Abstract
Individuals with type 2 diabetes mellitus (DM) are at increased risk of cardiovascular disease (CVD) and mortality. Beyond traditional CVD risk factors, novel measures reflecting additional aspects of disease pathophysiology, such as biventricular volume (BiVV), may be useful for risk stratification. This study examined the relationship between BiVV and risk for mortality in European Americans with type 2 DM from the Diabetes Heart Study. BiVV was calculated from 771 non-contrast computed tomography scans performed to image coronary artery calcified plaque (CAC). Relationships between BiVV and traditional CVD risk factors were examined. Cox proportional hazards regression was performed to determine risk for mortality (all-cause and CVD-mortality) associated with increasing BiVV. Area under the curve analysis was used to assess BiVV utility in risk prediction models. During 8.4 ± 2.4 years (mean ± SD) of follow-up, 23% of the sample were deceased. In unadjusted analyses, BiVV was significantly associated with increasing body mass index, height, CAC, history of hypertension and prior myocardial infarction (p<0.0001–0.012). BiVV was significantly associated with all-cause (HR: 2.45; CI: 1.06–5.67; p=0.036) and CVD-mortality (HR: 4.36; CI: 1.36–14.03; p=0.014) in models adjusted for other known CVD risk factors. Area under the curve increased from 0.76 to 0.78 (p=0.04) and 0.74 to 0.77 (p=0.02) for all-cause and CVD-mortality on inclusion of BiVV. In conclusion, in the absence of echocardiography or other noninvasive imaging modalities to assess ventricular volumes, or when such methods are contra-indicated, BiVV from computed tomography may be considered as a tool for stratification of high-risk individuals, such as those with type 2 DM.
Keywords: cardiovascular disease, heart size, diabetes, risk-prediction
Coronary artery calcified plaque scores (CAC), determined by computed tomography scanning, are an independent predictor of all-cause mortality1 and CVD-mortality2 in the Diabetes Heart Study, a family based study enriched for type 2 diabetes mellitus (DM). In addition to CAC, other reported cardiovascular-related predictors of all-cause and CVD-mortality include arterial stiffness3, conduction wave abnormalities (as determined by electrocardiography)4–6 and left ventricular hypertrophy.7–9 More recently, biventricular volume (BiVV) determined from non-contrast computed tomography scans (as used for measurement of CAC) has been identified as a safe, non-invasive and cost-effective surrogate of left ventricular mass with correlation coefficients >0.8 when compared with magnetic resonance imaging-derived left ventricular mass.10 Several methods have been described to calculate ventricular volumes11–13, however, relationships between BiVV and other traditional markers of CVD risk remains unclear. In addition, whether BiVV is similarly predictive of mortality has not been investigated. The aim of the current investigation was to assess the relationships between BiVV and traditional cardiac risk factors and to examine the ability of BiVV to predict risk for all-cause and CVD-mortality in patients with DM from the Diabetes Heart Study.
METHODS
This investigation included 771 self-described European American individuals (from 352 families) with overt type 2 DM from the Diabetes Heart Study. Briefly, the Diabetes Heart Study includes siblings concordant for type 2 DM without advanced renal insufficiency. Type 2 DM was clinically defined as diabetes developing after the age of 35 years and treated with insulin and/or oral agents, in the absence of historical evidence of ketoacidosis. Ascertainment and recruitment have been described in detail previously.14, 15
Study protocols were approved by the Institutional Review Board at Wake Forest School of Medicine, and all participants provided written informed consent prior to participation. Participant examinations were conducted in the General Clinical Research Center of the Wake Forest Baptist Medical Center, and included interviews for medical history and health behaviors, anthropometric measures and resting blood pressure were recorded, fasting blood sampling for laboratory analyses and spot urine collection. Standard laboratory analyses included fasting glucose, glycated hemoglobin, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides and C-reactive protein concentrations. Individuals reported history of prior CVD based on prior event (angina, myocardial infarction, stroke) and/or intervention (coronary angiography, coronary artery bypass grafting, carotid endarterectomy). Individuals were classified as hypertensive if prescribed anti-hypertensive medication or had blood pressure measurements exceeding 140 mmHg (systolic) or 90 mmHg (diastolic), and dyslipidemic based on the criteria established in the Third Report of the National Cholesterol Education Program Expert Panel Detection, Evaluation and Treatment in Adults (ATP III)16.
Participant examinations included measurement of carotid artery intima-media thickness by high-resolution B-mode ultrasonography with a 7.5-MHz transducer and a Biosound Esaote (AU5) ultrasound machine (Biosound Esaote, Inc., Indianapolis, IN) as previously described.17 In addition a resting 12-lead electrocardiography was performed using a Marquette MAC 500 ECG instrument (Marquette Electronics, Milwaukee, WI, USA) as described previously.18 All data were transmitted electronically to a central reading center for processing (EPICARE Inc., Winston-Salem, NC, USA). Electrocardiographic left ventricular hypertrophy (ECG-LVH) was determined according to the Minnesota code criteria (http://www.sph.umn.edu/epi/ecg/).
