Abstract
In the past four decades of cholinesterase (ChE) research, we have seen substantive evolution of the field from one centered around substrate and inhibitor kinetic profiles and compound characterizations to the analysis of ChE structure, first through the gene families and then by x-ray crystallographic determinations of the free enzymes and their complexes and conjugates. Indeed, these endeavors have been facilitated by recombinant DNA technologies, structure determinations and parallel studies in related proteins in the α/β-hydrolase fold family. This approach has not only contributed to a fundamental understanding of structure and function of a large family of hydrolase-like proteins possessing functions other than catalysis, but also has been used to develop new practical strategies for scavenging and antidotal activity in cases of organophosphate insecticide or nerve agent exposure.
1.1 Introduction
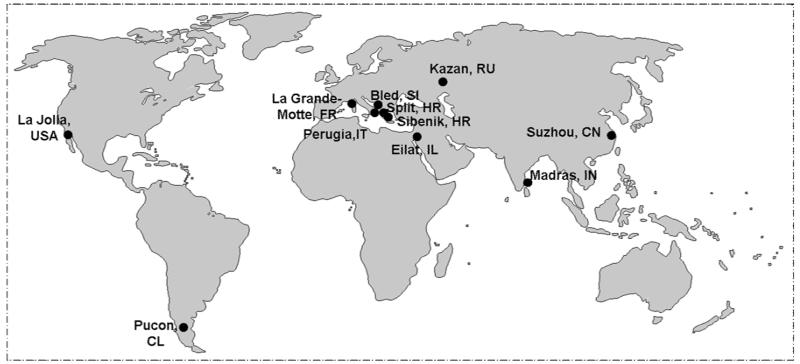
The cholinesterase (ChE) field has blossomed, in part, through triennial capsule meetings, first organized by the late Elsa Reiner in Split, in then Yugoslavia, in 1975. Although cholinesterases were Elsa Reiner’s abiding interest, the first meeting was indeed a wide ranging, cholinergic meeting, encompassing presentations on all molecules then known to interact at cholinergic synapses. In fact, it was that meeting that soon spawned others in the cholinergic field and on hydrolase enzymes related to ChEs. The ChE field has kept its pattern of regular and well focused meetings, deservedly so, since important new observations, strategic directions, and challenges emerge periodically in this field. Moreover, many of these ChE developments spill over into related fields. One often now hears about the ChE (or hydrolase) domain when considering the structure of neuroligin or thyroglobulin. Studies with ChE’s are indeed a world-wide endeavor as is evident from global locations of our meetings (Fig.1), and we are indeed indebted to colleagues in Russia and our host city of Kazan for sponsoring the XI International meeting. The wonderful location of the meeting, a rich tradition of landmark chemical studies in Kazan, and the recent work of our Russian colleagues all contribute to the global nature of our endeavors.
Figure 1.
Global Locations of the Cholinesterase Meetings. The map shows the locations of our meetings that have occurred recently on an approximate three year sequence starting with Split, Croatia (1975), Bled, Slovenia (1983) La Grand Motte, France (1990); Eliat, Israel (1992); Madras, India (1994); La Jolla, U.S.A. (1998); Pucon, Chile (2002); Perugia, Italy (2004); Suzhou, China (2007); Sibenik, Croatia (2009) and Kazan, Russia (2012). We are indeed indebted to our colleagues in Alacante, Spain who have agreed to sponsor the 12th International Meeting in the Fall of 2014.
1.2 The Cholinesterase Molecule and its Cousins
The fundamental ChE interests of investigators at the time of the first meeting were the physical and biochemical properties of the ChE’s. Extensive effort had gone into purification of acetylcholinesterase (AChE) from Electrophorus [1], but procedures for purification required proteolysis for dissociation of AChE from the membrane, and this precluded obtaining a high fraction of intact enzyme suitable for crystallography. Other studies, contemporary at the time, were directed to AChE from Torpedo sp. where Massoulie, Reiger and colleagues had identified a tail containing form from Torpedo mamorata [2] while we were engaged in characterizing a tetrameric assembly of subunits from Torpedo californica [3]. In fact, the Torpedo sp. became the staple for extensive biochemical characterizations [3], molecular cloning of the AChE [4] and the first crystallographic structure at high resolution [5]. What became evident at this initial meeting in 1975 was that studies of the cholinesterases had evolved from largely kinetic studies of substrate turnover and inhibition to investigations of the molecular species of the enzyme and the assembly of catalytic and structural subunits.
