Abstract
Depressed mothers show negatively biased responses to their infants’ emotional bids, perhaps due to faulty processing of infant cues. This study is the first to examine depression-related differences in mothers’ neural response to their own infant’s emotion faces, considering both effects of perinatal depression history and current depressive symptoms. Primiparous mothers (n = 22), half of whom had a history of major depressive episodes (with one episode occurring during pregnancy and/or postpartum), were exposed to images of their own and unfamiliar infants’ joy and distress faces during functional neuroimaging. Group differences (depression vs. no-depression) and continuous effects of current depressive symptoms were tested in relation to neural response to own infant emotion faces. Compared to mothers with no psychiatric diagnoses, those with depression showed blunted responses to their own infant’s distress faces in the dorsal anterior cingulate cortex. Mothers with higher levels of current symptomatology showed reduced responses to their own infant’s joy faces in the orbital-frontal cortex and insula. Current symptomatology also predicted lower responses to own infant joy–distress in left-sided prefrontal and insula/striatal regions. These deficits in self-regulatory and motivational response circuits may help explain parenting difficulties in depressed mothers.
Keywords: maternal depression, infant, faces, emotion, fMRI
Introduction
An important avenue to understanding the parenting difficulties of mothers suffering from depression—and ultimately their children’s risk for affective disorder (e.g., Goodman et al., 2011; Hammen et al., 2012)—is the identification of differential neural responses to infant cues (Squire & Stein, 2003). Although a pattern of insensitive, negatively biased responding to their infant’s emotional bids has long been observed among both currently depressed mothers and those with a depression history, it is still unclear exactly what drives these behaviors (Foster et al., 2008; Lovejoy et al., 2000). The present study aims to shed light on brain mechanisms underlying such response deficits by investigating depression-related differences in maternal neural response to their infant’s positive and negative emotion faces.
Normative emotional face processing involves a network of occipital, limbic/paralimbic, striatal, and prefrontal regions, with somewhat different emphases depending on the type of emotion target and characteristics of the respondent (Fusar-Poli et al., 2009). Individuals with major depressive disorder (MDD) have shown reduced activation to negative emotion (sad and/or angry) faces in orbital-frontal cortex (OFC), anterior cingulate cortex (ACC), and insula regions that play a critical role in generating and regulating emotional responses (Lee et al., 2008; Townsend et al., 2010). Even in depression remission, blunted OFC responses to negative emotion faces have been found, suggesting this may be a trait marker of MDD; at the same time, researchers found increased medial prefrontal cortex (mPFC) activation with longer periods of euthymia, pointing to dynamic recovery of certain aspects of neural function in the absence of current depressive symptoms (Kerestes et al., 2012). In response to positive emotion (happy) faces, reduced activation and/or functional amygdala connectivity has also been observed in several emotion-relevant prefrontal and ACC areas, at least for a subset of MDD patients (i.e., females, high ruminators)(Almeida et al., 2011; Thomas et al., 2011). The importance of restoring emotion face sensitivity in these regions is underlined by research showing increased ACC (to happy faces), insula, and OFC (to sad faces) activation in MDD patients undergoing antidepressant treatment (Anderson et al., 2011).
Although the bulk of depression research has not dealt with mothers in particular, a study of postpartum depression also revealed reduced prefrontal (mPFC) activation to negative emotion faces, suggesting a common emotion appraisal and/or regulation deficit in various forms of depression (Moses-Kolko et al., 2010). Another study indicated depression effects on paracingulate responses to the face of an attachment figure’s (the participant’s mother)(Zhang et al., 2011). However, none of these studies has examined neural response to participants’ own children’s faces, limiting conclusions that can be made about depression-related disturbances in response to offspring.
The emerging field of parental neuroimaging has shed light on neural circuits typically activated by visual and/or auditory infant stimuli; these comprise a range of subcortical and cortical (especially prefrontal) regions involved in motivation and emotional processing, including the regions discussed above (see reviews by Swain, 2011; Swain et al., 2007). Mothers have shown heightened activation to pictures of their own child, relative to unfamiliar children, in a number of brain areas implicated in emotional response and empathy—the insula, OFC, ACC, and striatum (caudate, putamen)(Bartels & Zeki, 2004; Leibenluft et al., 2004). Further research on maternal response to specific infant emotions has demonstrated activation to own compared to unfamiliar infant happy faces in reward-related striatal (putamen) and substantianigra regions, with progressively lower activations in these areas for neutral and sad face comparisons(Strathearn et al., 2008); however, these researchers found diminished ventral striatal activation to own infant happy faces in mothers with insecure/dismissing relative to those with secure adult attachment (Strathearn et al., 2009). There is also evidence that mothers’ positive mood while viewing their own infant’s (predominantly happy) faces is related to OFC activity (Nitschke et al., 2004). As outlined by Swain and colleagues (2007), the next step in this research involves going beyond normative maternal responses to test effects of maternal psychopathology on neural activation to their infants’ emotion cues. Subclinical levels of maternal anxiety and depression have been shown to relate to reduced amygdala activation to own infant positive emotion faces, suggesting mood-related blunting of normative maternal responses that could underlie difficulties in parenting and attachment formation (Barrett et al., 2012). However, these findings were limited to positive emotion stimuli in the early postpartum and did not involve diagnosable psychopathology, leaving significant questions about lasting impacts of maternal depression on a range of emotional responses.
