Abstract
Vitamin D has broad range of physiological functions and anti-tumor effects. 24-hydroxylase, encoded by the CYP24A1 gene, is the key enzyme for degrading many forms of vitamin D including the most active form, 1,25D3. Inhibition of CYP24A1 enhances 1,25D3 anti-tumor activity. In order to isolate regulators of CYP24A1 expression in prostate cancer cells, we established a stable prostate cancer cell line PC3 with CYP24A1 promoter driving luciferase expression to screen a small molecular library for compounds that inhibit CYP24A1 promoter activity. From this screening, we identified, 4,5,6,7-tetrabromobenzimidazole (TBBz), a protein kinase CK2 selective inhibitor as a disruptor of CYP24A1 promoter activity. We show that TBBz inhibits CYP24A1 promoter activity induced by 1,25D3 in prostate cancer cells. In addition, TBBz downregulates endogenous CYP24A1 mRNA level in TBBz treated PC3 cells. Furthermore, siRNA-mediated CK2 knockdown reduces 1,25D3 induced CYP24A1 mRNA expression in PC3 cells. These results suggest that CK2 contributes to 1,25D3 mediated target gene expression. Lastly, inhibition of CK2 by TBBz or CK2 siRNA significantly enhanced 1,25D3 mediated anti-proliferative effect in vitro and in vivo in a xenograft model. In summary, our findings reveal that protein kinase CK2 is involved in the regulation of CYP24A1 expression by 1,25D3 and CK2 inhibitor enhances 1,25D3 mediated anti-tumor effect.
Keywords: 1,25-dihydroxyvitamin D3; CYP24A1; protein kinase CK2; prostate cancer
Introduction
The most physiologically active form of the prohormone, vitamin D3 (cholecalciferol), is 1,25-dihydroxyvitamin D3 (1,25D3). 1,25D3 plays a key role in the regulation of calcium homeostasis and bone metabolism through effects on tissues such as bone, gut and kidney (1, 2). Non-classical roles for 1,25D3 including the regulation of proliferation, differentiation and immune function have now been identified in a variety of cell types (3). The serum level of 1,25D3 is highly regulated through synthesis facilitated by 1-alpha-hydroxylase (CYP27B1), and through inactivation by 24-hydroxylase (CYP24A1) (1, 2).
CYP24A1 is transcriptionally regulated by the interaction between the vitamin D receptor (VDR)-retinoid-X-receptor (RXR) heterodimer and vitamin D response elements (VDREs) on CYP24A1 gene (4–6). In the absence of 1,25D3, VDR/RXR hetero-dimers bind to these VDREs and repress transcription through interactions with a co-repressor complex that has histone de-acetylase activity (7). In the presence of 1,25D3, the co-repressor complex is released, permitting the recruitment of a co-activator complex that leads to the activation of the gene (8, 9). 1,25D3 also stimulates rapid non-genomic effects in some cell-types via the ERK1/ERK2/ERK5, PKC, or JNK MAP kinase modules through a cell-membrane-associated VDR (3).
High CYP24A1 expression level is a common feature of several solid tumors (3, 10–15) and is associated with poorer prognosis (10, 14, 16). The increased intra-tumoral levels of CYP24A1 would lead to rapid degradation of 1,25D3, thus, limiting the amount of 1,25D3 locally in the tumor cells and abrogating the anti-proliferative, or pro-differentiation effects of 1,25D3 (10, 16, 17). Inhibition of CYP24A1 is expected to slow the catabolism of 1,25D3, thereby enhancing the anti-proliferative effect of 1,25D3 (18–21). Administration of 1,25D3 in combination with a CYP24A1 inhibitor enhances the anti-tumor activity of 1,25D3 (19, 22). However, most of the current CYP24A1 inhibitors, such as ketoconazole, are relatively non-specific, and strikingly increase the CYP24A1 expression level compared to cells treated with 1,25D3 alone (19).
In the present study, we screened a small molecule library to identify novel CYP24A1 inhibitors using a CYP24A1 promoter-driving luciferase reporter assay. Furthermore, we expected that the new CYP24A1 inhibitor would enhance 1,25D3-mediated function by inhibiting CYP24A1 expression.
Materials and methods
Materials
1,25D3 was purchased from Tetrionics (Madison, WI). 25D3, LOPAC1280 and 4,5,6,7-tetrabromobenzimidazole (TBBz) were obtained from Sigma-Aldrich (St. Louis, MO). The dual-luciferase assay kit was supplied by Promega (Madison, WI). Mouse anti-CYP24 antibody was a gift from Cytochroma Inc. (Markham, Ontario, Canada). Anti-CK2α (H-286, sc-9030) antibody and anti-actin antibody were from Santa Cruz biotechnology (Santa Cruz, CA). Anti-cleaved Caspase-3 (Asp175, #9661) antibody was purchased from Cell Signaling Technology (Danvers, MA). Anti-Ki-67 antibody was purchased from Leica Microsystems (NCL-Ki67p; Buffalo Grove, IL). TaqMan® Gene Expression Assay for CYP24A1 (Hs00167999_m1), CSNK2A1 (Hs00751002_s1), CDKN1A (Hs00355782_m1), Growth arrest and DNA-damage-inducible protein 45a (GADD45A, Hs00169255_m1) and the transient receptor potential vanilloid type 6 gene (TRPV6, Hs00367960_m1) were purchased from Applied Biosystems (Foster City, CA). ON-TARGET plus SMARTpool siRNA specific for human CSNK2A1 (CK2α1, L-003475), ON-TARGET plus Non-targeting Pool (D-001810), and DharmaFECT 2 transfection reagents were purchased from Dharmacon (Thermo Fisher Scientific Dharmacon, Lafayette, CO). Human RNA from 30 paired human prostate normal and primary tumor lesions were obtained from Department of Pathology, Roswell Park Cancer Institute and approved by Institutional review board.
Cell lines
The prostate cancer cell lines DU145 and PC3 were purchased from American Type Culture Collection (ATCC) and used within 6 months after resuscitation. Cell lines were authenticated by ATCC with short tandem repeat (STR) DNA profiling and cytogenetic analysis. Cells were maintained in culture according to providers’ protocols for a maximum of 10 passages (one month).
Generation of stable reporter cell line
pGL4.21 vector expressing the firefly luciferase gene under the control of CYP24A1 promoter was constructed by the insertion of CYP24A1 promoter using NheI and XhoI restriction enzyme sites (23). A stable human prostate cancer PC3 cell line expressing CYP24A1 promoter-driving luciferase reporter (PC3/CYP24A1) was generated by transfection using lipofectamine 2000 followed by puromycin selection.
