Abstract
Behavioral evidence from the young suggests spatial cues that orient attention toward task relevant items in visual working memory (VWM) enhance memory capacity. Whether older adults can also use retrospective cues (“retro-cues”) to enhance VWM capacity is unknown. In the current event-related potential (ERP) study, young and old adults performed a VWM task in which spatially informative retro-cues were presented during maintenance. Young but not older adults’ VWM capacity benefitted from retro-cueing. The contralateral delay activity (CDA) ERP index of VWM maintenance was attenuated after the retro-cue, which effectively reduced the impact of memory load. CDA amplitudes were reduced prior to retro-cue onset in the old only. Despite a preserved ability to delete items from VWM, older adults may be less able to use retrospective attention to enhance memory capacity when expectancy of impending spatial cues disrupts effective VWM maintenance.
Introduction
Visual working memory (VWM) refers to the system in which we represent visual information after it is no longer available via sensory input. VWM is a capacity limited system and it is believed that only a few items can be maintained at any given time. The capacity limit varies across individuals and is believed to contribute to individual differences in episodic memory (Unsworth & Spillers, 2010), fluid intelligence (Oberauer, Schulze, Wilhelm, & Suss, 2005), focus switching (Unsworth & Engle, 2008), and visual search (Bleckley, Durso, Crutchfield, Engle, & Khanna, 2003). VWM capacity declines with healthy aging and may consequently contribute to age-related impairments in various cognitive measures (Verhaeghen & Salthouse, 1997). Although the exact nature of the working memory deficit in aging is unclear, the inhibitory deficit hypothesis of aging suggests that older adults’ reduced ability to inhibit processing of task-irrelevant information contributes to age-related impairments in various cognitive measures, including working memory capacity (Hasher & Zacks, 1988). That is, interference from irrelevant distracters reduces the ability to attend to and encode relevant items. Specifically, inhibition deficit theory proposes that working memory impairments may arise from dysfunction in multiple processes, including limiting memory access to task-relevant information, and deletion of information in working memory that is no longer relevant. Some researchers have claimed evidence exists for dysfunction in both processes across different tasks (Connelly & Hasher, 1993; Connelly, Hasher, & Zacks, 1991; Hasher, Quig, & May, 1997; Oberauer, Wendland, & Kliegl, 2003; Rozek, Kemper, & McDowd, 2012); but see (Kemper, McDowd, & Kramer, 2006; Verhaeghen, 2011). The extent to which age-related declines in both functions contribute to VWM within the same task is less clear.
Interestingly, some behavioral evidence from VWM studies suggests that presenting a retrospective spatial cue (“retro-cue”) during the delay period in order to indicate the location of the to-be-probed item enhances memory accuracy and capacity measures in young adults (Landman, Spekreijse, & Lamme, 2003; Lepsien & Nobre, 2007; Makovski & Jiang, 2007; Makovski, Sussman, & Jiang, 2008; Matsukura, Luck, & Vecera, 2007). By directing one’s attention toward the location of the critical item, the retro-cue acts to make all other items in VWM irrelevant thereby reducing the effective memory load. If items held in VWM compete with one another for attentional resources (Bahcall & Kowler, 1999), the retro-cue benefit in memory is most likely to be observed at higher memory loads where inter-item interference is high (Lepsien & Nobre, 2007). Whether older adults like the young can make use of retro-cues to enhance VWM capacity is not known. According to inhibition deficit theory, however, if older adults were incapable of deleting task-irrelevant items from working memory, then no retro-cue benefit would be predicted for the old regardless of memory load. A non-mutually exclusive explanation to the inter-item interference account of how retro-cues might enhance performance is that they may reduce the number of comparisons needing to be made between the probe item/s and representations in VWM (Makovski, et al., 2008). That is, in theory, only one comparison needs to be made at test; that between the retro-cued item and the test probe. Any interference caused by needing to make multiple comparisons between memory and probe items would be reduced.
Previous neuroimaging evidence suggests that maintenance of information in VWM is associated with persistent activity in sensory cortex during the delay period (Ester, Serences, & Awh, 2009; Gazzaley, Rissman, & D'Esposito, 2004; Postle & D'Esposito, 1999; Stokes, Thompson, Cusack, & Duncan, 2009). This activity is localized to regions selective for processing the specific categories of stimuli held in VWM (i.e. faces, objects), is subject to top-down control from the prefrontal cortex, and is believed to contribute to VWM accuracy. Event-related potentials (ERPs) may be useful for investigating the time course of VWM and the effect of retro-cues on delay period activity. Previous studies have identified one such ERP, the “contralateral delay activity” (CDA) that is believed to reflect the persistent delay period activity in the extrastriate cortex in VWM tasks (Anderson, Vogel, & Awh, 2011; McCollough, Machizawa, & Vogel, 2007; Vogel & Machizawa, 2004). The CDA is characterized by sustained, negative-going activity over posterior scalp electrodes. It is observed for task-relevant items held in VWM that were presented in the contralateral visual field with respect to the posterior electrode location. CDA amplitude is also positively correlated with the number of items stored in VWM, as well as individual working memory capacity.
Some recent work in young adults has examined the effect of retrospective spatial cues on the CDA and working memory performance (Kuo, Stokes, & Nobre, 2012). Consistent with the idea that spatial retro-cues act to filter items from VWM that are no longer relevant, the presentation of the cues during the maintenance period reduced the magnitude of the CDA, concomitant with an increase in working memory performance indices, including sensitivity and capacity. Moreover, as the effects of the retro-cue were particularly evident at high memory loads (4 > 2), these results are consistent with the hypothesis that retro-cues reduce inter-item competition, consequently reducing the effective memory load and enhancing the probability of memory success. Collectively, these previous ERP findings suggest that the CDA tracks the maintenance of information until a subset of the items held in WM are deemed irrelevant by attentional cues.
Few studies have investigated how aging affects the CDA (Jost, Bryck, Vogel, & Mayr, 2011; Sander, Werkle-Bergner, & Lindenberger, 2011) and no studies, to our knowledge, have investigated how aging affects the interaction between retrospective attention and VWM performance. If irrelevant information enters working memory due to impaired access limitation functions in the old, and the CDA reflects maintenance of task-relevant information, a reasonable prediction from inhibition theory would be that CDA amplitudes would be attenuated for the old compared to the young. That is, interference from irrelevant information (the contralaterally presented non-targets in a typical CDA study) might reduce efficient encoding and/or maintenance of task-relevant items. With regard to retrospective attention, in order to make use of an attentional cue, one must first have a representation of the to-be-probed item active in VWM and also be able to delete those items that are no longer relevant. It follows therefore that older adults may receive less of a performance benefit from spatial retro-cues than the young and show reduced modulation of CDA amplitudes by these cues due to an impaired ability to delete irrelevant items being held in VWM. Alternatively, it may be the case that older adults can filter irrelevant items from VWM but they do so more slowly than do the young. For example, in a recent study implementing a change detection task, older adults were shown to be able to filter irrelevant items from VWM, as evidenced by the amplitude of the CDA, but only later in the delay period (Jost, et al., 2011). In this previous study, however, participants were aware of which items were relevant and which were irrelevant, based on item color, at the time of initial encoding. Thus, it is unknown whether older adults, like the young, can exercise top-down control over VWM maintenance.
In the present study, we aimed to determine the extent to which age-related impairments in access limitation and deletion functions contribute to older adults’ decline in VWM performance. We used the CDA as a marker of VWM maintenance in order to assess the degree to which aging affects the ability to limit VWM access to task-relevant items, as well as the ability to delete items from VWM that are deemed irrelevant by retrospective attentional cues. Consistent with previous studies measuring the CDA, each trial began with a visual cue that indicated whether the participant should encode items in either the left or right visual hemifield. An array of colored squares was briefly presented in each hemifield. After a fixed delay period, a single central test probe item was presented for which participants decided whether it matched an item from the memory array. For half of the trials, a spatial cue was presented during the retention interval directing the participant’s attention toward the location of the to-be-probed item, consequently reducing the effective memory load to one. Given that these spatial “retro-cues” are presented hundreds of milliseconds after the offset of the memory array, they can impact the maintenance of information in VWM but not the initial encoding of items.