The imaging protocol has been reported previously.19 In brief, images to calculate BiVV were captured using fast-gated helical computed tomography. All cardiac computed tomography examinations were performed on a single-slice helical computed tomography or a four-channel multidetector computed tomography both with cardiac gating and capable of 500 ms temporal resolution (HiSpeed LX and LightSpeed QXi with the SmartScore Cardiac scan package; General Electric Medical Systems, Waukesha, WI). After a scout image of the chest, the heart was imaged during suspended respiration at end inspiration. Scan parameters were 3 mm slice thickness, 26 cm display field of view, retrospective cardiac gating, 120 KV, 240 mA, and computed tomography scan pitch adjusted to heart rate for the single-slice system and 2.5-mm slice thickness in four-slice mode, 26 cm display field of view, prospective cardiac gating at 50% of the RR interval, 120 KV, and 240 mA for the multidetector computed tomography. BiVV was calculated using the modified Simpson’s formula10:
(Max short axis area + Mid-ventricular area) * (Ventricular Length / 3) + (Area at apex X Ventricular Length2 X pi) / 108.
BiVV has been highly correlated (r=0.804) with left ventricular mass obtained from magnetic resonance imaging.10 Determinations of CAC, carotid artery calcified plaque (CarCP) and infra-renal abdominal aortic calcified plaque (AACP) were also made from computed tomography scan images as previously described20 and reported as Agatston scores.
For all participants, vital status was determined from the National Social Security Death Index maintained by the United States Social Security Administration. For participants confirmed as deceased, length of follow-up was determined from date of the initial study visit to date of death. For deceased participants, death certificates were obtained from relevant county Vital Records Offices to confirm cause of death. For all other participants length of follow-up was determined from the date of the initial study visit to the end of 2011. Cause of death was categorized based on information contained in death certificates as CVD-mortality (myocardial infarction, congestive heart failure, cardiac arrhythmia, sudden cardiac death, peripheral vascular disease, and stroke) or either cancer, infection, end-stage renal disease, accidental, or other (including obstructive pulmonary disease, pulmonary fibrosis, liver failure and Alzheimer’s dementia).
Summary statistics were calculated including means and standard deviations (SD) for continuous variables and count and percentages for categorical variables. Due to the inclusion of related individuals in this study, marginal models with incorporation of generalized estimating equations were used to describe associations between BiVV and cardiovascular risk factors. First, analysis of the simple univariate associations between BiVV and each individual risk factor/demographic trait were performed, where BiVV was treated as the outcome variable. Second, each of these associations was adjusted for age, sex and body mass index (BMI) (for the analysis with height the BMI adjustment was not performed). For the analysis with ECG-LVH, individuals with electrocardiography readings failing to meet the minimum accepted quality standard were excluded (n=13). Continuous variables (including both BiVV and CAC) were log-transformed to better approximate the normality assumption as appropriate. The statistical inference (i.e. p-value) was reported based on the log-transformed BiVV analysis, however, to allow easier interpretation, the beta coefficients for untransformed BiVV are reported. This approach is reasonable when the p-values from the transformed and untransformed analyses are similar.
To evaluate the association between BiVV and mortality, BiVV was considered as both a continuous (log-transformed) and categorical variable derived from quartile ranges (Q1–4 based on increasing BiVV). The curves of cumulative incidence of all-cause mortality and CVD-mortality for each quartile of BiVV were plotted for exploratory analysis. Cox proportional hazards models were used to assess the ability of BiVV to predict all-cause mortality and CVD-mortality. To account for the correlated family structure, a robust sandwich covariance estimate was calculated. The robust standard error for the parameter estimates were used for statistical inference. Follow-up duration started from the beginning of the study to the last updated assessment of vital status. When the endpoint is all-cause mortality, censoring may occur if one is alive. When the endpoint is CVD-mortality, censoring may occur if one is alive or the death is due to other causes. Models were adjusted for (i) age and sex (model 1) (ii) age, sex, height and known CVD risk factors including current smoking, hypertension, dyslipidemia, and history of prior myocardial infarction (MI) (model 2); and (iii) age, sex, height, known CVD risk factors, ECG-LVH and CAC (model 3). The test for trend across increasing BiVV quartiles was also performed. SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for the analysis.
Receiver operating characteristic (ROC) curves were computed for the basic (model 1) and fully adjusted models (model 3) and progressive addition of continuous measures of CAC and BiVV. The areas under the curves were used to assess the ability of BiVV to predict all cause mortality or CVD-mortality after adjusting for other confounding variables and the known predictor CAC. The difference in area under the curve between two models was tested using Delong’s method.21 Stata software, version 8.2 (StataCorp, College Station, TX), was used for the ROC analysis. Statistical significance was accepted at p<0.05.
