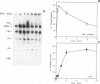
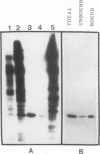
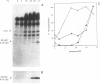
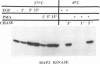
Abstract
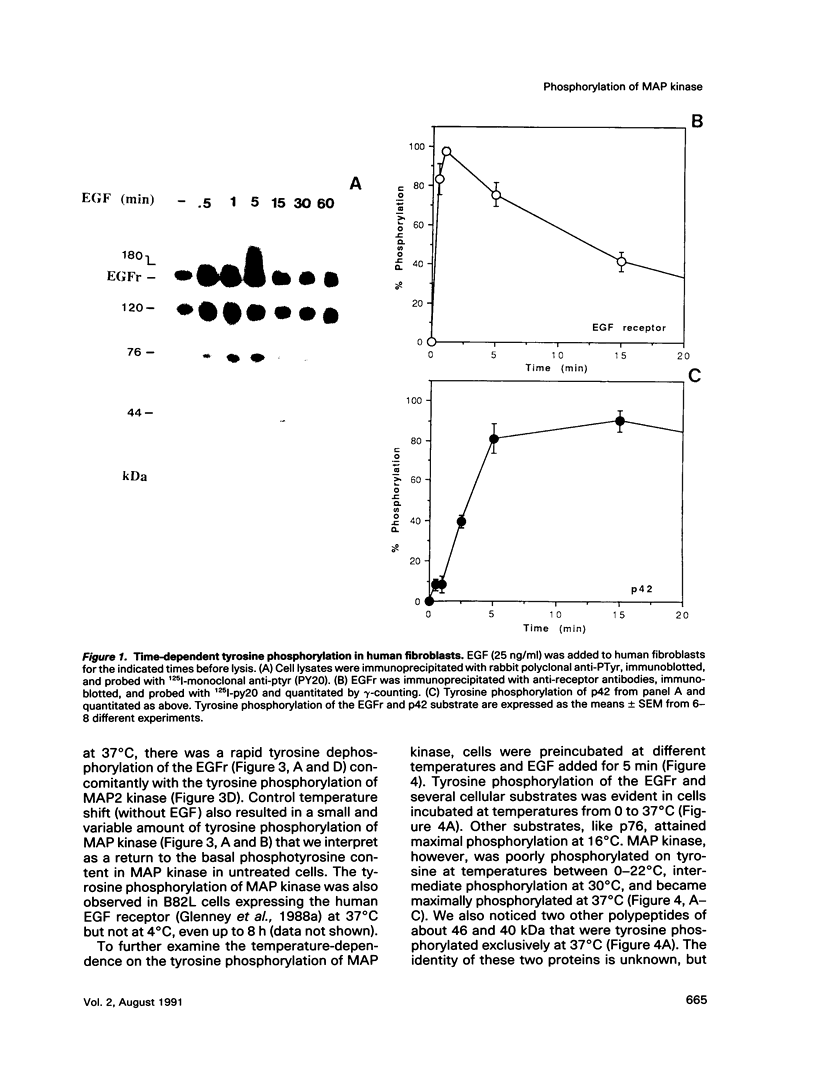
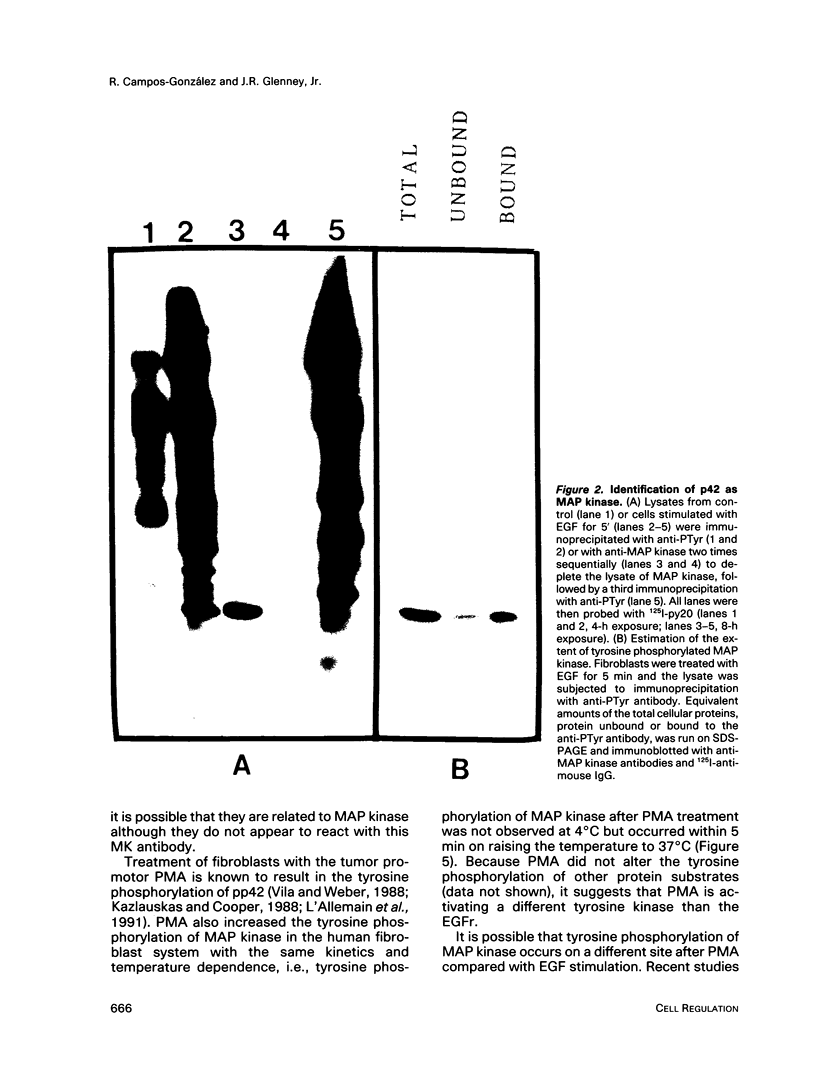
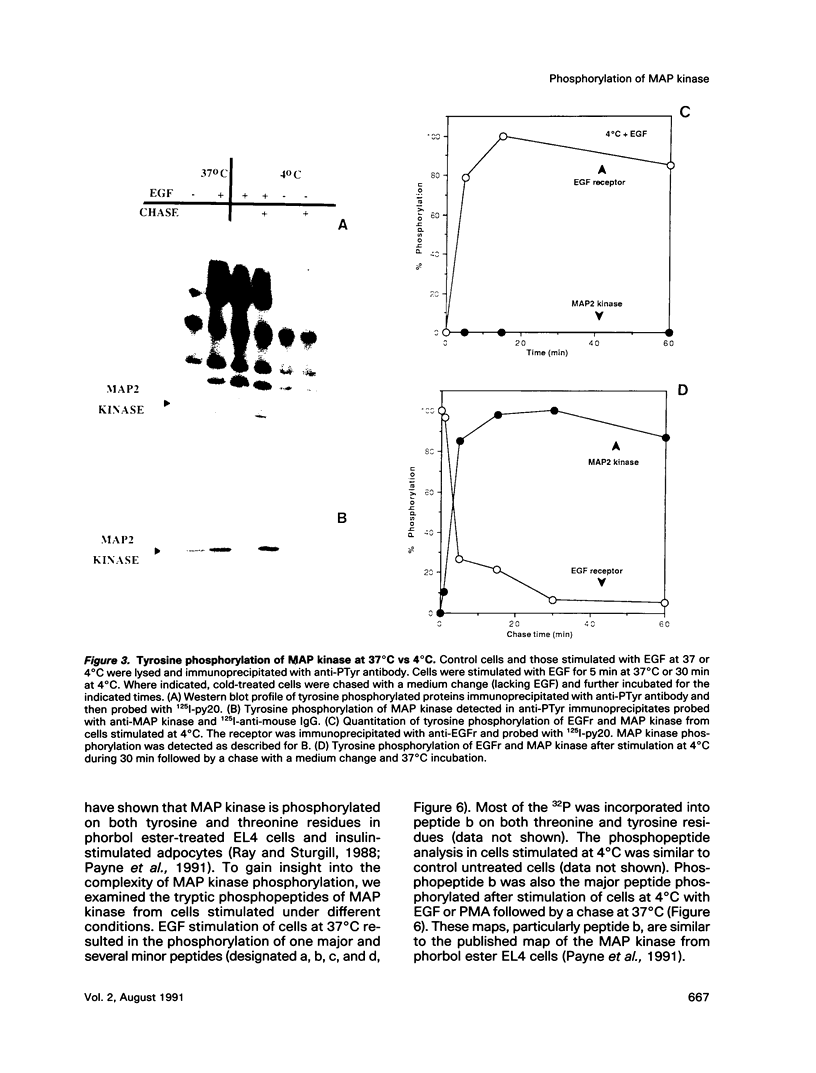
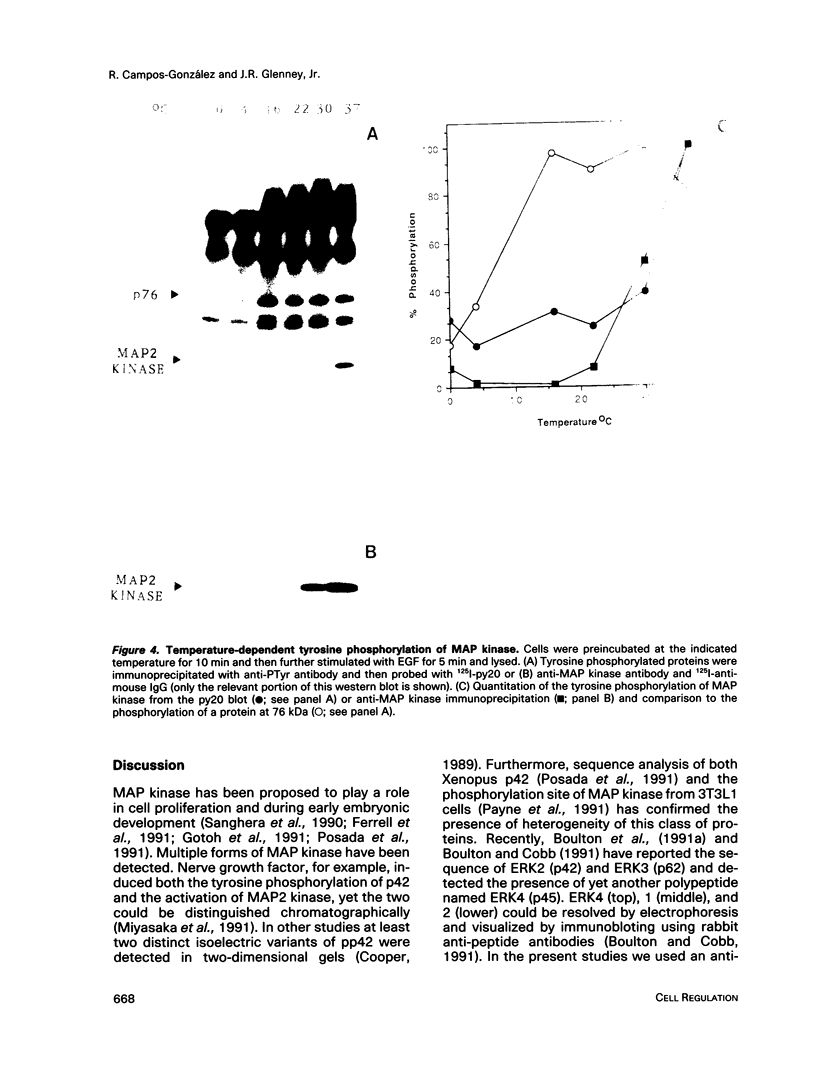
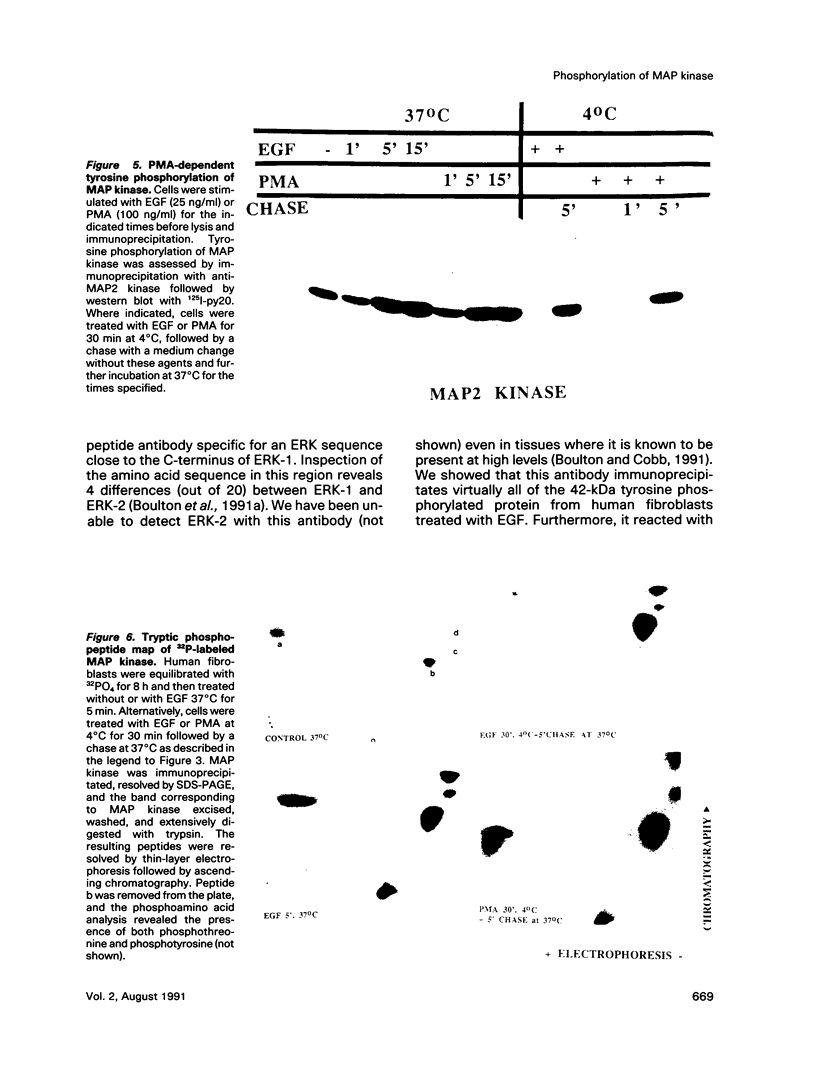
Treatment of normal human fibroblasts with epidermal growth factor (EGF) results in the rapid (0.5 min) and simultaneous tyrosine phosphorylation of the EGF receptor (EGFr) and several other proteins. An exception to this tyrosine phosphorylation wave was a protein (42 kDa) that became phosphorylated on tyrosine only after a short lag time (5 min). We identified this p42 kDa substrate as the microtubule-associated protein (MAP) kinase using a monoclonal antibody to a peptide corresponding to the C-terminus of the predicted protein (Science 249, 64-67, 1990). EGF treatment of human fibroblasts at 37 degrees C for 5 min resulted in the tyrosine phosphorylation of 60-70% of MAP kinase as determined by the percent that was immunoprecipitated with antiphosphotyrosine antibodies. Like other tyrosine kinase growth factor receptors, the EGFr is activated and phosphorylated at 4 degrees C but is not internalized. Whereas most other substrates were readily tyrosine phosphorylated at 4 degrees C, MAP kinase was not. When cells were first stimulated with EGF at 4 degrees C and then warmed to 37 degrees C without EGF, tyrosine phosphorylation of MAP kinase was again observed. Treatment of cells with the protein kinase C activator phorbol myristate acetate (PMA) also resulted in the tyrosine phosphorylation of MAP kinase, and again only at 