Abstract
Purpose
This phase I study was conducted to identify the MTD of alvocidib when combined vorinostat in patients with relapsed, refractory, or poor prognosis acute leukemia, or refractory anemia with excess blasts-2 (RAEB-2). Secondary objectives included investigating the pharmacokinetic and pharmacodynamic effects of the combination.
Experimental Design
Patients received vorinostat (200 mg orally, 3 times a day [TID], for 14 days), on a 21-day cycle, combined with 2 different alvocidib administration schedules: a 1-h intravenous infusion, daily x 5; or a 30-min loading infusion followed by a 4-h maintenance infusion, weekly x 2. The alvocidib dose was escalated using a standard 3+3 design.
Results
Twenty-eight patients were enrolled and treated. The alvocidib MTD was 20 mg/m2 (30-min loading infusion) followed by 20 mg/m2 (4-h maintenance infusion) on days 1 and 8, in combination with vorinostat. The most frequently encountered toxicities were cytopenias, fatigue, hyperglycemia, hypokalemia, hypophosphatemia, and QT prolongation. Dose limiting toxicities (DLTs) were cardiac arrhythmia-atrial fibrillation and QT prolongation. No objective responses were achieved, although 13 of 26 evaluable patients exhibited stable disease. Alvocidib appeared to alter vorinostat pharmacokinetics, whereas alvocidib pharmacokinetics were unaffected by vorinostat. Ex vivo exposure of leukemia cells to plasma obtained from patients after alvocidib treatment blocked vorinostat-mediated p21CIP1 induction and down-regulated Mcl-1 and p-RNA Pol II for some specimens, although parallel in vivo bone marrow responses were infrequent.
Conclusions
Alvocidib combined with vorinostat is well tolerated. Although disease stabilization occurred in some heavily pretreated patients, objective responses were not obtained with these schedules.
Keywords: Vorinostat, Alvocidib, Acute Leukemia, Clinical Trial, Phase I
INTRODUCTION
The outcome for adults with relapsed or refractory acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), high-risk myelodysplastic syndrome (MDS), and chronic myelogenous leukemia in blast crisis (CML-BC) remains poor. Twenty to 30% of patients never achieve complete remission (CR) and fewer than 35% are cured. Furthermore, cures are achieved in fewer than 10% patients at least 60 years of age. Current treatment strategies include chemotherapy, in which survival beyond 1 year is rare, or allogeneic stem cell transplant, for which there are few eligible patients, and long-term survival rates are 20–40% (1).
HDACIs act by modifying chromatin structure and gene expression (2). They kill transformed cells through diverse mechanisms, including induction of oxidative injury and death receptors, interference with chaperone protein function and DNA repair, and up-regulation of pro-apoptotic proteins (e.g., Bim), among others (3). Multiple HDACIs, representing diverse chemical classes, have been investigated as anticancer agents. Vorinostat is a pan-HDACI that received FDA approval for the treatment of patients with progressive, persistent, or recurrent cutaneous T-cell lymphoma (4). Vorinostat has shown some, albeit limited, single-agent activity in AML/MDS (5).
Alvocidib (flavopiridol) is a rohitukine alkaloid that inhibits cyclin-dependent kinases (CDKs) 1, 2, 4/6, 7, and 9 (6), and in some cell types, down-regulates expression of cyclin D1 (7). Alvocidib also acts as a transcriptional repressor by inhibiting the CDK9/cyclin T pTEFb (positive transcription elongation factor b) complex (8). Aside from inducing cell cycle arrest (9), alvocidib potently triggers apoptosis in tumor cells, particularly those of hematopoietic origin (10). Alvocidib down-regulates various anti-apoptotic proteins including p21CIP1, cyclin D1, Mcl-1, XIAP, and Bag-1 (11–15). In human leukemia cells, alvocidib-mediated apoptosis has been related to induction of mitochondrial injury, characterized by loss of mitochondrial membrane potential and release of cytochrome c into the cytosol (16). Preclinical studies have also demonstrated synergism between alvocidib and cytotoxic agents (17).
In preclinical studies involving human leukemia cells, coadministration of alvocidib with differentiating agents, such as the HDACIs sodium butyrate and vorinostat, potently induces leukemic cell apoptosis (11, 12) (18–21). This may reflect alvocidib-mediated transcriptional repression of several anti-apoptotic genes required for leukemic cell differentiation, including p21CIP1 and Mcl-1 (18). We have reported that in human leukemia cells, alvocidib blocks vorinostat-mediated p21CIP1 induction, diminishes expression of various anti-apoptotic proteins, and induces pronounced apoptosis (22).
