
| Characteristic, % (n) | Value |
|---|---|
| Male | 64 (38) |
| Age in years, mean (sd) | 46 (6.9) |
| White | 32 (19) |
| Black | 39 (23) |
| Methadone | 53 (31) |
| Buprenorphine | 47 (28) |
| % on OAT for ≥ 12 weeks | 80 (47) |
| Years of HIV diagnosis, median (range) | 19 (1–29) |
| Receiving ARV | 89 (49) |
| 100% adherence to ARVs, % (n/N) | 71 (35/49) |
| Detectable viral load | 32 (19) |
| CD4 count, median (range) | 433 (18–1744) |
Prevalence of paraphernalia sharing, unprotected sex, and ARV resistance
Sixty-six percent of the sample had urine toxicology results positive for ongoing illicit substance use and 14% (n=8) of the sample reported ongoing injection drug use with four subjects reporting sharing of needles/works for a total of 21 events in the past 90 days. Of these, 19 events occurred with HIV-uninfected or status unknown partners and two events occurred with HIV-infected partners. Fourteen percent of the sample reported engaging in unprotected sexual activity in the 90 days prior for a total of 102 events. Of these events, 55 occurred with HIV-uninfected or status unknown partners and 47 occurred with HIV-infected partners (Table 2)
Table 2.
Prevalence of paraphernalia sharing and unprotected sex, N=59
| Total % of sample # of events (n subjects) |
HIV-uninfected or status unknown partners # of events (n subjects) |
HIV-infected partners # of events (n subjects) |
|
|---|---|---|---|
| Sharing needles/works | 7% 21 (4) |
19 (2) |
2 (2) |
| Unprotected sex | 14% 102 (8) |
55(5) |
47 (4) |
Of the 59 subjects enrolled in the study, 32% (n=19) had a detectable HIV viral load (ranging from 57–167,000 copies/mL) amenable to resistance testing. Of these, 17% of the total sample had no evidence of resistance by standard sequencing techniques and 15% of the total sample had evidence of resistance; 7% had single class resistance, 3% had double class resistance, and 5% had triple class resistance (Table 3). Four subjects had resistance to the protease inhibitor class, 6 subjects had resistance to the nucleoside reverse transcriptase inhibitor class, and 7 subjects had resistance to the non-nucleoside reverse transcriptase inhibitor class. Of the 19 subjects with a detectable HIV viral load, 15 were on ARVs. Of the 9 subjects with evidence of ARV resistance by standard sequencing, all were on ARVs.
Table 3.
Prevalence of ARV Resistance by standard genotype testing, N=59
| Resistance | % (n) |