A significant breakthrough came with the determination of the sequence of cholinesterase and the cloning of the gene encoding the enzyme in Torpedo [4], for these studies showed that the cholinesterases, though serine hydrolases with the characteristic catalytic triad, were not related to other lower molecular weight serine hydrolase families, namely the chymotrypsin and subtilisin families. Rather they likely defined a new family of proteins, by virtue of homology to thyroglobulin and lack thereof to other known esterases. Once the crystal structure of Torpedo AChE was determined [5], the residues involved in the characteristic catalytic triad were defined and family identification became of importance. The initial sequence led to discovering other hydrolases that were homologous, and soon the concept of the α/β-hydrolase fold was proposed [6] showing commonality of structure of a new family of hydrolases, typically of higher molecular mass than those of other families.
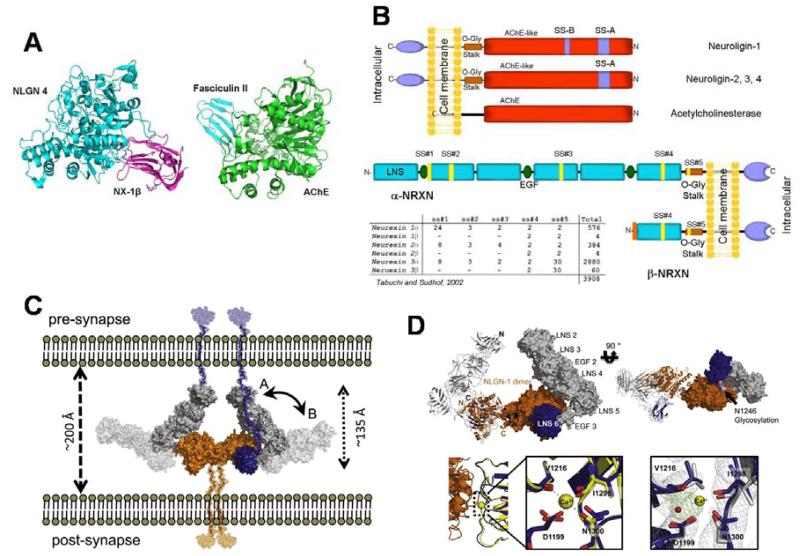
The lack of characterization of the large thyroglobulin molecule of known sequence left the question of what is its relationship to a family of hydrolases? Could this large molecule approaching 250 kDa contain hidden esterase or hydrolase activity? The mystery was further confounded with the discovery that neuroligin, a synaptic adhesion protein homologous to the cholinesterases [7], could then be classified as an α/β-hydrolase fold protein. Neither the portion of thyroglobulin deemed homologous to the cholinesterase, nor neuroligin, had correctly positioned aspartic acid, histidine and serine residues to serve as a catalytic triad. What the structural studies also revealed is that the adhesion contact area in neuroligin for neurexin association is on the opposite side of the molecule from the substrate entry portal to the catalytic triad (Figure 2a). and that the small contact area of neuroligin for β-neurexin and the far larger α-neurexin with its repeat domains might differ given the multiple LNS and EGF domains of α-neurexin differ (figures 2b,c,d). Moreover, it is the neuroligin dimer extending from the post-synaptic cell that forms the association base for this trans-synaptic interaction, since two β-neurexin monomers cluster around the neuroligin dimer (Figure 2d). Other proteins, such as the leucine-rich repeat transmembrane protein, also interact with neurexin, but this interaction site has not been characterized [8].
Figure 2.