Chemical library and high throughput screening
Screening was performed by Small Molecule Screening Core Facility (SMSC) at the Roswell Park Cancer Institute using LOPAC1280 library. PC3/CYP24A1 cells were seeded to 96-well plate (104/well) overnight. 120 nL of each compound or DMSO was added to the plate for 20 minutes using a JANUS robotic liquid handler (PerkinElmer) equipped with 96-pinn tool (V&P Scientific), followed by the addition of 1,25D3 to a final concentration of 100 nM. The final concentration of the library compounds in the media was 10 μM. After 24-h incubation, luciferase activity for each well was assayed using SteadyGlo kit (Promega) and luminescence measured using Envision multilabel plate reader (PerkinElmer). Hits were defined as over 50% inhibition of 1,25D3 mediated CYP24A1 promoter-driving luciferase reporter activity.
CK2 small interfering RNA (siRNA)
PC3 cells were plated in 6-well plates (105/well) overnight. Cells were transfected with 50 nM siRNA-CK2 or Non-targeting siRNA for 72 h using Dharma-FECT 2 transfection reagent following the manufacturer’s instruction. Following transfection, the cells were treated with vehicle EtOH or 1,25D3 for 6 h or 48 h and harvested for experiments as indicated.
Quantitative reverse transcriptase PCR (qRT-PCR)
Expression of CK2, CYP24A1, TRPV6, p21Waf1 and GADD45A mRNA was assessed by qRT-PCR using TaqMan® Gene Expression Assay and normalized to the human GAPDH and samples were analyzed in triplicate.
Immunoblotting analysis
Whole cell lysates were prepared and Western blot analysis performed as described previously (24).
Trypan blue exclusion assay
PC3 cells or PC3 cells transfected with siRNA-CK2 were plated in 6-well plates (3×104/well) for 24 h and treated with 5 μM of TBBz or/and 100 nM of 1,25D3 or 1000 nM of 25D3. Cells were trypsinized and viable cell count measured using ViCell XR (Beckman Coulter) on day 3, 6 and 9.
Tumor growth assay
PC3 cells (2×106) were inoculated subcutaneously into the right flank of male SCID mice (6–8 weeks old). At day 8–9 post implantation, when the tumors were palpable (6.5 × 5 mm), animals were treated with 1,25D3 (15.5 μg/kg/d × 3, i.p. weekly), TBBz three times weekly (15 mg/kg/d, i.p., every 2 days), or the combination for 2 weeks. Body weight was monitored twice a week. Tumor growth was assessed and calculated as described previously (22, 25). The mice protocols used in tumor growth assay were approved by the Institutional Animal Care and Use Committee at Roswell Park Cancer Institute.
Immunohistochemistry
Tissue staining with anti-Ki-67 and anti-cleaved Caspase-3 was conducted as described previously (22).
TUNEL assay
Nuclear DNA fragmentation in situ was detected using TACS-XL In Situ Apoptosis Detection Kit according to the manufacture’s instruction (R&D system, Minneapolis, MN).
Statistics
Statistical significance of data was determined by two-tailed Student’s t test. Wilcoxon Signed-Rank test was performed to compare the expression levels of CK2 in paired normal and tumor samples. 2×2 contingency table was performed to analyze the correlation between increased CYP24A1 expression and CK2 expression in paired normal and tumor human prostate samples.
Results
Screening of small molecules from LOPAC1280 Library by CYP24A1 promoter driving reporter assay
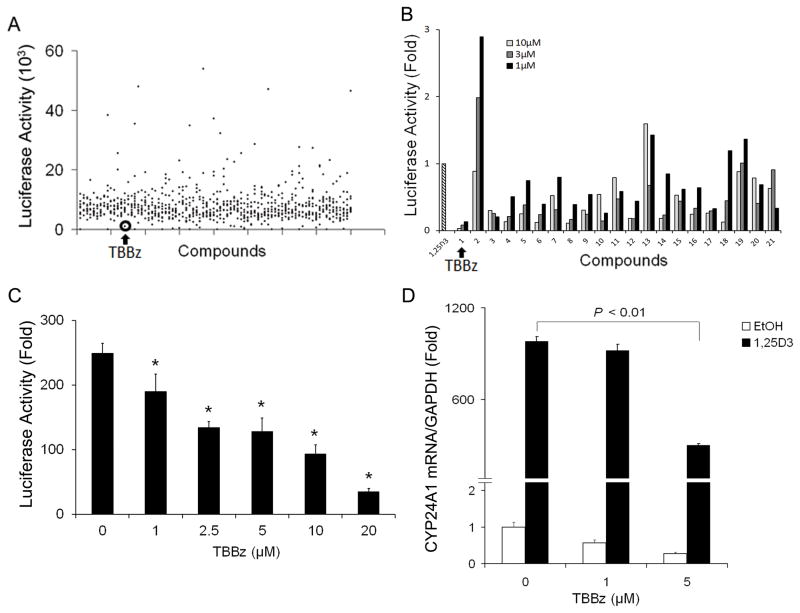
A stable human prostate cancer PC3 cell line expressing CYP24A1 promoter-driving luciferase reporter was generated by transfection using lipofectamine 2000 followed by puromycin selection. Screening of the LOPAC1280 library in this system resulted in the identification of 70 hits each of which had over 50% inhibition of 1,25D3-induced CYP24A1 promoter activity. (Fig. 1A) Excluding the hits with high toxicity, known from Small Molecule Screening Core (SMSC) database, twenty-one selected molecules were subjected to secondary dose-response experiments to confirm initial observations. Seventeen hits reduced 1,25D3-mediated CYP24A1 promoter activation (Fig. 1B). Among them, 4,5,6,7-tetrabromobenzimidazole (TBBz) displayed the strongest inhibitory effect and was chosen for further investigation (Fig. 1A and 1B).
Figure 1. Identification of CYP24A1 small molecular inhibitors by screening LOPAC compounds.
(A) PC3/CYP24A1 cells containing CYP24A1 promoter-driving luciferase were seeded into 96-well plates overnight. The LOPAC1280 library of pharmacologically active compounds was dispensed at a final concentration of 10 μM per compound followed by the addition of 100 nM 1,25D3 for 24 hours. Luciferase activity for each well was assayed and luminescence measured. Each dot represents the value of luminescence. (B) Excluding the hits with high toxicity, known from SMSC database, 21 selected compounds were subjected to secondary dose-response experiments to confirm initial observations.) PC3/CYP24A1 cells were treated with compounds at indicated concentration followed by 1,25D3. CYP24A1 promoter luciferase activity was measured and fold change of luciferase value was calculated for the ratio of (1,25D3-induced luciferase activity in the presence of the compound) to (1,25D3-induced luciferase activity in the absence of the compound). (C) PC3 cells were transfected with the CYP24A1 promoter constructs along with Renilla luciferase control construct. Twenty-four hours post transfection, cells were treated with TBBz as indicated and 1,25D3 (100 nM) for additional 24 hours and harvested, and luciferase activities were measured using the Dual-Luciferase Reporter Assay System. The experiment was repeated twice to confirm the reproducibility of results. (*, P < 0.05). (D) PC3 cells were treated with TBBz as indicated followed by 1,25D3 (100 nM). Expression of CYP24A1 mRNA was assessed by qRT-PCR and normalized to human GAPDH and all samples were analyzed in triplicate.