|---|---|
| Undetectable viral load | 68% (n=40) |
| Detectable viral load (range 57–167,000 copies/mL) |
32% (n=19) |
| ARV Resistance | |
| None | 17% (n=10) |
| Resistance | 15% (n=9) |
| Single class | 7% (n=4) |
| Double class | 3% (n=2) |
| Triple class | 5% (n=3) |
Prevalence of ARV resistance among subjects with paraphernalianelia sharing and unprotected sex
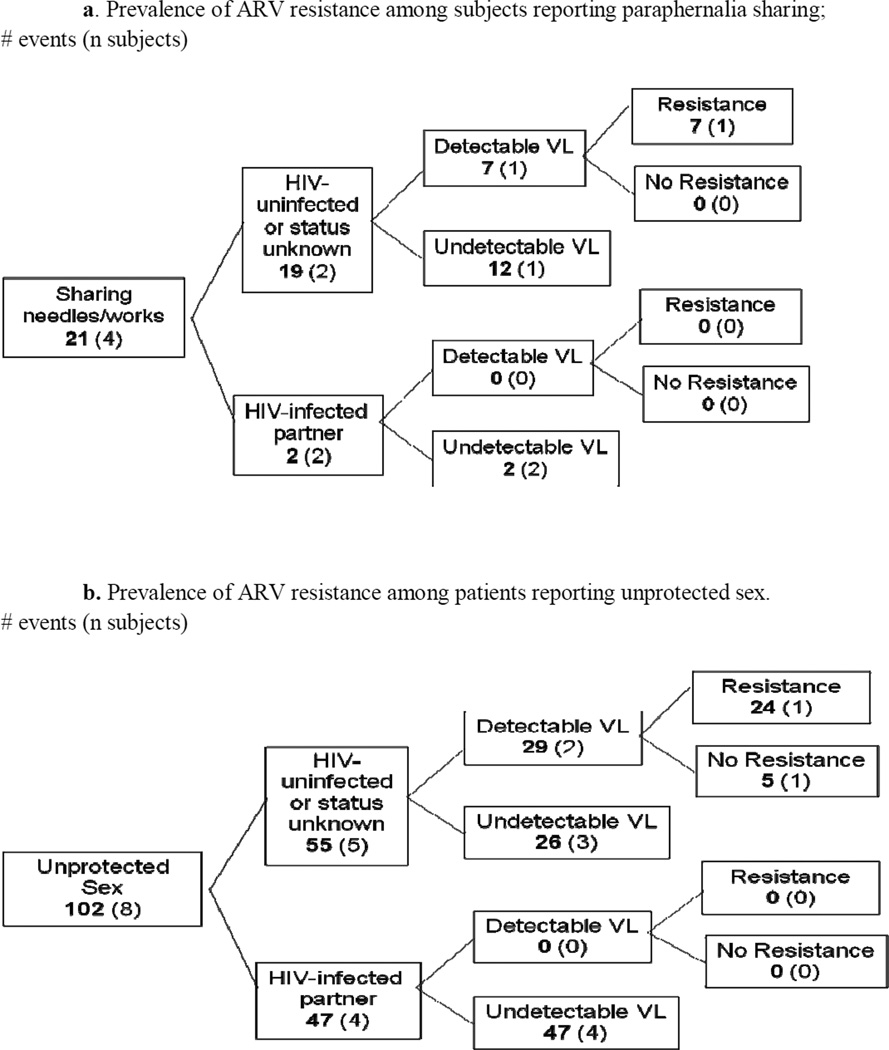
Of the 19 events of paraphernalia sharing occurring with HIV-uninfected or status unknown partners, seven events were reported by one subject with a detectable viral load and evidence of antiretroviral resistance. Of the two paraphernalia events occurring with HIV-infected partners, none of the events occurred among subjects with a detectable viral load.
Of the 55 unprotected sexual events occurring with HIV-uninfected or status unknown partners, 29 events were reported by subjects with a detectable viral load, and 24 events were reported by subjects with evidence of ARV resistance (Figures 1a and 1b).
Figure 1.
Finally, we found that 22% of subjects with evidence of risk behaviors had antiretroviral resistance while 14% of subjects with no risk behaviors had antiretroviral resistance (p=0.62).
Ultra-deep sequencing
Out of 19 subjects with a detectable viral load, we were able to obtain ultra-deep sequencing on 13 samples. Of these, compared to standard sequencing, ultra-deep sequencing detected an additional class resistance in five subjects (e.g., increase from double to triple class resistance), and seven subjects had increases in resistance to at least one new antiretroviral drug (Table 4).
Table 4.
Comparison of detection of class mutations by standard genotypic sequencing and ultra-deep sequencing; N=13
| Subject | Standard sequencing | Ultra-deep sequencing |
|---|---|---|
| 1 | WT | PI, NRTI, NNRTI |
| 2 | PI, NRTI, NNRTI | PI, NRTI* |
| 3 | NRTI | NRTI,NNRTI |
| 4 | WT | WT |
| 5 | NRTI | WT* |
| 6 | NNRTI | PI, NRTI, NNRTI |
| 7 | WT | WT |
| 8 | WT | WT |
| 9 | WT | NNRTI |
| 10 | NNRTI | NRTI, NNRTI |
| 11 | PI, NRTI, NNRTI | PI, NRTI, NNRTI |
| 12 | PI, NRTI, NNRTI | PI, NRTI, NNRTI |
| 13 | WT | WT |
Abbreviations used in table: WT=wildtype, PI=protease inhibitor mutation, NRTI=nucleoside reverse transcriptase inhibitor mutation, NNRTI=non-nucleoside reverse transcriptase inhibitor mutation.
Ultra-deep sequencing detected fewer mutations than standard sequencing in two samples.