The current investigation was designed to take this next step in maternal neuroimaging research by examining neural response to their 18-month-old infant’s joy and distress faces in a group of first-time mothers, half of whom had experienced several major depressive episodes including one episode during the perinatal period (pregnancy and/or postpartum), and half of whom had never experienced a major depressive episode. Given indications that both depression history and current level of symptoms may impact brain function (e.g., Kerestes et al., 2012; Liotti et al., 2002), both of these factors were considered as predictors of neural response. Based on the depression/parental neuroimaging literature reviewed above and our own previous findings in this sample for depression effects on response to cry sound (Laurent & Ablow, 2012), we predicted that mothers with a history of perinatal depression and/or higher current depressive symptoms would show lower activation to their own infant’s relative to other infants’ emotion faces in the OFC, insula, ACC, and striatum. We further hypothesized, in line with a general negativity bias in depression (e.g., Erickson et al., 2005; Murphy et al., 1999), that these depressed mothers would show reduced activation to positive vs. negative emotion in their own infants.
Method
Participants
A community sample of primiparous mothers of 15–18-month-old infants was recruited from the Women Infants Children (WIC) program. Mothers who indicated interest by responding to a flier were contacted for further screening. After giving written consent to participate (per University of Oregon IRB-approved guidelines), they were screened for MRI contraindications and psychopathology using the Structured Clinical Interview for the DSM-IV (SCID). To be in the “depressed” group, the mother had to meet criteria for a major depressive episode (MDE) during the perinatal period and report ongoing symptoms at least at the level of minor depression. To be in the “non-depressed” group, the mother could not meet criteria for any axis I disorder. The final sample (n = 22, 11 per group) represents the subset of those screened into the study (n = 34) who were eligible to complete the study; reasons for discontinuation included new pregnancy and failure to complete a well baby visit or lack of infant cry (required for another part of the study as reported in 19) at the visit.
Participants represented a low SES group (see Table 1, top panel). The majority of mothers (77%) were Caucasian (14% African American, 9% Latina). Most had experienced a vaginal delivery (18% caesarian section). Mothers tended to be young (M age = 24.1, SD = 4.1) and unmarried (64%). Non-depressed mothers did not differ from depressed mothers on any demographic variables, including marital status; however, non-depressed mothers were more likely to report being in a stable married or cohabiting relationship with the biological father of their infant (n = 8 vs. 3 of the depressed group, χ2[1] = 4.54, p = .03).
Table 1.
Sample Characteristics
| SES Variables | Percent of Total Sample |
|---|---|
| Education | |
| High School | 32% |
| Some College | 50% |
| College Graduate | 18% |
| Yearly Household Income | |
| < $5,000 | 9% |
| $5,000–$9,999 | 23% |
| $10,000–$20,000 | 14% |
| $20,001–$40,000 | 36% |
| $40,001–$60,000 | 14% |
| > $60,000 | 4% |
| Diagnostic Variables | M (SD), Range |
| MDD Onset Age | 14.3 (2.95), 11–21 |
| Number of MDE’s | 4.25 (2.36), 2–too many to count/indistinct |
| Comorbid Diagnoses | 2.36 (1.57), 1–6 |
| Past Substance Abuse/ Dependence | n = 8 |
| PTSD | n = 2 |
| Other Anxiety Disorder | n = 8 |
| Eating Disorder | n = 2 |
Note. MDD = major depressive disorder; MDE = major depressive episode. MDE descriptives are based on the 9 participants who offered a count (2 reported too many/indistinct episodes to count).
Mothers in the depressed group all reported MDD onset prior to the perinatal period; thus, they represent a group prone to recurrent depression, and symptoms were not unique to perinatal events (see Table 1, lower panel). During the perinatal period, half had a MDE during pregnancy (n = 3) or postpartum (n = 3) only, with the remainder depressed across both (n = 5). In keeping with the less controlled but ecologically valid community sampling approach, depressed mothers were allowed to have comorbidities, though MDD had to be the dominant current complaint.
Closer to scanning (within 1 week), mothers reported on current depressive symptoms using the Center for Epidemiologic Symptoms Depression (CESD) scale (Radloff, 1977). As expected, the depressed group scored significantly higher (M = 24.18, SD = 9.37) than non-depressed (M = 7.45, SD = 6.19). Yet a degree of intergroup overlap (non-depressed range 0–20, depressed range 10–32) suggested diagnostic status did not fully capture current depressive symptoms. Therefore, CESD scores were considered as an additional index of depression severity. At this assessment, 3 of the depressed mothers reported having begun antidepressant (SSRI) treatment (duration 2–4 weeks). Dropping these cases did not alter the pattern of results; in the interests of retaining balanced groups and reflecting a range of treated/untreated depression syndromes, they were kept in analyses.
Stimulus Collection and Presentation
Researchers attended participants’ 18-month well-baby visits and took digital pictures of the infant both prior to and immediately following standard immunization injections. The 3 images best representing the “Duchenne smile face” and the 3 best representing the “cry-distress face” as outlined by Oster’s (2006) Baby FACS manual were selected for the own infant joy and distress stimuli, respectively. In particular, smile faces were selected for lip corner and cheek raising criteria, and cry faces selected for brow knotting and cheek raising accompanied by nasiolabial furrow deepening. Each image was cropped closely to the infant’s head, and any distracting visual elements remaining in the picture (i.e., a parent’s hand) were edited out using GIMP photo editing tools. All final images were sized to 6 × 6 in. with 72 pixels per in. resolution. In addition to the own infant stimuli, photos of unfamiliar 18-month-old infants (from mothers not included in the study sample) were collected and edited using these procedures. The same set of other infant joy (3 images) and distress (3 images) faces was presented to all participants.
The stimulus protocol was created as a block design to be presented via the Presentation 10.0 program and consisted of two 9-minute runs. Each run contained 5 repetitions of own infant joy, other infant joy, own infant distress, other infant distress, each separated by rest (blank screen). Stimulus blocks were 18 seconds (6 second presentation of each picture) and rest blocks 10 seconds. Stimulus presentation order was counterbalanced within and across participants. Participants were simply instructed to look at the images to allow the most natural range of response to infant faces. Images were projected onto a screen at the back of the scanner, which participants could see via a mirror positioned over their eyes.