The current design also allowed us to examine ERPs related to the memory probe. Specifically, if retro-cues reduce the number of comparisons that need to be made at the time of test (Makovski, et al., 2008), we would predict ERPs reflecting processes contributing to memory decisions (i.e. stimulus evaluation and categorization, “updating” the contents of working memory) to be affected. Specifically, in order to assess the influence of spatial retro-cues on these probe-related processes, we measured the latency and amplitude of the parietal-maximal P300 or “P3b” typically observed between 300–700 ms, given its well established association with working memory updating (Donchin & Coles, 1988; Polich, 2007). When a memory representation has been more recently processed and is active in working memory, less updating is needed and the latency and amplitude of the P300 are reduced. According to this theory, it is reasonable to predict shorter latencies and smaller amplitudes for test probes following retro-cues. Importantly, this predicted pattern for the P300 is not mutually exclusive to the predictions for the CDA. Indeed, if retro-cues allow inter-item interference to be reduced, and consequently more attention to be devoted to the to-be-probed item, it is likely that both CDA and P300 modulations would be observed.
Method
Participants
18 young adults (ages 18–24) were recruited from Georgia Institute of Technology, as well as from the metropolitan Atlanta community, and 18 older adults (ages 62–75) were recruited from the community. Additional participants (3 young and 3 old) were excluded due to poor performance, excessive eye movements or other EEG artifacts (e.g. alpha). All participants were right-handed, with normal or corrected to normal vision, with no reports of psychiatric or neurological disorders, vascular disease, color blindness, or psychoactive drug use. None of the participants were taking CNS-active medications. All participants were paid $10 per hour for their time, or in the case of some of the young adults, given extra credit for a psychology class. All participants signed consent forms approved by the Georgia Institute of Technology Institutional Review Board. The total session duration, including time for consent forms, EEG setup and removal, experimental and neuropsychological testing was approximately 2.5 to 3 hours. Group characteristics are displayed in Table 1.
Table 1.
Group demographics and neuropsychological test performance
| Measure | Young | Old |
|---|---|---|
| Age | 19.4 (1.58) | 67.3 (4.04)* |
| Gender | 10/18 female | 9/18 female |
| Education | 13.67 (1.45) | 16.13 (3.05)* |
| Shipley vocabulary | 34.3 (9.06) | 38.2 (9.76) |
| Digit span forward | 7.71 (1.26) | 7.22 (1.26) |
| Digit span backward | 5.59 (1.50) | 5.61 (1.29) |
| Digit-symbol substitution | 62.4 (11.0) | 47.7 (7.62)* |
| WCST (# of errors) | 14.7 (6.68) | 26.6 (12.9)* |
Note: Standard deviations in parentheses. All neuropsychological test scores are reported as raw scores.
= significantly different from Young (p < 0.05).
Neuropsychological Testing
Immediately following EEG recording, all participants were administered a neuropsychological test battery. This battery assessed processing speed, working memory, executive function, and semantic memory and was administered to ensure no large group differences in performance due to cognitive impairment. The neuropsychological battery consisted of digit-symbol substitution and digit span forward and backward from the Wechsler Adult Intelligence Test (WAIS-R) (Wechsler, 1981), the Wisconsin Card Sorting Test (WCST) 64 card version (Lezak, 1995), and the Shipley Vocabulary Test (Shipley, 1946). This battery lasted approximately 20–30 minutes. Results are shown in Table 1.
Design and Procedure
Participants were provided with a short practice block containing 32 trials immediately prior to beginning the experiment. Care was taken to ensure that participants fully understood the task before beginning. If necessary, the practice block was repeated to achieve full competence. Participants were seated in a sound-attenuated and dimly lit room approximately 2 feet from the computer monitor. Participants were instructed to fixate centrally for the duration of the trial and to minimize head and eye movements. Participants were asked to respond as quickly and accurately as possible. Participants responded with the first two fingers of the right hand.
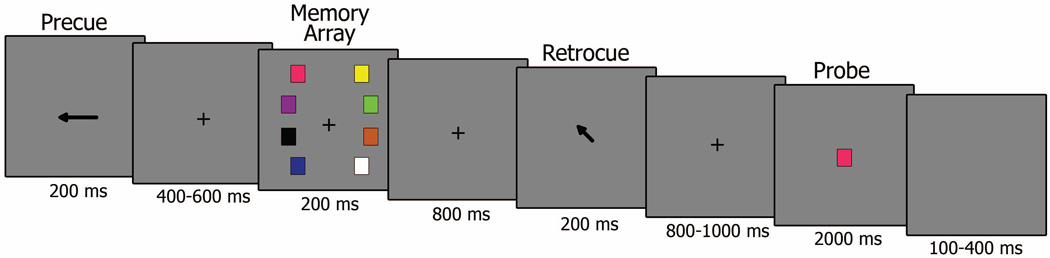
A schematic of the design is shown in Figure 1. All stimuli were presented against a gray background. The colored square memory stimuli were presented in one of 8 possible colors (pink, purple, black, blue, yellow, green, orange, and white) and each subtended 0.67° of visual angle. The experiment was divided into 12 blocks each containing 63 trials. Piloting results suggested that older adults were unable to effectively perform the task when load and cue type were randomized, as in some previous young adult studies (Kuo, et al., 2012). Consequently, we chose to present trials in separate blocks based on cue and load type. There were 2 blocks for each VWM load (2, 3, 4 items) and cue type (retro-cue, no cue), such that participants would execute 63 consecutive trials of only one load and cue type before proceeding to the next block. The order of blocks was randomized within and across participants. Each trial began with a precue that indicated the side of the screen to which participants should direct their attention. Half of the trials within each of the 12 blocks were presented with a left hemifield cue and half with a right hemifield cue in a random order. Following a randomized interstimulus interval (400–600 ms in 50 ms increments), the memory array was presented containing two, three or four colored squares in each hemifield. Participants were asked to try to remember as many squares as possible from the precued hemifield. There were 4 possible positions within each hemifield arranged in an ellipse 2.2° of visual angle from the center of the screen. After an 800 ms delay, for half of the trials, a spatial retro-cue was presented in which a centrally located arrow (0.87° of visual angle) pointed to one of the 4 possible locations in the precued hemifield. This spatial retro-cue always indicated the location of the item in VWM that would be subsequently tested (100% valid). In no cue trials, a centrally located cross was presented that provided no information about which item would subsequently be tested. Following a randomized interstimulus interval (800–1000 ms in 50 ms increments), a centrally located colored square was presented along with a response cue prompting participants to decide whether the square was presented in the previous memory array (50% target present) or not (50% target absent). The order of target present and target absent trials was randomly presented within each block. Lastly, a randomized intertrial interval (100–400 ms in 50 ms increments) was presented.
Figure 1.
Schematic of the experimental design showing example stimuli and task requirements.
EEG Acquisition
Scalp-recorded EEG data was collected from 32 Ag-Ag/Cl electrodes using an ActiveTwo amplifier system (Biosemi, Amsterdam, Netherlands). Two additional electrodes placed on the left and right mastoid processes were used as off-line references. Four additional electrodes were placed above and below the left eye to record vertical electrooculogram (VEOG) and on the outer canthi of the left and right eyes to record horizontal electrooculogram (HEOG). EEG was recorded with 24 bit resolution and a sampling rate of 1024 Hz. All channels were off-line digitally band-pass filtered between 30 Hz and 0.01 Hz. Prior to segmentation, both vertical and horizontal eye movements were removed from the data using a method based on principal component analysis (Berg & Scherg, 1994) as available in EMSE version 5.3 (http://www.sourcesignal.com/). Extensive analysis of this method determined that there was no reduction in waveform magnitude1. Epochs containing any uncorrected artifacts (±100 µV) were removed. Trials containing incorrect behavioral responses (misses, false alarms, more or less than 1 response, RTs less than 200 ms) were excluded from all analyses. Segments were averaged separately for each participant, electrode, cue type, memory load, and hemifield of target presentation. For all analyses, ERPs were collapsed across correct responses (hits, correct rejections) to target present and target absent conditions.
EEG Analysis
Our main objective was to determine whether spatial retro-cues modulate neural activity associated with VWM maintenance as indexed by the amplitude of the CDA, measured as the difference between contralateral and ipsilateral target activity (i.e. collapsed across left and target right visual fields), and whether healthy aging affects this modulation. To this end, EEG segments were created for the assessment of the CDA from 200 ms pre-memory array onset lasting until 1800 ms post memory array onset. Mean amplitudes of the CDA were computed for two time windows: 400–900 ms after the memory array onset (100 ms before the retro-cue onset), and 1300–1600 ms after the memory array onset (100–400 ms after the retro-cue offset).2 Mean amplitudes for these windows were averaged across both hemisphere and 3 electrode locations: (CP5/CP6; P3/P4; P7/P8), in order to increase sensitivity to detect CDA modulations in both age groups (Jost, et al., 2011). These time windows and electrodes were chosen based on similar previous studies (Jost, et al., 2011; Kuo, et al., 2012; McCollough, et al., 2007; Vogel & Machizawa, 2004) and where effects were most evident for both age groups in the current data. We were careful to select electrodes and time windows for analyses for which ERP effects were observed for both age groups given that aging may affect both the latency and distribution of ERP effects. Mean amplitudes of the delay period activity were submitted to Load (2, 3, 4 items) × Hemifield (ipsilateral, contralateral) × Cue (no cue, retro-cue) × Group (young, old) ANOVAs for each time window separately.
