Abstract
SS1P is a recombinant immunotoxin composed of an anti-mesothelin Fv fragment fused to a truncated portion of Pseudomonas exotoxin (PE) A. SS1P targets and kills mesothelin-expressing tumors, which include mesothlioma as well as ovarian, lung and pancreatic cancers. SS1P is currently in clinical trials in mesothelioma. Because insulin (INS) acting through the insulin receptor (IR, INSR) is a survival factor for many cancer cell lines, we explored how lowering IR level would affect the cytotoxic action of SS1P. We show here that siRNA knock-down of the IR enhanced the cytotoxic action of native PE and enhanced SS1P toxicity on several human cell lines, but did not affect the response to other cytotoxic agents such as TRAIL, etoposide and cycloheximide. To determine how IR knock-down enhances SS1P action, we analyzed various steps involved in cell killing. We found that IR knock down increases the cleavage of SS1P by furin, which allows more toxin to reach the cytosol and inactivate elongation factor 2. These findings indicate that the IR negatively regulates immunotoxin action.
Keywords: insulin receptor, immunotoxin, Pseudomonas toxin, furin cleavage
Introduction
Monoclonal antibodies (mAbs) are now widely used to treat cancer, but unfortunately many cancers are resistant to antibody treatment. To take advantage of the selective binding of antibodies to cancer cells, mAbs are now being used to deliver different types of cytotoxic agents to these cells (1). Immunotoxins are one such type of anti-cancer agent in which protein toxins are attached to mAbs (2, 3). We construct recombinant immunotoxins (RITs) by attaching a 38 kDa fragment of Pseudomonas exotoxin A (PE38) to the Fv portion of a mAb chosen to react selectively with cancer cells and not with essential normal tissues (3, 4). SS1P (anti-mesothelin Fv-PE38) is a RIT that targets mesothelin, a cell-surface protein that is highly expressed on mesothelioma cells, as well as pancreatic, ovarian, lung and other cancer cells (5, 6). After binding to mesothelin, SS1P enters cells by endocytosis where the furin protease cleaves the Fv from the toxin (7). The toxin then is transferred in a retrograde fashion to the endoplasmic reticulum from which it is translocated into the cytosol, there the toxin catalyzes the ADP-ribosylation and inactivation of elongation factor 2, eventually leading to apoptosis (4, 7). Despite the effectiveness of SS1P and other immunotoxins in killing cancer cells (8, 9), many of the mechanistic steps in immunotoxin action have not been established. Understanding more about these mechanisms could be useful in developing strategies to make immunotoxins more effective in killing cancer cells.
Insulin is an important ingredient in many types of tissue culture media, because activating the insulin receptor (IR) promotes cell growth and protects against loss of viability and apoptosis (10–12). Likewise, the presence of insulin- like growth factor 1 (IGF1), which also binds the IR, can promote growth and survival. We hypothesized that activities associated with the IR, including tyrosine kinase, could negatively regulate the ability of immunotoxins to kill target cells. Using siRNA technology to reduce expression, we show here that knock down of the IR enhances the cytotoxic action of immunotoxin SS1P on several human cancer cell lines. We provide evidence that the IR acts as an early step in immunotoxin action and regulates the cleavage of the immunotoxin by furin, perhaps by regulating immunotoxin trafficking. Of further interest, we report that other toxic agents, unrelated to PE, were not enhanced by the silencing of the IR.
Material and Methods
Reagents
Immunotoxins SS1P and HB21-PE40 were purified in our laboratory (13). AGL2263 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rapamycin, PD98059, LY294002, Anti-IR, anti-PARP, anti-cleaved caspase-3, anti-Bax, anti-Bak, anti-Bcl-xL, anti-Mcl-1 Abs were from Cell Signaling (Danvers, MA). Anti-furin Ab from Invitrogen (Carlsbad, CA) Insulin was purchased from Sigma (St. Louis, MO). 3H-Leucine was purchased from GE Healthcare (Piscataway, NJ).
Cell culture
A431/H9 is a human meso000thelin-transfected A431 cell (ATCC) HAL-01 (Germany, DSMZ). KB31 cells were provided by Michael Gottesman (NCI, Bethesda). M30 mesothelioma cell line is from Steven Albelda (University of Pennsylvania, Philadelphia, PA). A1847 is from Stuart Aaronson, (NCI, Bethesda, MD). All cells are cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin in humidified atmosphere of 5% CO2 at 37°C. HAL-01, KB31 and A431/H9 cells were authenticated within 1 year by short tandem repeat (STR) analysis; The M30 and A1847 were analyzed in 1 month by STR and no known matches were found.