Scanning
MR imaging was carried out with a 3T Siemens Allegra 3 magnet. A standard birdcage coil was used to acquire data from the whole brain. Sessions began with a shimming routine to optimize signal-to-noise ratio, followed by a fast localizer scan (FISP) and Siemens Autoalign routine, 2 functional runs not used in this study, an anatomical scan, and finally the 2 functional runs reported in this study.
Functional
T2*-weighted gradient echo sequence, 64 × 64 voxel matrix, TE = 30 ms, TR = 2000ms, flip angle = 80°, 32 contiguous slices thickness = 4 mm; 273 volumes per run.
Structural
T1-weighted 3D MP-RAGE sequence, TI = 1100ms, TR = 2500ms, TE = 4.4ms, 176 transverse slices 1.0 mm thick, 256 × 176 matrix FOV = 256 mm.
Data Analysis
Functional imaging data were analyzed with tools from the fMRIB Software Library (FSL v.4.1). Preprocessing steps included motion correction with MCFLIRT, nonbrain structure removal with BET, spatial smoothing using Gaussian kernel 5-mm FWHM, intensity normalization using grand mean scaling, and high-pass temporal filtering (sigma = 65s). Within-subject time series data were analyzed using FILM with local autocorrelation correction, and boxcar models describing onset/offset of each sound stimulus were convolved with a double-gamma basis function. Functional data were registered to the participant’s own high-resolution structural image (6 df) and to a standard brain (Montreal Neurological Institute template; 12 df) using FLIRT.
Within-participant and group-level analyses were carried out using FEAT v.5.98. For each participant, four explanatory variables (EVs) modeled signal associated with own infant joy, other infant joy, own infant distress, and other infant distress; zero for all 4 stimulus EVs corresponded to rest. Contrasts of parameter estimates (COPEs) for own joy > other joy and own distress > other distress tested primary hypotheses regarding response to own infant emotion faces. Own joy > distress was also tested to investigate differential response to positive vs. negative emotion in one’s own infant, and own joy/distress > rest contrasts were tested to describe signal change relative to baseline. First-level COPE images were averaged across runs using fixed-effects analysis. These served as inputs to higher-level group analyses, conducted using FLAME to model random-effects components of mixed-effects variance. AlphaSim was used to determine cluster size needed, in conjunction with intensity threshold p < .005, to achieve a false discovery rate (FDR) of .05 for whole-brain analyses (Cox, 1996). Using these criteria, activation clusters exceeding 16 voxels, or 615 mm3, were considered significant in group analyses.
Group-level analyses tested effects of depression on brain response in two ways. First, response to own infant joy and distress faces was compared between groups, i.e., non-depressed > depressed, depressed > non-depressed. Second, centered depressive symptom (CESD) scores were entered as a continuous predictor of brain response in the entire sample to investigate the explanatory power of current subjective depression, as opposed to diagnostic grouping (based more on history of a clinical syndrome). Finally, to visualize the data driving these effects, percent signal change associated with own infant visual stimuli compared to rest was computed within clusters showing significant depression-related differences.
Results
Associations among Self-report Variables
To better interpret effects of depressive symptoms—in particular, to determine whether current self-reported symptoms reflected the severity or chronicity of MDD—other diagnostic indicators were tested in relation to CESD scores. Mothers with more depressive symptoms tended to report a greater number of MDE’s (r = .35) and earlier age of onset (r = −.45), but no difference in the number of comorbid conditions. As mentioned above, demographic variables including SES indicators were unrelated to depression variables.1
Data Screening
Functional data were screened for artifacts and excessive motion (> 1 mm). One mother moved 1.24 mm during a functional run, and activations were compared with and without this participant’s data. An anterior prefrontal cluster related to depressive symptoms in both the own–other infant joy and distress face contrasts was found to be driven by this participant; this cluster was deemed unreliable and is not reported in the results below. Outlying data points were identified on the scatterplots described below; removal of such outliers only strengthened associations, so the full sample was retained in reported results.
Own Infant > Other Infant Joy Face Response
Mothers showed no significant between-group (non-depressed vs. depressed) differences in neural response to their own infant’s > other infants’ joy faces. However, mothers with lower current self-reported depressive symptoms showed greater activation in the right insula and the left inferior OFC (Table 2, panel 1; Figure 1). Plots of signal change (relative to resting baseline) associated with own infant joy in these clusters confirmed that whereas mothers with the fewest depressive symptoms tended to activate, those with the most depressive symptoms tended to deactivate to the cue.
Table 2.
Maternal Depression-Related Neural Activation to Infant Joy and Distress Face Images
| Region | BA | R/L | X | Y | Z | Size (mm3) | Peak Z |
|---|---|---|---|---|---|---|---|
| 1. Own > Other Infant Joy Activation inversely related to CESD |
|||||||
| Insula | R | 42 | −1 | 6 | 740 | 3.57 | |
| Orbital-Frontal Cortex | 11 | L | −18 | 37 | −24 | 711 | 3.81 |
| 2. Own > Other Infant Distress Non-depressed > depressed group activation |
|||||||
| Dorsal Anterior Cingulate – Paracingulate Cortex |
32 | L | −6 | 38 | 27 | 2416 | 3.55 |
| Occipital Pole | 18 | R | 18 | −87 | 16 | 697 | 3.52 |
| 3. Own Infant Joy > Distress Activation inversely related to CESD |
|||||||
| Insula extending to Putamen | L | −39 | 5 | −4 | 1421 | 3.26 | |
| Dorsal Anterior Cingulate - Supplementary Motor Cortex |
24/6 | R/L | 6 | 6 | 49 | 1115 | 3.31 |
| Inferior Frontal Gyrus – Frontal Operculum | 44 | L | −57 | 11 | 7 | 623 | 3.44 |
| Supramarginal Gyrus | 40 | L | −58 | −24 | 24 | 945 | 3.64 |
Note. Clusters met thresholding criteria based on whole-brain false discovery rate < .05. Coordinates based on Montreal Neurological Institute template. BA = putative Brodmann’s area.