37 degrees C. Tryptic phosphopeptide maps demonstrated that EGF and PMA both induced the phosphorylation of the same peptide on tyrosine and threonine. This temperature and PMA sensitivity distinguishes MAP kinase from most other tyrosine kinase substrates in activated human fibroblasts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Seger R., Bratlien R. L., Diltz C. D., Tonks N. K., Krebs E. G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991 Mar 5;266(7):4220–4227. [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Cobb M. H. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991 May;2(5):357–371. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T. G., Gregory J. S., Cobb M. H. Purification and properties of extracellular signal-regulated kinase 1, an insulin-stimulated microtubule-associated protein 2 kinase. Biochemistry. 1991 Jan 8;30(1):278–286. doi: 10.1021/bi00215a038. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Yancopoulos G. D., Gregory J. S., Slaughter C., Moomaw C., Hsu J., Cobb M. H. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990 Jul 6;249(4964):64–67. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976 Jun;88(2):227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Major substrate for growth factor-activated protein-tyrosine kinases is a low-abundance protein. Mol Cell Biol. 1985 Nov;5(11):3304–3309. doi: 10.1128/mcb.5.11.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Related proteins are phosphorylated at tyrosine in response to mitogenic stimuli and at meiosis. Mol Cell Biol. 1989 Jul;9(7):3143–3147. doi: 10.1128/mcb.9.7.