Abstract
Purpose
Epileptogenesis is the process by which a brain becomes hyperexcitable and capable of generating recurrent spontaneous seizures. In humans, it has been hypothesized that following a brain insult there are a number of molecular and cellular changes that underlie the development of spontaneous seizures. Studies in animal models have shown that an injured brain may develop epileptiform activity before appearance of epileptic seizures and that the pathophysiology accompanying spontaneous seizures is associated with a dysfunction of GABAergic neurotransmission. Here, we analyzed the effects of status epilepticus on the expression of GABAA receptors and scaffolding proteins involved in the regulation of GABAA receptor trafficking and anchoring.
Methods
Western blot analysis was used to determine the levels of proteins involved in GABAAR trafficking and anchoring in adult rats subjected to pilocarpine-induced SE and controls. Cell surface biotinylation using a cell membrane impermeable reagent was used to assay for changes in the expression of receptors at the plasma membrane. Finally, immunoprecipitation experiments were used to evaluate the composition of GABAA receptors. We examined for a correlation between total GABAAR subunit expression, plasma membrane expression and receptor composition.
Key Findings
Analysis of tissue samples from the CA1 region of hippocampus show that SE promotes a loss of GABAA receptor subunits and of the scaffolding proteins associated with them. We also found a decrease in the levels of receptors located at the plasma membrane and alterations in GABAA receptor composition.
Significance
The changes in protein expression of GABAA receptors and scaffolding proteins detected in these studies provide a potential mechanism to explain the deficits in GABAergic neurotransmission observed during the epileptogenic period. Our current observations represent an additional step towards the elucidation of the molecular mechanisms underlying GABAAR dysfunction during epileptogenesis.
Keywords: GABAA receptor, Gephyrin, Epileptogenesis, Scaffolding Proteins, Receptor Trafficking
INTRODUCTION
The process by which neuronal cells progressively form hyperexcitable networks leading to the manifestation of spontaneous seizures has been called epileptogenesis (Sharma et al., 2007; Curia et al., 2008; Fritschy 2008; Scorza et al., 2009). In humans, a head injury, prolonged febrile seizures, stroke or status epilepticus (SE) may precede the appearance of spontaneous seizures by months or years (Löscher & Brandt 2010). In experimental models, an initial brain injury is followed by a number of pathophysiological and structural alterations that increase the probability of seizure occurrence and favor the appearance of overt spontaneous seizures (Falconer et al., 1964; Herman 2002; Luscher & Keller 2004; McNamara et al., 2006). In the pilocarpine model of epilepsy, the CA1 region of hippocampus becomes hyperexcitable soon after the induction of SE and remains hyperexcitable during the chronic period. The origin of this hyperexcitability is unclear but a decrease in GABAergic drive and intrinsic cell excitability has been detected after SE induction. More importantly, it is possible that this increased hyperexcitability may contribute to the genesis and/or propagation of epileptic seizures. A transient decrease in GABAergic drive has been associated with the appearance of inter-ictal activity (3–5 days after SE) that evolves into epileptiform activity (7–10 days after) to culminate in the appearance of electrographic seizures (El-Hassar et al., 2007).
GABAA receptors (GABAAR) are heteropentameric ion channels assembled from at least 18 homologous subunits: α(1–6), β(1–3), γ(1–3), ε(1-3), δ, θ, and π (Macdonald & Olsen 1994; Sieghart et al., 1999; Chen et al., 2007; Jacob et al., 2008). Subunit composition of GABAAR governs physiological, pharmacological and targeting properties. Synaptic GABAAR contain γ subunits (phasic inhibition) whereas perisynaptic or extrasynaptic receptors contain δ subunits (tonic inhibition). Protein-protein interactions with scaffolding proteins also depend on subunit composition and modulate the proper delivery and anchorage of GABAAR (Luscher & Keller 2004; Chen & Olsen 2007; Jacob et al., 2008; Leidenheimer 2008). Most GABAAR are anchored to synaptic sites by gephyrin, a 93-kDa protein that appears to form a hexagonal lattice beneath the plasma membrane onto which inhibitory receptors are anchored (Jacob et al., 2005; Fritschy et al., 2008; Tretter et al., 2008). Elimination of gephyrin by gene targeting or siRNA strongly affects GABAAR clustering and inhibitory postsynaptic currents (Essrich et al., 1998; Yu et al., 2007; Fritschy et al., 2008).
In addition to gephyrin other proteins like the GABA receptor associated protein (GABARAP), N-ethylmaleimide-sensitive factor (NSF) and the glutamate receptor interacting protein (GRIP) bind to, colocalize with and play a role in the intracellular trafficking and plasma membrane expression of GABAAR (Wang et al., 1999; Kneussel et al., 2000; Charych et al., 2004; Chen & Olsen 2007; Yu et al., 2008). GABARAP is predominately enriched in intracellular membranes of the Golgi apparatus and regulates cell surface levels of GABAAR but has not been found at significant levels within inhibitory synapses (Chen & Olsen 2007; Michels & Moss 2007; Leidenheimer 2008). GABARAP binds to tubulin to presumably link microtubules and γ2 containing receptors. GABARAP also interacts with NSF and GRIP and might facilitate the association of proteins involved in the intracellular trafficking of GABAAR. NSF is an important cofactor that plays a central role in membrane fusion events underlying intracellular trafficking and may be involved in the intracellular trafficking of GABAAR containing β subunits. GRIP interacts with gephyrin and GABARAP and although its role at inhibitory synapses remains unclear, it has been suggested that GRIP may be involved in the formation of inhibitory synapses and the regulation of GABAAR function (Chen & Olsen 2007; Michels & Moss 2007; Leidenheimer 2008).
A rapid loss of GABAAR from the plasma membrane following SE induction has been associated with changes in trafficking and phosphorylation of GABAAR subunits (Goodkin et al., 2005; Naylor et al., 2005; Chen et al., 2007; Terunuma et al., 2008). During the chronic period, animals experiencing spontaneous seizures show altered inhibitory neurotransmission associated with abnormal composition and function of GABAAR in dentate gyrus (Brooks-Kayal et al., 1998). Despite these findings, the molecular remodeling that affects GABAergic neurotransmission during epileptogenesis in the CA1 region is not fully understood. Increased neuronal excitability during the epileptogenic period has been described in this region and might reflect compromised GABAergic neurotransmission that results from a lack of functional GABAAR and/or altered subunit expression (Leroy et al., 2004; El-Hassar et al., 2007; Fritschy 2008).