Transfection and cytotoxicity assays
To knock down the IR, 5000 cells were transfected via the addition of 3 μl of 20 μM siRNA, 3.5 μl of DharmaFECT Transfection Reagent 3 (Dhmarcon, Lafayette, CO) in 125 μl final volume per well for 96-well experiments. After 48 hours of transfection, the cells were treated with SS1P or other toxic agents at the indicated concentration for a further 72 hours. Cell viability was then measured by the ATP levels using CellTiter-Glo luminescent cell viability assay (Promega, Madison, WI). Viability is expressed as the percentage of luminance with SS1P compared to control without SS1P treatment. All siRNA experiments used an unrelated luciferase siRNA (GL2) as a negative control.
Inhibitor study
Before experiments were initiated, 5000 KB31 cells were seeded overnight in 96 well plates. Inhibitors were added and cells were incubated for 1 hour before the addition of SS1P. After 72 hours of incubation, cell viability was measured by ATP level. In some cases, inhibitors AGL2263 and Rapamycin were also added and cells were incubated for approximately 18 hours before SS1P addition; however, the effects on inhibition of SS1P activity were similar to the 1 hour inhibitor incubation at 37°C in culture media. All experiments were done 3–4 times with reproducible results.
Western blot analysis
Cells were washed in PBS and disrupted by the addition of lysis buffer (50mM Tris HCl, 150mM NaCl, 5 mM EDTA with 1% NP40, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 10 μM PMSF) on ice for 30 minutes. After high-speed centrifugation, 20–40 μg supernatant protein was analyzed by SDS-PAGE, transferred to a PVDF membrane and subjected to western blotting with detection by ECL or ECL plus (Amersham; Piscataway, NJ).
Internalization and FACS analysis
A431/H9 cells were transfected with siRNA for 48 hours in 6-well plates, then 1 μg/ml of SS1P-Alex-647 was added and cells were incubated at 37°C for the indicated times. After labeling, the cells were washed with PBS and stripped with glycine buffer containing 0.2mol/L glycine (pH2.5) and 1 mg/ml of bovine serum albumin to remove surface bound SS1P. Cells were then trypsinized, washed with FACS buffer (PBS with 5% FBS, plus 0.1%NaN3) and analyzed by FACS Calibur.
SS1P cleavage
A431/H9 cells were transfected with siRNA for 48 hours in 6-well plates, 1 μg/ml of SS1P was added to cells and incubated on ice for 30 minutes to saturate SS1P binding. Cells were changed to fresh media and incubated at 37°C for the indicated time before making a total cell lysate.
Real time PCR
RNA was isolated using the Trizol reagent (Invitrogen). Reverse transcription and cDNA synthesis were performed using a Quantitect Reverse transcription kit following the manufacturer’s instructions (Qiagen, Valencia, CA). Primers are listed in Table 1. PCR was performed using Quantifast SYBR green PCR master kits (Qiagen).
Table 1.
Oligo sequences used in this study.
| Name | Target | Sequence | Purpose |
|---|---|---|---|
| siCo | luciferase | cgtacgcggaatacttcga | Knock down |
| silR-1 | IR | gcaggtcccttggcgatgt | Knock down |
| silR-2 | IR | aaacgaggcccgaagattt | Knock down |
| silR-3 | IR | gaacaaggctcccgagagt | Knock down |
| silR-4 | IR | acggagacctgaagagcta | Knock down |
| silGFR | IGF-1R | ggactcagtacgccgttta | Knock down |
| IR-for | IR | cgaagatttccgagacctcagt | Real time PCR |
| IR-rev | IR | tcgaagatgaccagcgcgtag | Real time PCR |