Repression of CYP24A1 transcriptional activity by TBBz
To confirm the results from the screening, we examined the effect of various concentration of TBBz on CYP24A1 promoter activity in PC3/CYP24A1 cells. Results showed that TBBz inhibited CYP24A1 promoter activity in a dose dependent manner (Fig. 1C). We also tested the effect of TBBz on endogenous and 1,25D3-regulated CYP24A1 expression. PC3 cells were treated with 1 or 5 μM of TBBz alone or followed by 10 nM, 30 nM and 100 nM of 1,25D3. qRT-PCR results showed that PC3 cells displayed low endogenous CYP24A1 mRNA level and dose-dependent induction of CYP24A1 mRNA expression by 1,25D3 (Fig. 1D and Supplementary Fig. 1A). TBBz significantly (P < 0.01) reduced 1,25D3-induced CYP24A1 mRNA expression in a dose-dependent manner (Fig. 1D). Less induction of CYP24A1 expression by lower dose of 1,25D3 was relatively less influenced by TBBz (Supplementary Fig. 1A). These results indicate that TBBz inhibits endogenous and 1,25D3-induced CYP24A1 expression at the transcriptional level. Furthermore, we observed that 25D3, the precursor to 1,25D3 also induced CYP24A1 mRNA expression in PC3 cells and TBBz inhibits 25D3-induced CYP24A1 expression at the transcriptional level in PC3 cells (Supplementary Fig. 2A).
Reduction of CYP24A1 expression by siRNA-CK2
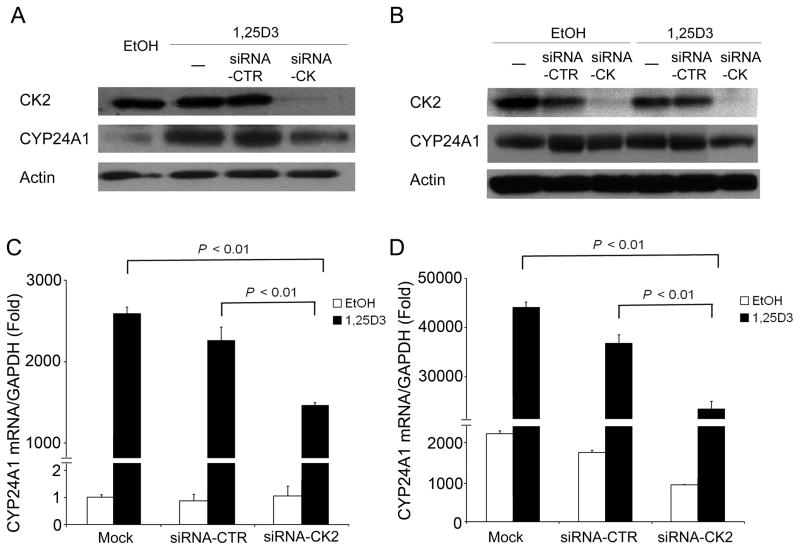
TBBz is a selective protein kinase CK2 inhibitor (26). To investigate whether CK2 plays a role in the regulation of CYP24A1 expression, siRNA-CK2 was transfected in prostate cancer PC3 or DU145 cells for 72 hours followed by the addition of 1,25D3. CYP24A1 mRNA and CYP24A1 protein were measured by qRT-PCR and Western blot, respectively. Results showed that PC3 and DU145 cells express CK2 and CK2 was effectively knocked down by siRNA-CK2 in PC3 and DU145 cells compared to control siRNA (Fig. 2A and 2B). 1,25D3 significantly (P < 0.01) increased CYP24A1 expression in PC3 cells, which express a low level of endogenous CYP24A1 (Fig. 2A and 2C, respectively), and in DU145 cells, which display a high level of endogenous CYP24A1 (Fig. 2B and 2D, respectively). Transfection with siRNA-CK2 significantly reduced 1,25D3-induced CYP24A1 expression at both mRNA and protein level in PC3 (Fig. 2A and 2C) and DU145 cells (Fig. 2B and 2D) as compared with the mock-transfected or siRNA control transfected samples. These results indicate that CK2 plays a role in regulation of 1,25D3-induced CYP24A1 expression.
Figure 2. siRNA-mediated silencing of CK2 reduces 1,25D3-induced CYP24A1 expression.
PC3 (A, C) or DU145 (B, D) cells were transfected with ON-TARGET plus SMARTpool siRNA-CK2 or siRNA control (siRNA-CTR) for 72 h. Cells were then treated with either vehicle EtOH or 1,25D3 (100 nM) for 24 h or 48 h and harvested for qRT-PCR (C, D) and immunoblotting analysis (A, B).
Differential effects of siRNA-CK2 on 1,25D3-induced TYPV6, p21Waf1and GADD45A mRNA expression
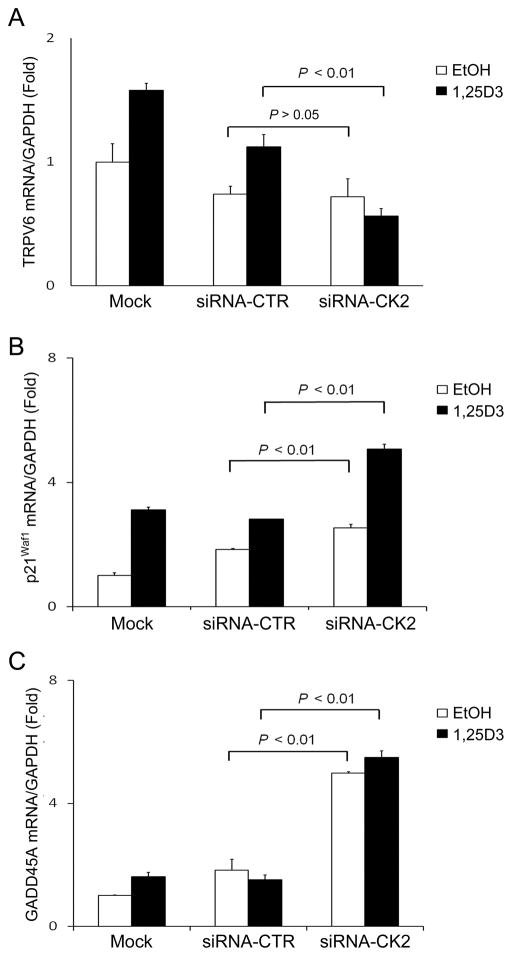
To investigate whether silencing of CK2 affects the expression of other vitamin D target genes, TRPV6, p21Waf1 and GADD45A mRNA expression was measured by qRT-PCR in CK2 knockdown PC3 cells. 1,25D3 induced TRPV6, p21Waf1 and GADD45A mRNA expression. siRNA-CK2 significantly (P < 0.01) reduced 1,25D3-induced TRPV6 mRNA expression as compared to the mock-transfected or siRNA control transfected cells (Fig. 3A). In contrast, knockdown of CK2 markedly increased p21Waf1 and slightly increased GADD45A mRNA expression (Fig. 3B and 3C). These results indicate that silencing of CK2 differentially affects the expression of vitamin D target genes.