Because it has been reported that the CA1 region of hippocampus becomes hyperexcitable during the epileptogenic period, we decided to characterize possible alterations in the expression of GABAergic proteins. The degree of cell loss typically detected in the CA1 region is not as extensive as that observed in the hilus or CA3 region of hippocampus (Esclapez et al., 1999; Houser & Esclapez 2003; Chauviere et al., 2009), allowing a better understanding of the impact of SE on the regulation of protein levels of GABAAR and scaffolding proteins in surviving cells. Thus in the present study, we characterized the levels of scaffolding proteins involved in the trafficking and anchoring of GABAAR and examined if a loss of scaffolding proteins is correlated with changes in GABAAR subunit expression, composition and plasma membrane localization. Analysis of tissue samples from the CA1 region of hippocampus showed a reduction in the total and plasma membrane expression of several GABAAR subunits that is concurrent with a loss of gephyrin. These observations represent an additional step towards the molecular characterization of the events underling abnormal GABAergic neurotransmission during epileptogenesis.
MATERIALS AND METHODS
Status Epilepticus Induction
Male Sprague Dawley rats (Charles River, Wilmington, MA) were housed in a temperature-controlled vivarium with food and water ad libitum. To minimize the peripheral effects of pilocarpine, rats were injected intraperitoneally with scopolamine methyl nitrate (1 mg/kg) 30 minutes before administration of pilocarpine hydrochloride (385 mg/kg). According to a standard protocol, if rats did not exhibit convulsive seizures within 1 h of pilocarpine injection a maximum of two subsequent doses of pilocarpine (192.5 mg/kg) were given in order to produce equivalence in seizures between animals (Brooks-Kayal et al., 1998; Shumate et al., 1998). Diazepam (6 mg/kg; Hospira, Lake Forest, IL) was administered 1 h after SE onset to stop seizure progression and additional doses (3 mg/kg) were administered every 2 h until rats stopped seizing. Rats used in these studies had confirmed stage 5 behavioral seizures and rats that did not exhibit SE were not included in this study. Control rats were handled similarly but treated with a subconvulsive dose of pilocarpine (38.5 mg/kg) and 1/10 of the dose of diazepam (0.6 mg/kg). There is evidence that SE rapidly alters the response of GABAA receptors to diazepam and produces a substantial reduction in diazepam potency for the termination of seizures (Kapur & Macdonald 1997). Since it is possible that a similar dose of diazepam could produce a greater effect on GABAA receptors in control rats than it does in rats undergoing SE, we chose a reduced dose of diazepam for the control rats in order to mitigate potential confounding effects while still controlling for the stress of repeated handling and injections. Rats were sacrificed at 1, 4, or 8 days after SE to prepare tissue samples for analysis. Animal procedures were performed in accordance with Institutional Animal Care and Use Committee regulations and approved protocols by the University of Colorado Anschutz Medical Campus.
Preparation of Total Tissue Lysates
Hippocampal slices (600 µM) were prepared using a McIlwan tissue chopper after rapid isolation of the whole hippocampus. The Cornus Ammonis 1 (CA1) was isolated from each slice as follows: first, the CA3 region was separated from CA1 and Dentate Gyrus (DG) and then CA1 was separated from DG through the hippocampal sulcus (Silva et al., 2001). All microdissected pieces of CA1 were pooled together. Lysates were prepared by brief sonication and agitation of the tissue at 4°C for 30 min in RIPA buffer (100 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 1% sodium deoxycholate) containing a mixture of protease and phosphatase inhibitors. Tissue lysates were cleared of cell debris by centrifugation at 15,000 × g for 20 min. Protein concentration was determined using the Bio-Rad RC/DC reagent kit (Bio-Rad Laboratories, Hercules, CA, USA).
Measurement of Receptor Cell Surface Expression by Biotinylation
This protocol is a modification of previously reported methods (Grosshans et al., 2002; Gonzalez et al., 2007; Holman & Henley 2007). Briefly, to label plasma membrane proteins, freshly prepared hippocampal slices (400 µM) were bathed in bubbled aCSF containing 1 mg/ml Sulfo-NHS-LC-Biotin (Thermo Scientific, Rockford, IL) for 30 min at 4°C with gentle agitation. After four rinses with aCSF containing 100 mM glycine, slices were incubated in this same buffer for 30 min at 4°C to eliminate unreacted biotin. Biotinylated slices were rinsed with ice-cold aCSF and microdissected to isolate the CA1. All CA1 pieces were pooled together and lysed by brief sonication and agitation at 4°C for 30 min in RIPA buffer containing protease and phosphatase inhibitors. Tissue lysates were cleared of cell debris by centrifugation at 15,000 × g for 20 min. An aliquot of lysate was mixed with a half volume of 4× Laemmli buffer and label as “lysate fraction”. A second lysate aliquot was mixed with an equal volume of Ultralink avidin-conjugated beads (Thermo Scientific, Rockford, IL) and incubated overnight at 4°C with constant agitation. Beads containing the biotinylated proteins were recovered by centrifugation and washed once with RIPA buffer, twice with a high-salt buffer (50 mM Tris, 5 mM EDTA, 500 mM NaCl, 0.1% Triton X-100, pH 7.5), and once with a no-salt buffer (50 mM Tris, pH 7.5). Biotinylated proteins were released by incubating the beads in 2× Laemmli buffer at 37°C for 30 min. The supernatant containing the biotinylated proteins was recovered after pelleting the beads by centrifugation. Proteins in the biotinylated fraction were diluted to the same extent than proteins in the total lysate, so that immunoreactivity in the lysate and biotinylated fractions is proportional when equal volumes of lysate and biotinylated fraction are analyzed.
Immunoisolation of GABAA Receptors
Microdissected CA1 was obtained as described above and dissociated in RIPA buffer by passing the tissue 25 times through a 21G needle. To obtain tissue lysates, dissociated tissue was shaken for 15 minutes at room temperature and 90 min at 4°C. Tissue lysates were then centrifuged at 15,000 × g for 20 min to remove cell debris. Lysates were pre-cleared with 40 µl of beads for 1 h at 4°C and shaking. A volume of lysate equivalent to 300–350 µg of protein was incubated with 5 µg of mouse monoclonal antibodies for α1 (NeuroMab, Davis, CA) or β2/3 (Millipore, Billerica, MA) subunits. 5 µg of non-immune mouse IgG (Santa Cruz Biotech, Santa Cruz, CA) was used as control. Following overnight incubation at 4°C, immune complexes were mixed with 25 µl of protein G-sepharose beads (GE Health Care, Piscataway, NJ) and incubated for 2 h at 4°C. Beads containing the immunoprecipitates were washed four times with RIPA buffer and immunoisolated proteins released in 25 µl of 2× Laemmli buffer after boiling for 3 min.