Discussion
ARV resistance is a public health threat for both HIV-infected patients and uninfected patients who are at risk of acquiring potentially resistant virus, especially in resource-limited settings. Opioid agonist treatment decreases HIV transmission risk and rates of HIV seroconversion. However, despite substantial efficacy in reducing risky behaviors, some HIV-infected patients receiving opioid agonist treatment still engage in sharing of drug use paraphernalia and unprotected sex, placing partners at-risk of acquiring resistant virus. We found that among HIV-infected patients receiving opioid agonist treatment, 14% continued to engage in unprotected sex, 7% continued to share drug use paraphernalia, 32% had a detectable viral load, and 15% had evidence of antiretroviral resistance by standard sequencing. Ultra-deep sequencing found additional class resistance in 5 subjects. Although there were no differences in evidence of resistance between those with or without risk behaviors, the relatively high prevalence of resistance in this sample of patients on opioid agonist treatment is concerning.
The global impact of injection drug related HIV is felt most in the areas of Central, South and Southeast Asia (e.g. China, the former Soviet Union, Russia, Vietnam). (World Health Organization, 2010) This treatment need is being met with improved access to ARVs in these areas. Similarly, access to opioid agonist treatment is increasing in China, Malaysia, countries of the former Soviet Union, and Vietnam among others. Rapid introduction of ARVs and opioid agonist treatment in developing countries without attention to the need to implement strategies to minimize the transmission of HIV resistance, may result in unintended consequences for resource-limited settings. (Sullivan, Metzger, Fudala, & Fiellin, 2005) Therefore, it is imperative to target public health measures at reducing the development and transmission of resistant virus—even among patients who are receiving opioid agonist treatment both domestically and, perhaps more importantly, globally.
Standard assays detect drug resistant HIV if it makes up at least 20% of the collection. Recent data suggest that minor resistant variants, constituting as little as 1% of the collection in a patient, are clinically important as they can rapidly proliferate after the introduction of a new antiretroviral medication and lead to treatment failure.(Kozal et al., 2006; Le et al., 2009; Li et al.; Simons et al., 2005) Techniques such as ultra-deep sequencing, used in this investigation, can detect these minor resistant variants.
Prior work has demonstrated the transmission of resistant virus among active injection drug users, among HIV-infected patients in clinical care, and among patients with multidrug resistant HIV. (Kozal, et al., 2005, 2006; Kozal, et al., 2004) Among 180 out of treatment IDUs, 31% reported IDU within the prior month and of these, 40% reported sharing paraphernalia, and 31% harbored resistant virus.(Kozal, et al., 2005) Similarly, among 333 HIV-infected patients in clinical care, 23% had unprotected sex in the three months prior, and 24% had resistant HIV infection.(Kozal, et al., 2004) Among 393 patients with multi-drug resistant HIV (defined as two or three medication class resistance), 45% of those engaging in sexual activity reported unprotected sex and 31% had ARV resistance.(Kozal, et al., 2006) Although, in two of these studies, as in the present study, a minority of subjects engaged in high-risk behavior, roughly 15–30% had resistant virus and exposed a substantial number of partners (both HIV-infected or HIV-uninfected or status unknown) to resistant virus.
One study investigated the resistance implications of directly observed antiretroviral treatment within a methadone maintenance treatment program compared with treatment as usual.(Moatti et al., 2000) Directly observed therapy improved ARV adherence, decreased HIV-viral load, and was not associated with increased ARV resistance as seen in the treatment as usual arm. A separate trial noted no difference in resistance with directly observed therapy although the drug use criteria for entry were minimal and the analysis used a modified intention-to-treat principles. Other investigations have focused on the integration of opioid agonist treatment and HIV care but have not necessarily focused on associations with ARV resistance.(Korthuis, et al., 2011; Rhee, et al., 2009; Simen, et al., 2009; Sullivan et al., 2006) In addition, these studies did not focus on the association between sharing drug use paraphernalia, unprotected sex, and antiretroviral resistance among patients maintained on opioid agonist treatment.