Figure 3. Effect of siRNA-CK2 on TYPV6, p21Waf1and GADD45A mRNA expression.
PC3 cells were transfected with siRNA-CK2 or siRNA-control for 72 h. Cells were then treated with either EtOH or 1,25D3 (100 nM) for 6 hours. TYPV6 (A), p21Waf1 (B) and GADD45A (C) mRNA expression were measured and normalized to human GAPDH and all samples were analyzed in triplicate.
Correlation of increased CYP24A1 expression with increased CK2 expression in human prostate tumors
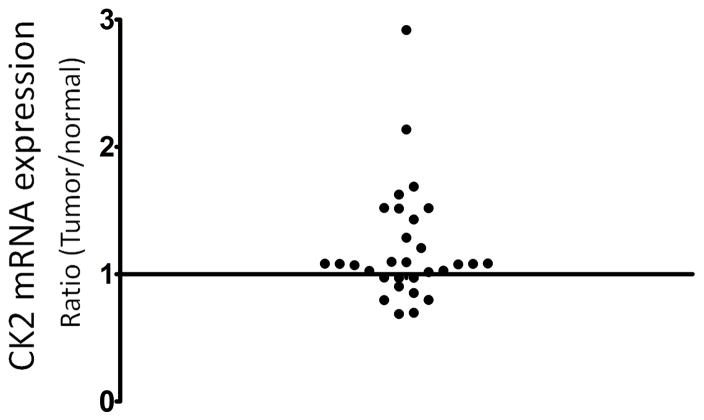
We analyzed mRNA expression of CYP24A1 and CK2 in 30 matched pair of human normal and tumor prostate samples by qRT-PCR. CK2 expression was significantly increased in prostate tumor lesions compared to normal lesions (P = 0.0224) (Fig. 4). There was no correlation of the level of CYP24A1 expression and CK2 expression with Gleason Score. To determine whether tumor samples with increased CYP24A1 expression correlated with increased CK2 expression compared to normal samples, we built a 2×2 contingency table by dividing the 30 samples based on the CYP24A1 expression change (≥ 1.5 fold up vs. other) and CK2 expression change (≥ 1.5 fold up vs. other). Seven samples display increased CYP24A1 expression in a total of 30 prostate tumors compared to matched normal prostate samples. Four of the 7 samples with increased CYP24A1 expression have increased CK2 expression. However, among the remaining 23 samples with low CYP24A1 expression, only 3 samples have high CK2 expression (Table 1). Fisher’s exact test shows that increased CYP24A1 expression is significantly associated with increased CK2 expression in tumor (P=0.0331). These data indicate that CK2 may be involved in regulation of increased CYP24A1 expression in prostate cancer.
Figure 4. CK2 expression in normal and tumor human prostate tissues.
CK2 mRNA expression in human matched prostate tumor and normal lesions was measured and normalized to human GAPDH by qRT-PCR. The difference of CK2 mRNA expression between matched tumor and normal lesions was represented as the ratio of CK2 expression of tumor to normal lesions. Each dot represents the ratio of CK2 expression in tumor to normal lesion.
Table 1.
mRNA expression of CYP24A1 and CK2 in 30 human prostate tumors compared to normal lesion
| Fold change | CK2 | |
|---|---|---|
| ≥ 1.5 | < 1.5 | |
| CYP24A1 | ||
| ≥ 1.5 | 4 | 3 |
| < 1.5 | 3 | 20 |
Enhancement of 1,25D3 anti-proliferative activity by TBBz or siRNA-CK2
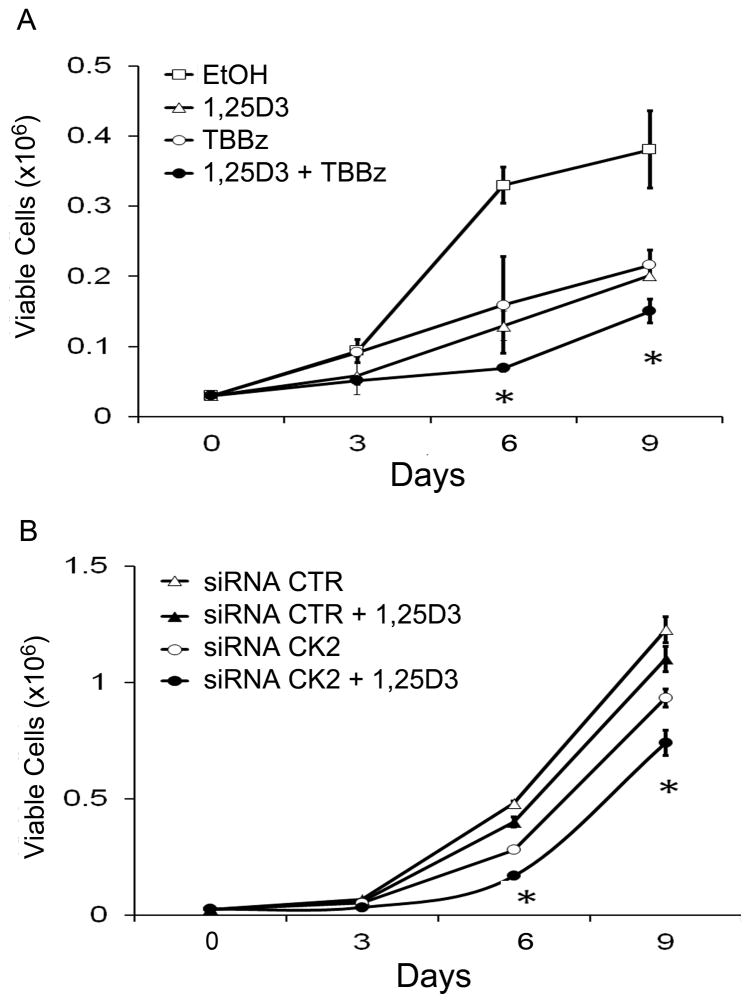
To ascertain the potential therapeutic role of CK2 inhibitors in 1,25D3 anti-tumor action, cell viability and cell proliferation was examined using the trypan blue exclusion assay after treatment with 1,25D3, TBBz, or the combination of 1,25D3 and TBBz for 9 days. Combination treatment of 1,25D3 and TBBz resulted in a significant (P < 0.05) enhancement of 1,25D3 anti-proliferative effect in PC3 cells (Fig. 5A). We also measured CYP24A1 mRNA expression on day 1 and day 9. We observed that CYP24A1 mRNA expression kept lower in PC3 cells treated with the combination of 1,25D3 and TBBz which showed the most antiproliferative activity compared to cells treated with 1,25D3 alone (Supplementary Fig. 1B). Instead of 1,25D3, 25D3 in combination of TBBz also caused a greater inhibition of proliferation in PC3 cells than when treated with either agent alone (Supplementary Fig. 2B).