These findings raised the possibility that alvocidib administered either prior to or concurrently with vorinostat might exert similar actions in vivo, resulting in enhanced anti-leukemic effects in patients with acute leukemia. The primary goal of this study was to establish the MTD for alvocidib when combined with vorinostat (200 mg orally, TID, for 14 days) in patients with relapsed, refractory or poor prognosis acute leukemia, or RAEB-2. Pharmacokinetic studies were performed to assess interactions between the drugs. In addition, pharmacodynamic studies were performed to determine whether patient plasma—after alvocidib treatment—could recapitulate known alvocidib actions (e.g., inhibition of p21CIP1 induction) in human leukemia cells (U937) exposed to vorinostat in vitro, and if analogous events occurred in leukemic bone marrow cells following in vivo administration of these agents to patients.
PATIENTS AND METHODS
Drug supply
Vorinostat (SAHA; NSC 701852) and alvocidib (flavopiridol; NSC 649890) were supplied by Merck and Co., Inc. and Sanofi-Aventis Pharmaceuticals, Inc., respectively, through the U.S. National Cancer Institute (NCI).
Patient eligibility
Eligible patients were at least 18 years of age with an Eastern Cooperative Oncology Group performance status ≤ 2 and a diagnosis of relapsed or primary refractory AML, ALL, acute leukemia following prior MDS, CML-BC following prior imatinib therapy, RAEB-2, or acute leukemia in patients 60 years or older (no requirement for prior therapy). Additional eligibility criteria included normal to near-normal kidney and liver function; no prior autologous or allogeneic bone marrow/stem cell transplantation; and a baseline white blood cell count (WBC) < 50,000/μL. Hydroxyurea and/or leukapheresis was permitted to reduce the WBC, but hydroxyurea must have been discontinued 24 h before study initiation.
Treatment plan
This phase I trial was a non-randomized, dose-escalation study to identify the MTD for alvocidib given in combination with vorinostat. Vorinostat was administered orally, at a dose of 200 mg TID, on days 1–14. Initially, alvocidib was given as a 1-h intravenous infusion on days 1–5 of a 21-day cycle. The starting dose was 10 mg/m2 and the dose was escalated by constant increments of 10 mg/m2, as permitted by toxicity.
After completing the evaluation of dose level 4 for this daily short infusion (DSI) schedule, the protocol was amended, based on recommendations of CTEP, to evaluate administration of alvocidib with a weekly loading-maintenance infusion (WLMI) schedule based upon promising evidence of activity in patients with chronic lymphocytic leukemia (CLL) (23), and also to improve the safety in patients receiving alvocidib. Specifically, a loading dose given as a 30-min intravenous infusion was followed immediately by a 4-h maintenance infusion on days 1 and 8 of a 21-day cycle. The dose levels (loading dose/maintenance dose, in mg/m2) were as follows: dose level 5, 20/20; dose level 6A, 30/20; dose level 6B, 30/30.
In general, patients were treated every 3 weeks. Disease status was assessed by bone marrow aspirate and biopsy prior to cycle 2 or as clinically indicated. Patients with no evidence of disease progression (reflected by an increase in bone marrow blasts in marrows exhibiting ≥ 50% cellularity) and no evidence of significant side effects, were eligible to receive multiple treatment courses without interruption.
All patients received full supportive care, including herpes zoster prophylaxis. With the WLMI schedule, QTc interval monitoring was included, due to vorinostat safety issues. Clinical issues unique to acute leukemia and alvocidib infusion, such as hyperacute tumor lysis syndrome (TLS) and cytokine release syndrome (characterized by fever, bronchospasm, altered blood pressure, myalgias, arthralgias, tumor pain and/or urticarial rash) (23), necessitated rigorous supportive care regimens with the WLMI schedule, i.e., prophylaxis, monitoring, and treatment for TLS during the first course (doses 1 and 2) of alvocidib and for subsequent infusions if clinically indicated. Patients also received oral dexamethasone, 20 mg prior to the first and second dose of alvocidib, to prevent cytokine release syndrome.
Study design, definition of DLT and identification of the MTD
This study used a 3+3 dose-escalation design, with expansion to 6 patients if a DLT was observed in any of the initial 3 patients evaluated. The MTD was defined as the highest dose level at which fewer than 2 of 6 patients experienced a DLT during the first cycle of therapy. DLT was defined as any of the following adverse events that were possibly, probably, or definitely related to study treatment: (1) any grade 4 non-hematological toxicity, except infection or hyperbilirubinemia; (2) any grade 3 non-hematological toxicity lasting longer than 7 days; (3) a grade 4 ANC or PLT toxicity persisting longer than 6 weeks, in the absence of leukemia.