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Ellis C., Moran M., McCormick F., Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990 Jan 25;343(6256):377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- Ely C. M., Oddie K. M., Litz J. S., Rossomando A. J., Kanner S. B., Sturgill T. W., Parsons S. J. A 42-kD tyrosine kinase substrate linked to chromaffin cell secretion exhibits an associated MAP kinase activity and is highly related to a 42-kD mitogen-stimulated protein in fibroblasts. J Cell Biol. 1990 Mar;110(3):731–742. doi: 10.1083/jcb.110.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L. Structure, expression, and regulation of protein kinases involved in the phosphorylation of ribosomal protein S6. J Biol Chem. 1991 Apr 5;266(10):6007–6010. [PubMed] [Google Scholar]
- Farese R. V., Nair G. P., Sierra C. G., Standaert M. L., Pollet R. J., Cooper D. R. Insulin-like effects of epidermal growth factor and insulin-like growth factor-I on [3H]2-deoxyglucose uptake, diacylglycerol generation and protein kinase C activation in BC3H-1 myocytes. Biochem J. 1989 Aug 1;261(3):927–934. doi: 10.1042/bj2610927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Wu M., Gerhart J. C., Martin G. S. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991 Apr;11(4):1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Chen W. S., Lazar C. S., Walton G. M., Zokas L. M., Rosenfeld M. G., Gill G. N. Ligand-induced endocytosis of the EGF receptor is blocked by mutational inactivation and by microinjection of anti-phosphotyrosine antibodies. Cell. 1988 Mar 11;52(5):675–684. doi: 10.1016/0092-8674(88)90405-9. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989 Jun;108(6):2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Matsuda S., Shiina N., Kosako H., Shiokawa K., Akiyama T., Ohta K., Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991 Jan 17;349(6306):251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- Hillman G. M., Schlessinger J. Lateral diffusion of epidermal growth factor complexed to its surface receptors does not account for the thermal sensitivity of patch formation and endocytosis. Biochemistry. 1982 Mar 30;21(7):1667–1672. doi: 10.1021/bi00536a030. [DOI] [PubMed] [Google Scholar]
- Hoshi M., Nishida E., Sakai H. Activation of a Ca2+-inhibitable protein kinase that phosphorylates microtubule-associated protein 2 in vitro by growth factors, phorbol esters, and serum in quiescent cultured human fibroblasts. J Biol Chem. 1988 Apr 15;263(11):5396–5401. [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Protein kinase C mediates platelet-derived growth factor-induced tyrosine phosphorylation of p42. J Cell Biol. 1988 Apr;106(4):1395–1402. doi: 10.1083/jcb.106.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M. Diverse mitogenic agents induce rapid phosphorylation of a common set of cellular proteins at tyrosine in quiescent mammalian cells. J Biol Chem. 1985 Feb 10;260(3):1771–1779. [PubMed] [Google Scholar]
- L'Allemain G., Sturgill T. W., Weber M. J. Defective regulation of mitogen-activated protein kinase activity in a 3T3 cell variant mitogenically nonresponsive to tetradecanoyl phorbol acetate. Mol Cell Biol. 1991 Feb;11(2):1002–1008. doi: 10.1128/mcb.11.2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Yeung Y. G., Stanley E. R. Tyrosine phosphorylation of a common 57-kDa protein in growth factor-stimulated and -transformed cells. J Biol Chem. 1991 Apr 15;266(11):6808–6814. [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meier K. E., Licciardi K. A., Haystead T. A., Krebs E. G. Activation of messenger-independent protein kinases in wild-type and phorbol ester-resistant EL4 thymoma cells. J Biol Chem. 1991 Jan 25;266(3):1914–1920. [PubMed] [Google Scholar]