Structure of the neurexin-neuroligin complex, the fasciculin-AChE complex and the α-and β-neurexin molecules showing interaction points in the structures: A. Association positions of fasciculin with the rim of the active center gorge of AChE [20] and β-neurexin with neuroligin [21]. Neuroligin and AChE are homologous α/β-hydrolase fold proteins, yet the association of fasciculin, a peptide of ~7,000 Daltons and neurexin a protein of 22,000 Daltons occur at opposite faces of the respective α/β-hydrolase fold proteins. B. Relationship of the structures of the cholinesterases, neuroligins and thyroglobulins obtained from molecular cloning. C. Proposed organization of the neurexins and neuroligins in the synapse (Adapted from reference [22]) D. Structure of α-neurexin. α-Neurexin contains 6 LNS domains with 3 intervening EGF domains. The sixth LNS domain is identical to the LNS domain of β-neurexin [22] (Adapted from reference [22]).
What has emerged since these early explorations has been systematically tabulated through the Esther data base http://bioweb.ensam.inra.fr/ESTHER/general?what=index). It reveals that the α/β-hydrolase fold superfamily is a large one, both in families and members, incorporating not only hydrolase functions for a variety of substrates including esters, amides, peptides, phosphoesters and halides, but also extending to other functions related to adhesion, chaperoning protein folding and trafficking, and structural subunit attachment, [9]. Considerations of residue conservation and homology become important for analyzing aberrations that result from mutations. In the case of neuroligin 3 and butyrylcholinesterase (BChE), we find a common mutation of a conserved arginine that is mutated to a cysteine in both of these proteins, resulting in deficiencies of folding of the respective molecules [10] For neuroligin this mutation was noted to be present in twin sets and linked with the autism spectrum disorders [11]. With this mutation neuroligin is still formed; trafficking to its extracellular location is compromised, but not completely blocked [10, 12, 13]. These considerations may have a practical outcome, since in the case of BChE, we find mutations in a single gene giving rise to a deficiency of expression of an enzyme, BChE, that is without obvious phenotypic consequence in man in the absence of administering certain ester drugs, such as succinylcholine and bambuterol. Animals or human subjects with the BChE mutation (Arg386Cys) common to neuroligin might form the basis for a plasma assay for compounds that ameliorate the trafficking deficiencies found with the mutant gene products. If suitable compounds can be found, they may serve a role in the treatment of certain autism spectrum disorders associated with neuroligin trafficking Recent studies with the cystic fibrosis transmembrane regulator (CTFR) mutations have shown compromised expression can be reversed with compounds that appear to augment trafficking of this integral membrane protein selectively [14, 15].
1.3 α/β-Hydrolase-fold Functions
There are at least four distinct recognition functions of the α/β-hydrolase-fold family of proteins, and they are outlined in Table 1. The most widely recognized is the catalytic function of hydrolysis, shared by other serine, cysteine and aspartate hydrolases. However, the cholinesterases lead the pack in rapid catalysis, and one might speculate on the structural basis for this. Obviously, it will take a larger protein to build a deep gorge lined with aromatic residues, thereby creating a sequestered environment for hydrolytic catalysis. The distance traveled to the catalytic triad in the active center, some 18-20 Å from the rim, must be compensated by other catalytic attributes that may relate to minimizing hydrogen bonding of water in the gorge and imparting solution flexibility of gorge dimensions.
Table 1.
Structural Domains and Functions of the α/β-hydrolase fold proteins.