Toxicity evaluation
Adverse events were characterized in terms of attribution, severity, and study treatment relatedness according to the NCI-Common Terminology Criteria for Adverse Events version 3.0.
Response evaluation
Response criteria were as described by Cheson et al (24, 25). Patients who did not experience a CR during the treatment course were allowed to continue on study treatment if they did not experience significant toxicity in the absence of disease progression.
Pharmacokinetic studies
The effect of vorinostat on the plasma pharmacokinetics of alvocidib was assessed during the first cycle of therapy by comparing data for the initial dose of alvocidib to that for a subsequent dose, administered after patients had received alvocidib for either 3 or 7 days, depending upon the alvocidib dosing schedule. For the DSI schedule, blood specimens were obtained before dosing, then at 30, 55 and 70 min, and 2, 3, 4, 8, and 24 h after starting the infusion, on days 1 and 4. For the WLMI schedule, blood specimens were obtained before dosing, then at 15 and 25 min, and 1, 2, 3, 4, 5, 6, 8, and 24 h after starting the infusion, on days 1 and 8. Blood samples (6 mL) were drawn from a peripheral arm vein into sodium heparin collection tubes and centrifuged (1,100–1,300 x g, 4°C, 10 min). The plasma was stored at −70°C until assayed.
Plasma samples (100 μL) were prepared for the analysis of alvocidib by adding 7 μL of internal standard (IS) working solution (150-ng/mL bis(O-methyl)flavopiridol in acetonitrile) and 200 μL of acetonitrile to plasma, with vigorous mixing (26). After centrifugation (10,000 x g, 5 min), the supernatant (200 μL) was diluted with 25-mM ammonium formate buffer, pH 2.75 (200 μL), whereupon 100 μL was injected onto a 150 × 4.6-mm Luna 5-μm C8 HPLC column (Phenomenex) and eluted with acetonitrile/25-mM ammonium formate buffer, pH 2.75 (30:70 v/v) at 1.0 mL/min. An Agilent Technologies 1100 Series LC/MSD system with an electrospray ionization interface was used for detection. Nitrogen was used as the nebulizing (50 p.s.i.) and drying gas (12 L/min, 350°C). Positive ions corresponding to the [M+H]+ ions of alvocidib at m/z 402.1 and the IS at 430.1 were measured by selected-ion monitoring with a 2,500 V capillary voltage, 130 V fragmentor voltage, and 289 msec dwell time. Extracted ion chromatograms were integrated to provide peak areas. The analytical method was validated and applied to the routine analysis of study samples according to published guidelines (27). Calibration standards of alvocidib in human donor plasma had concentrations ranging from 1–100 ng/mL (2.3–228 nM). Samples were assayed in 28 runs during a period of 24 months. The calibration curves had correlation coefficients ≥ 0.998. Interday accuracy was within ± 4.4% of the known concentration of the calibration standards and quality control solutions with a precision ≤ 7.7%.
Blood samples to define the plasma profile for the first dose of vorinostat were collected before dosing and 0.5, 1, 2, 2.5, 3, 4, and 8 h after dosing. Sampling according to this schedule was also performed for the morning dose of vorinostat when given together with alvocidib on day 4 for the DSI schedule and day 8 for the WLMI schedule. Serum was harvested from the blood and assayed for vorinostat, vorinostat glucuronide (VG), and 4-anilino-4-oxobutanoic acid (VA) using a validated liquid chromatography-electrospray ionization tandem mass spectrometry method as previously described by (28, 29).
Plasma concentration-time curves for each patient were analyzed by standard noncompartmental methods using WinNonlin Professional 5.0 software (Pharsight Corp.) (30). Mean values of the pharmacokinetic variables were calculated as the geometric mean ± SD of the individual patient values (31, 32). The paired two-tailed t-test was used to compare the overall mean total body clearance (CL) for the first dose of alvocidib to the doses given on day 4 for the DSI schedule) or day 8 for the WLMI schedule using logarithmically transformed data. P < 0.05 was considered to be significantly different.
Pharmacodynamics
Collection of plasma and bone marrow samples
Bone marrow aspirate and peripheral blood (3–5 mL) samples were obtained before treatment and on day 2, approximately 24 h after the first dose of alvocidib. Blood samples were processed to obtain plasma and stored at −80°C prior to use for ex vivo studies. Bone marrow mononuclear cells were isolated from the aspirates with the Accuspin System-Hisopaque-1077 (Sigma-Aldrich), according to manufacturer instructions, and stored at −80°C for Western blot analysis. Only bone marrow samples with a blast count of at least 59% were assayed to avoid problems in interpretation due to cellular heterogeneity.