Western Blot
Protein samples were separated in SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Blots were blocked for 1 h at room temperature with 5% non-fat dry milk in Tris-buffered saline (pH 7.4) plus 0.05% Tween 20. Blots were then incubated overnight at 4°C with primary antibodies diluted in 1% non-fat dry milk. After washing the primary antibody, blots were incubated with secondary antibody for 1 h at room temperature. Immunoreactive bands were visualized using Super Signal West Pico chemiluminescent substrate (Pierce, Rockford, IL, USA) and film. After scanning the films, immunoreactive bands of the appropriate size were quantified using Image J software (National Institutes of Health, Bethesda, MD, USA). To estimate potential variability in protein content and loading, blots were stripped and probed with an anti-actin antibody from Sigma (St. Louis, MO). Immunoreactivity for the protein of interest was normalized to actin immunoreactivity and compared to control values. Monoclonal mouse antibodies anti-gephyrin, anti-NSF and anti-GRIP were from BD Biosciences (Bedford, MA). Monoclonal mouse anti-GABARAP was from MBL International Corporation (Woburn, MA). Monoclonal mouse anti-β2/3 (clone 62-3G1) and polyclonal rabbit antibodies anti-α1 and anti-α4 were from Millipore (Billerica, MA). Polyclonal rabbit anti-γ2 was from Phosphosolutions (Aurora, CO). Anti-mouse or anti-rabbit secondary antibodies conjugated to horseradish peroxidase were from Jackson Immunoresearch laboratories (West Grove, PA) or GE Health Care (Piscataway, NJ), respectively.
Immunofluorescence and confocal microscopy
Rats were deeply anesthetized and perfused transcardially with 4% PFA in 0.1 M phosphate buffer pH 7.4. Brains were post-fixed, cryoprotected frozen in OCT. Coronal sections (40 µM) were obtained using a cryostat and placed in PBS for the staining procedure. For consistency control and SE brains were processed and stained in parallel. Floating sections were blocked for at least one hour in PBS containing 5% normal goat serum and 0.3% Triton X-100. Sections were incubated overnight in PBS containing 5% goat serum and 0.2% Triton X-100 and a mouse monoclonal anti-neuronal nuclei antibody (NeuN, Millipore, 1:1500) or a mix of purified mouse monoclonal anti-gephyrin (mAb7a, synaptic systems, 1:500) and guinea pig polyclonal anti-vesicular GABA transporter (VGAT, synaptic systems, 1:300). The next day, slices were washed and incubated with the appropriate highly cross-absorbed goat secondary antibodies: alexa fluor 488 anti-mouse or alexa fluor 568 anti-guinea pig (Invitrogen, 1:750) in PBS containing 5% goat serum and 0.2% Triton X-100. After washing, tissue sections were mounted on slides using Vectorshield (Vector Laboratories, Burlingame, CA, USA). Controls where the primary antibodies were omitted were run to confirm that the staining was dependent on the primary antibody. Low-resolution images were obtained using a Nikon Eclipse microscope and digital images of double-labeled sections were optically sectioned and scanned using a Zeiss LSM 510 META confocal microscope.
Statistical analysis
Data are presented as the mean ± SEM. The differences between groups were assessed by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. A paired student’s t-test was used when only two groups of data were compared. In all cases, p values < 0.05 were considered significant. GraphPad InStat (GraphPad Software, Inc., San Diego, CA, USA) was used to perform the statistical analysis.
RESULTS
Within minutes, status epilepticus triggers a rapid removal of GABAARs from the plasma membrane, a phenomenon associated with changes in phosphorylation of individual receptor subunits (Goodkin et al., 2008; Terunuma et al., 2008). Because proper anchoring and trafficking of GABAAR is dependent upon a number of GABAAR associated proteins (Chen & Olsen 2007; Jacob et al., 2008; Luscher et al., 2011), we investigated whether expression of the scaffolding proteins associated with GABAAR is altered as a consequence of status epilepticus. The protein expression of gephyrin, GRIP, NSF and GABARAP was analyzed in tissue samples from the CA1 region of hippocampus obtained at three time points after SE induction: 1 day (during the acute injury), 4 days (when inter-ictal activity can be readily detected) and 8 days (when inter-ictal activity becomes established and spontaneous seizures might start to emerge).
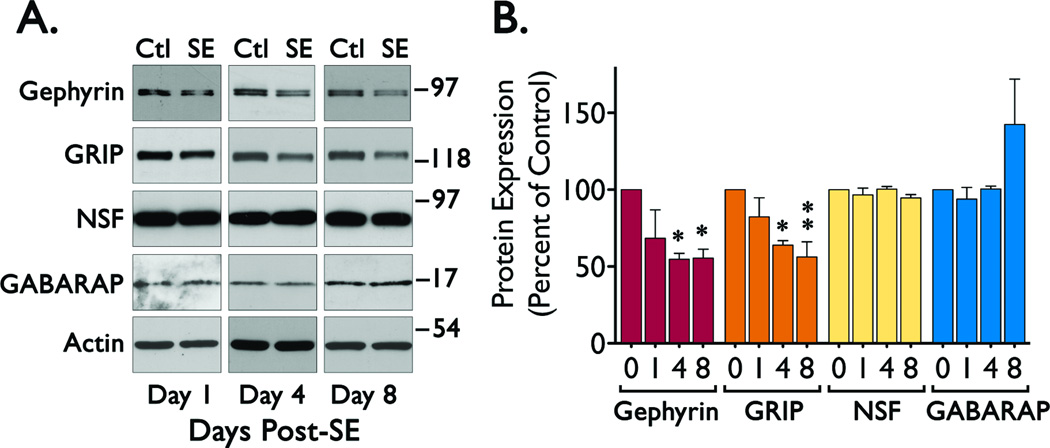
Western blot analysis of samples from the CA1 region of hippocampus showed a significant reduction in gephyrin (ANOVA, F = 4.604, p < 0.0229) and GRIP (ANOVA, F = 5.845, p < 0.0106) immunoreactivity in samples obtained 4 and 8 days after induction of SE (Figure 1). In contrast, immunoreactivity for NSF and GABARAP did not show a significant change. GABARAP expression shows a non-significant trend towards an increase in samples obtained 8 days post-SE (ANOVA, F = 2.140, p < 0.1484).
Figure 1. Expression of scaffolding proteins in CA1 during the epileptogenic period.
Hippocampal slices (600 µm) were obtained from control (Ctl, day 0) and SE animals (1, 4 or 8 days after SE induction) to microdissect the CA1 region and prepare tissue lysates for Western blot analysis. (A) Representative blots of samples from the CA1 region probed with antibodies for gephyrin, GRIP, NSF, GABARAP or actin. (B) Densitometric analysis of the immunoreactivity for each scaffolding protein normalized to actin immunoreactivity. Data was obtained from four independent experiments and it is presented as the mean ± SEM (*p < 0.05 or **p < 0.01 compared to control by ANOVA followed by Bonferroni post hoc test). The overall values for the ANOVA analysis were: Gephyrin (F = 4.604, p < 0.0229), GRIP (F = 5.845, p < 0.0106), and GABARAP (F = 2.140, p < 0.1484).