Figure 5. Enhancement of inhibitory effect of 1,25D3 in prostate cancer cells by TBBz or siRNA-CK2.
(A) PC3 cells were treated with TBBz (5 μM), 1,25D3 (100 nM) or the combination of TBBz and 1,25D3. Viable cells were determined using trypan blue exclusion assay on day 3, 6 and 9. (B) PC3 cells were transfected with siRNA-CK2 or siRNA control for 72 h. Following transfection, cells were treated with EtOH or 1,25D3 (100 nM). Viable cells were determined on day 3, 6 and 9. (*, P < 0.01)
To more specifically investigate the importance of CK2 in 1,25D3 anti-proliferative effect, siRNA-CK2 was employed. siRNA-CK2 significantly (P < 0.05) enhanced 1,25D3 anti-proliferative effect in PC3 cells (Fig. 5B). This indicates that CK2 inhibition is anti-proliferative and enhances 1,25D3 anti-proliferative effect.
Enhancement of 1,25D3 anti-tumor activity by TBBz in vivo
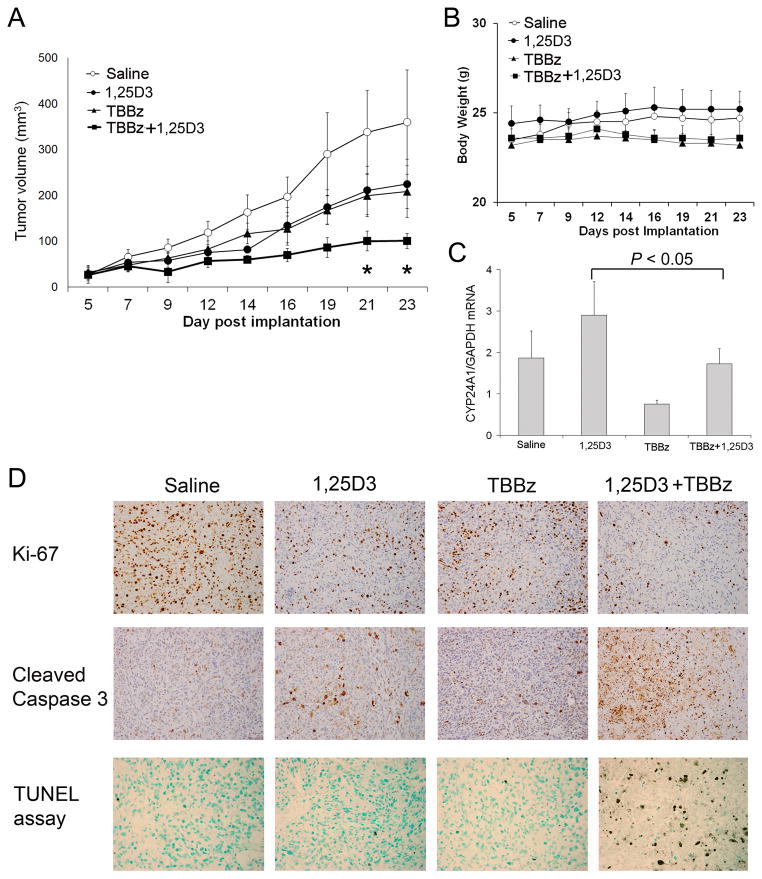
Having demonstrated the efficacy of the combination treatment of 1,25D3 and TBBz in vitro, we next assessed both toxicity and efficacy of the combination treatment in a PC3 prostate tumor xenograft mouse model. We observed a marked inhibition of tumor growth by the combination of 1,25D3 and TBBz, compared to 1,25D3 or TBBz alone (Fig. 6A). Mice grew normally without suffering from weight loss at a dose sufficient to induce anti-tumor effect (Fig. 6B). These results indicate that CK2 inhibitor TBBz enhances 1,25D3 anti-tumor activity in vivo.
Figure 6. TBBz enhances 1,25D3 anti-tumor effect in PC3 xenograft mouse model.
PC3 prostate cancer cells were inoculated subcutaneously into the right flank of male SCID mice. When the tumors were palpable, animals were treated intraperitoneally with saline, 1,25D3, TBBz or the combinations of 1,25D3 and TBBz as described in Material and Methods. (A) Tumor growth was monitored by measuring tumor size three times per week. Tumor volumes were calculated by (length × width2)/2. (*, P < 0.01). (B) Mouse weight was measured three times per week. (C) PC3 tumors were harvested after the treatment, and CYP24A1 mRNA expression in tumor tissues was determined by qRT-PCR. (D) PC3 tumors were harvested after the treatment, and immunohistochemical staining of Ki-67 and cleaved Caspase-3 in tissues was performed. Nuclear DNA fragmentation in situ was detected using TACS-XL In Situ Apoptosis Detection Kit in tumor tissues (× 200).
Effect of the combination of 1,25D3 and TBBz on tumor cell CYP24A1 expression, proliferation and apoptosis in in vivo
To investigate the effect of TBBz on CYP24A1 expression in vivo, tumor tissues were harvested at the end of the treatment described in Fig. 5A, and CYP24A1 mRNA expression was measured by qRT-PCR. 1,25D3 increased CYP24A1 expression and TBBz reduced CYP24A1 expression in tumors as compared to saline group (Fig. 6C). Furthermore, TBBz significantly (P < 0.05) reduced 1,25D3-induced CYP24A1 expression (Fig. 6C). These observations were consistent with the results obtained in the in vitro study.