Vorinostat and alvocidib ex vivo studies
U937 human leukemia (myelomonocytic) cells were obtained from the American Type Culture Collection and cultured, as previously reported (33). Cells (0.2 × 106/mL) were treated with 1-μM vorinostat in the presence of 90% patient plasma obtained before or 24 h after treatment with alvocidib and stored at −80°C for Western blot analysis. See Supplementary Methods for details.
Human investigation studies
These studies were performed after Institutional Review Board approval and in accordance with an assurance filed with and approved by the Department of Health and Human Services. Informed consent was obtained from each subject.
RESULTS
Patient characteristics
A total of 14 patients, 4 male and 10 female, were enrolled for treatment with the alvocidib DSI schedule (Table 1). The median age of the patients was 63 years (range: 20–78). Thirteen patients had AML, 1 patient had ALL. The median number of prior regimens was 2.5 (range: 1–5). The patients received a median of 1.5 courses of study treatment (range: 1–4).
Table 1.
Patient enrollment and characteristics
| DSI schedule | WLMI schedule | |
|---|---|---|
| Gender (number of patients) | ||
|
| ||
| Male | 4 | 8 |
| Female | 10 | 6 |
| Total | 14 | 14 |
|
| ||
| Age (years) | ||
|
| ||
| Median | 63 | 66 |
| Range | 20–78 | 24–76 |
| ≥ 60 years old (number of patients) | 10 | 12 |
|
| ||
| Performance Status (number of patients) | ||
|
| ||
| 0 | 3 | 8 |
| 1 | 7 | 5 |
| 2 | 4 | 1 |
| Total | 14 | 14 |
|
| ||
| Diagnosis (number of patients) | ||
|
| ||
| AML | 13 | 14 |
| ALL | 1 | 0 |
| CML-BC | 0 | 0 |
| RAEB-2 | 0 | 0 |
| Total | 14 | 14 |
|
| ||
| Prior Treatment (number of regimens) | ||
|
| ||
| Median | 2.5 | 3 |
| Range | 1–5 | 1–5 |
| >2 prior regimens (no. of patients) | 4 | 8 |
|
| ||
| Study Treatment Received (number of courses) | ||
|
| ||
| Mean | 2.07 | 2.21 |
| Median | 1.5 | 1.5 |
| Range | 1–4 | 1–10 |
A total of 14 patients, 8 male and 6 female, were treated with the alvocidib WLMI schedule (Table 1). The median age of the patients was 66 years (range: 24–76). All 14 patients enrolled to the WLMI schedule had AML. The median number of prior regimens was 3 (range: 1–5). The patients received a median of 1.5 courses of study treatment (range: 1–10).
Safety and tolerability
Overall, the treatment was well tolerated, with toxicities that were transient and/or manageable, and generally as expected for an acute leukemia regimen (Table 2). Fatigue was the most common non-hematological toxicity. Hyperacute TLS was not seen but aggressive prophylaxis and monitoring were integral to the treatment plan for the WLMI schedule. There were no episodes of cardiac arrhythmias. Ten patients died while enrolled in the study and all deaths were reported as being unrelated to treatment.
Table 2.
Toxicities possibly, probably, or definitely related to study treatment occurring during any treatment course
| Nature | DSI schedule (number of patients/%)
|
WLMI schedule (number of patients/%)
|
||
|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Hematologic | ||||
| Hemoglobin | 8 (57%) | 3 (21%) | 3 (21%) | 1 (7%) |
| Leukopenia | 7 (50%) | 6 (43%) | 6 (43%) | 7 (50%) |
| Lymphopenia | 1 (7%) | 1 (7%) | 8 (57%) | 4 (29%) |
| Neutropenia | 2 (14%) | 4 (29%) | 3 (21%) | 5 (36%) |
| Thrombocytopenia | 8 (57%) | 8 (57%) | 7 (50%) | 7 (50%) |
| Non-Hematologic | ||||
|
| ||||
| Anorexia | 3 (21%) | 0 | 1 (7%) | 0 |
| Diarrhea | 1 (7%) | 0 | 3 (21%) | 0 |
| Dyspnea | 2 (14%) | 0 | 0 | 0 |
| Fatigue | 4 (29%) | 1 (7%) | 4 (29%) | 2 (14%) |
| Febrile neutropenia | 3 (21%) | 0 | 0 | 0 |
| Hyperbilirubinemia | 2 (14%) | 0 | 0 | 0 |
| Hyperglycemia | 3 (21%) | 1 (7%) | 2 (14%) | 0 |
| Hypokalemia | 2 (14%) | 1 (7%) | 4 (29%) | 1 (7%) |
| Hypophosphatemia | 4 (29%) | 0 | 2 (14%) | 1 (7%) |
| Prolonged QTc | NOT MONITORED* | NOT MONITORED* | 5 (36%)** | 0** |
QTc monitoring was not required by protocol, however, all patients underwent evaluation for risks of QTc prolongation in the context of certain CYP modulating agents in combination with vorinostat.