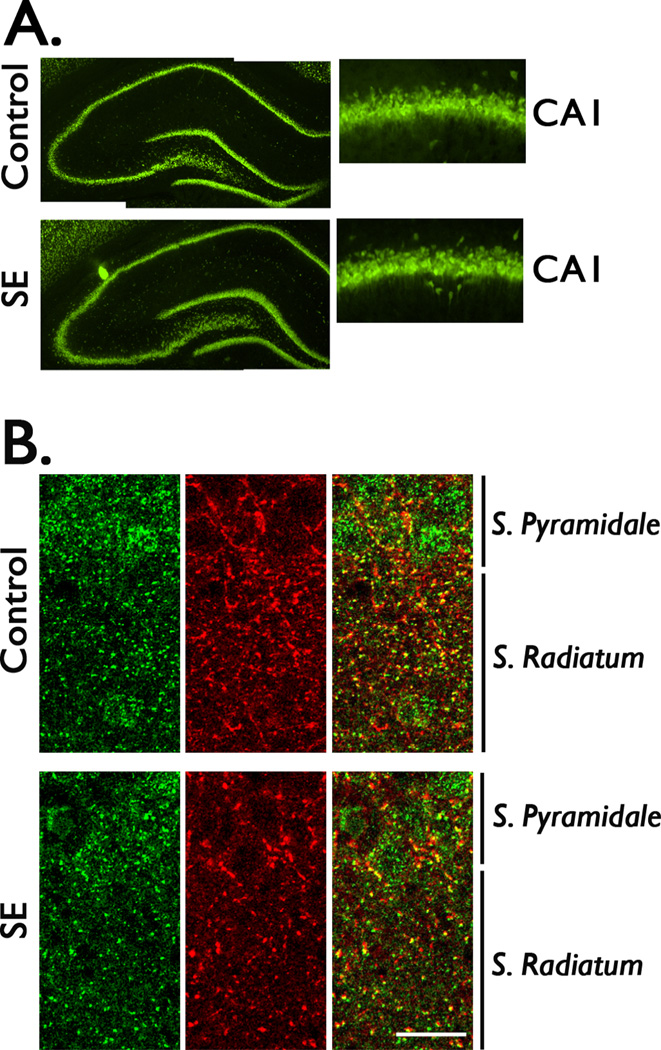
Previous studies have shown that after pilocarpine-induced SE there is extensive cell loss in several regions of the brain but the CA1 region is relatively well preserved (Esclapez et al., 1999; Houser & Esclapez 2003; Chauviere et al., 2009). Using the neuronal marker, NeuN, we assessed the pattern of neuronal damage in the CA1 region at 4 days post-SE. Compared to control, there were no obvious changes in the anatomical distribution of neuronal bodies in this hippocampal region, suggesting that if there is some neuronal loss this change is not sufficiently large to be detectable by standard light microscopy (Figure 2A). Brain sections were also stained with gephyrin and VGAT antibodies to obtain a qualitative assessment of gephyrin distribution in the CA1 region. In control tissue, gephyrin immunoreactivity was clearly detected on the cell bodies of pyramidal cells and was surrounded by VGAT staining (Figure 2B). A punctate distribution of gephyrin staining that often colocalized with VGAT punctae was observed in stratum radiatum. In contrast, the distribution of gephyrin and VGAT appears more diffuse throughout the cell bodies and projections of pyramidal cells in tissue obtained after SE induction (Figure 2B).
Figure 2. Detection of gephyrin distribution in the CA1 region of hippocampus.
Brain sections (40 µM) were collected from control (Ctl) and SE animals at 4 days post-SE to qualitatively evaluate the distribution of neuronal profiles and gephyrin staining. (A) Representative images of hippocampal sections of control and SE animals stained with the neuronal marker, NeuN. Low magnification images (4X) of the hippocampal structure do not show an evident alteration in the neuronal layers. Images of the CA1 region at a higher magnification (20X) show a similar distribution of neuronal profiles in control and SE tissue. (B) Double labeled immunofluorescence of brain sections with anti-gephyrin (green) and anti-VGAT (red) antibodies were used to qualitatively assess the distribution of gephyrin in the CA1 region of hippocampus. Confocal images show that gephyrin staining (green) at the cell bodies is surrounded by the VGAT staining (red) that delineate the cell bodies, some colocalization (yellow) is observed at both the cell bodies and stratum radiatum. Both gephyrin and VGAT staining appears to be more disperse in tissue obtained from SE animals. Images are representative of four independent experiments. Scale bar represents 20 µM.
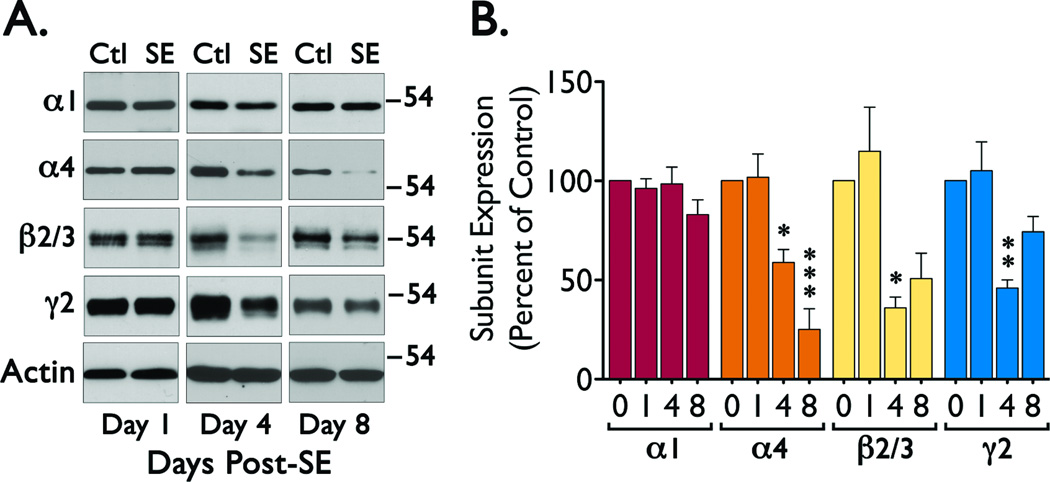
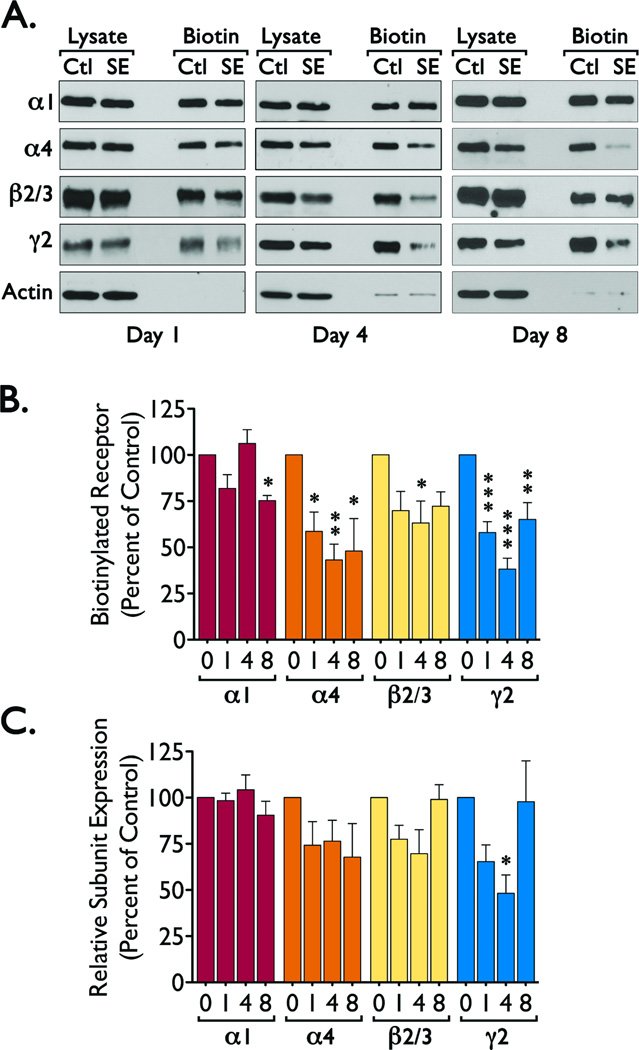
There is evidence that a loss of scaffolding proteins may result in a loss of GABAAR expression and vice versa (Essrich et al., 1998; Yu et al., 2007). Thus, we examined if the reduced expression of scaffolding proteins (particularly the reduction in gephyrin expression) observed after status epilepticus might correlate with a loss of specific GABAAR subunits. We detected a significant reduction in α4 (ANOVA, F = 18.361, p < 0.0001), β2/3 (β2/3 ANOVA, F = 8.396, p < 0.0028) and γ2 (ANOVA, F = 10.098, p < 0.0013) immunoreactivity at 4 days after SE induction, with a persistent reduction in α4 at 8 days after SE (Figure 3). The changes in GABAAR expression parallel the loss of gephyrin, suggesting that the network of proteins anchoring GABAAR at the plasma membrane might be disrupted during the epileptogenic period and could have an impact on the number of functional receptors located at the plasma membrane. To analyze for this possibility, we used a cell-surface