Figure 1.
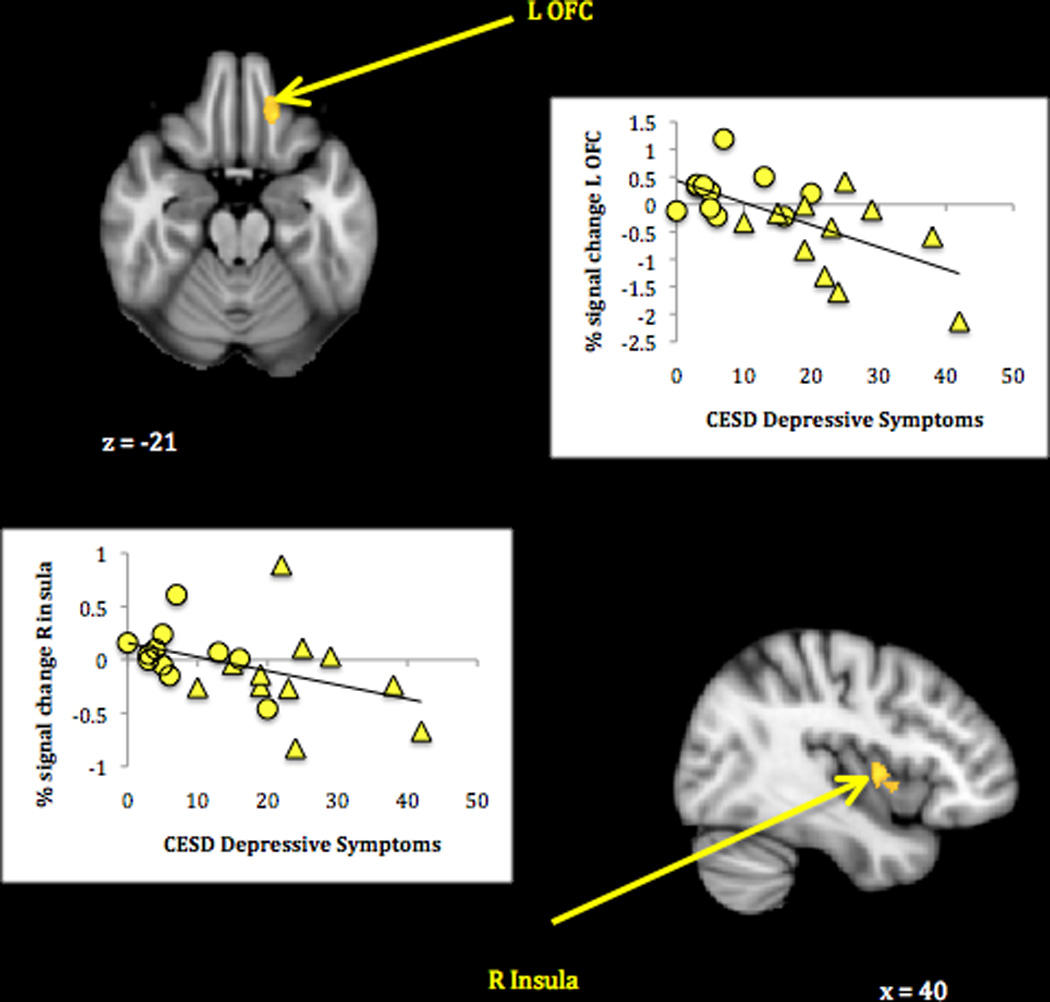
Maternal neural response to own infant compared to other infant joy faces related to lower depressive symptoms.
Note. Activations thresholded at whole-brain FDR = .05 with voxel-level threshold of Z = 2.6, p < .005. Scatterplots show BOLD signal change (compared to rest) associated with own infant joy faces in relation to mothers’ self-reported depressive symptoms. Circles represent non-depressed group mothers; triangles represent depressed group mothers.
Own Infant > Other Infant Distress Face Response
Mothers with no depression history, compared to those with MDD, showed a greater response to their own infant’s > other infants’ distress faces. In particular, the non-depressed group showed greater activation in the left dorsal ACC extending to paracingulate cortex and in the right occipital pole (Table 2, panel 2; Figure 2). An examination of signal change associated with own infant distress in this cluster showed that whereas the non-depressed mothers activated, the depressed mothers deactivated. There were no neural response differences associated with current depressive symptoms.
Figure 2.