Revised protocol criteria for QTc eligibility and monitoring were initiated based on findings of QTc changes in one subject which, due to the known risks of QTc prolongation with CYP modulating agents in combination with vorinostat, underwent QTc monitoring at initiation of study treatment.
DLT and MTD
An MTD was not reached for the alvocidib DSI schedule before the protocol was amended to evaluate the WLMI schedule for all additional patients enrolled into the study.
Four patients were initially treated at dose level 5 (20 mg/m2 loading infusion; 20 mg/m2 maintenance infusion). No DLTs were seen in 3 of the patients, and one patient died during cycle 1 due to a fungal infection considered unrelated to treatment. A single patient was treated at dose level 6 (30 mg/m2 loading infusion; 20 mg/m2 maintenance infusion) without a DLT. This dose level was later noted to have been erroneously defined by the dose-escalation schema. This level was re-designated 6A and no further patients enrolled. Dose Level 6B was added, with the corrected dose combination of 30 mg/m2 loading infusion and 30 mg/m2 maintenance infusion. Six patients were treated on dose level 6B. Two of these patients experienced a DLT: a grade 3 cardiac arrhythmia-atrial fibrillation in one and a grade 3 QT prolongation in the other. The toxicities resolved with discontinuation of vorinostat but recurred when vorinostat was restarted. Dose level 6B was deemed to have exceeded the MTD. Consequently, dose level 5 was expanded. None of the 3 additional patients treated at dose level 5 experienced a DLT, establishing it as the MTD (Table 3).
Table 3.
Dose levels and dose limiting toxicities (DLTs)
| DSI Schedule
| |||||
|---|---|---|---|---|---|
| Dose level | Vorinostat (mg total dose) orally, TID Days 1–14 | Alvocidib loading dose | Alvocidib (mg/m2) 1-h intravenous infusion days 1–5 | Patients treated/DLT | DLT |
| 1 | 200 | ---- | 10 | 4/0 | |
| 2 | 200 | ---- | 20 | 3/0 | |
| 3 | 200 | ---- | 30 | 4/0 | |
| 4 | 200 | ---- | 40 | 3/1 | Gr 4 infection with neutropenia (sepsis >7d) |
| WLMI Schedule
| |||||
|---|---|---|---|---|---|
| Dose level | Vorinostat (mg total dose) orally, TID Days 1–14 | Alvocidib (mg/m2) 30-min intravenous loading dose days 1, 8 | Alvocidib (mg/m2) 4-h intravenous infusion days 1, 8 | Patients treated/DLT | DLT |
| 5* | 200 | 20 | 20 | 7/0 | |
| 6A† | 200 | 30 | 20 | 1/NE†† | |
| 6B** | 200 | 30 | 30 | 6/2 | Gr 3 atrial fibrillation Gr 3 QTc prolongation (intermittent, precluded vorinostat dosing for >7d) |
Defined as the MTD.
Exceeded the MTD.
Level defined by clerical error in study protocol and was aborted after 1 patient enrolled.
Patient did not complete cycle 1 of treatment so not evaluable (NE) for DLT.
Disease response
All but two treated patients were evaluable for response. One patient treated with the alvocidib WLMI schedule expired due to fungal infection prior to the disease assessment time point and the second patient treated with the DSI schedule was removed from the study due to adverse events and did not complete disease assessment. None of the 26 evaluable patients achieved a CR. Disease stabilization was evident in 6 patients treated with the alvocidib DSI schedule (median number of treatment cycles: 3; range: 2–5) and 7 patients receiving the WLMI schedule (median number of treatment cycles: 2; range: 2–10). One heavily pretreated patient (7+3, 5+2, FLAG, Mylotarg) with refractory AML remained on treatment for 11 cycles before death due to infection (Supplementary Table 1).