A second objective was to determine whether spatial retro-cues modulate activity related to VWM memory decision operations. To this end, EEG segments were created from 200 ms pre-memory probe onset lasting until 2000 ms post-probe onset. Preliminary analysis of probe-related activity confirmed that there were no reliable differences between contralateral and ipsilateral conditions across the entire 2000 ms epoch. Consequently, ERPs for correct trials (collapsed across hits and correct rejections) were collapsed across visual field of target presentation. Mean amplitudes and peak latencies were computed for the time period between 300 and 1000 ms at the Pz electrode location based on similar previous studies and where effects were most evident for both groups in the current data. Mean amplitudes were submitted to a Load (2, 3, 4 items) × Cue (no cue, retro-cue) × Group (young, old) ANOVA.
Topographic maps of surface potentials, calculated by spherical spline interpolation (Perrin, Pernier, Bertrand, & Echalher, 1989) were used to display the scalp distributions of the CDA and P3b effects. For all analyses, p-values reflect the Huynh-Feldt correction where appropriate. Significant interactions at an alpha level of 0.05 were followed up with subsidiary ANOVAs to determine the source of the effects when necessary.
Results
Neuropsychological Test Results
Group characteristics and neuropsychological test scores are shown in Table 1. Older adults showed significant declines in tests of processing speed (Digit-symbol substitution), executive function and potentially working memory (WCST) [t(34)’s > 3.48, p’s < 0.001]. The older adults were more educated than the young adults [t(34) = 3.10, p = 0.004]. There were no other significant group differences [t(34)’s < 1.28].
Behavioral Results
Mean proportions of hit rates for target present trials and correct rejection rates for target absent trials are shown for no cue and retro-cue conditions for each load in Table 2. Hits and misses were defined as “match” and “non-match” responses, respectively, to target present trials while correct rejections and false alarms were defined as “non-match” and “match” responses, respectively to target absent trials. Memory accuracy measures are also shown in Table 2. Specifically, “Pr” discrimination measures calculated as hit rate – false alarm rate along with memory capacity measures “Pashler-Cowan K” calculated as N (N= set size of memory array) × (hit – false alarm rate) (Cowan, 2001; Pashler, 1988). Mean response times (RTs) for correct responses (collapsed across hit and correct rejections) are also shown in Table 2.
Table 2.
Response proportions, performances indices, and response times for each load and cue type.
| Young | Old | |||||
|---|---|---|---|---|---|---|
| Load 2 | Load 3 | Load 4 | Load 2 | Load 3 | Load 4 | |
| No Cue | ||||||
| Hit | 0.93 (0.04) | 0.82 (0.11) | 0.76 (0.12) | 0.93 (0.06) | 0.81 (0.11) | 0.67 (0.14) |
| Correct rejection | 0.97 (0.02) | 0.93 (0.08) | 0.90 (0.09) | 0.96 (0.05) | 0.91 (0.07) | 0.83 (0.12) |
| Pr | 0.90 (0.06) | 0.75 (0.17) | 0.66 (0.18) | 0.89 (0.11) | 0.72 (0.16) | 0.49 (0.20) |
| K | 1.81 (0.11) | 2.27 (0.49) | 2.62 (0.73) | 1.78 (0.21) | 2.17 (0.48) | 1.97 (0.82) |
| Response time | 656 (80.0) | 728 (79.0) | 761 (111.2) | 826 (98.3) | 940 (121.9) | 995 (131.3) |
| Retro-cue | ||||||
| Hit | 0.95 (0.05) | 0.93 (0.04) | 0.80 (0.10) | 0.94 (0.06) | 0.80 (0.09) | 0.61 (0.11) |
| Correct rejection | 0.98 (0.02) | 0.98 (0.03) | 0.95 (0.05) | 0.97 (0.02) | 0.94 (0.06) | 0.85 (0.12) |
| Pr | 0.93 (0.07) | 0.91 (0.06) | 0.75 (0.14) | 0.91 (0.07) | 0.73 (0.15) | 0.46 (0.19) |
| K | 1.81 (0.13) | 2.71 (0.19) | 2.99 (0.57) | 1.46 (0.29) | 2.20 (0.44) | 1.82 (0.79) |
| Response time | 606 (82.9) | 587 (106.7) | 607.7 (87.8) | 724 (124.8) | 824 (185.4) | 907 (161.3) |
Note: Standard deviations in parentheses.
First, in order to assess the effect of retro-cueing on response times, we conducted a Cue (no cue, retro-cue) × Load (2, 3, 4) × Group (Young, Old) ANOVA on RTs shown in Table 2. Main effects of Load [F(2,68) = 53.5, p < 0.001], Cue [F(1,34) = 40.3, p < 0.001], Group [F(1,34) = 56.6, p < 0.001] and a Load × Group interaction [F(2,68) = 15.7, p < 0.001] were observed. As can be seen in the table, the main effect of Load reflects the fact that both groups were slower to respond as load increased while the main effect of Cue confirms that retro-cueing reduced response times to targets in both groups. Importantly, the lack of Cue × Group interaction [F(1,34) < 1] suggests that both age groups received a similar response time benefit. The same ANOVA was additionally conducted on the log-transformed RTs in order to ensure that the Load × Group interaction was not simple an artifact of multiplicative slowing (Faust, Balota, Spieler, & Ferraro, 1999). A reliable Load × Group interaction remained after the log transformation [F(2,68) = 10.5, p < 0.001]. Although young adults were faster to respond than the older adults overall, the Load × Group interaction confirms that age group differences in RT increased with load. Subsidiary ANOVAs for each group separately revealed a Cue × Load interaction for the young [F(2,34) = 4.26, p = 0.02] but not the old [F(2,34) < 1]. As can be seen in the table, the retro-cueing benefit in RT increased with load in the young only.
In order to assess the effect of retro-cueing on memory accuracy as a function of memory load, we conducted a Cue (no cue, retro-cue) × Load (2, 3, 4) × Group (Young, Old) ANOVA on both Pr and K memory measures. Significant effects of Load [F(2,68)’s > 44.2, p’s < 0.001] and Group [F(1,34)’s > 10.9, p’s < 0.002], were observed for both Pr and K measures. As can be seen in Table 2, as load increased, VWM accuracy decreased and K increased across groups, and both measures were generally reduced in the old compared to the young. Load × Group [F(2,68)’s > 12.3, p’s < 0.001] and Cue × Group [F(1,34)’s > 9.29, p’s < 0.004] interactions reflect the fact that retro-cueing effects were largest for the young and that age-related memory impairments increased with higher loads. Subsidiary ANOVAs revealed Cue × Load interactions [F(2,34)’s > 6.86, p’s < 0.003] for the young but no effects involving Cue in the old [F’s < 2.5, p’s > 0.1] for either memory measure. The interactions in the young reflect the fact that retro-cueing enhanced memory accuracy and capacity for loads 3 and 4 [t(17)’s > 3.2, p’s < 0.001] but not reliably for load 2 [t(17)’s < 1.9, p’s > 0.09]. As can be seen in the table, ceiling effects in the young may contribute to the lack of substantial retro-cueing benefit for set size 2 trials. These results suggest that young but not older adults were able to effectively use retro-cues to filter irrelevant items from VWM and enhance working memory capacity.
One potential issue with analyzing retro-cue effects according to objective load is that older adults generally have lower levels of capacity compared to the young. Thus, the observed group differences in retro-cue processing may be due to differences in baseline capacity rather than aging per se. To address this potential confound, we reanalyzed the behavioral data (K and RTs) as a function of individual estimates of maximum capacity (i.e. subjective memory load) rather than set size (i.e. objective memory load). The same approach has been used in an aging study previously (Schneider-Garces et al., 2010). To this end, we identified the maximum capacity (K) reached for each individual for the no cue condition, regardless of set size, and analyzed the effect of retro-cueing on performance indices for the objective load level nearest to that maximum K in addition to the next largest load level3. For example, for a participant achieving a maximum K of 1.8 at set size 3, RT, and K estimates would be computed for set sizes 2 (“capacity”) and 3 (“capacity +1”). K estimates and RTs for capacity and capacity +1 set sizes and the results of the ANOVAs for these data are shown in Supplemental Information. The retro-cue effects were largely consistent with those presented above; retro-cue benefits were observed for all behavioral measures in the young and for RTs only in older adults. Two interesting findings did diverge from those of the objective load analyses, however. First, age-related impairments in memory accuracy did not increase as subjective load increased (Load × Group interactions: [F’s < 2.0, p’s > 0.16]). Second, retro-cue benefits were equal across capacity levels for all behavioral measures in the young and for RTs in the old (Cue × Load interactions: [F’s < 1.3, p’s > 0.3]).