To further elucidate the molecular mechanisms for the anti-tumor activity of 1,25D3 and TBBz in vivo, we examined the proliferation marker Ki-67 and apoptosis marker cleaved caspase-3 as well as in situ DNA fragmentation (TUNEL) in tumor tissues (Fig. 6D). The results showed that saline-treated tumor tissue had strong Ki-67 staining, 1,25D3 or TBBz reduced Ki-67 staining (Fig. 6D). The combination of 1,25D3 and TBBz further reduced Ki-67 staining (Fig. 6D). We also observed that saline group did not have positive cleaved caspase-3 staining, whereas 1,25D3 or TBBz treatment alone induced caspase-3 cleavage in the tumor tissue (Fig. 6D). The combination of 1,25D3 and TBBz further enhanced caspase-3 cleavage (Fig. 6D). The effect of 1,25D3 and TBBz on apoptosis was further confirmed by TUNEL assay (Fig. 6D). These results clearly indicate the potential usefulness of the combination of 1,25D3 and CK2 inhibitors in prostate cancer therapy.
Discussion
The majority of CYP24A1 inhibitors developed so far target the enzyme activity. However, decreased enzyme activity with current CYP24A1 inhibitors is often associated with increased enzyme expression which negatively impacts on the vitamin D-mediated anti-tumor activity (19, 21, 27). In this study, the strategy we utilized to identify new CYP24A1 inhibitors differs from previously described (28). We established a stable PC3 cell line, which express luciferase driven by CYP24A1 promoter, to screen a small molecular library containing 1280 compounds. We identified 17 new CYP24A1 inhibitors, TBBz being the strongest was selected for further characterization, which revealed a new CYP24A1 expression regulating molecule, protein kinase CK2. Analysis of 30 paired normal and tumor human prostate samples showed that increased CYP24A1 expression is related to increased CK2 expression in tumor. Moreover, we observed a significant enhancement of 1,25D3 anti-tumor activity by inhibiting CK2 in vitro or in vivo. The effects were associated with the reduction of CYP24A1 expression, inhibition of proliferation and the induction of apoptosis in tumors.
Protein kinase CK2 is an evolutionarily conserved serine/threonine kinase which is ubiquitously expressed in human tissues. CK2 is located both in cytosol and nucleus (29, 30). Overexpression of CK2 has been noted in a variety of human cancers including prostate cancer and correlates with a poor clinical outcome (31–34). Inhibition of CK2 activity reduced cell proliferation in prostate cancer cells (35, 36). The role for CK2 in the regulation of CYP24A1 gene expression in tumor has not been described before. Our study shows that CK2 positively regulates CYP24A1 expression. We further show that CK2 expression was higher in tumor lesions compared to normal lesions (P = 0.0224) (Fig. 4). Increase of CK2 expression was significantly (P=0.0331) associated with increased CYP24A1 expression in these prostate tumor samples. These observations suggest that CK2 may serve as a mechanism for controlling CYP24A1 expression in human cancers, and therefore supporting the use of CK2 inhibitors for cancer treatment in combination with 1,25D3.
CYP24A1 expression is heterogeneous in prostate cancer (23). It is noteworthy that three human prostate tumor samples with high CK2 expression did not express high level of CYP24A1 and three prostate tumor samples with high CYP24A1 expression did not express high level of CK2 compared to normal lesions (table 1). These data suggest that the level of CK2 expression does not entirely account for the level of CYP24A1 expression in human prostate tumor. Previous studies indicated multiple events are associated with CYP24A1 expression in cancer, such as methylation and histone modification associated with the CYP24A1 promoter (3, 23, 37), amplification at the CYP24A1 locus (13) and miRNA regulation (38).
We also observed that the silencing of CK2 differentially affects vitamin D target genes. We observed the significant reduction of 1,25D3-induced TRPV6 in siRNA-CK2 transfected PC3 cells. Up-regulation of TRPV6 by 1,25D3 in prostate cancer cells is considered to be pro-proliferative by increasing Ca2+-uptake (39, 40). On the other hand, siRNA-CK2 enhanced 1,25D3-mediated induction of p21Waf1 and GADD45A. p21Waf1 is accounted in part for the anti-proliferative effects of VDR ligands on some cell types, such as prostate cancer (19, 41–43). GADD45A is identified as a primary target gene for 1,25D3 in ovarian, testicular and prostate cancer cells (19, 44, 45). The increase in GADD45A expression leads to a decrease of cyclin B and induces G2/M cell cycle arrest (45, 46). In the present study, the reduction of CYP24A1 and TRPV6 expression and the increase in the p21waf1 and GADD45A expression by the combination of 1,25D3 and siRNA-CK2 may be reflective of the co-operative growth inhibition observed from the cell-proliferation assay.
However, the exact mechanisms underlying the effect of CK2 on 1,25D3-mediated CYP24A1 induction remain unclear despite reports of CK2-mediated phosphorylation of purified VDR at serine208 and VDRE construct transactivation in COS-7 kidney cells co-transfected with VDR and CK2 (47–49). Studies have shown that phosphorylation of hVDR at serine208 does not affect the ability of VDR to bind to DNA and is not obligatory for 1,25D3 action, but may contribute to the modulation of the affinity of VDR for the vitamin D interacting protein (DRIP) complex, therefore increasing its ability to transactivate target promoters (50). At present, we cannot rule out additional mechanisms in the interaction between CK2 and vitamin D target genes as differential effect was observed on TRPV6, p21Waf1 and GADD45A.
In summary, we developed a new strategy to identify novel CYP24A1 inhibitors. Furthermore, we found that protein kinase CK2 is involved in the regulation of CYP24A1 and other vitamin D target genes. CK2 inhibitor TBBz significantly enhances 1,25D3 anti-tumor activity in vitro and in vivo. These findings provide support for the combination treatment of CK2 inhibitor and vitamin D in prostate cancer therapy.
Supplementary Material
Acknowledgments
Financial support: This study was supported by NIH/NCI grants CA067267, CA085142 and CA095045.
We thank Dr. Adam Karpf and Dr. Elizabeth A Griffiths for helpful discussions, Yan Li for statistical assistance, Mrs. Rui-Xian Kong for her excellent technical assistance, and Ms. Ellen Karasik for her excellent technical assistance in immunohistochemistry study.