Pharmacokinetic studies
Pharmacokinetic parameters for both the initial dose of alvocidib and a subsequent dose given to the same patient after receiving vorinostat (200 mg orally, TID) for either 3 or 7 days were determined for a total of 18 patients. Data were obtained from 9 patients receiving the DSI schedule and 9 patients treated with the WLMI schedule. The more protracted infusion schedule resulted in a lower maximum concentration of the drug in plasma (Cmax) although the duration of time that drug levels remained near the Cmax before decay was prolonged. Mean (± SD) values of the Cmax and CL of alvocidib at each dose level evaluated in the study are presented in Supplementary Table 2. Drug accumulation upon repeated dosing was insignificant for the daily and weekly infusion schedules as the drug concentration in plasma samples obtained immediately before dosing on days 4 or 8 was <1% of the subsequent Cmax, on average. The overall mean CL of alvocidib for the initial doses in the DSI (13.0 ± 4.8 L/h/m2) and WLMI (12.2 ± 5.3 L/h/m2) schedules were not significantly different (P = 0.75). The mean CLs for the dose given on day 4 of the DSI schedule (9.9 ± 5.4 L/h/m2; P = 0.15) and day 8 of the WLMI schedule (11.0 ± 3.9 L/h/m2; P = 0.26) were not significantly different from the mean CL for the initial dose of the respective infusion schedule.
Pharmacokinetic data for vorinostat and its major circulating metabolites, VG and VA, for the initial dose of the drug were obtained from 28 patients (Supplementary Table 3). When vorinostat was administered with alvocidib using the DSI schedule, there was no change in Tmax, Cmax, and AUC0-8h for vorinostat, VA, or VG on day 1 or day 4 when alvocidib was escalated over the 10–40 mg/m2 dose range. Administration of vorinostat with alvocidib using the WLMI schedule resulted in 25–50% higher vorinostat Cmax and AUC values when compared to the DSI schedule. In contrast, the Cmax, and AUC of VG were 50% lower when given together with the 60 mg/m2 alvocidib dose compared to the 40 mg/m2 dose using the WLMI schedule.
Pharmacodynamic studies
In vivo changes in p21CIP1, Mcl-1, and p-RNA Pol II expression
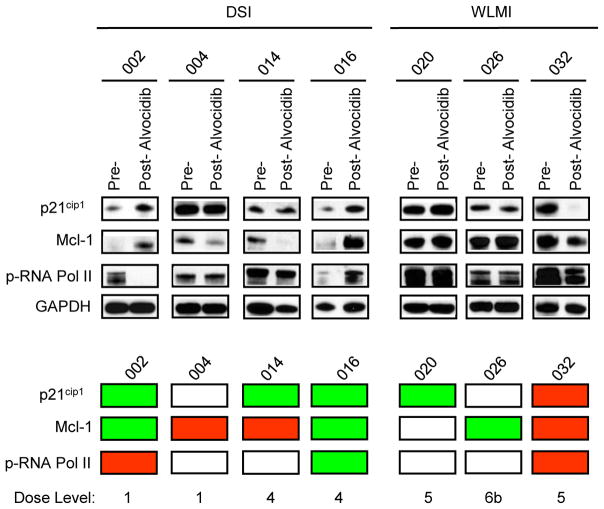
Protein levels of p21CIP1, Mcl-1, and phosphorylated RNA polymerase II (p-RNA Pol II), which have been shown to be down-regulated in leukemia cells exposed to vorinostat and alvocidib in vitro (18, 19), were monitored in bone marrow mononuclear cells from 7 patients, prior to and approximately 24 h after the first dose of alvocidib (Figure 1). Changes in the pharmacodynamic markers measured were variable and did not correlate with the DSI versus the WLMI schedules of alvocidib, with p21CIP1down-regulation observed in 1 patient (14.3%), no change in 2 patients, and up-regulation of p21CIP1 in 4 patients. For Mcl-1 protein levels, 3 samples (42.9%) exhibited down-regulation post-alvocidib, no change was observed in 1 sample, and Mcl-1 up-regulation was detected in 3 samples (Figure 1). Of note, changes in p21CIP1 and Mcl-1 were concordant in some samples (e.g., #32) but discordant in others (e.g., #14). p-RNA Pol II down-regulation post-alvocidib treatment was observed in 2 samples (28.6%), no change in 4 samples, and 1 sample displayed p-RNA Pol II up-regulation. Of note, concordance was observed between changes in p-RNA Pol II and p21CIP1/Mcl-1 expression in some samples (e.g., #32) but not others (e.g., #2). Down-regulation of all 3 assayed proteins occurred in only 1 of 7 patients (14.3%) i.e., #32 (WLMI schedule). Conversely, up-regulation of all 3 assayed proteins also occurred in 1 of 7 patients (14.3%) i.e., #16 (DSI schedule). No clear relationship between dose level and in vivo expression of these proteins could be discerned. Collectively, these findings indicate that in vivo administration of vorinostat and alvocidib results in highly variable and in some cases discordant changes in the expression of p21CIP1, Mcl-1, and p-RNA Pol II in leukemic bone marrow cells, and that in vitro perturbations observed in preclinical studies, e.g., down-regulation of these proteins (18, 19), are recapitulated in only a minority of patients.