ERP Results
Memory array ERPs
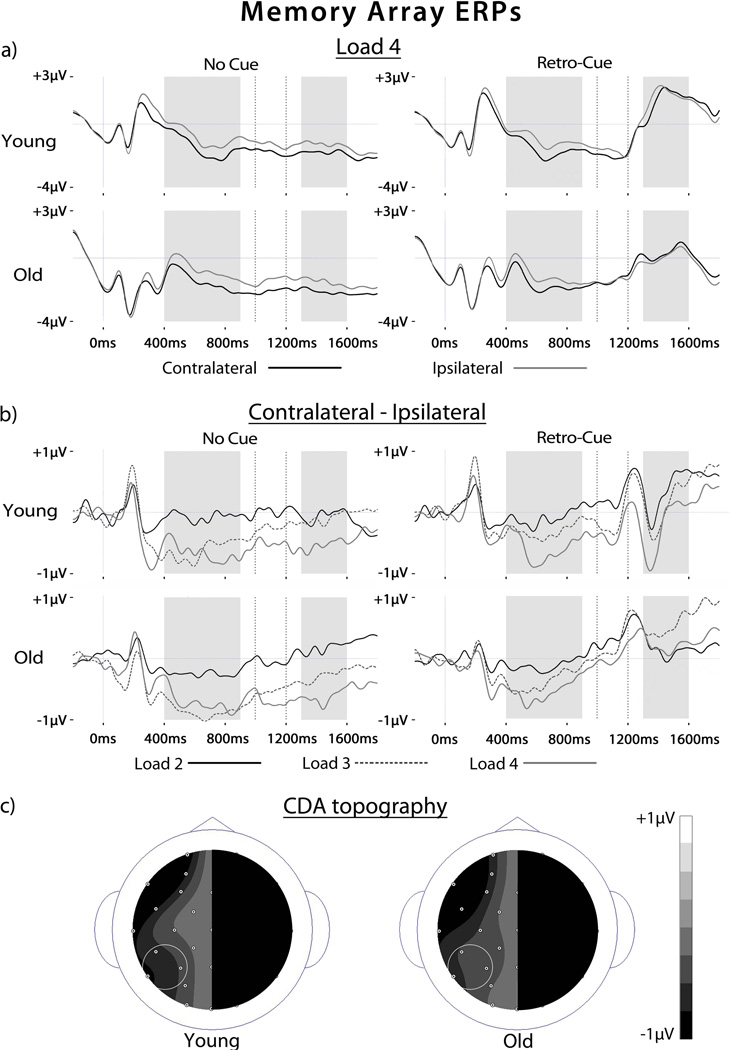
ERPs associated with contralateral and ipsilateral targets for no cue and retro-cue conditions for load 4, at which CDA effects were most robust, are shown in Figure 2A. Visually-evoked ERPs associated with the onset of the retro-cue (1000 ms) can be observed in the waveforms. For both groups, CDA effects were evident with greater negative-going activity for contralateral relative to ipsilateral trials beginning at roughly 300 ms post-memory array onset. The CDA difference waves (contralateral-ipsilateral) are shown for each load, cue type, and age group in Figure 2B. As can be seen in the figure, CDA amplitude increased as load increased and was reduced and somewhat reversed after the presentation of the retro-cue in both age groups. As can be seen on the scalp topographic maps in Figure 2C, the CDA was maximal at lateral temporal posterior sites in both groups
Figure 2.
ERPs to memory arrays. (A) ERPs are shown for no cue and retro-cue targets over contralateral and ipsilateral scalp sites averaged across 3 posterior electrode locations for set size 4 for each age group. The greater negative magnitude for contralateral than ipsilateral targets represents the contralateral delay activity (CDA). Gray shading indicates the time windows for CDA analysis. Dotted vertical lines indicate the retro-cue presentation period. (B) Difference waves representing the CDA for set sizes 2, 3 and 4 for no cue and retro-cue targets for each age group. Gray shading indicates the time windows for CDA analysis. Dotted vertical lines indicate the retro-cue presentation period. (C) Scalp topographies of the CDA collapsed across load and cue type are shown for each age group. Only one side of the scalp is shown as the CDA represents activity averaged across hemispheres. Small circles represent electrode locations as viewed from above. The circled locations indicate the electrodes chosen for CDA analysis (CP5/CP6; P3/P4; P7/P8).
First, we determined the reliability of the CDA in the period prior to the cue (400–900 ms). A Load × Hemifield × Cue × Group ANOVA revealed a main effect of Hemifield [F(1,34) = 19.7, p < 0.001] that was modified by an interaction with Load [F(2,68) = 7.4, p = 0.002]. As can be seen in Figure 2B, CDAs were evident and similarly modulated by load for both age groups (4 > 3 > 2). Additionally, marginal Cue × Hemifield [F(1,34) = 3.89, p = 0.057] and Cue × Group interactions were also observed [F(1,34) = 3.27, p = 0.07]. A subsidiary ANOVA for the young adults only revealed no effects involving Cue [F(1,17)’s < 1] suggesting that cue type did not influence the magnitude of the CDA for any load. By contrast, the same ANOVA for the old showed a Cue × Hemifield interaction [F(1,17) = 7.11, p = 0.01], reflecting the fact that CDA magnitudes were attenuated for retro-cue trials for each load in the time period prior to the onset of the cue, as seen in Figure 2B.
Second, we tested whether the CDA index of VWM capacity would be modulated by the presentation of the retro-cue in both age groups. To this end, a Load × Hemifield × Cue × Group ANOVA was conducted for the period directly following the presentation of the retro-cue (1300–1600 ms). Cue × Hemifield [F(1,34) = 9.19, p = 0.005] and Load × Hemifield [F(2,68) = 3.24, p = 0.04] interactions were observed, but no effects involving Group [F’s < 1.1, p’s > 0.3]. A subsidiary ANOVA for the no cue trials revealed a marginal main effect of Hemifield [F(1,34) = 3.15, p = 0.08] that was modified by an interaction with Load [F(1,34) = 3.46, p = 0.04]. As can be seen in Figure 2B, CDA magnitudes were generally smaller than in the previous time window but still modulated by load (4 > 3 > 2). By contrast, no effects involving Hemifield or Load were observed for retro-cue trials [F’s < 1.3, p’s > 0.24]. As is evident from Figure 2, CDA amplitudes were reduced for both groups after the presentation of the retro-cue, effectively removing the influence of load on this ERP index of VWM capacity 4.
Finally, we analyzed the effects of age group and cue on the CDA while controlling for individual estimates of maximum capacity, as discussed above for the behavioral data. The results of the ANOVAs for these ERP data are shown in Supplemental Information. The results were largely consistent with those presented above, namely that CDA amplitudes were attenuated for retro-cue relative to no cue trials prior to the onset of the cue in the old only but for both age groups after the presentation of the cue. One interesting difference from the analysis of objective load was the lack of load modulation for CDA magnitudes for both no cue and retro-cue trials in both time windows and age groups (effects including Load and Hemifield [F’s < 2.0, p’s > 0.15]). What this suggests is that CDA amplitude did not increase for set sizes greater than an individual’s VWM capacity.