| act-for | actin | ccggccagccaggtccagac | Real time PCR |
| act-rev | actin | ccaaggccaaccgcgagaagat | Real time PCR |
Results
IR knock down
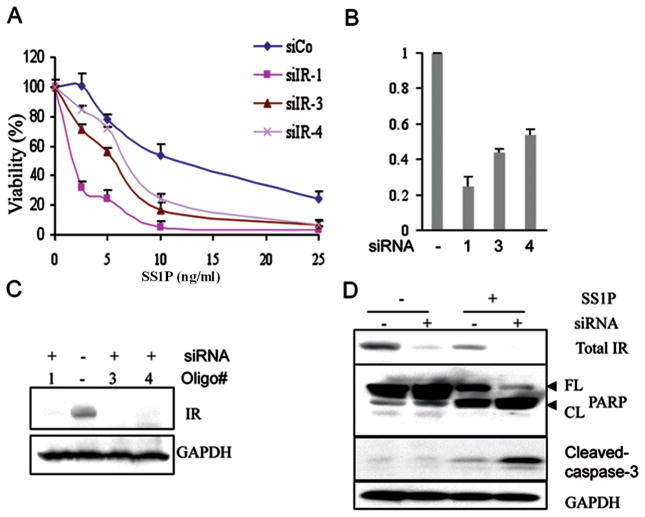
Our strategy assumed that the IR would be activated in cells growing in serum-containing media because serum contains large amounts of IGF1 that, like insulin, can activate this receptor. To investigate its role in immunotoxin response, we used siRNA to knock down the mRNA encoding the IR and then determined how effectively the immunotoxin SS1P, killed various types of mesothelin-expressing cells. To measure cell viability we used the CellTiter-Glo assay, which measures cellular ATP levels. In early experiments we used KB31 cells, which express IR and are sensitive to killing by SS1P. We evaluated four different siRNA oligos targeting the IR: designated siIR-1, siIR-2, siIR-3, and siIR-4. As shown in Fig. 1A we found that 3 of these (siIR-1, -3 and -4) increased the cytotoxic effect of SS1P (Fig. 1A) with siRNA-1 being the most effective. We then measured the levels of IR mRNA and protein after transfection with siIRs-1, -3 and -4 and found that siIR-1 caused the greatest reduction in both mRNA and protein levels, which also produced the greatest increase in SS1P cytotoxicity (Fig. 1A–C). We have seen siIR-1 knock down IR protein level by 80–90% consistently and the IC50 value decreased 3–5 fold in KB cells. Transfection with siIR-2, which did not knock down IR RNA or protein levels, did not increase SS1P toxicity (Supplementary Fig. S1). These results show that knock down of the IR specifically increases the ability of SS1P to kill a mesothelin-expressing cell line.
Figure 1.
A. Silencing of IR via RNAi alters SS1P-induced toxicity. A. A viability assay was conducted after the knock down of the IR using 3 individual siRNA oligos as listed in Table 1. RNAs were transfected into KB31 cells and after 48 hours SS1P was added and viability determined by measuring ATP levels. B. After 48 hours of transfection with 3 individual siRNA oligos, the mRNA level for the IR was measured by real time PCR. The relative expression of IR was calculated using actin as the internal control. C. Western blot analysis of IR expression after siRNA transfection for 48 hours. GAPDH is the loading control. D. Knock down of IR stimulated SS1P-mediated apoptosis. After 48 hours of IR knock down, the cells were treated with 10 ng/ml of SS1P; 24 hours later total cell lysates were analyzed with anti-IR, anti-PARP and anti-cleaved-caspase-3 by western blot. FL, full-length PARP; CL, cleaved PARP. GAPDH is the loading control
To verify that the fall in ATP induced by SS1P in IR knock down cells was associated with an increase in markers of apoptosis, we performed western blots on siIR-1-treated cells. Fig. 1D shows that the level of intact PARP was decreased and the levels of cleaved PARP and cleaved caspase-3 were increased relative to cells treated with SS1P alone (Fig. 1D). This result shows that apoptosis is being simulated. In addition, we observed that SS1P treatment of control cells decreased the IR level (Fig. 1D, lane 1 vs 3). We believe this occurs because SS1P inhibits overall protein synthesis. In addition, we also found treatment of cells with HB21-PE40 that targets the transferrin receptor caused a fall of IR protein level (Supplementary Fig. S2). When SS1P was combined with siIR-1, IR protein was undetectable (Fig. 1D, lane 3 vs 4).