Figure 1.
Western blot analysis of protein expression of p21CIP1, Mcl-1, and p-RNA Pol II in bone marrow samples containing ≥ 59% blasts obtained from patients, before and 24 h after alvocidib treatment. Patient samples are divided into 2 groups according to the 2 alvocidib infusion schemes (DSI and WLMI) employed in this study. GAPDH is used as an internal loading control. The panel below provides a color scheme comparing results for the pre-treatment sample to results for the post-treatment sample. Red = down-regulation, green = up-regulation, and white = no change.
Ex vivo studies
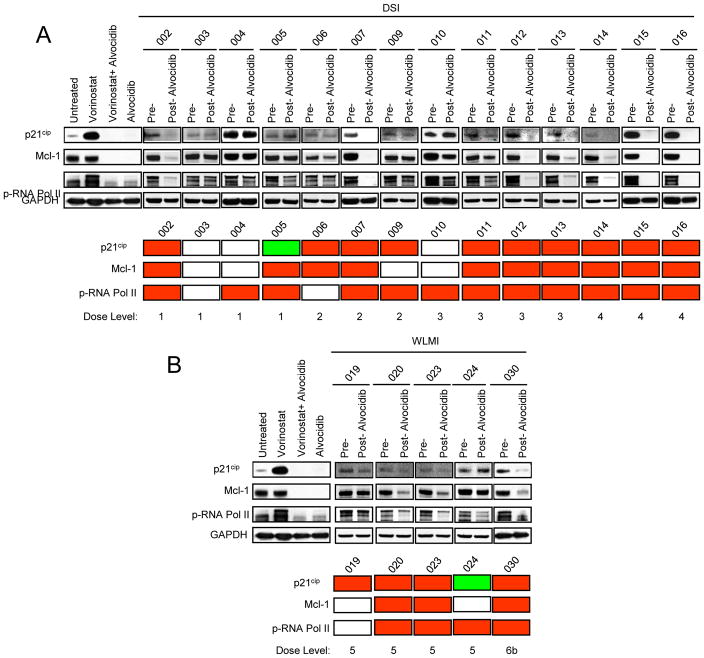
Alvocidib is highly bound to plasma proteins (34), raising the possibility that free levels of the compound achieved in the plasma of patients treated at MTDs of the drug may be insufficient to recapitulate the pharmacological effects observed in vitro (18, 19). Plasma samples from 19 patients were collected prior to treatment and approximately 24 h after the first dose of alvocidib. U937 cells were treated with 1-μM vorinostat in the presence of 90% patient pre- or post-alvocidib treatment plasma, after which effects on p21CIP1, Mcl-1, and p-RNA Pol II expression were monitored (Figure 2). Results for U937 cells treated (24 h) in vitro with 1-μM vorinostat ± 150-nM alvocidib, demonstrated virtually complete down-regulation of p21CIP1, Mcl-1, and p-RNA Pol II as previously reported (18, 19). Interestingly, p21CIP1, Mcl-1, and p-RNA Pol II expression was down-regulated by post-treatment plasma (compared to expression in the presence of pre-treatment plasma) in a subset of patients (i.e., 73.7, 68.4, and 84.2% respectively), regardless of the alvocidib schedule. In some of the samples (e.g., #16), discordance was observed with respect to the ex vivo effects of plasma on U937 cell expression of p21CIP1, Mcl-1, and p-RNA Pol II (i.e., down-regulation) and in vivo effects on bone marrow mononuclear cells (i.e., up-regulation of each of the proteins; Figure 1). The discordance between perturbations in expression of p21CIP1, Mcl-1, or p-RNA Pol II were less pronounced than that observed in post-treatment bone marrow samples. As in the case of the in vivo studies, ex vivo effects on these proteins were not clearly associated with dose level. Together, these findings suggest that in a subset of patients, plasma alvocidib concentrations can be achieved that are sufficient to mimic previously described in vitro actions (down-regulation of p21CIP1, Mcl-1, and p-RNA Pol II), (18, 19), but raise the possibility that yet to be determined factors limit the ability of alvocidib to recapitulate these actions in bone marrow leukemia cells following in vivo administration of vorinostat and alvocidib to patients.