- Miyasaka T., Sternberg D. W., Miyasaka J., Sherline P., Saltiel A. R. Nerve growth factor stimulates protein tyrosine phosphorylation in PC-12 pheochromocytoma cells. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2653–2657. doi: 10.1073/pnas.88.7.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. D., Martinez R., Weber M. J. Tyrosine phosphorylation of specific proteins after mitogen stimulation of chicken embryo fibroblasts. Mol Cell Biol. 1983 Mar;3(3):380–390. doi: 10.1128/mcb.3.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Meier K. E., Krebs E. G. Rapid microassay for protein kinase C translocation in Swiss 3T3 cells. Biochemistry. 1986 Dec 30;25(26):8348–8353. doi: 10.1021/bi00374a002. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S., Paddon H. B., Quayle K. A., Brownsey R. W. Identification of a major maturation-activated acetyl-CoA carboxylase kinase in sea star oocytes as p44mpk. Biochem J. 1991 Mar 15;274(Pt 3):759–767. doi: 10.1042/bj2740759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada J., Sanghera J., Pelech S., Aebersold R., Cooper J. A. Tyrosine phosphorylation and activation of homologous protein kinases during oocyte maturation and mitogenic activation of fibroblasts. Mol Cell Biol. 1991 May;11(5):2517–2528. doi: 10.1128/mcb.11.5.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1502–1506. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghera J. S., Paddon H. B., Bader S. A., Pelech S. L. Purification and characterization of a maturation-activated myelin basic protein kinase from sea star oocytes. J Biol Chem. 1990 Jan 5;265(1):52–57. [PubMed] [Google Scholar]