IR knock down on other cancer cell types
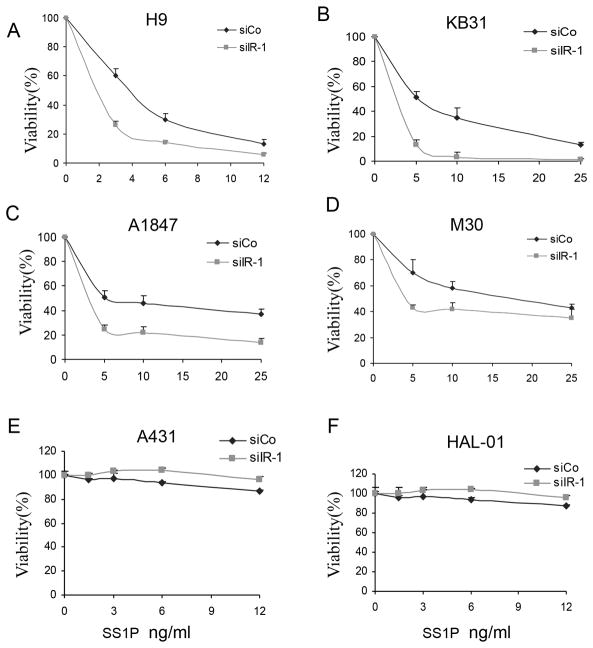
To determine if lowering IR affected SS1P toxicity in other cancer cell types, we examined mesothelin expressing cell lines: A431/H9 cells, which are an epidermoid carcinoma cell line transfected with mesothelin, A1847 ovarian cancer cells and M30 mesothelioma cells. Fig. 2 shows that knock down of the IR increased the cytotoxic effect of SS1P in all 3 cell lines as well as in KB31 cells (Fig. 2A–D). IR knock down did not enhance the killing by SS1P of cell lines, A431 and HAL-01, that do not express mesothelin. This indicates that IR knock down does not promote non-specific killing by SS1P (Fig. 2E–F). We also found that the growth rate of the cell lines was not reduced by IR knock down indicating the effect on SS1P toxicity is independent of cell growth rate (data not shown).
Figure 2.
Knock down of the IR enhanced SS1P toxicity in several cell lines. A431/H9 (A), KB31 (B), A1847 (C), M30 (D) A431(E) and HAL-01(F) cells were transfected with siIR-1 (siIR) or control siRNA GL2 (siCO) for 48 hours in 96 well plates. SS1P was then added for 72 hours. Cell viability was measured by an ATP assay.
Immunotoxin specificity
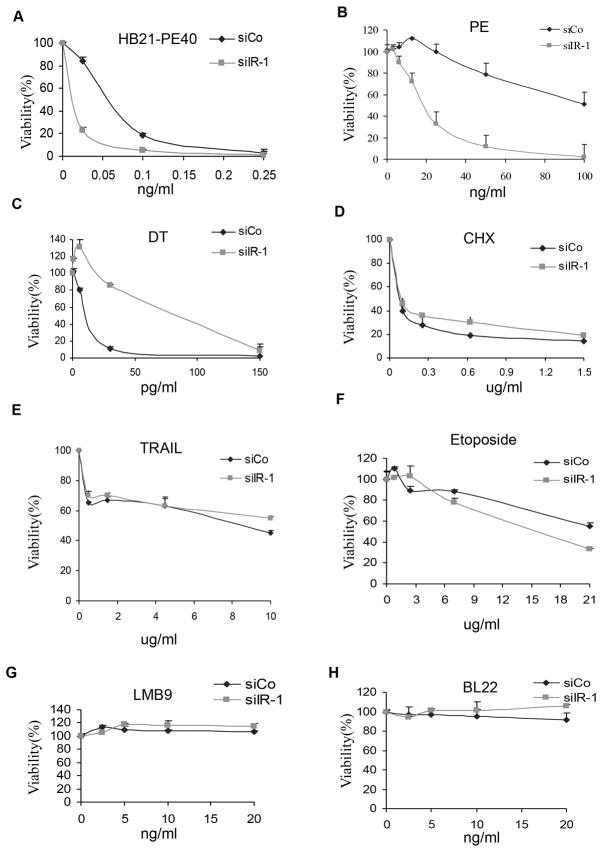
To determine if the IR knock down effect was specific for an immunotoxin targeting mesothelin, we examined the effects on a PE-containing immunotoxin (HB21-PE40) targeting the human transferrin receptor and found that its cytotoxic activity was also greatly increased (Fig. 3A). We also tested the effects of IR knock down on immunotoxins that do not target receptors on KB31 cells and found that neither LMB9 which targets Lewis Y antigen, or BL22 which targets CD22, had any toxic activity (Fig. 3G and H), demonstrating that knock down IR did not enhance non-specific internalization.
Figure 3.
Knock down of IR affects PE-immunotoxins or native PE but not other cytotoxins. KB31cells were transfected with siIR-1 (siIR) or siCo GL2 for 48 hours in 96 well plates, apoptosis inducers or toxins at indicated concentrations were added to the culture medium for 72 hours. Cell viability was measured by ATP assay.