RESULTS
The demographic and clinical characteristics of the participants are presented in Table 1. A predominance of known CVD risk factors, including high body mass, hypertension and abnormal lipid concentrations was evident. Vascular calcified plaque scores for CAC, CarCP and AACP reflect a substantial burden of subclinical CVD in these diabetes-affected individuals. Based on available data, 4.3% of the sample had ECG-LVH defined using Minnesota Code criteria. The cohort was followed for 8.4 ± 2.4 years (mean ± SD) with 180 participants found to be deceased (23.3% of the sample) during this period. Among those individuals with documented cause of death information (n=152), 82 individuals (53.9%) had a documented CVD related cause of death.
Table 1.
Demographic Information for n=771 European Americans with type 2 diabetes mellitus and available Biventricular Volume from the Diabetes Heart Study.
| Variable | Mean ± SD (range; median*) or % | ||
|---|---|---|---|
| All (n=771) | Living (n=591) | Deceased (n=180) | |
| Demographic Information | |||
| Age (years) | 61.9 ± 8.9 (36.3–86.0) | 60.5 ± 8.4 | 66.7 ± 9.0 |
| Male | 49.2% | 47.2% | 55.6% |
| Smoking (current or prior) | 44.0% | 43.5% | 45.6% |
| Metabolic Syndrome | 89.0% | 89.0% | 88.9% |
| Diabetes Duration (years) | 10.6 ± 7.1 (1.0–41.1) | 9.6 ± 6.3 (1.0–30.8) | 13.6 ± 8.5 (1.0–41.1) |
| Self-reported CVD History | 44.0% | 38.9% | 60.6% |
| Self reported prior MI | 21.4% | 16.4% | 37.8% |
| Deceased – CVD causes | 9.3% | n/a | 40.0% |
| Body Composition | |||
| Height (cm) | 168.8 ± 9.7 (122.8–195.8) | 168.8 ± 9.8 (122.8–195.8) | 168.8 ± 9.5 (147.4–189.0) |
| Weight (kg) | 92.3 ± 20.1 (40.8–209.2) | 92.8 ± 20.0 (44.1–209.2) | 90.5 ± 20.6 (40.8 152.1) |
| Body Mass Index (kg/m2) | 32.4 ± 6.6 (17.1–58.0) | 32.6 ± 6.5 (17.1–58.0) | 31.7 ± 6.8 (17.5–56.9) |
| Blood Pressure | |||
| Systolic BP (mmHg) | 140 ± 19 (98–260) | 140 ± 17 (98–214) | 141 ± 23 (99–260) |
| Diastolic BP (mmHg) | 72 ± 10 (37–102) | 73 ± 10 (37–102) | 69 ± 10 (45–101) |
| Pulse Pressure (mmHg) | 68 ± 17 (28–159) | 67 ± 16 (28–124) | 71 ± 19 (32–159) |
| Hypertension | 88.7% | 87.1% | 93.9% |
| Anti-hypertensive Use | 78.7% | 76.1% | 87.2% |
| Blood Biochemistry | |||
| Fasting Glucose (mg/dL) | 150 ± 57 (16–463) | 148 ± 54 (16–463) | 156 ± 68 (46–436) |
| HbA1c (%) | 7.5 ± 1.6 (4.3–16.6) | 7.4 ± 1.5 (4.6–15.4) | 7.8 ± 1.8 (4.3–16.6) |
| Cholesterol (mg/dL) | 186 ± 43 (74–427) | 186 ± 43 (74–427) | 187 ± 43 (105–386) |
| LDL (mg/dL) | 103 ± 32 (14–236) | 103 ± 32 (14–236) | 104 ± 32 (33–228) |
| HDL (mg/dL) | 43 ± 12 (8–98) | 43 ± 12 (18–98) | 41 ± 12 (8–86) |
| Triglycerides (mg/dL) | 208 ± 133 (30–1068) | 204 ± 127 (30–1068) | 221 ± 152 (38–1065) |
| Dyslipidemia | 94.3% | 93.6% | 96.7% |
| C-reactive protein (mg/L) | 6.3 ± 9.7 (0.05–96) | 5.7 ± 8.3 (0.05–96) | 8.4 ± 12.8 (0.08–90) |
| Cardiovascular Disease Severity | |||
| CAC>0 | 96.0 % | 94.6% | 98.9% |
| Mean CAC | 2096 ± 3557 (0–50415; 604) | 1640 ± 3412 (0–50415; 374) | 3586 ± 3622 (0–21725; 2506) |
| CarCP>0 | 79.3% | 74.6% | 93.9% |
| Mean CarCP | 390 ± 745 (0–6122; 97) | 296 ± 656 (0–6122; 51) | 692 ± 916 (0–5605; 299) |
| AACP>0 | 96.7% | 96.2% | 98.3% |
| Mean AACP | 13644 ± 17113 (0–94156; 6766) | 10998 ± 15046 (0–75567; 4525) | 23482 ± 20497 (0–94156; 18732) |
| Carotid IMT (mm) | 0.687 ± 0.137 (0.451–1.569) | 0.671 0. ± 132 (0.451–1.569) | 0.739 ± 0.140 (0.498–1.316) |
| Ventricular Volume | |||
| Biventricular Volume (mL) | 384.5 ± 112.1 (156.6–884.5) | 374.6 ± 105.5 (156.6–884.5) | 416.8 ± 128.7 (188.1–798.3) |
| Biventricular Volume Quartile | |||
| Ranges | |||
| Q1 | 156.6–300.9 | 156.6–297.5 | 188.1–332.2 |
| Q2 | 300.9–375.6 | 297.5–364.1 | 332.2–395.9 |
| Q3 | 375.6–448.8 | 364.1–438.6 | 395.9–495.3 |
| Q4 | 448.8–884.5 | 438.6–884.5 | 495.3–798.3 |
CVD = cardiovascular disease; MI = myocardial infarction; BP = blood pressure; HbA1c = glycated hemoglobin; LDL = low density lipoprotein cholesterol; HDL = high density lipoprotein cholesterol; CAC = coronary artery calcified plaque; CarCP = carotid artery calcified plaque; AACP = abdominal aortic calcified plaque; IMT = intima-media thickness.