biotinylation assay to selectively label the population of GABAAR located at the plasma membrane. These studies revealed that when compared to control, SE induction promotes a decrease in the levels of α4 (ANOVA, F = 6.597, p < 0.0046), β2/3 (ANOVA, F = 3.471, p < 0.0430) and γ2 (ANOVA, F = 20.422, p < 0.0001) subunits located at the cell surface (Figure 4). For α4 and γ2 subunits these changes can be clearly observed as soon as 1 day after seizure induction (Figure 4). Because we observed that over time there is a significant change in the total expression of GABAAR subunits, we also normalized the immunoreactivity detected in the biotinylated fraction to the signal detected in the total lysate to estimate the proportion of receptor subunits located at the plasma membrane. Using this normalization, the fraction of γ2 subunits located at the plasma membrane was significantly decreased (ANOVA, F = 4.801, p < 0.0154, Bonferroni post hoc test, *p < 0.05 compared to control) at 4 days after SE (Figure 4C). Other subunits (α4 and β2/3) did not show a statistically significant decrease. A parallel loss of γ2 subunits from the cell surface and a reduction in gephyrin expression suggests that gephyrin levels may directly impact the stability of γ2-containing receptors and may also be directly correlated with the differential changes in the total expression of individual GABAAR subunits.
Figure 3. Expression of GABAAR subunits in CA1 during the epileptogenic period.
Tissue samples of CA1 region were obtained at 0, 1, 4 or 8 days after SE and analyzed by western blot. (A) Representative blots for the expression of α1, α4, β2/3, γ2 and actin in CA1 samples. (B) Summary of the immunoreactivity detected for each GABAAR subunit normalized to actin immunoreactivity. Data was obtained from four independent experiments and is presented as the mean ± SEM. (*p < 0.05, **p < 0.01 or ***p < 0.01 compared to control by ANOVA followed by Bonferroni post hoc test). The overall values for the ANOVA analysis were: α1 (F = 1.575, p < 0.2469), α4 (F = 18.361, p < 0.000), β2/3 (F = 8.396, p < 0.0028), and γ2 (F = 10.098, p < 0.0013).
Figure 4. Cell surface expression of GABAAR subunits during the epileptogenic period.
Freshly prepared hippocampal slices (400 µM) from control (Ctl, day 0) or SE animals (at 1, 4 or 8 after SE) were labeled using a cell impermeable biotinylation reagent (sulfo-NHS-LC-biotin). All data are presented as mean ± SEM of four to five independent experiments. (A) Representative blots showing the immunoreactivity of α1, α4, β2/3, γ2 or actin in total lysates and biotinylated fraction. (B) Densitometric analysis for each GABAAR subunit in the biotinylated fraction, immunoreactivity in the biotinylated fraction of SE samples was compared to immunoreactivity in control samples by ANOVA with a Bonferroni post hoc test (*p < 0.05, **p < 0.01 or ***p < 0.001 compared to control). The overall values for the ANOVA analysis were: α1 (F = 6.307, p < 0.0056), α4 (F = 6.597, p < 0.0046), β2/3 (F = 3.471, p < 0.0430), and γ2 (F = 20.422, p < 0.0001). (C) Immunoreactivity for GABAAR subunits in the biotinylated fraction (plasma membrane) was normalized to immunoreactivity in the lysate (total protein expression) and compared control values to determine the fraction of each receptor subunit located at the plasma membrane. The overall values for the ANOVA analysis were: α1 (F = 0.9445, p < 0.4439), α4 (F = 1.424, p < 0.2750), β2/3 (F = 3.079, p < 0.0596), and γ2 (F = 4.801, p < 0.0154). Bonferroni post hoc test *p < 0.05 compared to control).