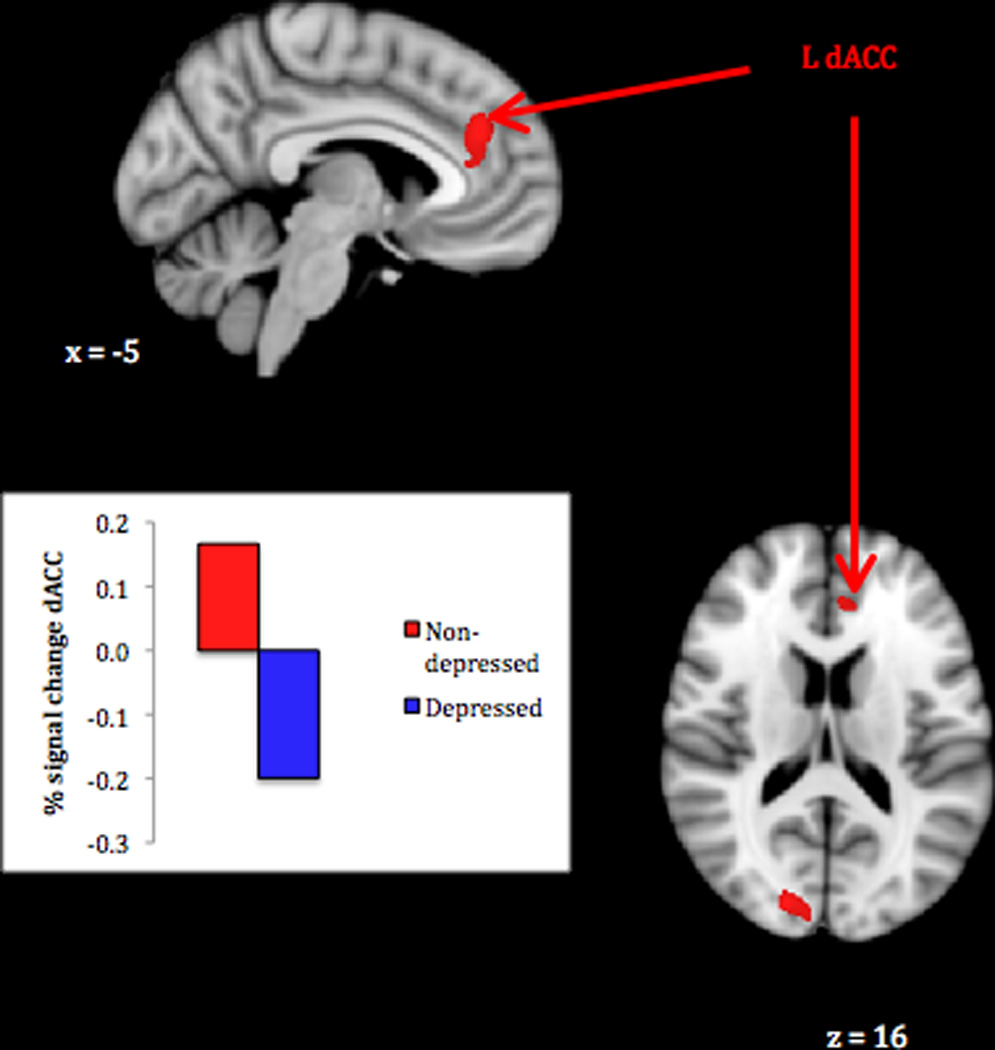
Greater maternal neural response to own infant compared to other infant distress faces in never-depressed vs. perinatal depression group.
Note. Activations thresholded at whole-brain FDR = .05 with voxel-level threshold of Z = 2.6, p < .005. Bar graph show mean BOLD signal change (compared to rest) associated with own infant distress faces in depressed and non-depressed groups.
Own Infant Joy > Distress Face Response
In a direct comparison of response their own infant’s joy > distress faces, mothers with lower current depressive symptoms showed greater activation across a set of left-sided clusters (Table 2, panel 3; Figure 3). These included the insula extending into the putamen, a caudal portion of the dorsal ACC extending to supplementary motor cortex, the inferior frontal gyrus (frontal operculum), and the anterior supramarginal gyrus. Plots of signal change associated with both the own infant joy and distress cues in these clusters revealed that whereas mothers with the fewest depressive symptoms tended to activate to the former more than the latter, those with the most depressive symptoms tended to deactivate to the former more than the latter. Again, there were no significant group differences in neural response.
Figure 3.
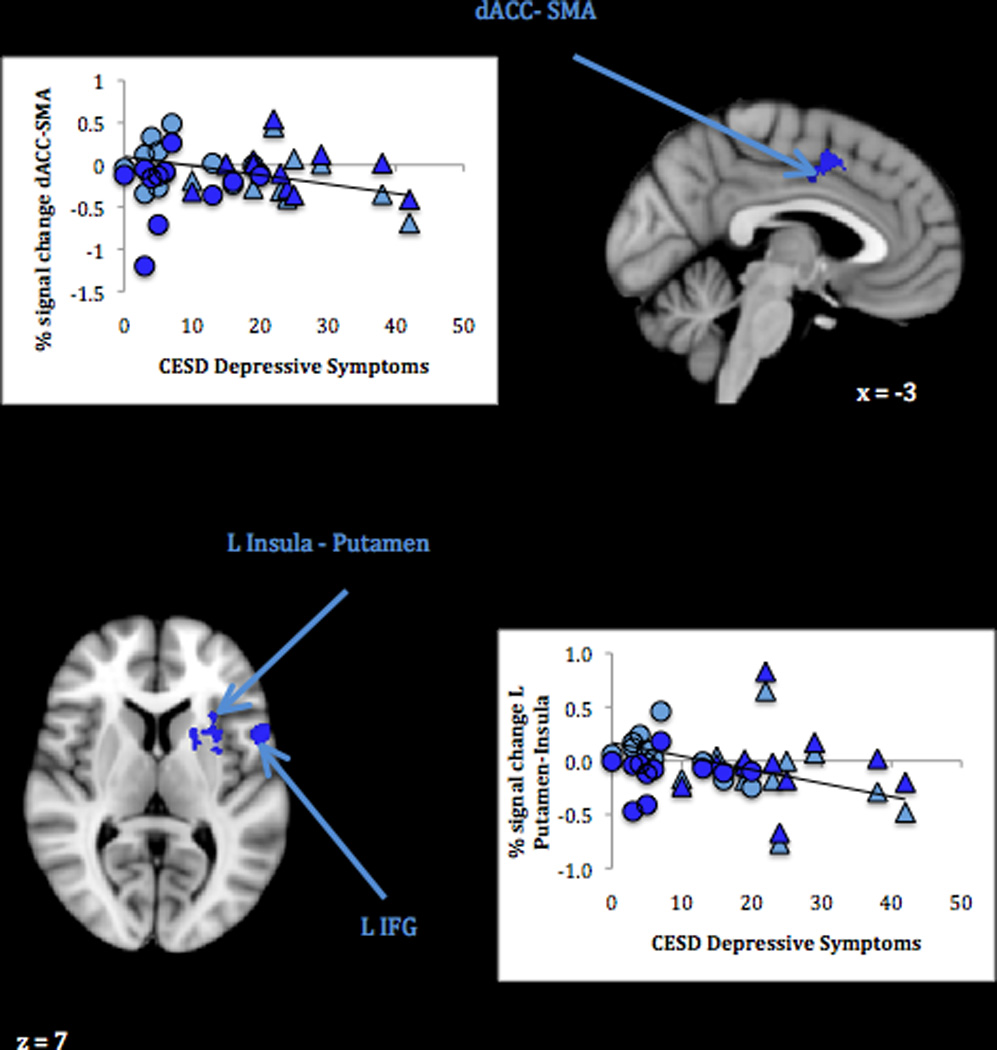
Maternal neural response to own infant joy compared to distress faces related to lower depressive symptoms.