median values included only for measures of vascular calcification
In unadjusted analyses BiVV was significantly associated with increasing BMI, height, CAC, and histories of hypertension and prior MI (Table 2). Following adjustment for age, gender and BMI, BiVV remained associated (p<0.01) with hypertension, prior MI, CAC and left ventricular hypertrophy (Table 2). BiVV was not significantly associated with dyslipidemia or current smoking status.
Table 2.
Associations between Biventricular Volume and cardiovascular disease risk factors in unadjusted and adjusted models.
| Unadjusted | Adjusted (age, sex, BMI) | |||
|---|---|---|---|---|
| β-coefficient | p-value | β-coefficient | p-value | |
| Age | −1.13 | 0.01 | n/a | n/a |
| Gender (male) | 119.33 | <0.0001 | n/a | n/a |
| BMI | 2.77 | <0.0001 | n/a | n/a |
| Height | 5.96 | <0.0001 | 2.82 | <0.0001 |
| Hypertension | 32.97 | 0.01 | 34.98 | 0.0003 |
| Current Smoking | 5.64 | 0.61 | −7.94 | 0.37 |
| Dyslipidemia | 5.92 | 0.41 | −2.05 | 0.89 |
| Prior Myocardial Infaction | 54.05 | <0.0001 | 28.05 | 0.0024 |
| Coronary Artery Calcium | 13.66 | <0.0001 | 7.12 | 0.0010 |
| ECG-LVH | 47.31 | 0.07 | 56.41 | 0.0074 |
BMI = body mass index; ECG-LVH = Electrocardiographic left ventricular hypertrophy
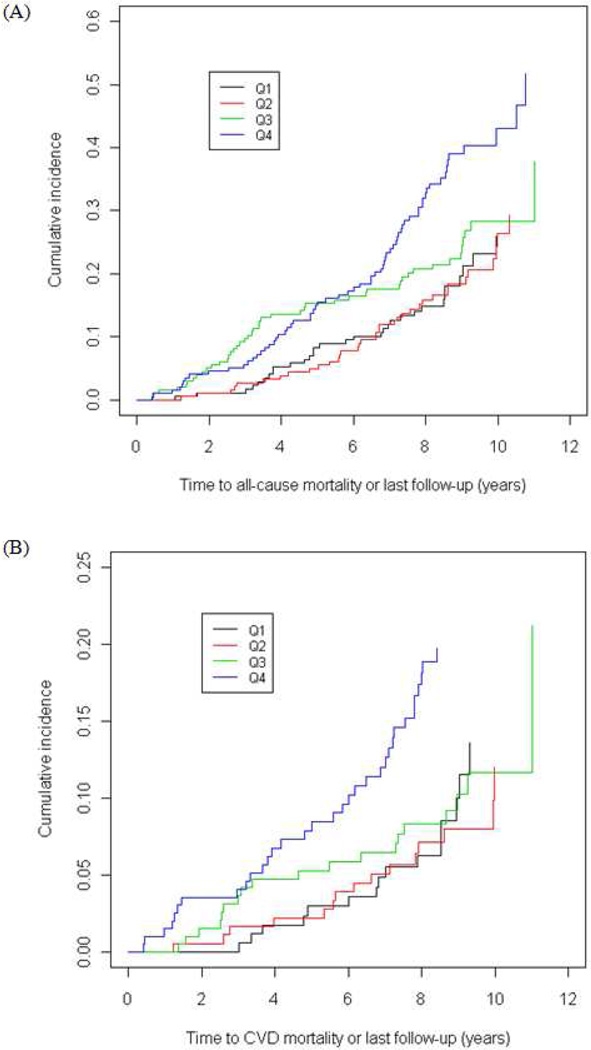
Cumulative incidence of all-cause and CVD-mortality increased across increasing BiVV quartiles (Figure 1). A linear relationship was observed where risk for all-cause and CVD-mortality increased with greater heart size, although only the comparison between the highest quartile of BiVV values versus the lowest quartile is significant (Table 3). A formal test for trend of BiVV as an ordinal measure (using quartiles as cut-points) supported an overall trend for increasing BiVV values (based on quartiles) to be associated with increased risk for both all-cause (HR: 1.28; 95% CI: 1.12–1.47; p=0.0005) and CVD mortality (HR: 1.31; CI: 1.07–1.61; p=0.011) in a univariate analysis.