Memory probe ERPs
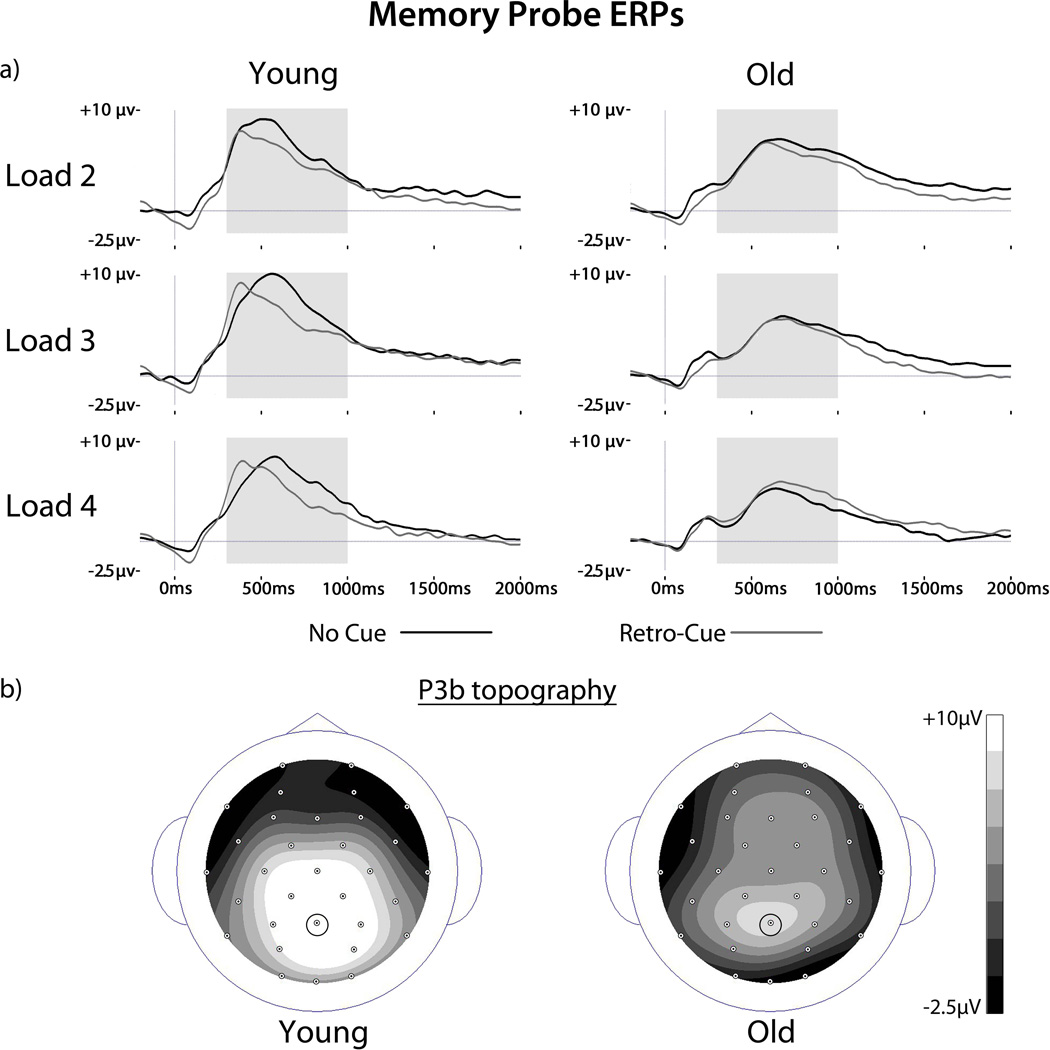
ERPs associated with targets for no cue and retro-cue conditions for each load are shown in Figure 3A. P3b components were evident for both cue types and age groups beginning between ~400–700 ms and lasting for several hundred milliseconds. A sustained positivity lasting until the end of the recording epoch is also visible5. P3bs associated with no cue trials peaked later (~60–90 ms) and were generally sustained relative to retro-cue trials particularly for young adults. As can be seen in the figure, P3b magnitudes were modulated by load in both groups, appearing most robust for loads 3 and 2 for the young and old, respectively. As can be seen in Figure 3B, topographic maps show that the P3b was maximal at centroposterior sites in both age groups.
Figure 3.
ERPs to memory probes. (A) ERPs are shown for no cue and retro-cue targets for each set size and age group at electrode Pz. The P3b component is clearly visible for all conditions and age groups beginning at ~300 ms post probe onset. Gray shading indicates the time windows for P3b analysis. (B) Scalp topographies of the P3b collapsed across load and cue type are shown for each age group. Small circles represent electrode locations as viewed from above. The Pz electrode chosen for P3b analysis is indicated.
We first tested whether the retro-cue affected the peak latency of the P3b. A Load × Cue × Group ANOVA for the peak latencies of the P3b between 300–1000 ms revealed main effects of Cue [F(1,34) = 7.67, p = 0.009] and Age [F(1,34) = 44.4, p < 0.001] but no effects of Load [F’s < 2.1, p’s > 0.12]. As can be seen in Figure 3A, the P3b for retro-cue trials peaked earlier than for no cue trials across load type, and was generally delayed for older adults compared to the young across both load and cue type. Subsidiary ANOVAs for each group separately showed that the effect of Cue was driven by the young [F(1,17) = 13.9, p = 0.002] but not the old [F(1,17) < 1]. As is evident from the figure, the latency shift for retro-cue trials was observed primarily in the young.
We next assessed the effect of the retro-cue on the magnitude of the P3b. A Load × Cue × Group ANOVA for the mean amplitudes in the 300–1000 ms time window revealed a main effect of Load [F(2,68) = 5.27, p = 0.008] that was modulated by an interaction with Group [F(2,68) = 5.53, p = 0.006]. Subsidiary ANOVAs for each group separately confirmed that the P3b was larger for load 3 than loads 2 or 4 [F(1,17)’s > 4.52, p’s < 0.04] in the young, and larger for load 2 than loads 3 or 4 in the old [F(1,17)’s < 6.45, p’s < 0.02]. Subsidiary analyses additionally revealed a main effect of Cue for the young [F(1,17) = 4.26, p < 0.05] but not the older adults [F(1,17) < 1]. As can be seen in Figure 3A, the effect of Cue reflects the fact that P3b amplitudes were sustained for no cue trials relative to retro-cue trials in the young while this difference was not reliable in the old.
Finally, we analyzed the effects of age group and cue on the CDA while controlling for individual estimates of maximum capacity. The results of the ANOVAs for these ERP data are shown in Supplemental Information. The results were largely consistent with those presented above, namely that retro-cues affected P3bs for young adults only.
Correlations between performance and ERPs
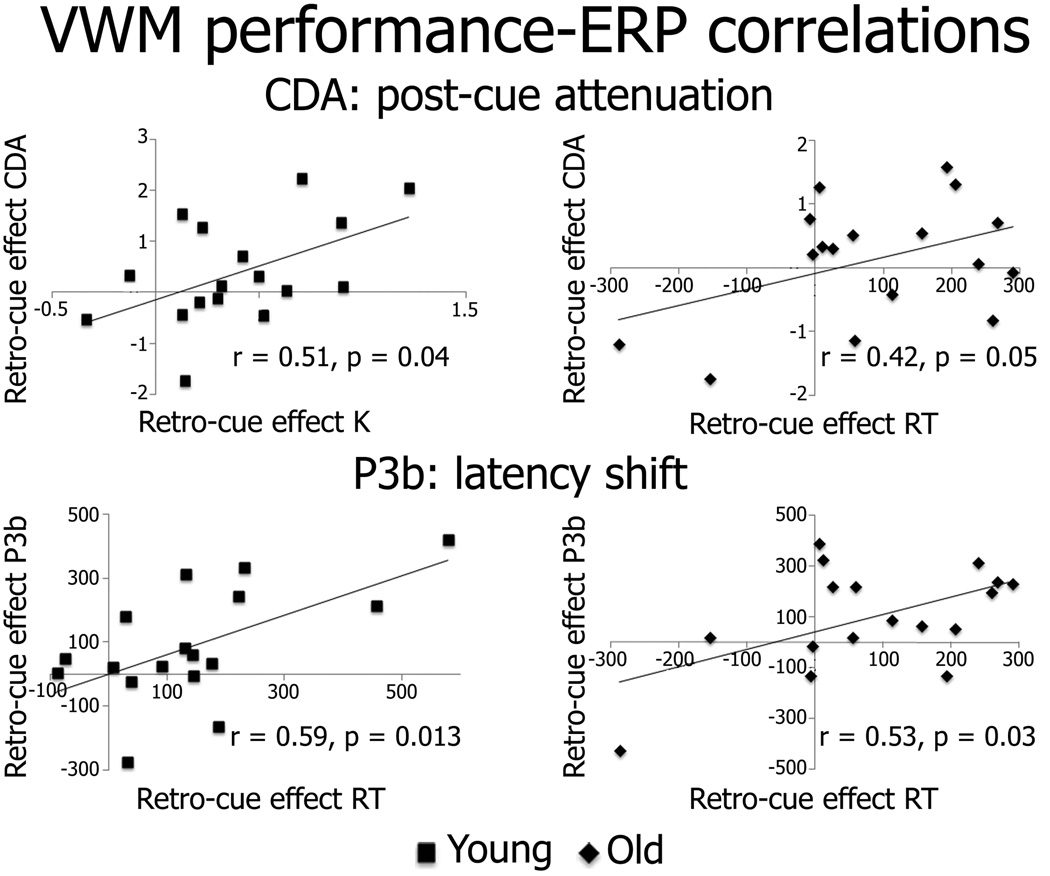
In order to more directly assess the relationship between working memory performance and associated ERPs, we examined correlations between the size of the retro-cue benefits for RT and K estimates with the size of the retro-cue effects for the CDA magnitude and P3b magnitude and latency for subjective load levels of capacity and capacity +1. First, as can be seen in Figure 4, significant correlations were observed between the post-cue attenuation of the CDA and the retro-cue benefit for K estimates at capacity +1 in the young adults [r(15) = 0.51, p = 0.04] and for RTs at capacity +1 in the older adults [r(15) = 0.42, p = 0.05]. Second, as seen in the figure, significant correlations were observed between the retro-cue related P3b latency shifts and the retro-cue benefits for RTs at both set sizes in the young [r(15)’s > 0.47, p’s < 0.05] and in the old [r(15)’s > 0.46, p’s < 0.05]. Significant correlations between P3b latency shifts and retro-cue benefits for K estimates were additionally observed in the older adults [r(15)’s > 0.43, p’s < 0.05]. No other significant correlations between performance and ERPs or between the CDA and P3b effects were observed. Collectively, these results suggest that the cue-induced modulations of the CDA and P3bs were positively related to retro-cue benefits in performance for both age groups.