Note. Activations thresholded at whole-brain FDR = .05 with voxel-level threshold of Z = 2.6, p < .005. Scatterplots show BOLD signal change (compared to rest) associated with own infant joy faces (light blue) and distress faces (dark blue) in relation to mothers’ self-reported depressive symptoms. Circles represent non-depressed group mothers; triangles represent depressed group mothers. Trendlines depict the relation between CESD scores and signal change for own infant joy faces; CESD scores were unrelated to signal change for own infant distress faces.
Discussion
This study confirmed predictions that mothers affected by depression would show reduced neural activation to their own infant’s emotion faces in key emotional response and regulation circuits. In particular, we found that a history of depression resulted in blunted maternal responses to their own infant’s distress faces in a region of the ACC that was also found less responsive to infant cry sound. Current depressive symptoms were further associated with suppressed maternal responses to their own infant’s joy faces in parts of the insula and OFC. Mothers with fewer depressive symptoms responded more to their own infant’s positive compared to negative facial expressions. Below, we relate these findings to previous work on emotion processing and consider implications for depressed mothers’ parenting.
Mothers reporting fewer depressive symptoms activated more to their own infant’s compared to other infants’ joy faces in neural circuits for processing emotionally relevant sensory information and generating a reward response. The insula is part of a functional “salience network” integrating emotion-relevant cues and activating emotional responses via extensive limbic and paralimbic connections (Seeley et al., 2007), and the right insula in particular is involved in implicit emotion processing (Fusar-Poli et al., 2009). Frequently implicated in empathy (Decety & Meyer, 2008), insula activation may translate the sensory information yielded by an image of her smiling infant into a felt emotional response on the mother’s part. The OFC activity accompanying this response further suggests a mutually positive emotional state, given this region’s role in valence evaluation and reward detection (Cox et al., 2005; Liu et al., 2007). A recent synthesis of parental (and non-parental) caregiving research points to the integral role of prefrontal and insula regions in compassion and caregiving behaviors (Swain et al., 2012). In mothers struggling with depression, the lack of such an empathic response to their infant’s joy may make the experience of parenting less rewarding and limit opportunities for shared positive emotion.
In response to their own infant’s compared to other infants’ distress faces, mothers with a history of major depressive episodes activated less than their never-depressed counterparts in a critical emotional judgment and decision-making node. The dACC plays an important role in the self-regulation of behavior through goal-directed action selection and performance monitoring (Ridderinkhof et al., 2004). This region is also a part of the “salience network” and can influence emotional responses via connections with paralimbic structures (Margulies et al., 2007). Mothers recruiting the dACC in response to their infant’s distress may thus be better prepared to evaluate and take control of the situation, selecting appropriate strategies to soothe their infant while remaining calm themselves. On the other hand, the lack of such a response may leave mothers with a recent depression history feeling helpless in the face of their infant’s distress, manifesting behaviorally as withdrawal or over-intrusive engagement.
In response to their own infant’s emotion faces, mothers with fewer depressive symptoms demonstrated greater activation to joy compared to distress faces in a set of left-sided regions suggestive of motivated approach, including supplementary motor and striatal areas (Harsay et al., 2011). This pattern—which overlaps with areas of normative own infant positive-negative emotion response in a previous study (Barrett et al., 2012)—implies a general readiness to respond to their infant’s positive emotions that may reinforce the more frequent positive emotional exchanges characteristic of well adjusted mother-infant dyads. Differences in striatal activation echo previous findings of dampened activity in depressed mothers responding to cry sound (Laurent & Ablow, 2012) and in insecure/dismissing mothers responding to infant faces (Strathearn et al., 2009), highlighting this region as a potentially critical component of engaged parenting. Although highly variable responses within a relatively small sample limit conclusions we can make based on the present study alone, striking convergence with brain areas previously found to be impaired by depression and/or involved in normative parental response adds confidence that the blunted responses found here help to explain parenting difficulties experienced by depressed new mothers.
Together, these activations may support the emotional mirroring observed in optimal mother-infant interactions but often lacking in exchanges between a depressed mother and her infant. It has been suggested that parents modulate their infant’s affective state by partially reflecting the infant’s facial and vocal emotion cues, supporting the infant’s recognition and regulation of emotion states (Fonagy, Gergely, & Target, 2007; Gergely & Watson, 1996). Such reflection may be facilitated by neural “mirroring”; a recent meta-anlysis highlighted mirror neuron properties in the insula, ACC, and ventral premotor cortex for emotion tasks (Molenberghs, Cunnington, & Mattingley, 2012), and mirror neuron activation has been related to individual differences in social connection and empathy (Hooker, Verosky, Germine, Knight, & D’Esposito, 2010; Iacoboni & Dapretto, 2006; Keysers & Fadiga, 2008). The integrity of several of the regions identified above appears to depend, in turn, on the quality of the mother’s own early care experiences (Kim et al., 2010), suggesting an intergenerational cascade of poor parenting and associated emotional dysregulation. That is, problematic childhood experiences that made mothers vulnerable to depression may also inhibit neurobehavioral mirroring responses to their infant’s emotional bids, restricting the infants’ developing sensitivity to their own (and down the line, their offspring’s) emotions. Interventions that enhance maternal sensitivity to a range of their own and their infant’s emotions could be especially useful in counteracting these deficits and should be investigated in relation to maternal neurobehavioral responses.