Native PE receptor LRP1B is expresses in KB cells (Supplementary Fig. S3). We also investigated if IR knock down affected the activity of native PE and found, as shown in Fig. 3B, that PE toxicity was increased 5-fold. This finding indicates that the effect of IR knock down is not restricted to an immunotoxin targeting mesothelin.
We then tested other toxic agents such as diphtheria toxin (DT) and cycloheximide, which inhibit protein synthesis, the extrinsic apoptosis inducer TRAIL, and etoposide, that acts through an intrinsic pathway. Silencing the IR inhibited cell killing by DT (Fig. 3C) and did not cause significant changes in the cell killing activities of cycloheximide (Fig. 3D), TRAIL or etoposide (Fig. 3E and F). This data indicates that the IR plays a specific regulatory role on immunotoxins containing PE or on native PE.
Knock down of IR affects SS1P processing
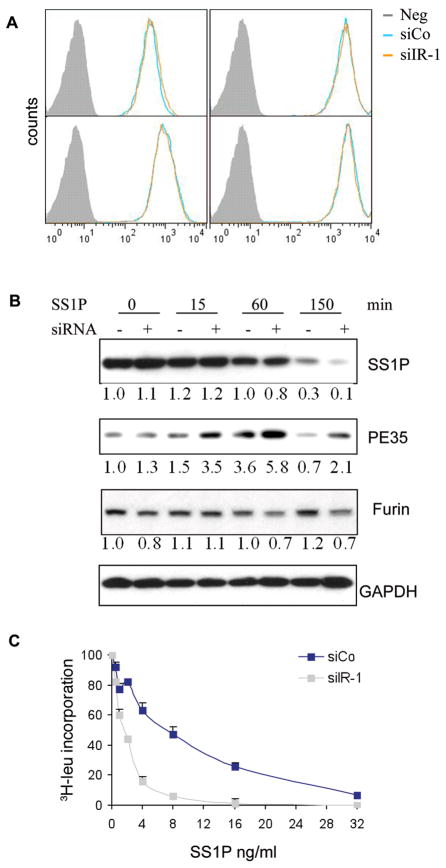
To investigate the mechanism by which the IR controls SS1P toxicity, we measured the internalization of SS1P by exposing cells to SS1P labeled with Alexa-647 for 5–120 minutes and using flow cytometry to assess the amount of cell-associated SS1P in A431H9 cells. Compared to the control, there was no increase in the rate or amount of SS1P taken up by siIR treated cells (Fig. 4A). We also demonstrated that SS1P internalization did not increase in KB cells after siIR-1 treatment (Supplementary Fig. S4).
Figure 4.
A. SS1P internalization was not enhanced after IR knock down. A431/H9 cells were transfected with siIR-1 for 48 hours and SS1P internalization was determined as described in Material and Methods. B. A431/H9 cells were transfected with siIR-1 for 48 hours, 1 μg/ml of SS1P was incubated on ice for 30 minutes then chased at 37°C for the indicated times. Total cell lysates were analyzed with anti-PE and anti-furin antibodies via western blot. PE35 is the cleaved form of SS1P. The blots were scanned and quantified by Image J software and the relative values are normalized with the value of GAPDH level. C. Protein synthesis inhibition assay: A431H9 cells were transfected with siIR-1 or control siCo for 48 hours; SS1P was then added for 20 hours and protein synthesis was measured by 3H-leucine incorporation.
After internalization, the next step in SS1P action is processing by furin. Cleavage by furin separates the Fv from the toxin and generates a 35 kD toxin fragment. As shown in Fig. 4B, in IR knock down cells there was an increase in the amount of the 35 kD fragment detected at 15, 60 and 150 minutes. By 150 minutes over 90% of SS1P was degraded (Note regarding 4B - at 60 minutes there is more PE35 in IR knock down but the amount of full sized SS1P is the same for either sample. This is consistent with interference of a degradation step - i.e. PE35 is not degraded but is stabilized. However, at 150 minutes there is less full sized SS1P in IR knock down and more PE35 suggesting a precursor product relationship presumably with furin). To determine if an increase in furin levels was responsible for the increase in SS1P cleavage, furin levels were measured using western blot analysis and were not found to increase after knock down of IR (Fig. 4B). Because furin is the only known cellular protease that cleaves PE-related immunotoxins and because furin cleavage is rate-limiting (see Discussion), these results indicate that lowering IR expression increases the processing of SS1P by furin and generates an increased amount of the toxin fragment. As a consequence there should be more free toxin fragment available to move to the endoplasmic reticulum, translocate to the cytosol, inactivate elongation factor 2 and arrest protein synthesis (7). To evaluate if the rate of protein synthesis was affected we incubated cells for 20 hours with SS1P after IR knock down and measured 3H-Leu incorporation into protein. Fig. 4C shows that IR knock down greatly enhanced the ability of SS1P to inhibit leucine incorporation.