- Sanghera J. S., Paddon H. B., Pelech S. L. Role of protein phosphorylation in the maturation-induced activation of a myelin basic protein kinase from sea star oocytes. J Biol Chem. 1991 Apr 15;266(11):6700–6707. [PubMed] [Google Scholar]
- Sengupta A., Liu W. K., Yeung Y. G., Yeung D. C., Frackelton A. R., Jr, Stanley E. R. Identification and subcellular localization of proteins that are rapidly phosphorylated in tyrosine in response to colony-stimulating factor 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8062–8066. doi: 10.1073/pnas.85.21.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik E. Y., Margolis B., Mohammadi M., Lowenstein E., Fischer R., Drepps A., Ullrich A., Schlessinger J. Cloning of PI3 kinase-associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell. 1991 Apr 5;65(1):83–90. doi: 10.1016/0092-8674(91)90410-z. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M., Carpenter G. Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol. 1984 Mar;98(3):1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takishima K., Griswold-Prenner I., Ingebritsen T., Rosner M. R. Epidermal growth factor (EGF) receptor T669 peptide kinase from 3T3-L1 cells is an EGF-stimulated "MAP" kinase. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2520–2524. doi: 10.1073/pnas.88.6.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Vila J., Weber M. J. Mitogen-stimulated tyrosine phosphorylation of a 42-kD cellular protein: evidence for a protein kinase-C requirement. J Cell Physiol. 1988 May;135(2):285–292. doi: 10.1002/jcp.1041350216. [DOI] [PubMed] [Google Scholar]