Figure 1.
Cumulative incidence curves for (A) all-cause and (B) CVD-mortality based on increasing BiVV quartiles (Q1–Q4).
Table 3.
Relationship between Biventricular volume quartiles and risk for all-cause and cardiovascular disease (CVD) mortality using Cox proportional hazard models partially and fully adjusted for known CVD risk factors.
| Unadjusted | Partially adjusted* | Fully Adjusted† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mortality | Quartile | # Events | Events / 100 person years |
HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
| All-cause | Q1 (Ref) | 31 | 2.1 | 1.00 | - | 1.00 | - | 1.00 | - |
| Q2 | 36 | 2.2 | 1.05 (0.63–1.72) | 0.86 | 1.05 (0.64–1.73) | 0.85 | 0.93 (0.56–1.56) | 0.80 | |
| Q3 | 46 | 2.8 | 1.34 (0.84–2.11) | 0.22 | 1.47 (0.84–2.57) | 0.18 | 1.22 (0.64–2.30) | 0.55 | |
| Q4 | 67 | 4.1 | 1.98 (1.29–3.05) | 0.002 | 2.31 (1.35–3.97) | 0.002 | 1.82 (1.02–3.25) | 0.04 | |
| CVD | Q1 (Ref) | 15 | 1.0 | 1.00 | 1.00 | - | 1.00 | - | |
| Q2 | 15 | 0.9 | 0.90 (0.44–1.83) | 0.77 | 0.85 (0.42–1.71) | 0.64 | 0.69 (0.32–1.47) | 0.33 | |
| Q3 | 19 | 1.1 | 1.136 (0.59–2.19) | 0.70 | 1.08 (0.50–2.35) | 0.84 | 0.90 (0.36–2.25) | 0.81 | |
| Q4 | 33 | 2.0 | 2.014 (1.11–3.64) | 0.02 | 2.19 (1.10–4.37) | 0.03 | 1.78 (0.83–3.83) | 0.14 | |
adjustment for age, sex, height, current smoking, hypertension, dyslipidemia, prior myocardial infarction.
adjustment for age, sex, height, current smoking, hypertension, dyslipidemia, prior myocardial infarction, electrocardiographic left ventricular hypertrophy and coronary artery calcium.
When treated as a continuous measure, BiVV was associated with a significant increase in risk for both all-cause and CVD-mortality in univariate models; each SD unit increase in log transformed BiVV was associated with a >2.5-fold increase in all-cause mortality and a >3.5-fold increase in risk for CVD-mortality (Table 4, unadjusted). BiVV remained associated with CVD-mortality following adjustment for age and sex (Table 4, model 1), and other known CVD risk factors (Table 4, model 2). In models adjusted for known CVD risk factors, CAC (predictor of mortality in T2D1) and ECG-LVH, BiVV remained a significant predictor of both all-cause and CVD-mortality (Table 4, model 3).
Table 4.
Biventricular volume prediction of all-cause and cardiovascular (CVD) mortality using Cox proportional hazard models.
| All-cause Mortality | CVD-Mortality | |||
|---|---|---|---|---|
| Models | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Unadjusted | 2.80 (1.56–5.01) | 0.0005 | 3.85 (1.64–9.05) | 0.002 |
| 1 | 4.17 (1.980–8.78) | 0.0002 | 6.83 (2.443–19.08) | 0.0002 |
| 2 | 3.63 (1.674–7.86) | 0.001 | 5.66 (1.993–16.10) | 0.001 |
| 3 | 2.45 (1.06–5.67) | 0.036 | 4.36 (1.356–14.03) | 0.014 |
Models adjusted as follows (1) age and sex; (2) age, sex, height, current smoking, hypertension, dyslipidemia, prior myocardial infarction; (3) age, sex, height, current smoking, hypertension, dyslipidemia, prior myocardial infarction, electrocardiographic left ventricular hypertrophy and coronary artery calcium.