IR Knock down did not change proapoptotic or antiapoptotic protein levels
Since the IR plays an important role in cancer cell growth and apoptosis, we determined if knock down of the IR would alter the levels of several pro-apoptotic or anti-apoptotic proteins. As shown in Fig. 5, Mcl-1, which has been shown to play an important role in immunotoxin killing (14), was not changed in knock down cells compared with control cells. Bax and xIAP (data not shown) also did not change. We observed a small but not significant reduction in Bcl-xl and Bak, but this small change would not explain the stimulation of SS1P induced toxicity and the reduction in DT induced toxicity. Together, these data indicate the effect of the IR is likely at the level of trafficking and not on proteins regulating apoptosis.
Figure 5.
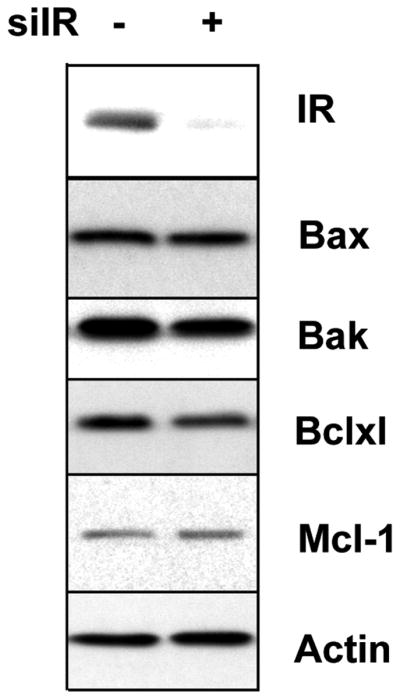
Knock down of IR did not affect the levels of apoptotic proteins. KB31 cells were transfected with siIR (+) or siCo (−) for 72 hours. The cell lysates were analyzed by western blot with antibodies for IR, Bax, Bak, Bclxl, Mcl-1 or actin.
IR level, but not IGF-1R, correlates with SS1P toxicity
IR and IGF-1R are very homologous and utilize similar signaling pathways (12). Fig. 6A shows that IGF-1R knock down slightly decreases SS1P toxicity, an effect totally opposite to that of IR knock down. However, when the two siRNAs were combined, there was still stimulation of SS1P activity although less than with IR knock down alone.
Figure 6.
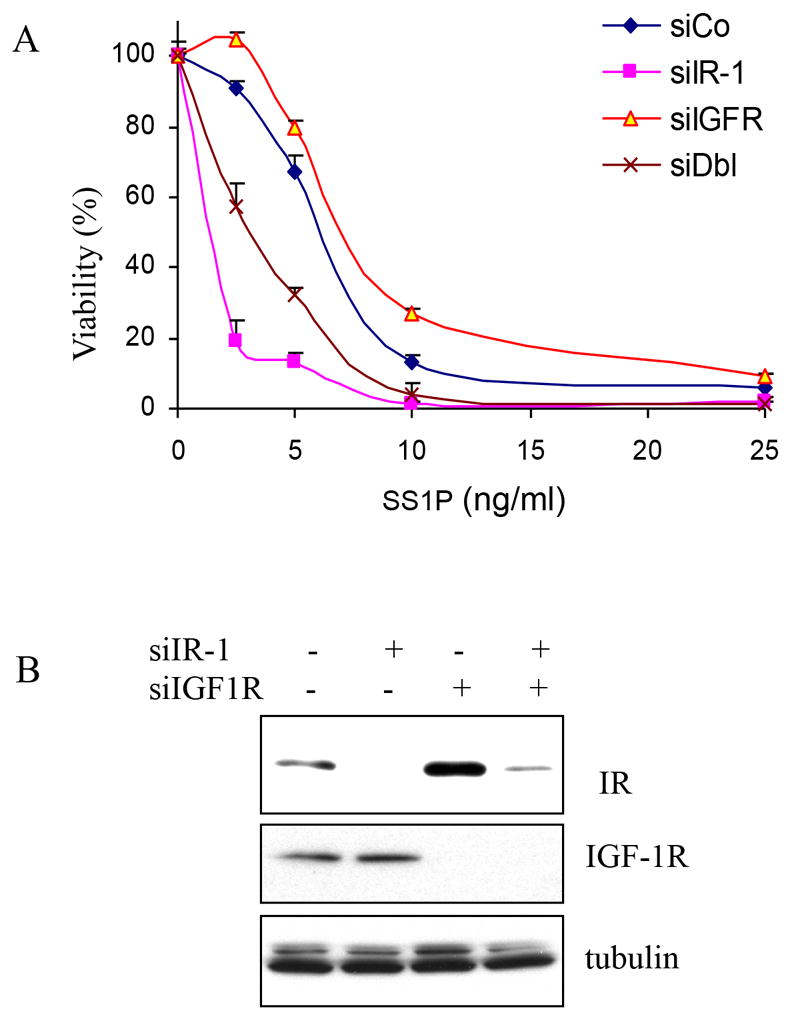
Knock down IGF-1R did not increase SS1P toxicity. A. KB31 cells were transfected with control siRNA (siCo), siIR-1, siIGF-1R (siIGFR) or both siIR-1 and siIGF-1R (siDbl) for 48 hours. SS1P was added at indicated concentrations for 72 hours. Cell viability was measured by ATP assay. B. KB31 cells were transfected with siIR-1 or siIGF-1R for 48 hours. Cell lysates were analyzed using anti-IR, anti-IGF-1R or anti-β-tubulin by western blot.