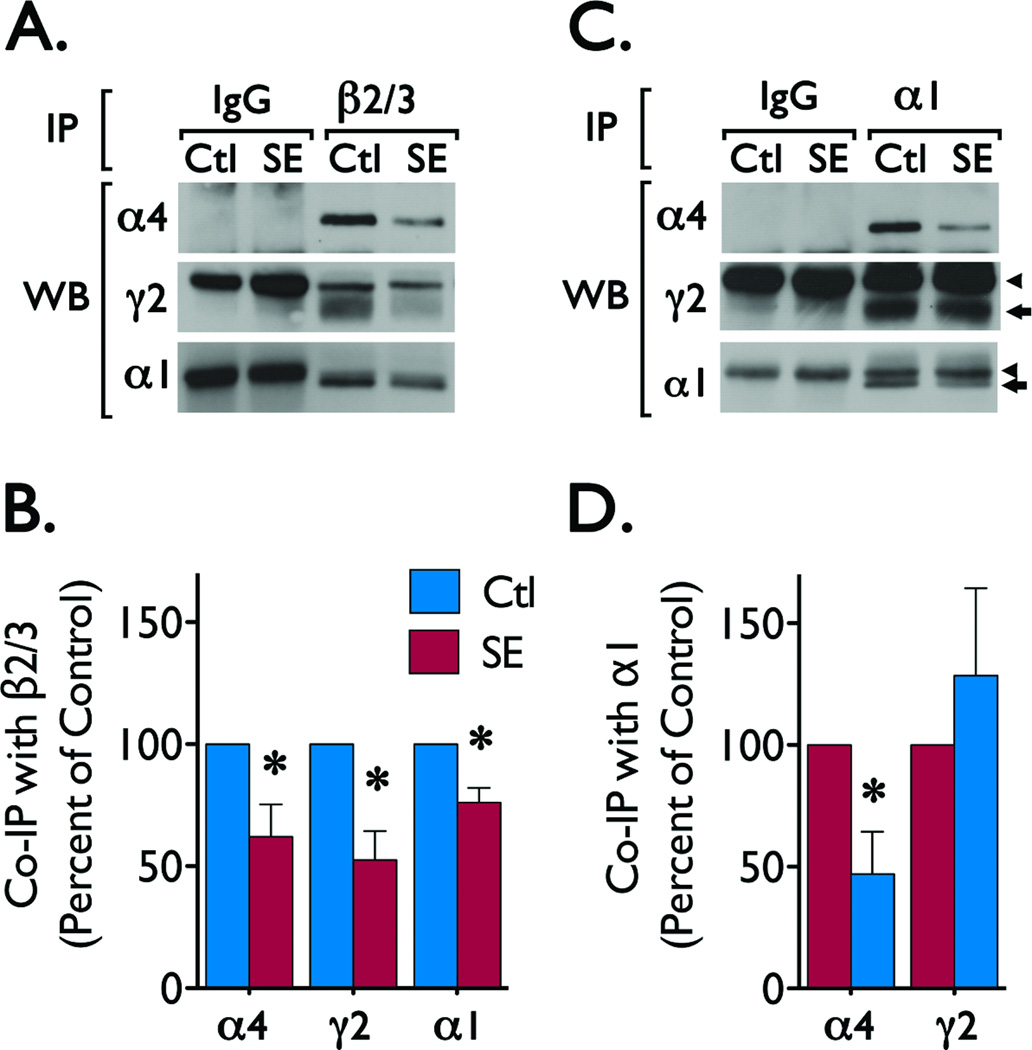
Subunit composition governs protein-protein interactions that regulate the trafficking and distribution of GABAAR. Previous studies have found that within 24 hours of SE induction the composition of GABAAR is rapidly altered in the dentate gyrus (Lund et al., 2008). Because we observed that γ2 subunits and gephyrin are regulated in the CA1 region, we analyzed whether alterations in GABAAR assembly preceded the changes in total protein expression of GABAAR subunits and gephyrin. For this, we immunoisolated receptors from samples obtained 1 day after induction of SE, a time point when the changes in total protein expression are not yet apparent (Figure 3). Immunoprecipitation experiments using anti-β2/3 antibody revealed a decrease in the levels of α1 (t = 3.899, p < 0.0176), α4 (t = 2.846, p < 0.0466) and γ2 (t = 4.007, p < 0.0279) immunoreactivity associated with β2/3 subunits (Figure 5A, 5B) and suggests an overall reduction in the abundance of assembled receptors containing β2/3 subunits. Receptors isolated using an anti-α1 antibody contained similar levels of γ2 immunoreactivity (t = 0.7874, p < 0.4751) in control and SE samples (Figure 5C, 5D), suggesting that SE does not alter abundance of α1γ2-containing receptors in the CA1. These results are in line with the reduced levels of plasma membrane receptors and suggest that following SE β2/3 subunits are no longer associated with subunits necessary for proper anchoring (γ2 and α1), resulting in less stable receptors. On the other hand, α1-containing receptors maintain a more typical receptor composition and remain more stable because of the presence of key subunits involved in trafficking and anchoring (like γ2). Together, our observations suggest that status epilepticus alters the expression of GABAAR subunits and scaffolding proteins in the CA1 region of hippocampus, potentially contributing to the hyperexcitability observed in this region during the epileptogenic period.
Figure 5. GABAAR subunit composition following SE induction.
GABAAR were immunoprecipitated from samples obtained 1 day after SE induction. Pre-cleared lysates (300–350 µg of protein) of whole tissue obtained from control (Ctl) and SE animals were incubated with 5 µg of mouse monoclonal antibodies directed to β2/3 or α1 subunits or with non-immune mouse IgG (used as control). Immune complexes were separated by SDS-PAGE and probed with α4, γ2 or α1 antibodies to determine the immunoreactivity associated to ββ2/3 or α1 subunits. (A) Representative blots of the α4, γ2 and α1 immunoreactivity detected in β2/3 immunoprecipitates. (B) Analysis of α4, γ2 and α1 immunoreactivity associated to β2/3 subunits. Data is presented as mean ± SEM of four to five independent experiments. *p < 0.05 compared with immunoreactivity in control samples by paired students t-test. The values for the t-test were: α4 (t = 2.846, p < 0.0466), α1 (t = 3.899, p < 0.0176), and γ2 (t = 4.007, p < 0.0279). (C) Representative blots showing the α4, γ2, α1 immunoreactivity detected in immunoprecipitates prepared using an α1 antibody. (D) Analysis of α4 and γ2 immunoreactivity detected in α1 immunoprecipitations. Data is presented as mean ± SEM of five independent experiments. *p < 0.05 compared with immunoreactivity in control samples by students t-test. The values for the t-test were: α4 (t = 3.027, p < 0.0389) and γ2 (t = 0.7874, p < 0.4751). (◄) Points to the signal produced by the heavy chains of the control IgG or antibodies for β2/3 or α1 subunits. ( ) Points to the immunoreactivity of α4, γ2 or α1 subunits associated with β2/3 or α1 subunits.
) Points to the immunoreactivity of α4, γ2 or α1 subunits associated with β2/3 or α1 subunits.
DISCUSSION
Gephyrin and other scaffolding proteins like GABARAP, GRIP and NSF form a network of proteins that facilitate the intracellular trafficking and anchoring of GABAAR at the plasma membrane (Chen & Olsen 2007; Kneussel & Loebrich 2007; Fritschy et al., 2008; Jacob et al., 2008; Luscher et al., 2011). Gephyrin facilitates the accumulation and clustering of GABAAR at synaptic sites within the GABAergic synapse and a reduction in gephyrin protein expression disrupts GABAAR clustering and reduces inhibitory postsynaptic currents (Fritschy et al., 2008). Because loss of GABAAR-γ2 subunits results in a reduction of gephyrin clustering and reduced GABAergic innervation (Kneussel et al., 1999; Li et al., 2005), it has been suggested that the levels of GABAARs might be affected by the loss of scaffolding proteins.