| Structural Domain | Molecular Steps | Overall Function | Proteins |
|---|---|---|---|
| Active Center Gorge Base |
Catalysis of substrates by trans esterification of the serine and subsequent H2O mediated catalysis |
Several metabolic processes ranging from neurotransmission to metabolic detoxification |
Many esterases, amidases, peptidases phosphatases, and dehalogenases that function as hydrolases |
| Side Opposite the Active Center Gorge |
Adhesion with pre- synaptic partner proteins in the synapse |
Adhesion and recognition in neurohumoral secretory function |
The tactin molecules in insects, procaryotics and the neuroligin family in mammals |
| Unknown portion of the ChEL domain in thyroglobulin |
Trafficking to the thyrocyte and storage of thyroglobulin |
Formation of the precursors to T3 and T4: thyroid hormone |
The ChEL domain in thyroglobulin |
| Carboxyl terminal region following the four helix bundle |
Linkage of the catalytic and structural subunits of AChE |
Disposition of cholinesterases in plasma and basement membranes |
Tetrameric subunit assembly and linkage of the catalytic and structural subunits |
A second function is that of adhesion. Here a different domain of the α/β-hydrolase fold is employed where coordination through extracellular calcium appears to be critical for the association template [16].
A third function appears to be a chaperone or chaperone assistant function, where the cholinesterase domain, found C-terminal to the 1, 2, 3 region of thyroglobulin is responsible for the proper trafficking of the molecule to the thyrocyte. Quite remarkably, the cholinesterase-like domain serves as a chaperone for a region some five times its molecular weight. Here again oligomerization may come into play where the assembling molecule oligomerizes in the trafficking and storage process. Less is known about thyroglobulin intramolecular interactions engaged in trafficking. Nevertheless, a similar principle prevails where mutations in the ChEL domain can be replicated in the smaller proteins giving rise to similar deficiencies in trafficking for all members of the family [17]. If the same principle holds for neuroligin and thyroglobulin, then a common mutation at a conserved residue position could affect thyroid hormone production through a polymorphism of the thyroglobulin gene and the autism spectrum disorders through a neuroligin gene polymorphism [18]
A fourth function is related to the capacity of AChE to exhibit alternative mRNA processing giving rise to three distinct carboxy-termini. The first is a non-spliced read-through sequence giving rise to monomeric AChE, the second involves splicing to the most proximal exon giving rise to a sequence determinant for adding a glycophospholipid link and tethering to AChE catalytic subunits to the outer leaflet of the membrane. The third, and more distal, splice variant gives rise to a proline-rich attachment domain sequence that allows attachment through disulfide bonding of one of two subunits that are rich in proline and either partition into the membrane or attach a collagen like subunit that associates with the basal lamina in the neuromuscular junction. This association requires the formation of a tetramer where four helices associate with the proline rich region of the structural subunit [19].
1.4 Concluding Perspective
Certainly in the next decade, much remains to be investigated in the α/β -hydrolase fold family, particularly in the neuroligin and thyroglobulin arenas, where the macromolecular associations between α-neurexin and neuroligin and the domains of thyroglobulin should be studied in detail, particularly at the interaction surfaces. This will bring us to a half century of progress at the molecular level, employing both a genomic and proteomic perspective.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leuzinger W, Baker AL, Cauvin E. Acetylcholinesterase. II. Crystallization, absorption spectra, isoionic point. Proc Natl Acad Sci U S A. 1968;59:620–623. doi: 10.1073/pnas.59.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massoulie J, Rieger F. Acetylcholinesterase of fish electric organs (torpedo and electric eel); membrane complexes. Eur J Biochem. 1969;11:441–455. doi: 10.1111/j.1432-1033.1969.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 3.Taylor P, Jones JW, Jacobs NM. Acetylcholinesterase from Torpedo: characterization of an enzyme species isolated by lytic procedures. Mol Pharmacol. 1974;10:78–92. [PubMed] [Google Scholar]