To examine this finding further, we performed western blot analysis and found that treatment with siIGF-1R not only completely reduced IGF-1R level (Fig. 6B, lane 1 vs 3 and 4), but also significantly increased the IR level. Furthermore, the IR level correlated with the SS1P toxicity effect (compare Fig. 6A and 6B).
Discussion
We show here that lowering IR levels in various cancer cell lines using siRNA knock down increases the ability of immunotoxins containing PE or of native PE to kill target cells. This novel function of the IR affects PE based toxins whether they enter the cell bound to an antibody to mesothelin or to an antibody to the transferrin receptor or through the native receptor for PE. We were surprised to find that IR knock down did not enhance the cytoxicity of DT, which like PE inactivates elongation factor 2, but instead protected cells from DT induced cell death. This is probably because PE and DT have different trafficking pathways and reach the cytosol by different mechanisms. PE must be cleaved by furin and transported to the endoplasmic reticulum before reaching the cytosol (15), whereas DT can be transferred directly to the cytosol from the endocytic compartment (7, 16). In addition, furin is the only cellular protease known to cleave PE and PE-immunotoxins, suggesting IR knock down is altering furin function but not other proteases.
It is known that one major action of insulin is the regulation of the transport of GLUT4 containing vesicles causing them to fuse with the plasma membrane and increase the amount of GLUT4 on the cell surface. Under conditions of low insulin, GLUT4 is sequestered in intracellular vesicles in muscle and fat cells. Insulin induces IR activation and through a complex cascade of signal transduction events increases the uptake of glucose by inducing the translocation of GLUT4 from these vesicles to the plasma membrane (17, 18). It is possible that some of the same proteins that control PE trafficking also control the trafficking of the IR. This area is under investigation.
Several compounds have been found to inhibit the action of insulin on target cells. We have investigated these agents to determine if they regulate the increase in PE toxicity produced by IR knock down. As shown in Supplementary Fig. S5 and Supplementary Table S1, none of these agents enhanced SS1P cell killing and some of them actually protected against cell killing when combined with SS1P. The compounds investigated include the IR/IGF-1R substrate inhibitor AGL2263, the mTOR inhibitor Rapamycin, the PI3K inhibitor LY294002, the MEK1 inhibitor PD98059 and a combination of both LY294002 and PD98059 (Supplementary Table S1). Furthermore, addition of insulin to the cells did not protect cells from killing by SS1P (Supplementary Fig. S1D). We have considered the possibility that the IR works through another pathway to regulate intracellular trafficking. Boucher et al. have presented evidence that the IR has a role in apoptosis regulation that is independent of its kinase activity (19). However, our findings here indicate the apoptotic protein levels did not change before or after insulin knock down (Fig. 5). It is possible the IR function here also controls membrane trafficking. A kinase independent mechanism of IR action could include interaction with a scaffolding protein that controls trafficking to lysosomes or retrograde trafficking of proteins leading to the accumulation of active PE35 in the cytosol.