Figure 4.
Correlations between retro-cue effects in performance and retro-cue effects in ERPs. Correlations for capacity +1 objective load levels are shown.
Discussion
In the present experiment, we examined the effect of aging on the use of top-down control to regulate the access into and the maintenance of information in visual working memory. Specifically, we used an ERP index of working memory maintenance, the CDA, to assess whether aging affects both the ability to limit the access to working memory to task-relevant information, and the ability to delete items from working memory that are deemed no longer relevant by retrospective attentional cues. There were four major findings. First, we showed that spatial retro-cues improved working memory accuracy in young adults but not older adults. Interestingly, however, retro-cueing benefits were observed for response times for both age groups. Second, both groups showed a CDA that was modulated by load consistent with an intact access function in older adults, somewhat inconsistent with inhibition deficit theory. However, age group differences were observed for the CDA but only in the period prior to the onset of the retro-cue, in which CDA amplitudes were reduced for the old, consistent with their lack of retro-cue induced accuracy benefit. Third, spatial retro-cues reduced the amplitude of the CDA, effectively mitigating the impact of memory load on this sustained activity in both young and older adults. Finally, spatial retro-cues resulted in earlier onsetting and abbreviated P3b components for young adults only. These results suggest that retrospective attentional cueing may enhance WM performance by reducing both inter-item interference during maintenance and the degree of probe-related evaluation and updating during test; and furthermore, that aging impairs the ability to effectively use retrospective attention to modulate the contents of working memory in a way that benefits memory accuracy. These results and their implications are discussed in more detail below.
Behavioral Results
Although older adults were slower than the young overall, as would be predicted for almost any aging comparison, we found equivalent retro-cueing benefits in response times for both groups. Similarly, for young but not older adults retro-cues enhanced memory accuracy for both Pr and K estimates. Consistent with previous findings in young adults (Kuo, et al., 2012; Lepsien & Nobre, 2007) these performance benefits increased with load but only in the young. This pattern of results supports the idea that for young adults retrospective attentional cues enhance VWM in part by reducing inter-item interference, which increases with load. If older adults can use retrospective attention to enhance response speed, potentially via the same mechanism as the young, why are similar benefits not observed for accuracy? One possibility is that interference produced by VWM competitors, particularly evident at higher set sizes, makes it difficult for older adults with capacity limitations to successfully maintain the array. Indeed, consistent with this idea, disproportionate age-related accuracy impairments were observed at larger set sizes.
In order to determine whether low levels of VWM capacity could explain the age group differences in retro-cueing, we analyzed retro-cue effects as a function of individual estimates of capacity (i.e. subjective memory load) rather than set size (objective memory load) for each group. A similar approach has been used in a previous fMRI study investigating working memory maintenance in the young and old (Schneider-Garces, et al., 2010). Interestingly, results were largely similar to those described above, namely that retro-cue benefits were observed for all behavioral measures in the young and for RTs only in older adults. A couple of differences did emerge from this analysis, however. First, age-related impairments in memory accuracy did not increase as subjective load increased. This suggests that once individual differences in capacity were accounted for, there was little evidence supporting the idea that increased susceptibility to inter-item interference could explain the lack of retro-cue accuracy benefits in the older adults. Second, retro-cue benefits did not increase with increases in subjective memory load in either age group. That is, once the level of capacity was reached, retro-cue benefits were stable and this pattern was similar for the young and the old, potentially suggesting that both age groups may use retro-cues to reduce inter-item interference in VWM.
Another possible explanation for the age group differences in retro-cue effects may relate to the fact that trials were blocked by cue type in the current design in order to ease exposition of the task for older adults. By contrast, in some previous target detection studies in which temporal (Zanto, Toy, & Gazzaley, 2011) or spatial (Hoyer & Familiant, 1987) orienting cues indicating the impending visual target were randomly presented, response time benefits were reduced or absent in the old. What these results may suggest is that age-related deficits in utilizing expectancy to enhance performance may be particularly evident when there is no prior knowledge of the attentional cue and mobilization of attention must occur quickly. However, when prior knowledge of the cue exists, older adults can make use of this knowledge to benefit performance, potentially via priming of perceptual and motor networks associated with target detection and response execution. Moreover, it is known that the anticipation of new perceptual information can disrupt memory for information being maintained in VWM (Chun & Potter, 1995). Thus, any benefit to performance resulting from expectancy mechanisms and/or suppression of inter-item interference may have been negated by the perceptual interference caused by the retro-cue itself. As discussed below, the ERP evidence offers some support for this hypothesis. We believe that the most parsimonious explanation for the group differences in retro-cue processing is that while both young and older adults can use retro-cues to reduce VWM interference, interference from the cue to which older adults may be particularly susceptible limits the potential benefits to performance.
Aging Affects Working Memory Maintenance, but Only for Retro-cue Trials
Our results showing that CDA amplitude varied in strength as a function of memory load before the onset of the retro-cues are consistent with previous findings suggesting that the CDA reflects maintenance of information in VWM (Anderson, et al., 2011; McCollough, et al., 2007; Vogel & Machizawa, 2004). Importantly, reliable CDA effects that were similarly modulated by load were also observed for the older adults. If, as suggested by inhibition deficit theory (Hasher & Zacks, 1988), the older adults’ working memory performance decrements were due to an impaired ability to limit WM access to task-relevant items, it is likely they would have encoded items from both hemifields (relevant and irrelevant) and the magnitude of the CDA would have been greatly attenuated or absent (McCollough, et al., 2007). Instead, these data suggest that regardless of age, participants were able to make use of simple spatial cues (pre-cues) to orient their attention to and encode exclusively the task-relevant items. It is also worth noting that CDA magnitude did not reliably increase once individual estimates of capacity had been reached for both young and older adults. This finding extends previous findings from young adults suggesting that the CDA reaches asymptote approximately at one’s VWM capacity (McCollough, et al., 2007; Vogel & Machizawa, 2004) and reflects the number of items being accurately maintained across the lifespan.
These results are however, inconsistent with two previous studies showing a smaller load-related modulation of CDA magnitude in the old (Jost, et al., 2011; Sander, et al., 2011). Sander and colleagues speculated that because aging impairs control mechanisms that operate on the contents of working memory, which are also known to affect CDA amplitude (Voytek & Knight, 2010), age group differences in the modulations of the CDA would be most pronounced when the demands on top-down control are high. In these previous studies, set size was randomized trial-to-trial and memory items (colored squares and rectangles) were presented in random positions on each side of the screen while in the current study, trials were blocked by set size and positions of the squares for each set size were fixed. Consequently, the demands on top-down control or perhaps sustained attention needed to encode memory items were arguably greater in these prior studies than in our own. Given that CDA magnitude is stable once one’s maximum capacity has been reached, it is possible that older adults’ capacity limitations may have contributed to their smaller load-related modulation of the CDA in the studies by Jost and Sander and colleagues. Future research assessing the CDA as a function of individual estimates of VWM capacity and task difficulty will be necessary to determine the conditions under which aging affects the ability to utilize top-down control over working memory.