Different effects associated with depression history vs. current depressive symptoms promise to advance understanding of trait vs. state depression costs. In line with mainstream depression research showing response deficits to negative emotion faces in remitted patients, an inadequate response to distress cues may represent a lasting marker of depression affecting mothers even after acute episodes have resolved. A substantial body of research suggests depressive episodes exert lasting effects on the structure and function of the ACC, which in turn predict a more chronic, treatment-resistant course of depression (e.g., Caetano et al., 2006, Liotti et al., 2002; Mayberg et al., 1997; Takami, Okamoto, Yamashita, Okada, & Yamawaki, 2007). In this sample of repeatedly depressed mothers, reduced ACC response may thus represent a trait-like characteristic limiting recovery efforts. On the other hand, diminished response to joy cues appears to be a more malleable, state-dependent marker of mothers’ current mood states. Reduced volume and/or activity in prefrontal, insula, and striatal regions related to depression severity may contribute to symptom-related functional impairment (Kimbrell et al., 2002; Lacerda et al., 2003; Li et al., 2010; Shah, Glabus, Goodwin, & Ebmeier, 2002), but not represent the same sort of lingering depression scar. In light of current findings, the latter could be more amenable to immediate treatment targeting—i.e., mothers recovering from depression could be taught to attend to and enjoy situations affording positive interactions with their infants—whereas the work of building self-regulatory resources to better handle negative situations may take longer to effect lasting change. Further study is needed to determine exactly when such depression effects emerge and how long they can be expected to last in the course of the mother-infant relationship, guiding recommendations for intervention.
Limitations of this study point to avenues for future research. As acknowledged above, the current sample was relatively small and had limited power to detect response variants related to depression course and comorbidities. Similar research in a larger sample that includes both repeated and first-episode depression, and homogenous psychopathology subgroups, could enable detection of finer-grained response differences than we were able to observe. Mothers were recruited according to criteria that ensured uniformly low SES and (in the depressed group) chronic depression. Sampling a greater range of these characteristics would allow researchers to address independent and synergistic contributions of these risk factors to neural hypo response. It would also be important to establish whether these effects apply to mothers experiencing a first-episode perinatal depressive episode, or whether they are confined to women with a more extensive depression history. The emotion stimuli to which mothers responded were limited to two prototypical expressions identified in infant emotion research (i.e., Duchenne smile, cry face), and it could be informative to examine maternal response to more variable infant emotions and/or emotion blends while also assessing mothers’ perceptions of the infant’s state. Finally, mothers were assessed relatively late in the postnatal period (18 months). A prospective longitudinal design starting prenatally or in the early postpartum is needed to discern when depression-related response differences arise, perhaps pointing to a “critical period” for maternal depression effects.
Despite these limitations, the present study—the first to investigate neural response to infant emotion faces in depressed mothers—represents an important step toward illuminating the brain basis of maternal depression effects on parent-infant interaction. It is hoped that by identifying and remedying the roots of poor maternal responsiveness during the earliest periods of life, intergenerational cycles of social-affective dysregulation can be interrupted.
Acknowledgments
Support was provided by a National Institute of Mental Health postdoctoral fellowship (F32MH083462-02) to HL, a pilot grant from the University of Oregon Brain Biology Machine Initiative, and a grant from the National Science Foundation (0643393). The authors also wish to thank the participants and research assistants who made this research possible.
Footnotes
In addition to primary tests of depression group and symptom effects, potential impacts of depression chronicity (number of episodes, age of onset) and comorbidities were probed. These tests yielded nonsignificant results but may have been underpowered (n = 11 for testing psychopathology-related effects). SES variables (i.e., household income, maternal education) were tested as controls in depression analyses; the only difference these made in results reported below was that the occipital cluster of non-depressed > depressed response to own > other infant distress faces disappeared.