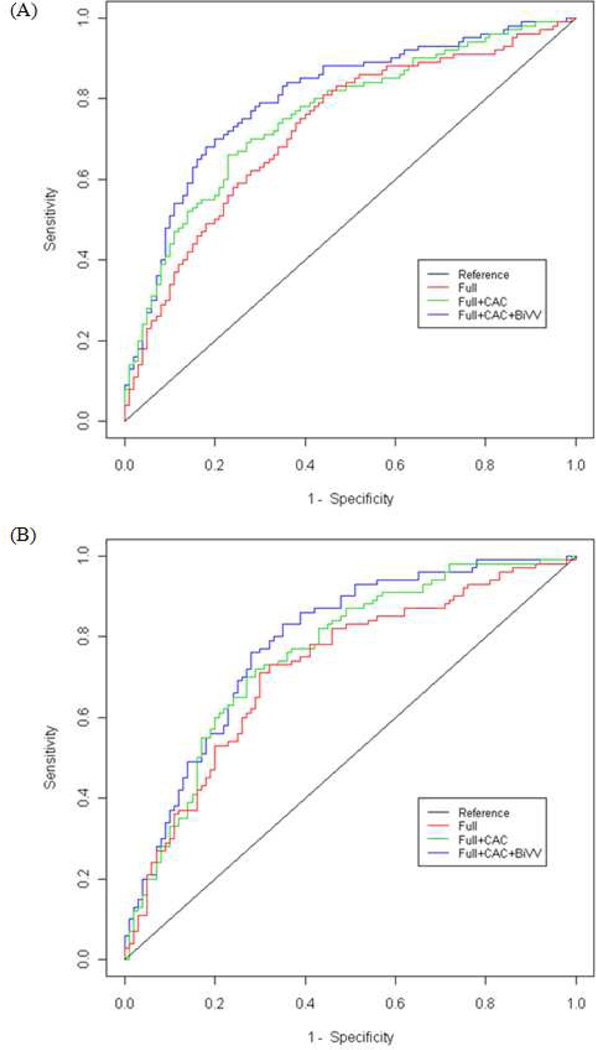
Area under the curve analysis confirmed the utility of BiVV in models for risk prediction (Figure 2 and Table 5). For models including age, sex and CAC only, the area under the ROC curve for prediction of all-cause mortality increased from 0.76 to 0.78 (p=0.04) for all-cause mortality and from 0.74 to 0.77 (p=0.02) for CVD-mortality upon inclusion of BiVV. For the fully adjusted models including CAC, the area under the curve increased from 0.79 to 0.80 (p=0.04) for all-cause mortality and from 0.77 to 0.78 (p=0.12) for CVD-mortality upon with inclusion of BiVV.
Figure 2.
Receiver operator characteristic curves for (A) all-cause and (B) CVD-mortality for fully adjusted (age, sex, height, hypertension, current smoking, dyslipidemia, prior MI, and LVH) models with sequential additional of CAC and BiVV.
Table 5.
Area under the curve (AUC) analysis for basic (age and sex) and fully adjusted (age, sex, height, current smoking, hypertension, dyslipidemia, prior myocardial infarction, and electrocardiographic left ventricular hypertrophy) models predicting all-cause and cardiovascular disease (CVD) mortality with sequential addition of coronary artery calcium (CAC) and biventricular volume (BiVV).
| Models | All-cause mortality | CVD-mortality | ||
|---|---|---|---|---|
| AUC | p-value* | AUC | p-value* | |
| Basic | 0.71 | - | 0.69 | - |
| Basic + CAC | 0.76 | <0.0001 | 0.74 | 0.0005 |
| Basic + CAC + BiVV | 0.78 | 0.0001 | 0.77 | 0.005 |
| Full | 0.76 | - | 0.75 | - |
| Full + CAC | 0.79 | 0.004 | 0.77 | 0.031 |
| Full + CAC + BiVV | 0.80 | 0.001 | 0.78 | 0.058 |
p-values are for comparisons of subsequent models to either basic or full models without CAC and BiVV
DISCUSSION
Individuals with type 2 DM, have elevated rates of cardiovascular complications and CVD-mortality. Improved risk prediction may better inform clinical management. This study was designed to evaluate the relationships between BiVV and traditional CVD risk factors, including CAC. We have previously shown that CAC is a powerful predictor of mortality in this cohort1, improves prediction compared to conventional risk factors and reclassifies a significant percentage of type 2 DM subjects to higher or lower risk categories2. Non-contrast computed tomography scans to determine CAC are increasingly common and we evaluated the ability of BiVV, derived from the computed tomography scans for CAC, to add to prediction of all-cause and CVD-mortality. BiVV was associated with conventional CVD risk factors: male sex, BMI, hypertension, prior MI, CAC and ECG-LVH. Accounting for these relationships BiVV was an independent predictor of both all-cause and CVD-mortality in this high-risk sample with type 2 DM.