- 4.Schumacher M, Camp S, Maulet Y, Newton M, MacPhee-Quigley K, Taylor SS, Friedmann T, Taylor P. Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. Nature. 1986;319:407–409. doi: 10.1038/319407a0. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]
- 5.Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991;253:872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 6.Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al. The alpha/beta hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- 7.Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. doi: 0092-8674(95)90396-8 [pii] [DOI] [PubMed] [Google Scholar]
- 8.de Wit J, Sylwestrak E, O’Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR, 3rd, Comoletti D, Taylor P, Ghosh A. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. doi: S0896-6273(09)01009-5 [pii] 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchot P, Chatonnet A. Enzymatic activity and protein interactions in alpha/beta hydrolase fold proteins: moonlighting versus promiscuity. Protein Pept Lett. 2012;19:132–143. doi: 10.2174/092986612799080284. doi: BSP/ PPL/ E pub/0407 [pii] [DOI] [PubMed] [Google Scholar]
- 10.De Jaco A, Lin MZ, Dubi N, Comoletti D, Miller MT, Camp S, Ellisman M, Butko MT, Tsien RY, Taylor P. Neuroligin trafficking deficiencies arising from mutations in the alpha/beta-hydrolase fold protein family. J Biol Chem. 2010;285:28674–28682. doi: 10.1074/jbc.M110.139519. doi: M110.139519 [pii] 10.1074/jbc.M110.139519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. doi: 10.1038/ng1136 ng1136 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Jaco A, Comoletti D, Kovarik Z, Gaietta G, Radic Z, Lockridge O, Ellisman MH, Taylor P. A mutation linked with autism reveals a common mechanism of endoplasmic reticulum retention for the alpha, beta-hydrolase fold protein family. J Biol Chem. 2006;281:9667–9676. doi: 10.1074/jbc.M510262200. doi: M510262200 [pii] 10.1074/jbc.M510262200. [DOI] [PubMed] [Google Scholar]
- 13.Comoletti D, DeJaco A, Jennings L, et al. The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein ptocessing. J. Neurosci. 2004;24:4889–4893. doi: 10.1523/JNEUROSCI.0468-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Accurso FJ, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A. 2011;108:18843–18848. doi: 10.1073/pnas.1105787108. doi: 1105787108 [pii] 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leone P, Comoletti D, Ferracci G, Conrod S, Garcia SU, Taylor P, Bourne Y, Marchot P. Structural insights into the exquisite selectivity of neurexin/neuroligin synaptic interactions. EMBO J. 2010;29:2461–2471. doi: 10.1038/emboj.2010.123. doi: emboj2010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Lee J, Di Jeso B, Treglia AS, Comoletti D, Dubi N, Taylor P, Arvan P. Cis and trans actions of the cholinesterase-like domain within the thyroglobulin dimer. J Biol Chem. 2010;285:17564–17573. doi: 10.1074/jbc.M110.111641. doi: M110.111641 [pii]10.1074/jbc.M110.111641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Jaco A, Comoletti D, Dubi N, Camp S, Taylor P. Processing of cholinesterase-like alpha/beta-hydrolase fold proteins: alterations associated with congenital disorders. Protein Pept Lett. 2012;19:173–179. doi: 10.2174/092986612799080103. doi: BSP/ PPL/ E pub/0411 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dvir H, Harel M, bon S, Liu WQ, Vidal M, Garbay C, Sssman JL, Massoullie J, Silman I. The synaptic acetylcholinesterase tetramer assembles around a polyproline helix. EMBO J. 2004;23:4394–4405. doi: 10.1038/sj.emboj.7600425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourne Y, Taylor P, Marchot P. Acetylcholinesterase inhibition by fasciculin: crystal structure of the complex. Cell. 1995;83:503–512. doi: 10.1016/0092-8674(95)90128-0. doi: 0092-8674(95)90128-0 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Fabrichny IP, Leone P, Sulzenbacher G, Comoletti D, Miller MT, Taylor P, Bourne Y, Marchot P. Structural analysis of the synaptic protein neuroligin and its beta-neurexin complex: determinants for folding and cell adhesion. Neuron. 2007;56:979–991. doi: 10.1016/j.neuron.2007.11.013. doi: S0896-6273(07)00962-2 [pii] 10.1016/j.neuron.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MT, Mileni M, Comoletti D, Stevens RC, Harel M, Taylor P. The crystal structure of the alpha-neurexin-1 extracellular region reveals a hinge point for mediating synaptic adhesion and function. Structure. 2011;19:767–778. doi: 10.1016/j.str.2011.03.011. doi: S0969-2126(11)00135-3 [pii] 10.1016/j.str.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]