Our study has some limitations. First, the sample size and recruitment from only two sites may limit generalizability and the ability to note statistical significance in some of the findings. Although we did not meet our recruitment goals for this study, we were able to gain valuable information regarding the prevalence of ARV resistance among subjects reporting risk behaviors. Additionally, our study was not designed to determine differences in risk behaviors or ARV resistance between those receiving methadone or buprenorphine. Because of the cross-sectional nature of the study, we were only able to assess associations between sharing of drug use paraphernalia, unprotected sex, and ARV resistance among patients on opioid agonist treatment and can not speculate on causality. Although our assessment of ARV adherence is an accepted standard(Terry Beirn Community Programs for Clinical Research on AIDS, 2004), it is self-reported and may not reflect the true nature of adherence. Despite these limitations, we did find that HIV-infected, opioid dependent individuals on opioid agonist treatment continued to engage in drug use paraphernalia sharing and unprotected sex and have evidence of ARV resistance placing several partners at risk of acquiring resistant virus. Noted strengths of our study include the long duration of HIV disease among subjects, detailed information about injection and sexual risk behaviors, inclusion of archived resistance patterns based on historic genotypes, and use of ultra-deep sequencing techniques to examine minor resistant variants.
Conclusions
In conclusion, we found that ongoing risk behaviors place HIV-uninfected partners at risk of acquiring resistant virus and HIV-infected individuals at risk of acquiring new viral strains. Improved prevention and treatment efforts may be needed for HIV-infected opioid dependent patients receiving opioid agonist treatment to decrease transmission of antiretroviral resistance, especially in the resource-limited settings-- such as the 15 resource-limited countries with high HIV/AIDS prevalence rates highlighted in the President’s Emergency Plan for AIDS Relief (PEPFAR) initiative-- often where ARVs and opioid agonist treatments are being introduced simultaneously.
Acknowledgements
This work was supported by Yale University's Center for Interdisciplinary Research on AIDS (CIRA) through a grant from the National Institute of Mental Health (No. P30 MH 62294) and was presented at the College on Problems of Drug Dependence (CPDD) Annual Meeting on June 16, 2010 in Scottsdale, AZ and at the Society of General Internal Medicine (SGIM) Annual Meeting on April 30, 2010 in Minneapolis, MN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Fisher JD, Cornman DH, Norton WE, Fisher WA. Involving behavioral scientists, health care providers, and HIV-infected patients as collaborators in theory-based HIV prevention and antiretroviral adherence interventions. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2006;43(Suppl 1):S10–S17. doi: 10.1097/01.qai.0000248335.90190.f9. [DOI] [PubMed] [Google Scholar]
- Fisher JD, Fisher WA, Cornman DH, Amico RK, Bryan A, Friedland GH. Clinician-delivered intervention during routine clinical care reduces unprotected sexual behavior among HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2006;41(1):44–52. doi: 10.1097/01.qai.0000192000.15777.5c. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. [Meta-Analysis Review] Cochrane Database of Systematic Reviews. 2011;(8):CD004145. doi: 10.1002/14651858.CD004145.pub4. [DOI] [PubMed] [Google Scholar]
- Korthuis PT, Tozzi MJ, Nandi V, Fiellin DA, Weiss L, Egan JE, Botsko M, Acosta A, Gourevitch MN, Hersh D, Hsu J, Boverman J Altice F.L. for the BHIVES Collaborative. Improved Quality of Life for Opioid-Dependent Patients Receiving Buprenorphine Treatment in HIV Clinics. JAIDS Journal of Acquired Immune Deficiency Syndromes, 56 Supplement 1, Integration of Buprenorphine/Naloxone Treatment into HIV Clinical(Care) 2011:S39–S45. doi: 10.1097/QAI.0b013e318209754c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozal MJ. Drug-resistant human immunodefiency virus. Clinical Microbiology & Infection. 2009;15(Suppl 1):69–73. doi: 10.1111/j.1469-0691.2008.02687.x. [DOI] [PubMed] [Google Scholar]