There was one interesting, albeit unpredicted group difference for the CDA. Specifically, the CDA was attenuated for retro-cue relative to no cue trials prior to cue onset in older adults only. It is not clear how such a pattern could be explained by an access limitation deficit, which as discussed above, would likely result in attenuated CDAs for all conditions. Importantly, this effect cannot be explained by capacity limitations in the old as the same age-related difference was observed after accounting for individual differences in capacity. As already noted, trials were blocked by cue type such that participants were aware of whether a retro-cue would or would not be presented. We speculate that the ability to make use of expectancy mechanisms to enhance response speed may have come at the cost of continued maintenance of relevant items in working memory in the older adults. Although the neural substrates underlying the CDA have not yet been specified, it is believed that sustained activation in extrastriate cortex during the delay period contributes to this ERP. Some recent fMRI evidence suggests that anticipatory attention to an imminent visual stimulus can modulate the level of activity in the extrastriate regions sensitive to that stimulus category (Lepsien, Thornton, & Nobre, 2011). Thus, we tentatively suggest that anticipation of the retro-cue influenced the tonic level of activity in extrastriate regions underlying the CDA and that this disruption may have negatively impacted working memory accuracy in the older adults such that no retro-cueing benefit was observed. Why this same pattern was not also observed for the young adults isn’t entirely clear but if expectancy of the retro-cue distracted from the perceived ability to maintain items in VWM, young adults may have been more capable of suppressing anticipatory attention. Although resolution of this issue would require further study, it is interesting to note that many of the older adults, but none of the young, reported that they found retro-cues to detract from their ability to perform the task.
Retro-cues Attenuate the Effect of Memory Load on the CDA in Both Age Groups
Our ERP results showed that the magnitude of the CDA was reduced by the presentation of the spatial retro-cue to the same degree in both the young and the old effectively mitigating the impact of memory load on this persistent activity. A similar finding has been shown recently in young adults (Kuo, et al., 2012) but the current findings suggest that the ability to use top-down control to modify the contents of VWM may be preserved in aging. Examining these ERP results in isolation might lead one to predict retro-cueing benefits in WM performance for both young and older adults but as already discussed, there was no benefit to indices of working memory accuracy or capacity in the old. We hypothesize that the effect of anticipation of the retro-cue, as evidenced by attenuated CDA magnitudes prior to cue onset, may have negatively impacted the representations in VWM such that any potential benefit of using the retro-cue to reduce inter-item interference may have been nullified. We further hypothesize that if the retro-cue presentation could not be anticipated, the preserved ability of older adults to use the retro-cue to delete no longer relevant items from VWM would manifest as greater memory accuracy for the relevant cued items. Thus, we believe that these data in combination with some recent evidence showing that older adults can filter task-irrelevant items from working memory, though somewhat later during retention than they young (Jost, et al., 2011), do not fully support the idea that a deletion function impairment is necessarily the rule in healthy aging (Hasher & Zacks, 1988).
Before moving on, it is worth discussing some alternative interpretations for the retro-cue induced attenuation of the CDA. We were careful to present retro-cues centrally in order to avoid confounding modulations of the CDA due to changes in demands on VWM maintenance with modulations due to lateralized visually-evoked potentials. Although some previous CDA studies have employed laterally presented probe stimuli (Ikkai, McCollough, & Vogel, 2010; Jost, et al., 2011; McCollough, et al., 2007; Sander, et al., 2011; Vogel & Machizawa, 2004), single probe stimuli were presented centrally for both no cue and retro-cue conditions in our study, thus spatial anticipation of the probes could not have affected the CDA and certainly can not explain the selective attenuation for retro-cue trials. It is possible, however, that the presentation of any visual stimulus could have contributed to some of the attenuation of the CDA observed after the retro-cue. Specifically, Kuo and colleagues (2012) showed that compared to no cue trials, spatially neutral cues also produce some attenuation of the CDA. These authors speculated that perceptual interference produced by visual stimuli might affect the tonic activity in extrastriate cortex that underlies the CDA. While our design does not allow us to separate the influence of retrospective attention from perceptual interference on the CDA, we reasoned that a direct relationship between the post-cue CDA attenuation and memory performance could not be easily explained by a visual interference account. Indeed, we observed significant correlations between the size of the CDA attenuation and the size of the retro-cue benefit for K estimates in the young and RTs in the old. Thus, we feel that these ERP findings are best explained by the top-down modulation of VWM maintenance by retro-cues.
Retro-cues Reduce Demands on Probe-related Processing for the Young Only
Age-related delays in the latency of the target-related P300 or “P3b” have often been observed in previous studies (Fogelson, Shah, Bonnet-Brilhault, & Knight, 2010; Polich, 1996, 2007) and we observed the same result here. More interesting for the current study is the differential impact of retro-cueing on the P3b in the young and the old. The P3b has been elicited by probe stimuli in previous working memory studies and the most widely held theory is that it reflects “updating” of the contents of working memory updating (Donchin & Coles, 1988; Polich, 2007). In short, after initial sensory processing, the stimulus is compared with representations held in working memory, categorized (match/non-match), and the contents of working memory are updated or changed, in preparation for the next stimulus. P3b latency and amplitude increase as a function of degree of engagement of these evaluative processes contributing to memory decisions (Kok, 2001; Polich, 2007 for reviews). Accordingly, our finding of earlier onsetting and shorter-lived P3b components following retro-cues in the young adults is consistent with the idea that retro-cues contribute to memory enhancements in part by reducing the demands on comparison and updating processes at the time of test (Makovski, et al., 2008).
The lack of reliable retro-cue induced modulations of the P3b for the older adults, despite the modulations for the CDA, may be the result of reduced maintenance related activity prior to the cue onset. As discussed above, the effect of retro-cue anticipation may have negatively impacted the representations in VWM despite a relatively spared ability to use the spatially informative cues to reduce interference. Consequently, decision difficulty was ultimately similar for no cue and retro-cue trials and the latency and amplitude of the P3b were unaffected. Another possible alternative explanation for the lack of P3b variation could be capacity limitations in the old, however, we observed the same general pattern of results after controlling for individual differences in VWM capacity. It is also possible that the lack of P3b variation is that aging is associated with intractable deficits in context updating mechanisms such that decision efficiency may never be increased. We feel that this explanation is unlikely, however, given that factors including working memory capacity (Peltz, Gratton, & Fabiani, 2011) and aerobic fitness (McDowell, Kerick, Santa Maria, & Hatfield, 2003; Pontifex, Hillman, & Polich, 2009) have been shown to mitigate the impact of aging on the P3b. Importantly, we identified significant correlations between P3b latency shifts and retro-cue benefits for RTs in the young and for RTs and K estimates in the older adults. Thus, despite older adults not benefitting to the same extent as the young from the retro-cues for the reasons already described, retro-cue modulations of the P3b likely support performance in both age groups.
Finally, although the P3b was not reliably modulated by retro-cues in the old, P3b amplitude was affected by memory load in both age groups, with maximal P3bs displayed for set size 3 in the young and set size 2 in the older adults. The effect of load in the absence of interactions with cue for this ERP was not of particular interest in the current study and so we do not want to speculate at length about these results. It is worth mentioning, however, that P3b amplitude, albeit measured during encoding, has been shown to be sensitive to memory load in previous working memory studies (reviewed in Kok, 2001).
Conclusions
These data are consistent with the idea that retrospective spatial attention can modify the representations held in VWM in a manner that benefits memory performance in young adults. Our ERP results suggest that these memory benefits are likely the result of both a reduction of inter-item competition that effectively reduces the memory load, as well as a reduction in the number of comparisons between the probe and working memory representations that need to be made at test. These data further suggest that despite preserved abilities to both limit working memory access to task-relevant information and to delete items from working memory deemed irrelevant by retrospective spatial cues, older adults may be less able to use retrospective attention to enhance working memory performance. We speculate that older adults’ performance does not benefit to the same degree as that of the young because expectancy mechanisms related to impending spatial cues negatively impact working memory representations in the old. Future studies that manipulate the influence of expectancy during working memory maintenance will be important for determining the extent to which older adults can use top-down attentional orienting to regulate the contents of VWM and enhance working memory performance.
Supplementary Material
Acknowledgements
This research was supported by NIA 2R01AG016201-11A2.
Footnotes
It is important to verify that artifact rejection methods remove the artifacts but leave the remaining EEG intact (Picton et al., 2000). To this end, we computed ERPs both with and without the artifact correction to determine whether the magnitudes of the ERP waveforms of interest were attenuated by the correction procedure (data not shown).
Analyses were also conducted for the last 200 ms of the epoch and revealed a similar pattern of results, thought slightly less robust, than those observed for the 1300–1600 ms window.
One young adult with a capacity of 4 and one older adult with a capacity of 1 were excluded from this analysis.
The post-cue CDA attenuation appears largest for load 3 in the older adults. We did not predict this pattern and given that the effect of Load was not reliable in this time period, we do not feel it prudent to speculate as to its meaning.
The same ANOVAs were conducted for the sustained positivity (1000–2000 ms) and results were similar to those observed for the P3b.