Two splice variants of the IR exist in mammalian cells: IR-A, lacking exon 11, and full-length IR-B. IR-A predominates in fetal tissues and is often up-regulated in many cancer cells; IR-B is present in adult muscle and fat cells and is responsible for glucose regulation (20, 21). Our finding of an IR function in cellular trafficking may apply to both isoforms, because the siRNAs used target both isoforms and enhance SS1P killing of KB cells, which express both IR-A and IR-B, and of A1847 cells which express predominantly IR-A (Supplementary Fig. S6). Additionally, mouse brown adipocytes, which predominantly expresse the IR-B isoform, when IR gene is deleted are more sensitive to PE than wild type cells (data not shown). We conclude both isoforms are important for the IT or PE toxicity. The IR is elevated and activated in several human maligancies including breast, colon and lung cancer (20, 22) and functionally enhances tumor progression (23). Our finding of a novel IR function that regulates membrane trafficking may contribute to the understanding of insulin action and the IR in cancer and other human diseases.
Supplementary Material
Acknowledgments
We thank Dr. Ron Kohn’s group for providing IR knock-out brown adipocytes and helpful discussion. The authors also thank YuJian Zhang for providing SS1P-Alexa 647. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
The authors declare no conflict of interest
References
- 1.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–57. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 2.Wu AM, Senter PD. Arming antibodies: prospects and changes for immunoconjugates. Nat Biotechnol. 2005;23:1137–46. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 3.FitzGerald DJ, Wayne AS, Kreitman RJ, Pastan I. Treatment of hematologic malignancies with immunotoxins and antibody-drug conjugates. Cancer Res. 2011;71:6300–9. doi: 10.1158/0008-5472.CAN-11-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 5.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–42. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 6.Hassan R, Laszik ZG, Lerner M, Raffield M, Postier R, Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–45. [PubMed] [Google Scholar]
- 7.Weldon JE, Pastan I. Guide to taming a toxin – recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J. 2011;278:4683–700. doi: 10.1111/j.1742-4658.2011.08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kinderler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 9.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupont J, Leroith D. Insulin and insulin-like growth factor I receptors: similarity and differences in signal transduction. Horm Res. 2001;55:22–6. doi: 10.1159/000063469. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 12.Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, et al. The role of insulin receptor and IGF-1 receptor in cancer and other disease. Arch Physiol Biochem. 2008;114:23–37. doi: 10.1080/13813450801969715. [DOI] [PubMed] [Google Scholar]
- 13.Pastan I, Beers R, Bera TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol. 2004;248:503–18. doi: 10.1385/1-59259-666-5:503. [DOI] [PubMed] [Google Scholar]
- 14.Du X, Youle RJ, Fitzgerald DJ, Pastan I. Pseudomonas exotoxin A-mediated apoptosis is Bak dependent and preceded by the degradation of Mcl-1. Mol Cell Biol. 2010;30:3444–52. doi: 10.1128/MCB.00813-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiron MF, Fryling CM, Fitzgerald DJ. Furin-mediated cleavage of Pseudomonas exotoxin-derived chimeric toxins. J Biol Chem. 1997;272:3107–11. doi: 10.1074/jbc.272.50.31707. [DOI] [PubMed] [Google Scholar]
- 16.Spooner RA, Watson P. Drug targeting: learning from toxin entry and trafficking in mammalian cells. Curr Opin Drug Discov Devel. 2010;13:86–95. [PubMed] [Google Scholar]
- 17.Kao AW, Ceresa BP, Santeler SR, Pessin JE. Expression of a dominant interfering dynamin mutant in 3T3L1 adipocytes inhibits GLUT4 endocytosis without affecting insulin signaling. J Biol Chem. 1998;273:25450–7. doi: 10.1074/jbc.273.39.25450. [DOI] [PubMed] [Google Scholar]
- 18.Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;4:267–77. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- 19.Boucher J, Macotela Y, Bezy O, Mori MA, Kriauciunas K, Kahn CR. A kinase-independent role of unoccupied insulin and IGF-1 receptors in the control of apoptosis. Sci Signal. 2010;3:ra87. doi: 10.1126/scisignal.2001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonino B, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocrine Reviews. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 21.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 22.Sciacca L, Costantino A, Pandini G, Mineo r, Frasca F, Scalia P, et al. Insulin receptor activation by IGF-II in breast cancer: evidence for a new autocrine/paracrine mechanism. Oncogene. 1999;18:2471–9. doi: 10.1038/sj.onc.1202600. [DOI] [PubMed] [Google Scholar]
- 23.Ulanet D, Ludwig D, Kahn CR, Hanahan D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc Natl Acad Sci USA. 2010;107:10791–8. doi: 10.1073/pnas.0914076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.