BiVV has been well correlated previously with left ventricular mass.10 Left ventricular hypertrophy is a predictor of adverse cardiovascular outcomes including sudden arrhythmic death9, congestive heart failure22, 23, MI24 and stroke.25 Diverse mechanisms have been proposed to explain the relationship between left ventricular hypertrophy and adverse CVD outcomes including a causal role of left ventricular hypertrophy via contributions to diastolic dysfunction9 and reduced coronary flow reserve.8 Alternatively left ventricular hypertrophy may simply reflect underlying disease processes such as hypertension8, valvular heart disease7 or atherosclerosis.9 Whether BiVV, as an alternative measure of left ventricular mass, similarly reflects elements of the underlying pathophysiology contributing to CVD risk remains unclear. In this study BiVV was associated with a 2.4 and 4.4-fold increased risk of all-cause and CVD-mortality, respectively. These associations persisted following adjustment for traditional CVD risk factors and CAC, suggesting an independent contribution of heart size to adverse CVD outcomes in type 2 DM, and supporting the utility of BiVV in risk stratification models.
Although BiVV was strongly correlated with left ventricular hypertrophy in this study, it is not a surrogate for left ventricular hypertrophy and may be capturing other underlying processes. The magnitude of increased risk conferred by BiVV for mortality is consistent with prior examinations of left ventricular hypertrophy as a risk factor for cardiac death. Increased left ventricular hypertrophy has been associated with an approximate 2-fold greater risk for all-cause mortality and an approximate 3-fold increase in risk for sudden arrhythmic death in the largely male, U.S. Heart and Soul Study.9 Likewise, in the Framingham cohort, left ventricular hypertrophy was associated with an approximate 1.5-fold increased risk of sudden death8 with hazard ratios between 1.5 and 2.0 also reported in the National Health and Nutrition Examination Survey II.7
We do not know if these observations are generalizable to other population samples, e.g. other ethnicities, non-diabetic individuals. Traditional CVD risk factors are increased in the Diabetes Heart Study and likely contribute to increased BiVV. Further, the electrocardiography determination of LVH described here is not the gold standard for determination of LVH and may have contributed additional variance to the reported risk prediction. While there are limitations to our study design an important counterpoint is that it likely reflects a general population of type 2 DM-affected individuals in the U.S., and thus the underlying CVD risk for such individuals.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported in part by R01 HL67348, R01 HL092301, R01 NS058700 (to DWB), F32 DK083214 (to CEH) and T35 DK007400 (supporting PTW), and the General Clinical Research Centre of the Wake Forest School of Medicine (M01 RR07122, F32 HL085989). The authors thank the other investigators, the staff, and the participants of the Diabetes Heart Study for their valuable contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Agarwal S, Morgan T, Herrington DM, Xu J, Cox AJ, Freedman BI, Carr JJ, Bowden DW. Coronary calcium score and prediction of all-cause mortality in diabetes: The Diabetes Heart Study. Diabetes Care. 2011;34:1219–1224. doi: 10.2337/dc11-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal S, Cox AJ, Herrington DM, Jorgensen MS, Xu J, Freedman BI, Carr JJ, Bowden DW. Coronary calcium score predicts cardiovascular mortality in diabetes: Diabetes heart study. Diabetes Care. 2012 doi: 10.2337/dc12-1548. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoji T, Maekawa K, Emoto M, Okuno S, Yamakawa T, Ishimura E, Inaba M, Nishizawa Y. Arterial stiffness predicts cardiovascular death independent of arterial thickness in a cohort of hemodialysis patients. Atherosclerosis. 2010;210:145–149. doi: 10.1016/j.atherosclerosis.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Beckerman J, Yamazaki T, Myers J, Boyle C, Chun S, Wang P, Froelicher V. T-wave abnormalities are a better predictor of cardiovascular mortality than ST depression on the resting electrocardiogram. Ann Noninvasive Electrocardiol. 2005;10:146–151. doi: 10.1111/j.1542-474X.2005.05607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaykha A, Myers J, Desser KB, Laufer N, Froelicher VF. The prognostic importance of isolated p-wave abnormalities. Clin Cardiol. 2010;33:E87–E93. doi: 10.1002/clc.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porthan K, Viitasalo M, Jula A, Reunanen A, Rapola J, Vaananen H, Nieminen MS, Toivonen L, Salomaa V, Oikarinen L. Predictive value of electrocardiographic QT interval and T-wave morphology parameters for all-cause and cardiovascular mortality in a general population sample. Heart Rhythm. 2009;6:1202–1208. doi: 10.1016/j.hrthm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. 2000;140:848–856. doi: 10.1067/mhj.2000.111112. [DOI] [PubMed] [Google Scholar]