References
- Anderson DE, Vogel EK, Awh E. Precision in visual working memory reaches a stable plateau when individual item limits are exceeded. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1128–1138. doi: 10.1523/JNEUROSCI.4125-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bahcall DO, Kowler E. Attentional interference at small spatial separations. Vision research. 1999;39:71–86. doi: 10.1016/s0042-6989(98)00090-x. [DOI] [PubMed] [Google Scholar]
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalogr Clin Neurophysiol. 1994;90:229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Bleckley MK, Durso FT, Crutchfield JM, Engle RW, Khanna MM. Individual differences in working memory capacity predict visual attention allocation. Psychonomic bulletin & review. 2003;10:884–889. doi: 10.3758/bf03196548. [DOI] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. J Exp Psychol Hum Percept Perform. 1995;21:109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Connelly SL, Hasher L. Aging and the inhibition of spatial location. Journal of experimental psychology. Human perception and performance. 1993;19:1238–1250. doi: 10.1037//0096-1523.19.6.1238. [DOI] [PubMed] [Google Scholar]
- Connelly SL, Hasher L, Zacks RT. Age and reading: the impact of distraction. Psychology and aging. 1991;6:533–541. doi: 10.1037//0882-7974.6.4.533. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. The Behavioral and brain sciences. 2001;24:87–114. doi: 10.1017/s0140525x01003922. discussion 114-185. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating. Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Ester EF, Serences JT, Awh E. Spatially global representations in human primary visual cortex during working memory maintenance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:15258–15265. doi: 10.1523/JNEUROSCI.4388-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: implications for group differences in response latency. Psychol Bull. 1999;125:777–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Fogelson N, Shah M, Bonnet-Brilhault F, Knight RT. Electrophysiological evidence for aging effects on local contextual processing. Cortex; a journal devoted to the study of the nervous system and behavior. 2010;46:498–506. doi: 10.1016/j.cortex.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, D'Esposito M. Functional connectivity during working memory maintenance. Cognitive, affective & behavioral neuroscience. 2004;4:580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- Hasher L, Quig MB, May CP. Inhibitory control over no-longer-relevant information: adult age differences. Memory & cognition. 1997;25:286–295. doi: 10.3758/bf03211284. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks R. Working memory, comprehension, and aging: A review and a new view. In: Bower G, editor. The psychology of learning and motivation. New York: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hoyer WJ, Familiant ME. Adult age differences in the rate of processing expectancy information. Cognitive Development. 1987;2:59–70. [Google Scholar]
- Ikkai A, McCollough AW, Vogel EK. Contralateral delay activity provides a neural measure of the number of representations in visual working memory. Journal of neurophysiology. 2010;103:1963–1968. doi: 10.1152/jn.00978.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost K, Bryck RL, Vogel EK, Mayr U. Are old adults just like low working memory young adults? Filtering efficiency and age differences in visual working memory. Cerebral cortex. 2011;21:1147–1154. doi: 10.1093/cercor/bhq185. [DOI] [PubMed] [Google Scholar]
- Kemper S, McDowd J, Kramer A. Eye movements of young and older adults while reading with distraction. Psychology and aging. 2006;21:32–39. doi: 10.1037/0882-7974.21.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Kuo BC, Stokes MG, Nobre AC. Attention modulates maintenance of representations in visual short-term memory. Journal of cognitive neuroscience. 2012;24:51–60. doi: 10.1162/jocn_a_00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman R, Spekreijse H, Lamme VA. Large capacity storage of integrated objects before change blindness. Vision research. 2003;43:149–164. doi: 10.1016/s0042-6989(02)00402-9. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Nobre AC. Attentional modulation of object representations in working memory. Cerebral cortex. 2007;17:2072–2083. doi: 10.1093/cercor/bhl116. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Thornton I, Nobre AC. Modulation of working-memory maintenance by directed attention. Neuropsychologia. 2011;49:1569–1577. doi: 10.1016/j.neuropsychologia.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd edition. New York: 1995. p. 1026. [Google Scholar]
- Makovski T, Jiang YV. Distributing versus focusing attention in visual short-term memory. Psychonomic bulletin & review. 2007;14:1072–1078. doi: 10.3758/bf03193093. [DOI] [PubMed] [Google Scholar]
- Makovski T, Sussman R, Jiang YV. Orienting attention in visual working memory reduces interference from memory probes. Journal of experimental psychology. Learning, memory, and cognition. 2008;34:369–380. doi: 10.1037/0278-7393.34.2.369. [DOI] [PubMed] [Google Scholar]
- Matsukura M, Luck SJ, Vecera SP. Attention effects during visual short-term memory maintenance: protection or prioritization? Perception & psychophysics. 2007;69:1422–1434. doi: 10.3758/bf03192957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollough AW, Machizawa MG, Vogel EK. Electrophysiological measures of maintaining representations in visual working memory. Cortex; a journal devoted to the study of the nervous system and behavior. 2007;43:77–94. doi: 10.1016/s0010-9452(08)70447-7. [DOI] [PubMed] [Google Scholar]
- McDowell K, Kerick SE, Santa Maria DL, Hatfield BD. Aging, physical activity, and cognitive processing: an examination of P300. Neurobiology of aging. 2003;24:597–606. doi: 10.1016/s0197-4580(02)00131-8. [DOI] [PubMed] [Google Scholar]
- Oberauer K, Schulze R, Wilhelm O, Suss HM. Working memory and intelligence--their correlation and their relation: comment on Ackerman, Beier, and Boyle (2005) Psychological bulletin. 2005;131:61–65. doi: 10.1037/0033-2909.131.1.61. author reply 72-65. [DOI] [PubMed] [Google Scholar]
- Oberauer K, Wendland M, Kliegl R. Age differences in working memory--the roles of storage and selective access. Memory and Cognition. 2003;31:563–569. doi: 10.3758/bf03196097. [DOI] [PubMed] [Google Scholar]
- Pashler H. Familiarity and visual change detection. Perception & psychophysics. 1988;44:369–378. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- Peltz CB, Gratton G, Fabiani M. Age-related changes in electrophysiological and neuropsychological indices of working memory, attention control, and cognitive flexibility. Frontiers in psychology. 2011;2:190. doi: 10.3389/fpsyg.2011.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin E, Pernier J, Bertrand O, Echalher J. Spherical splines for scalp potential and current source density mapping. Electroencephalogr Clin Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, Taylor MJ. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Polich J. Meta-analysis of P300 normative aging studies. Psychophysiology. 1996;33:334–353. doi: 10.1111/j.1469-8986.1996.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [pii] 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, Hillman CH, Polich J. Age, physical fitness, and attention: P3a and P3b. Psychophysiology. 2009;46:379–387. doi: 10.1111/j.1469-8986.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, D'Esposito M. "What"-Then-Where" in visual working memory: an event-related fMRI study. Journal of cognitive neuroscience. 1999;11:585–597. doi: 10.1162/089892999563652. [DOI] [PubMed] [Google Scholar]
- Rozek E, Kemper S, McDowd J. Learning to ignore distracters. Psychology and aging. 2012;27:61–66. doi: 10.1037/a0025578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander MC, Werkle-Bergner M, Lindenberger U. Contralateral delay activity reveals life-span age differences in top-down modulation of working memory contents. Cerebral cortex. 2011;21:2809–2819. doi: 10.1093/cercor/bhr076. [DOI] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Fabiani M. Span, CRUNCH, and beyond: working memory capacity and the aging brain. Journal of cognitive neuroscience. 2010;22:655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley W. Institute of living scale. Los Angeles: Western Psychological Services; 1946. [Google Scholar]
- Stokes M, Thompson R, Cusack R, Duncan J. Top-down activation of shape-specific population codes in visual cortex during mental imagery. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:1565–1572. doi: 10.1523/JNEUROSCI.4657-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. Speed and accuracy of accessing information in working memory: an individual differences investigation of focus switching. Journal of experimental psychology. Learning, memory, and cognition. 2008;34:616–630. doi: 10.1037/0278-7393.34.3.616. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Spillers GJ. Variation in working memory capacity and episodic recall: the contributions of strategic encoding and contextual retrieval. Psychonomic bulletin & review. 2010;17:200–205. doi: 10.3758/PBR.17.2.200. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P. Aging and Executive Control: Reports of a Demise Greatly Exaggerated. Current Directions in Psychological Science. 2011;20:174–180. doi: 10.1177/0963721411408772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: estimates of linear and nonlinear age effects and structural models. Psychological Bulletin. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Voytek B, Knight RT. Prefrontal cortex and basal ganglia contributions to visual working memory. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18167–18172. doi: 10.1073/pnas.1007277107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence ScaleRevised. New York: Psychological Corporation; 1981. [Google Scholar]
- Zanto TP, Toy B, Gazzaley A. Delays in neural processing during working memory encoding in normal aging. Neuropsychologia. 2011;48:13–25. doi: 10.1016/j.neuropsychologia.2009.08.003. [pii] 10.1016/j.neuropsychologia.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.