References
- 1.Morris HA. Vitamin D: a hormone for all seasons--how much is enough? Clin Biochem Rev. 2005;26:21–32. [PMC free article] [PubMed] [Google Scholar]
- 2.Omdahl JL, Morris HA, May BK. Hydroxylase enzymes of the vitamin D pathway: expression, function, and regulation. Annu Rev Nutr. 2002;22:139–66. doi: 10.1146/annurev.nutr.22.120501.150216. [DOI] [PubMed] [Google Scholar]
- 3.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nature reviews Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 4.Vaisanen S, Dunlop TW, Sinkkonen L, Frank C, Carlberg C. Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of 1alpha,25-Dihydroxyvitamin D3. J Mol Biol. 2005;350:65–77. doi: 10.1016/j.jmb.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 5.Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3) Endocrinology and metabolism clinics of North America. 2010;39:255–69. doi: 10.1016/j.ecl.2010.02.007. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson PD, Jurutka PW, Haussler CA, Whitfield GK, Haussler MR. Heterodimeric DNA binding by the vitamin D receptor and retinoid X receptors is enhanced by 1,25-dihydroxyvitamin D3 and inhibited by 9-cis-retinoic acid. Evidence for allosteric receptor interactions. J Biol Chem. 1998;273:8483–91. doi: 10.1074/jbc.273.14.8483. [DOI] [PubMed] [Google Scholar]
- 7.Dwivedi PP, Muscat GE, Bailey PJ, Omdahl JL, May BK. Repression of basal transcription by vitamin D receptor: evidence for interaction of unliganded vitamin D receptor with two receptor interaction domains in RIP13delta1. J Mol Endocrinol. 1998;20:327–35. doi: 10.1677/jme.0.0200327. [DOI] [PubMed] [Google Scholar]
- 8.Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord. 2001;2:203–16. doi: 10.1023/a:1010062929140. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald PN, Baudino TA, Tokumaru H, Dowd DR, Zhang C. Vitamin D receptor and nuclear receptor coactivators: crucial interactions in vitamin D-mediated transcription. Steroids. 2001;66:171–6. doi: 10.1016/s0039-128x(00)00200-2. [DOI] [PubMed] [Google Scholar]
- 10.Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother Pharmacol. 2006;57:234–40. doi: 10.1007/s00280-005-0059-7. [DOI] [PubMed] [Google Scholar]
- 11.Bareis P, Bises G, Bischof MG, Cross HS, Peterlik M. 25-hydroxy-vitamin d metabolism in human colon cancer cells during tumor progression. Biochem Biophys Res Commun. 2001;285:1012–7. doi: 10.1006/bbrc.2001.5289. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich M, Rafi L, Mitschele T, Tilgen W, Schmidt W, Reichrath J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent Results Cancer Res. 2003;164:239–46. doi: 10.1007/978-3-642-55580-0_17. [DOI] [PubMed] [Google Scholar]
- 13.Albertson DG, Ylstra B, Segraves R, Collins C, Dairkee SH, Kowbel D, et al. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nature genetics. 2000;25:144–6. doi: 10.1038/75985. [DOI] [PubMed] [Google Scholar]
- 14.Mimori K, Tanaka Y, Yoshinaga K, Masuda T, Yamashita K, Okamoto M, et al. Clinical significance of the overexpression of the candidate oncogene CYP24 in esophageal cancer. Ann Oncol. 2004;15:236–41. doi: 10.1093/annonc/mdh056. [DOI] [PubMed] [Google Scholar]
- 15.Horvath HC, Lakatos P, Kosa JP, Bacsi K, Borka K, Bises G, et al. The candidate oncogene CYP24A1: A potential biomarker for colorectal tumorigenesis. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2010;58:277–85. doi: 10.1369/jhc.2009.954339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Kim SH, King AN, Zhao L, Simpson RU, Christensen PJ, et al. CYP24A1 is an independent prognostic marker of survival in patients with lung adenocarcinoma. Clin Cancer Res. 2011;17:817–26. doi: 10.1158/1078-0432.CCR-10-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller GJ, Stapleton GE, Hedlund TE, Moffat KA. Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1alpha,25-dihydroxyvitamin D3 in seven human prostatic carcinoma cell lines. Clin Cancer Res. 1995;1:997–1003. [PubMed] [Google Scholar]
- 18.Schuster I, Egger H, Herzig G, Reddy GS, Schmid JA, Schussler M, et al. Selective inhibitors of vitamin D metabolism--new concepts and perspectives. Anticancer Res. 2006;26:2653–68. [PubMed] [Google Scholar]
- 19.Yee SW, Campbell MJ, Simons C. Inhibition of Vitamin D3 metabolism enhances VDR signalling in androgen-independent prostate cancer cells. J Steroid Biochem Mol Biol. 2006;98:228–35. doi: 10.1016/j.jsbmb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Parise RA, Egorin MJ, Kanterewicz B, Taimi M, Petkovich M, Lew AM, et al. CYP24, the enzyme that catabolizes the antiproliferative agent vitamin D, is increased in lung cancer. Int J Cancer. 2006;119:1819–28. doi: 10.1002/ijc.22058. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Kanterewicz B, Buch S, Petkovich M, Parise R, Beumer J, et al. CYP24 inhibition preserves 1alpha,25-dihydroxyvitamin D(3) anti-proliferative signaling in lung cancer cells. Mol Cell Endocrinol. 2012;355:153–61. doi: 10.1016/j.mce.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muindi JR, Yu WD, Ma Y, Engler KL, Kong RX, Trump DL, et al. CYP24A1 inhibition enhances the antitumor activity of calcitriol. Endocrinology. 2010;151:4301–12. doi: 10.1210/en.2009-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo W, Karpf AR, Deeb KK, Muindi JR, Morrison CD, Johnson CS, et al. Epigenetic regulation of vitamin D 24-hydroxylase/CYP24A1 in human prostate cancer. Cancer Res. 2010;70:5953–62. doi: 10.1158/0008-5472.CAN-10-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y, Yu WD, Kong RX, Trump DL, Johnson CS. Role of nongenomic activation of phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase 1/2 pathways in 1,25D3-mediated apoptosis in squamous cell carcinoma cells. Cancer Res. 2006;66:8131–8. doi: 10.1158/0008-5472.CAN-06-1333. [DOI] [PubMed] [Google Scholar]
- 25.Yu WD, Ma Y, Flynn G, Muindi JR, Kong RX, Trump DL, et al. Calcitriol enhances gemcitabine anti-tumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle. 2010;9:3022–9. doi: 10.4161/cc.9.15.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szyszka R, Grankowski N, Felczak K, Shugar D. Halogenated benzimidazoles and benzotriazoles as selective inhibitors of protein kinases CK I and CK II from Saccharomyces cerevisiae and other sources. Biochem Biophys Res Commun. 1995;208:418–24. doi: 10.1006/bbrc.1995.1354. [DOI] [PubMed] [Google Scholar]