- 8.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 9.Turakhia MP, Schiller NB, Whooley MA. Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from the Heart and Soul Study) Am J Cardiol. 2008;102:1131–1135. doi: 10.1016/j.amjcard.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel KR, Bertoni AG, Ding J, Johnston S, Budoff MJ, Bluemke DA, Carr JJ. Comparison of methods to measure heart size using noncontrast-enhanced computed tomography: Correlation with left ventricular mass. J Comput Assist Tomogr. 2008;32:934–941. doi: 10.1097/RCT.0b013e318159a49e. [DOI] [PubMed] [Google Scholar]
- 11.Raman SV, Shah M, McCarthy B, Garcia A, Ferketich AK. Multi-detector row cardiac computed tomography accurately quantifies right and left ventricular size and function compared with cardiac magnetic resonance. Am Heart J. 2006;151:736–744. doi: 10.1016/j.ahj.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Yamaoka O, Yabe T, Okada M, Endoh S, Nakamura Y, Mitsunami K, Kinoshita M, Mori M, Murata K, Morita R. Evaluation of left ventricular mass: Comparison of ultrafast computed tomography, magnetic resonance imaging, and contrast left ventriculography. Am Heart J. 1993;126:1372–1379. doi: 10.1016/0002-8703(93)90536-i. [DOI] [PubMed] [Google Scholar]
- 13.Bleiweis MS, Mao SS, Brundage BH. Total biventricular volume and total left ventricular volume by ultrafast computed tomography: Prediction of left ventricular mass. Am Heart J. 1994;127:667–673. doi: 10.1016/0002-8703(94)90678-5. [DOI] [PubMed] [Google Scholar]
- 14.Bowden DW, Cox AJ, Freedman BI, Hugenschimdt CE, Wagenknecht LE, Herrington D, Agarwal S, Register TD, Maldjian JA, Ng MC, Hsu FC, Langefeld CD, Williamson JD, Carr JJ. Review of the Diabetes Heart Study (DHS) family of studies: A comprehensively examined sample for genetic and epidemiological studies of type 2 diabetes and its complications. Rev Diabet Stud. 2010;7:188–201. doi: 10.1900/RDS.2010.7.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowden DW, Lehtinen AB, Ziegler JT, Rudock ME, Xu J, Wagenknecht LE, Herrington DM, Rich SS, Freedman BI, Carr JJ, Langefeld CD. Genetic epidemiology of subclinical cardiovascular disease in the Diabetes Heart Study. Ann Hum Genet. 2008;72:598–610. doi: 10.1111/j.1469-1809.2008.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.Lange LA, Bowden DW, Langefeld CD, Wagenknecht LE, Carr JJ, Rich SS, Riley WA, Freedman BI. Heritability of carotid artery intima-medial thickness in type 2 diabetes. Stroke. 2002;33:1876–1881. doi: 10.1161/01.str.0000019909.71547.aa. [DOI] [PubMed] [Google Scholar]
- 18.Lehtinen AB, Daniel KR, Shah SA, Nelson MR, Ziegler JT, Freedman BI, Carr JJ, Herrington DM, Langefeld CD, Bowden DW. Relationship between genetic variants in myocardial sodium and potassium channel genes and QT interval duration in diabetics: The Diabetes Heart Study. Ann Noninvasive Electrocardiol. 2009;14:72–79. doi: 10.1111/j.1542-474X.2008.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: Standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 20.Carr JJ, Crouse JR, Goff DC, Jr, D'Agostino RB, Jr, Peterson NP, Burke GL. Evaluation of subsecond gated helical CT for quantification of coronary artery calcium and comparison with electron beam CT. AJR Am J Roentgenol. 2000;174:915–921. doi: 10.2214/ajr.174.4.1740915. [DOI] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 22.Gerdts E, Okin PM, Boman K, Wachtell K, Nieminen MS, Dahlof B, Devereux RB. Association of heart failure hospitalizations with combined electrocardiography and echocardiography criteria for left ventricular hypertrophy. Am J Hypertens. 2012;25:678–683. doi: 10.1038/ajh.2012.31. [DOI] [PubMed] [Google Scholar]
- 23.de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: The Cardiovascular Health Study. Eur Heart J. 2008;29:741–747. doi: 10.1093/eurheartj/ehm605. [DOI] [PubMed] [Google Scholar]
- 24.Lemmert ME, de Vreede-Swagemakers JJ, Eurlings LW, Kalb L, Crijns HJ, Wellens HJ, Gorgels AP. Electrocardiographic predictors of out-of-hospital sudden cardiac arrest in patients with coronary artery disease. Am J Cardiol. 2012;109:1278–1282. doi: 10.1016/j.amjcard.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Bots ML, Nikitin Y, Salonen JT, Elwood PC, Malyutina S, Freire de Concalves A, Sivenius J, Di Carlo A, Lagiou P, Tuomilehto J, Koudstaal PJ, Grobbee DE. Left ventricular hypertrophy and risk of fatal and non-fatal stroke. Eurostroke: A collaborative study among research centres in europe. J Epidemiol Community Health. 2002;56(Suppl 1):8–13. doi: 10.1136/jech.56.suppl_1.i8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.