In the present study we found that expression of key scaffolding proteins associated with GABAAR are regulated during epileptogenesis. SE triggers a reduction in the expression of gephyrin and GRIP in the CA1 region 4–8 days after the insult but expression of NSF and GABARAP did not change significantly during this period of time. Recent studies have found a similar alteration in gephyrin clustering and expression during epileptogenesis in other hippocampal regions (Thind et al., 2010; Fang et al., 2011). Thind and coworkers found that during the epileptogenic period (5 days post-SE) there is a reduction in the number of gephyrin clusters and GABAergic synapses in dentate gyrus. Once rats become epileptic (during the chronic period) there is an increase in the number of gephyrin punctae (Thind et al., 2010). Fang et al., found that in the neocortex gephyrin expression gradually decreases during the epileptogenic period and appears to return to control levels during the chronic phase (Fang et al., 2011). Our current findings are in agreement with both studies and support the concept that gephyrin dysfunction may be a consistent component of the epileptogenic process. A possible consequence of gephyrin down-regulation is that the network of proteins that control GABAAR trafficking and clustering may become unstable, amplifying the deficit in GABAergic neurotransmission.
Previous studies demonstrating that genetic reduction of gephyrin expression disrupts GABAAR clustering and inhibitory postsynaptic currents suggest that gephyrin and GABAAR subunits may be concomitantly regulated (Essrich et al., 1998; Yu et al., 2007). Our data are consistent with this concept, as we found that in the CA1 region of hippocampus there is a significant reduction in the expression of α4, β2/3 and γ2 subunits that occurs in parallel with the loss of gephyrin and GRIP. Immunocytochemical studies using the kainate model of epilepsy have similarly shown a wide spread loss GABAAR subunits (α1, α2, α4, α5, β1-3 and γ2) in the CA1 region of hippocampus (Schwarzer et al., 1997). All together, these observations suggest that SE may have specific effects on the expression of GABAAR subunits.
Due to the considerable cell loss observed in some models of epilepsy, a reduction in protein expression has been directly attributed to cell loss (Schwarzer & Sperk 1995). However, some studies have shown that although cells are lost in the hilus and CA3 regions of hippocampus, the degree of cell loss in the CA1 region is not extensive (Esclapez et al., 1999; Houser & Esclapez 2003; Chauviere et al., 2009). Using the neuronal marker, NeuN, we assessed the gross anatomical pattern of neuronal cell bodies in the CA1 at 4 days post-SE and there was no apparent reduction in neuronal density, suggesting that the degree of neuronal loss at this time point is relatively limited. Thus, although a slight reduction (~10%) in the number of cells in CA1 (Chauviere et al., 2009) may contribute to the loss of protein expression, cell loss does not appear to be the only factor contributing to the marked reduction of GABAAR subunits and scaffolding proteins observed in this study (~50%). Another factor arguing against cell loss being the only cause of the reduction in scaffolding proteins and GABAAR subunits is the selective nature of the changes observed. Immunoreactivity for NSF, GABARAP and the α1 subunit remained relatively unchanged.
The presence of GABAAR subunits at the cell surface was analyzed to establish a possible correlation between the loss of scaffolding proteins and the stability of receptors at the plasma membrane. In these experiments, we found that cell surface availability of α4 and γ2 subunits is reduced as early as 1 day after SE induction (acute injury) and becomes more evident at 4 days post-SE (epileptogenic period). These alterations in cell surface expression might reflect changes in gephyrin expression, suggesting GABAAR might be persistently internalized during epileptogenesis and result in a long-term deficit of functional receptors. Increased excitability has been detected in the CA1 region shortly after SE induction (El-Hassar et al., 2007), and it is feasible that loss of the network of proteins involved in the trafficking and anchoring of GABAAR may produce a deficit in inhibitory neurotransmission that contributes to hyperexcitability. In the current study we did not directly address the fate of the receptors that are internalized as a consequence of seizure induction, but it has been proposed that after internalization receptors are relocated to intracellular compartments and targeted for degradation (Chen et al., 2007). Our findings are consistent with this hypothesis.
Rearrangement of receptor composition due to altered expression of GABAAR subunits appears to underlie some of the alterations in receptor function seen in dentate gyrus during the chronic stages of epilepsy (Gibbs et al., 1997; Brooks-Kayal et al., 1998). More recently, it was found that within 24 hours of seizure induction the abundance of GABAAR containing 〈α1γ2 subunits is decreased and the number of GABAAR containing α4γ2 was increased in dentate gyrus (Lund et al., 2008). In our current study, we found a reduction in the levels of α4β2/3γ2 receptors and no obvious loss of α1β2/3γ2 receptors in the CA1 region within 24 hours of SE induction. These alterations were accompanied by a reduction in the availability of receptors at the cell surface and a loss in the total expression of α4, β2/3 and γ2 subunits. These observations suggest that in CA1 some receptor subunits targeted for down regulation (α4, β2/3, γ2) might form less-stable receptors while the more stable subunits (α1) might be rearranged into more stable receptor compositions. These findings, along with previous observations (Lund et al., 2008), suggest that a rapid rearrangement of GABAAR composition may be triggered by SE and might directly influence the trafficking and anchoring of GABAAR.
The functional impact of gephyrin loss from hippocampal CA1 tissue during the epileptogenic period remains to be elucidated. The characterization of a functional interaction between GABAAR and gephyrin is suggestive of the direct involvement of gephyrin in the trafficking and/or post-synaptic localization of GABAAR at GABAergic synapses. Unfortunately, the direct involvement of gephyrin in the regulation of GABAAR at GABAergic synapses has been harder to demonstrate since the biochemical characterization of an interaction between GABAAR and gephyrin remains elusive. Only recently, the association of gephyrin and GABAAR containing α1 subunits has been demonstrated in cultured neurons (Mukherjee et al., 2011) and thus the functional impact of scaffolding proteins on normal GABAAR function is still poorly understood.
In summary, we have found alterations in the expression of GABAAR subunits and GABAAR scaffolding proteins that correlate with changes in the plasma membrane expression and assembly of GABAAR. These alterations are evident during the epileptogenic period and offer a molecular substrate to explain the alterations in GABAAR function previously characterized by other laboratories. These observations represent an additional step towards a fuller characterization of the molecular events behind the abnormalities in GABAergic neurotransmission detected during the epileptogenic period.
ACKNOWLEDGMENTS
The National Institutes of Health Grants K01-NS069583 to MIG and R01-NS051710 to ABK supported this work. NIH/NCRR Colorado CCTSI Grant Number UL1 RR025780 supported a co-pilot award to MIG.