- Kozal MJ, Chiarella J, St. John EP. Prevalence of Low-level HIV-1 Variants with Reverse Transcriptase Mutation K65R Among Different HIV Subtypes and the Effects of Antiretroviral Drug Exposure on Variant Levels. Antiviral Therapy. 2011;16(6):925–929. doi: 10.3851/IMP1851. [DOI] [PubMed] [Google Scholar]
- Kozal MJ, Amico KR, Chiarella J, Cornman D, Fisher W, Fisher J, Friedland G. HIV drug resistance and HIV transmission risk behaviors among active injection drug users. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2005;40(1):106–109. doi: 10.1097/01.qai.0000159666.95455.d2. [DOI] [PubMed] [Google Scholar]
- Kozal MJ, Amico KR, Chiarella J, Cornman D, Fisher W, Fisher J, Friedland G. A population-based and longitudinal study of sexual behavior and multidrug-resistant HIV among patients in clinical care. Medgenmed [Computer File]: Medscape General Medicine. 2006;8(2):72. [PMC free article] [PubMed] [Google Scholar]
- Kozal MJ, Amico KR, Chiarella J, Schreibman T, Cornman D, Fisher W, Fisher J, Friedland G. Antiretroviral resistance and high-risk transmission behavior among HIV-positive patients in clinical care. AIDS. 2004;18(16):2185–2189. doi: 10.1097/00002030-200411050-00011. [DOI] [PubMed] [Google Scholar]
- Kozal MJ, Hullsiek KH, Macarthur RD, Berg-Wolf M, Peng G, Xiang Y, Baxter JD, Uy J, Telzak EE, Novak RM Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) The Incidence of HIV drug resistance and its impact on progression of HIV disease among antiretroviral-naive participants started on three different antiretroviral therapy strategies. HIV Clinical Trials. 2007;8(6):357–370. doi: 10.1310/hct0806-357. [DOI] [PubMed] [Google Scholar]
- Kozal MJ, Shah N, Shen N, Yang R, Fucini R, Merigan TC, Richman DD, Morris D, Hubbell E, Chee M, Gingeras TR. Extensive polymorphisms observed in HIV-1 clade B protease gene using high-density oligonucleotide arrays. [Research Support, U.S. Gov't, Non-P.H.S. Research Support U.S. Gov't PHS. Nature Medicine. 2(7):753–759. doi: 10.1038/nm0796-753. [DOI] [PubMed] [Google Scholar]
- Lataillade M, Chiarella J, Yang R, Schnittman S, Wirtz V, Uy J, Kozal M. Prevalence and clinical significance of HIV drug resistance mutations by ultra-deep sequencing in antiretroviral-naive subjects in the CASTLE study. [Research Support, Non-U.S. Gov't] PLoS ONE [Electronic Resource] 2010;5(6):e10952. doi: 10.1371/journal.pone.0010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T, Chiarella J, Simen BB, Hanczaruk B, Egholm M, Landry ML, Dieckhaus K, Rosen MI, Kozal MJ. Low-abundance HIV drug-resistant viral variants in treatment-experienced persons correlate with historical antiretroviral use. [Research Support N.I.H., ExtramuralResearch Support U.S. Gov't, Non-P.H.S.] PLoS ONE [Electronic Resource] 2009;4(6):e6079. doi: 10.1371/journal.pone.0006079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JZ, Paredes R, Ribaudo HJ, Svarovskaia ES, Metzner KJ, Kozal MJ, Hullsiek KH, Balduin M, Jakobsen MR, Geretti AM, Thiebaut R, Ostergaard L, Masquelier B, Johnson JA, Miller MD, Kuritzkes DR. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure: a systematic review and pooled analysis. [Meta-Analysis Research Support N.I.H. Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Review] JAMA. 305(13):1327–1335. doi: 10.1001/jama.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru DS-R, Kozal MJ, Bruce RD, Springer SA, Altice FL. Directly administered antiretroviral therapy for HIV-infected drug users does not have an impact on antiretroviral resistance: results from a randomized controlled trial. [Comparative Study Randomized Controlled Trial Research Support, N.I.H. Extramural] Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2007;46(5):555–563. doi: 10.1097/qai.0b013e318158c0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. [Research Support, U.S. Gov't, Non-P.H.S.] Journal of Nervous & Mental Disease. 168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Woody GE, McLellan AT, O'Brien CP, Druley P, Navaline H, DePhilippis D, Stolley P, Abrutyn E. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. Journal of Acquired Immune Deficiency Syndromes. 1993;6(9):1049–1056. [PubMed] [Google Scholar]