- 27.Beumer JH, Parise RA, Kanterewicz B, Petkovich M, D’Argenio DZ, Hershberger PA. A local effect of CYP24 inhibition on lung tumor xenograft exposure to 1,25-dihydroxyvitamin D(3) is revealed using a novel LC-MS/MS assay. Steroids. 2012;77:477–83. doi: 10.1016/j.steroids.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahraman M, Sinishtaj S, Dolan PM, Kensler TW, Peleg S, Saha U, et al. Potent, selective and low-calcemic inhibitors of CYP24 hydroxylase: 24-sulfoximine analogues of the hormone 1alpha,25-dihydroxyvitamin D(3) J Med Chem. 2004;47:6854–63. doi: 10.1021/jm040129+. [DOI] [PubMed] [Google Scholar]
- 29.Krek W, Maridor G, Nigg EA. Casein kinase II is a predominantly nuclear enzyme. J Cell Biol. 1992;116:43–55. doi: 10.1083/jcb.116.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faust M, Montenarh M. Subcellular localization of protein kinase CK2. A key to its function? Cell Tissue Res. 2000;301:329–40. doi: 10.1007/s004410000256. [DOI] [PubMed] [Google Scholar]
- 31.Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC. Protein kinase CK2 in mammary gland tumorigenesis. Oncogene. 2001;20:3247–57. doi: 10.1038/sj.onc.1204411. [DOI] [PubMed] [Google Scholar]
- 32.Laramas M, Pasquier D, Filhol O, Ringeisen F, Descotes JL, Cochet C. Nuclear localization of protein kinase CK2 catalytic subunit (CK2alpha) is associated with poor prognostic factors in human prostate cancer. Eur J Cancer. 2007;43:928–34. doi: 10.1016/j.ejca.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Oc P, Rusch V, Talbot SG, Sarkaria I, Viale A, Socci N, et al. Casein kinase II alpha subunit and C1-inhibitor are independent predictors of outcome in patients with squamous cell carcinoma of the lung. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:5792–803. doi: 10.1158/1078-0432.CCR-03-0317. [DOI] [PubMed] [Google Scholar]
- 34.Gapany M, Faust RA, Tawfic S, Davis A, Adams GL, Ahmed K. Association of elevated protein kinase CK2 activity with aggressive behavior of squamous cell carcinoma of the head and neck. Mol Med. 1995;1:659–66. [PMC free article] [PubMed] [Google Scholar]
- 35.Gotz C, Bachmann C, Montenarh M. Inhibition of protein kinase CK2 leads to a modulation of androgen receptor dependent transcription in prostate cancer cells. The Prostate. 2007;67:125–34. doi: 10.1002/pros.20471. [DOI] [PubMed] [Google Scholar]
- 36.Hessenauer A, Montenarh M, Gotz C. Inhibition of CK2 activity provokes different responses in hormone-sensitive and hormone-refractory prostate cancer cells. International journal of oncology. 2003;22:1263–70. [PubMed] [Google Scholar]
- 37.Khorchide M, Lechner D, Cross HS. Epigenetic regulation of vitamin D hydroxylase expression and activity in normal and malignant human prostate cells. J Steroid Biochem Mol Biol. 2005;93:167–72. doi: 10.1016/j.jsbmb.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Komagata S, Nakajima M, Takagi S, Mohri T, Taniya T, Yokoi T. Human CYP24 catalyzing the inactivation of calcitriol is post-transcriptionally regulated by miR-125b. Mol Pharmacol. 2009;76:702–9. doi: 10.1124/mol.109.056986. [DOI] [PubMed] [Google Scholar]
- 39.Lehen’kyi V, Raphael M, Oulidi A, Flourakis M, Khalimonchyk S, Kondratskyi A, et al. TRPV6 determines the effect of vitamin D3 on prostate cancer cell growth. PLoS One. 2011;6:e16856. doi: 10.1371/journal.pone.0016856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehen’kyi V, Flourakis M, Skryma R, Prevarskaya N. TRPV6 channel controls prostate cancer cell proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene. 2007;26:7380–5. doi: 10.1038/sj.onc.1210545. [DOI] [PubMed] [Google Scholar]
- 41.Ly LH, Zhao XY, Holloway L, Feldman D. Liarozole acts synergistically with 1alpha,25-dihydroxyvitamin D3 to inhibit growth of DU 145 human prostate cancer cells by blocking 24-hydroxylase activity. Endocrinology. 1999;140:2071–6. doi: 10.1210/endo.140.5.6698. [DOI] [PubMed] [Google Scholar]
- 42.Zhao XY, Ly LH, Peehl DM, Feldman D. Induction of androgen receptor by 1alpha,25-dihydroxyvitamin D3 and 9-cis retinoic acid in LNCaP human prostate cancer cells. Endocrinology. 1999;140:1205–12. doi: 10.1210/endo.140.3.6561. [DOI] [PubMed] [Google Scholar]
- 43.Guzey M, DeLuca HF. A group of deltanoids (vitamin D analogs) regulate cell growth and proliferation in small cell carcinoma cell lines. Research communications in molecular pathology and pharmacology. 1997;98:3–18. [PubMed] [Google Scholar]
- 44.Bremmer F, Thelen P, Pottek T, Behnes CL, Radzun HJ, Schweyer S. Expression and function of the vitamin D receptor in malignant germ cell tumour of the testis. Anticancer research. 2012;32:341–9. [PubMed] [Google Scholar]
- 45.Jiang F, Li P, Fornace AJ, Jr, Nicosia SV, Bai W. G2/M arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through the induction of GADD45 via an exonic enhancer. J Biol Chem. 2003;278:48030–40. doi: 10.1074/jbc.M308430200. [DOI] [PubMed] [Google Scholar]
- 46.Tront JS, Hoffman B, Liebermann DA. Gadd45a suppresses Ras-driven mammary tumorigenesis by activation of c-Jun NH2-terminal kinase and p38 stress signaling resulting in apoptosis and senescence. Cancer Res. 2006;66:8448–54. doi: 10.1158/0008-5472.CAN-06-2013. [DOI] [PubMed] [Google Scholar]
- 47.Jurutka PW, Hsieh JC, MacDonald PN, Terpening CM, Haussler CA, Haussler MR, et al. Phosphorylation of serine 208 in the human vitamin D receptor. The predominant amino acid phosphorylated by casein kinase II, in vitro, and identification as a significant phosphorylation site in intact cells. J Biol Chem. 1993;268:6791–9. [PubMed] [Google Scholar]
- 48.Jurutka PW, Terpening CM, Haussler MR. The 1,25-dihydroxy-vitamin D3 receptor is phosphorylated in response to 1,25-dihydroxy-vitamin D3 and 22-oxacalcitriol in rat osteoblasts, and by casein kinase II, in vitro. Biochemistry. 1993;32:8184–92. doi: 10.1021/bi00083a019. [DOI] [PubMed] [Google Scholar]
- 49.Jurutka PW, Hsieh JC, Nakajima S, Haussler CA, Whitfield GK, Haussler MR. Human vitamin D receptor phosphorylation by casein kinase II at Ser-208 potentiates transcriptional activation. Proc Natl Acad Sci U S A. 1996;93:3519–24. doi: 10.1073/pnas.93.8.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arriagada G, Paredes R, Olate J, van Wijnen A, Lian JB, Stein GS, et al. Phosphorylation at serine 208 of the 1alpha,25-dihydroxy Vitamin D3 receptor modulates the interaction with transcriptional coactivators. J Steroid Biochem Mol Biol. 2007;103:425–9. doi: 10.1016/j.jsbmb.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.