Contributor Information
Heidemarie K. Laurent, University of Wyoming
Jennifer C. Ablow, University of Oregon
References
- Almeida JRC, Kronhaus DM, Sibille EL, Langenecker SA, Versace A, LaBarbara EJ, et al. Abnormal left-sided orbitomedial prefrontal cortical-amygdala connectivity during happy and fear face processing: A potential neural mechanism of female MDD. Front Psychiatry. 2011;2:1–14. doi: 10.3389/fpsyt.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IM, Juhasz G, Thomas E, Downey D, McKie S, Deakin JFW, et al. The effect of acute citalopram on face emotion processing in remitted depression: A pharmacoMRI study. Eur Neuropsychopharmacol. 2011;21:140–148. doi: 10.1016/j.euroneuro.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Barrett J, Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, Fleming AS. Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Social Neuroscience. 2012;7:252–268. doi: 10.1080/17470919.2011.609907. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Kaur S, Brambilla P, Nicoletti M, Hatch JP, Sassi RB, Mallinger AG, Keshevan MS, Kupfer DJ, Frank E, Soares JC. Smaller cingulate volumes in unipolar depressed patients. Biol Psychiatry. 2006;59:702–706. doi: 10.1016/j.biopsych.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox SML, Andrade A, Johnsrude IS. Learning to like: A role for human orbitofrontal cortex in conditioned reward. J Neurosci. 2005;25:2733–2740. doi: 10.1523/JNEUROSCI.3360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Meyer M. From emotion resonance to empathic understanding: A social developmental neuroscience account. Dev Psychopathol. 2008;20:1053–1080. doi: 10.1017/S0954579408000503. [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets WC, Clark L, Cannon DM, Bain EE, Zarate CA, et al. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am J Psychiatry. 2005;162:2171–2173. doi: 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- Fonagy P, Gergely G, Target M. The parent-infant dyad and the construction of the subjective self. J Child Psychol Psychiatry. 2007;48:288–328. doi: 10.1111/j.1469-7610.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- Foster CJ, Garber J, Durlak JA. Current and past maternal depression, maternal interaction behaviors, and children’s externalizing and internalizing symptoms. J Abnorm Child Psychol. 2008;36:527–537. doi: 10.1007/s10802-007-9197-1. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Gergely G, Watson JS. The social biofeedback theory of parental affect-mirroring: The development of emotional self-awareness and self-control in infancy. Int J Psychoanal. 1996;77:1181–1212. [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: A meta-analytic review. Clin Child Fam Psych Rev. 2011;14:1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Hammen C, Hazel NA, Brennan PA, Najman J. Intergenerational transmission and continuity of stress and depression: Depressed women and their offspring in 20 years of follow-up. Psychol Med. 2012;42:931–942. doi: 10.1017/S0033291711001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay HA, Cohen MX, Oosterhof NN, Forstmann BU, Mars RB, Ridderinkhof KR. Functional connectivity of the striatum links motivation to action control in humans. J Neurosci. 2011;31:10701–10711. doi: 10.1523/JNEUROSCI.5415-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D’Esposito M. Neural activity during social signal perception correlates with self-reported empathy. Brain Res. 2010;1308:100–113. doi: 10.1016/j.brainres.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Bhagwagar Z, Nathan PJ, Meda SA, Ladouceur CD, Maloney K, et al. Prefrontal cortical response to emotional faces in individuals with major depressive disorder in remission. Psychiatry Res. 2012;202:30–37. doi: 10.1016/j.pscychresns.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Fadiga L. The mirror neuron system: New frontiers. Soc Neurosci. 2008;3:193–198. doi: 10.1080/17470910802408513. [DOI] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Newman MA, Feldman R, Swain JE. Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Dev Sci. 2010;13:662–673. doi: 10.1111/j.1467-7687.2009.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrell TA, Ketter TA, George MS, Little JT, Benson BE, Willis MW, Herscovitch P, Post RM. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51:237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- Lacerda AL, Nicoletti MA, Brambilla P, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshevan MS, Soares JC. Anatomical MRI study of basal ganglia in major depressive disorder. Psychiatry Res. 2003;124:129–140. doi: 10.1016/s0925-4927(03)00123-9. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. A cry in the dark: Depressed mothers show reduced neural activation to their own infant’s cry. SCAN. 2012;7:125–134. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Seok J, Lee B, Cho SW, Yoon B, Lee K, et al. Neural correlates of affective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:778–785. doi: 10.1016/j.pnpbp.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Li CT, Lin CP, Chou KH, Chen IY, Hsieh JC, Wu CL, Lin WC, Su TP. Structural and cognitive deficits in remitting and non-remitting recurrent depression: A voxel-based morphometric study. Neuroimage. 2010;50:347–356. doi: 10.1016/j.neuroimage.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: Mood challenges in patients with remitted unipolar depression. Am J Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, Neuman G : Maternal depression and parenting behavior: A meta-analytic review. Clin Psychol Rev. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: A potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev. 2012;36:341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167:1373–1380. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, et al. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. NeuroImage. 2004;21:583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Oster H. Baby FACS: Facial Action Coding System for Infants and Young Children. Unpublished monograph and coding manual. New York University; 2006. [Google Scholar]
- Radloff The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PJ, Glabus MF, Goodwin GM, Ebmeier KP. Chronic, treatment-resistant depression and right fronto-striatal atrophy. Br J Psychiatry. 2002;180:434–440. doi: 10.1192/bjp.180.5.434. [DOI] [PubMed] [Google Scholar]
- Squire S, Stein A. Functional MRI and parental responsiveness: A new avenue into parental psychopathology and early parent-child interactions? Br J Psychiatry. 2003;183:481–483. doi: 10.1192/bjp.183.6.481. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacol. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague R. What’s in a smile? Maternal brain responses to infant facial cues. Pediatr. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE. The human parental brain: In vivo neuroimaging. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1242–1254. doi: 10.1016/j.pnpbp.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Konrath S, Brown SL, Finegood E, Akce LB, Dayton CJ, Ho SS. Parenting and beyond: Common neurocircuits underlying parental and altruistic caregiving. Parent Sci Pract. 2012;12:115–123. doi: 10.1080/15295192.2012.680409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami H, Okamoto Y, Yamashita H, Okada G, Yamawaki S. Attenuated anterior cingulate activation during a verbal fluency task in elderly patients with a history of multiple-episode depression. Am J Geriatr Psychiatry. 2007;15:594–603. doi: 10.1097/01.JGP.0b013e31802ea919. [DOI] [PubMed] [Google Scholar]
- Thomas EJ, Elliott R, McKie S, Arnone D, Downey D, Juhasz G, et al. Interaction between a history of depression and rumination on neural response to emotional faces. Psychol Med. 2011;41:1845–1855. doi: 10.1017/S0033291711000043. [DOI] [PubMed] [Google Scholar]
- Townsend JD, Eberhart NK, Bookheimer SY, Eisenberger NI, Foland-Ross LC, Cook IA, et al. fMRI activation in the amygdala and the orbitofrontal cortex in unmedicated subjects with major depressive disorder. Psychiatry Res: Neuroimaging. 2010;183:209–217. doi: 10.1016/j.pscychresns.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yaseen ZS, Galynker II, Hirsch J, Winston A. Can depression be diagnosed by response to mother’s face? A personalized attachment-based paradigm for diagnostic fMRI. PLoS ONE. 2011;6:e77253. doi: 10.1371/journal.pone.0027253. [DOI] [PMC free article] [PubMed] [Google Scholar]