- Metzger DS, Woody GE, O'Brien CP. Drug treatment as HIV prevention: a research update. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 55(Suppl 1):S32–S36. doi: 10.1097/QAI.0b013e3181f9c10b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moatti JP, Carrieri MP, Spire B, Gastaut JA, Cassuto JP, Moreau J. Adherence to HAART in French HIV-infected injecting drug users: the contribution of buprenorphine drug maintenance treatment. The Manif 2000 study group. [Clinical Trial Research Support, Non-U.S. Gov't] AIDS. 2000;14(2):151–155. doi: 10.1097/00002030-200001280-00010. [DOI] [PubMed] [Google Scholar]
- Novak RM, Chen L, MacArthur RD, Baxter JD, Huppler Hullsiek K, Peng G, Xiang Y, Henely C, Schmetter B, Uy J, van den Berg-Wolf M, Kozal M. Prevalence of antiretroviral drug resistance mutations in chronically HIV-infected, treatment-naive patients: implications for routine resistance screening before initiation of antiretroviral therapy. Clin Infect Dis. 2005;40(3):468–474. doi: 10.1086/427212. [DOI] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2011. Jan 10, [Google Scholar]
- Rhee S-Y, Fessel WJ, Liu TF, Marlowe NM, Rowland CM, Rode RA, Vandamme AM, Van Laethem K, Brun-Vezinet F, Calvez V, Taylor J, Hurley L, Horberg M, Shafer RW. Predictive value of HIV-1 genotypic resistance test interpretation algorithms. [Research Support, N.I.H. ExtramuralResearch Support, Non-U.S. Gov't] Journal of Infectious Diseases. 2009;200(3):453–463. doi: 10.1086/600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen BB, Simons JF, Hullsiek KH, Novak RM, Macarthur RD, Baxter JD, Huang C, Lubeski C, Turenchalk GS, Braverman MS, Desany B, Rothberg JM, Egholm M, Kozal MJ Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) Low-abundance drug-resistant viral variants in chronically HIV-infected, antiretroviral treatment-naive patients significantly impact treatment outcomes. Journal of Infectious Diseases. 2009;199(5):693–701. doi: 10.1086/596736. [DOI] [PubMed] [Google Scholar]
- Simons JF, Egholm M, Lanza J, Truenchalk G, Desany B, Ronan M, Kozal M. Ultradeep sequencing of HIV clinical samples; Paper presented at the XIV International HIV Drug Resistance Workshop; Quebec City, Quebec, Canada. 2005. [Google Scholar]
- Stanford University. HIV Drug Resistance Database. 2009 Retrieved Accessed July 5, 2009, from http://hivdb.stanford.edu/
- Sullivan LE, Barry D, Moore BA, Chawarski MC, Tetrault JM, Pantalon MV, O'Connor PG, Schottenfeld RS, Fiellin DA. A trial of integrated buprenorphine/naloxone and HIV clinical care. Clinical Infectious Diseases. 2006;43(Suppl 4):S184–S190. doi: 10.1086/508182. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Metzger DS, Fudala PJ, Fiellin DA. Decreasing international HIV transmission: the role of expanding access to opioid agonist therapies for injection drug users. Addiction. 2005;100(2):150–158. doi: 10.1111/j.1360-0443.2004.00963.x. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Moore BA, Chawarski MC, Pantalon MV, Barry D, O'Connor PG, Schottenfeld RS, Fiellin DA. Buprenorphine/naloxone treatment in primary care is associated with decreased human immunodeficiency virus risk behaviors. Journal of Substance Abuse Treatment. 2008;35(1):87–92. doi: 10.1016/j.jsat.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LE, Moore BA, O'Connor PG, Barry DT, Chawarski MC, Schottenfeld RS, Fiellin DA. The association between cocaine use and treatment outcomes in patients receiving office-based buprenorphine/naloxone for the treatment of opioid dependence. [Research Support, N.I.H. Extramural] American Journal on Addictions. 2010;19(1):53–58. doi: 10.1111/j.1521-0391.2009.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry Beirn Community Programs for Clinical Research on AIDS. Form 646: Antiretroviral Medication Self Report. 2004. [Google Scholar]
- World Health Organization. HIV/AIDS Data and Statistics. 2010 Retrieved February 2, 2011 www.who.int/hiv/data.