Footnotes
DISCLOSURE
None of the authors has any conflict of interest to disclose. The authors have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004;90:173–189. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- Chauviere L, Rafrafi N, Thinus-Blanc C, Bartolomei F, Esclapez M, Bernard C. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J Neurosci. 2009;29:5402–5410. doi: 10.1523/JNEUROSCI.4699-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JW, Naylor DE, Wasterlain CG. Advances in the pathophysiology of status epilepticus. Acta Neurol Scand Suppl. 2007;186:7–15. [PubMed] [Google Scholar]
- Chen ZW, Olsen RW. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem. 2007;100:279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hassar L, Esclapez M, Bernard C. Hyperexcitability of the CA1 hippocampal region during epileptogenesis. Epilepsia. 2007;48 Suppl 5:131–139. doi: 10.1111/j.1528-1167.2007.01301.x. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C. Newly formed excitatory pathways provide a substrate for hyperexcitability in experimental temporal lobe epilepsy. J Comp Neurol. 1999;408:449–460. doi: 10.1002/(sici)1096-9861(19990614)408:4<449::aid-cne1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Falconer M, Serafetinides E, Corsellis J. Etiology and pathogenesis of temporal lobe epilepsy. Arch Neurol. 1964;10:233–248. doi: 10.1001/archneur.1964.00460150003001. [DOI] [PubMed] [Google Scholar]
- Fang M, Shen L, Yin H, Pan YM, Wang L, Chen D, Xi ZQ, Xiao Z, Wang XF, Zhou SN. Downregulation of gephyrin in temporal lobe epilepsy neurons in humans and a rat model. Synapse. 2011;65:1006–1014. doi: 10.1002/syn.20928. [DOI] [PubMed] [Google Scholar]
- Fritschy JM. Epilepsy, E/I Balance and GABA(A) Receptor Plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31:257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Shumate M, Coulter D. Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. Journal of Neurophysiology. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Krizman-Genda E, Robinson MB. Caveolin-1 regulates the delivery and endocytosis of the glutamate transporter, excitatory amino acid carrier 1. J Biol Chem. 2007;282:29855–29865. doi: 10.1074/jbc.M704738200. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. Analysis of glutamate receptor surface expression in acute hippocampal slices. Sci STKE. 2002;2002:l8. doi: 10.1126/stke.2002.137.pl8. [DOI] [PubMed] [Google Scholar]
- Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59:S21–S26. doi: 10.1212/wnl.59.9_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- Holman D, Henley JM. A novel method for monitoring the cell surface expression of heteromeric protein complexes in dispersed neurons and acute hippocampal slices. J Neurosci Methods. 2007;160:302–308. doi: 10.1016/j.jneumeth.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Downregulation of the alpha5 subunit of the GABA(A) receptor in the pilocarpine model of temporal lobe epilepsy. Hippocampus. 2003;13:633–645. doi: 10.1002/hipo.10108. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Haverkamp S, Fuhrmann JC, Wang H, Wassle H, Olsen RW, Betz H. The gamma-aminobutyric acid type A receptor (GABAAR)-associated protein GABARAP interacts with gephyrin but is not involved in receptor anchoring at the synapse. Proc Natl Acad Sci U S A. 2000;97:8594–8599. doi: 10.1073/pnas.97.15.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Loebrich S. Trafficking and synaptic anchoring of ionotropic inhibitory neurotransmitter receptors. Biol Cell. 2007;99:297–309. doi: 10.1042/BC20060120. [DOI] [PubMed] [Google Scholar]
- Leidenheimer NJ. Regulation of excitation by GABA(A) receptor internalization. Results Probl Cell Differ. 2008;44:1–28. doi: 10.1007/400_2007_039. [DOI] [PubMed] [Google Scholar]
- Leroy C, Poisbeau P, Keller AF, Nehlig A. Pharmacological plasticity of GABA(A) receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J Physiol. 2004;557:473–487. doi: 10.1113/jphysiol.2003.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Yu W, Christie S, Miralles CP, Bai J, Loturco JJ, De Blas AL. Disruption of postsynaptic GABA receptor clusters leads to decreased GABAergic innervation of pyramidal neurons. J Neurochem. 2005;95:756–770. doi: 10.1111/j.1471-4159.2005.03426.x. [DOI] [PubMed] [Google Scholar]
- Löscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev. 2010;62:668–700. doi: 10.1124/pr.110.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal. 2008;1:ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006;2006:re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- Michels G, Moss SJ. GABAA receptors: properties and trafficking. Crit Rev Biochem Mol Biol. 2007;42:3–14. doi: 10.1080/10409230601146219. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, Davies PA, Triller A, Schindelin H, Moss SJ. The residence time of GABA(A)Rs at inhibitory synapses is determined by direct binding of the receptor alpha1 subunit to gephyrin. J Neurosci. 2011;31:14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C, Sperk G. Hippocampal granule cells express glutamic acid decarboxylase-67 after limbic seizures in the rat. Neuroscience. 1995;69:705–709. doi: 10.1016/0306-4522(95)00348-m. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Tsunashima K, Wanzenbock C, Fuchs K, Sieghart W, Sperk G. GABA(A) receptor subunits in the rat hippocampus II: altered distribution in kainic acid-induced temporal lobe epilepsy. Neuroscience. 1997;80:1001–1017. doi: 10.1016/s0306-4522(97)00145-0. [DOI] [PubMed] [Google Scholar]
- Scorza FA, Arida RM, Naffah-Mazzacoratti Mda G, Scerni DA, Calderazzo L, Cavalheiro EA. The pilocarpine model of epilepsy: what have we learned? An Acad Bras Cienc. 2009;81:345–365. doi: 10.1590/s0001-37652009000300003. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW. Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol. 2007;35:984–999. doi: 10.1080/01926230701748305. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, Adamiker D. Structure and subunit composition of GABA(A) receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Silva AP, Carvalho AP, Carvalho CM, Malva JO. Modulation of intracellular calcium changes and glutamate release by neuropeptide Y1 and Y2 receptors in the rat hippocampus: differential effects in CA1, CA3 and dentate gyrus. J Neurochem. 2001;79:286–296. doi: 10.1046/j.1471-4159.2001.00560.x. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind KK, Yamawaki R, Phanwar I, Zhang G, Wen X, Buckmaster PS. Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J Comp Neurol. 2010;518:647–667. doi: 10.1002/cne.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- Yu W, Charych EI, Serwanski DR, Li RW, Ali R, Bahr BA, De Blas AL. Gephyrin interacts with the glutamate receptor interacting protein 1 isoforms at GABAergic synapses. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jiang M, Miralles CP, Li RW, Chen G, de Blas AL. Gephyrin clustering is required for the stability of GABAergic synapses. Mol Cell Neurosci. 2007;36:484–500. doi: 10.1016/j.mcn.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]