Figure 2.
Analysis of expression of p21CIP1, Mcl-1, and p-RNA Pol II in U937 cells treated with 1-μM vorinostat in the presence of 90% patient human plasma collected before and 24 h after alvocidib treatment. Samples are divided into 2 groups according to the 2 alvocidib infusion schemes used in this study i.e., (A) DSI and (B) WLMI. As a control, results of an in vitro experiment with U937 cells treated (24 h) with vorinostat (1 μM) and/or alvocidib (150 nM) are also shown. GAPDH is used as an internal loading control. The panel below represents a color scheme comparing results for the pre-treatment sample to results for the post-treatment sample. Red = down-regulation, green = up-regulation, and white = no change.
DISCUSSION
The results of this study demonstrate that vorinostat can be safely administered in combination with alvocidib—administered according to the WLMI schedule—in patients with relapsed/refractory leukemias; whether tolerable doses will be therapeutically relevant remains to be determined. The MTD for this regimen was vorinostat at 200 mg TID and alvocidib administered as a 1-h intravenous loading infusion at a dose of 20 mg/m2 followed by a 20 mg/m2 4-h infusion on days 1 and 8 of a 21 day cycle. Fatigue was the most common non-hematologic toxicity and myelosuppression was the most common hematologic toxicity. Notably, TLS, which has been reported in high-count CLL patients receiving a WLMI alvocidib schedule (35) was not observed. Nevertheless, given the potentially lethal consequences of this syndrome, aggressive monitoring and prophylaxis appears warranted. In a recently completed phase I study of vorinostat and alvocidib in solid tumor patients, the MTD was determined to be 800 mg of vorinostat on days 1–3 and 15–17 with alvocidib given by the WLMI schedule at a dose of 30 mg/m2, delivered during the initial 30 min infusion and another 30 mg/m2 given over 4 h on days 2 and 16, with cycles repeated every 28 days (36). The principal toxicity of this regimen was myelosuppression as observed in the present study.
The concurrent oral administration of vorinostat at a dose of 200 mg TID did not appear to modify alvocidib pharmacokinetics. The CL of alvocidib, determined after patients received vorinostat for either 3 or 7 days, was in agreement with the mean values previously reported for clinical studies of single-agent alvocidib given as a daily 1-h intravenous infusion or a weekly 30-min loading and 4-h maintenance infusion (37–39). As observed previously (36), concomitant administration with alvocidib appeared to modify the pharmacokinetics of vorinostat when alvocidib was administered using the WLMI schedule, but not with the DSI schedule, and was most apparent when the combined alvocidib dose was increased to 60 mg/m2. As the increased vorinostat Cmax and AUC values were accompanied by a concomitant reduction in VG, the glucoronide metabolite of vorinostat, this interaction may be attributed to inhibition of one ormore glucuronosyl transferases that metabolize both alvocidib and vorinostat (40, 41).
Although for various reasons (e.g., limited patient numbers, treatment with different drug doses), phase I trials are not equipped to assess regimen efficacy, an attempt was made to describe clinical responses. The best response in this heavily pretreated patient population with relapsed or refractory leukemia was stable disease (Supplementary Table 1). Nevertheless, several patients with highly refractory disease were able to continue treatment with stable disease for up to 11 months. Although single-agent vorinostat has some, albeit limited, activity in AML (5), evidence of alvocidib activity in this disease, in contrast to CLL (35), is lacking. Whether alvocidib can enhance vorinostat anti-leukemic activity in vivo, as observed in vitro (15), remains to be determined, e.g., through the conduct of an appropriately powered phase II trial involving uniform drug doses and a larger number of patients. In this context, encouraging results in AML have recently been reported for a regimen in which alvocidib was combined with cytotoxic chemotherapy (mitoxantrone and ara-C (34). In light of preclinical evidence that HDACIs can increase the activity of genotoxic agents in AML (42), the concept of combining the vorinostat/alvocidib regimen with such agents warrants consideration, if justified by preclinical evidence of efficacy.
Although there were exceptions, the effects of the vorinostat/alvocidib regimen on bone marrow mononuclear cell expression of p21CIP1 (11, 12), Mcl-1 (13, 14), and p-RNA Pol II (43, 44) revealed a lack of down-regulation of these proteins in most cases (Figure 1). Although such findings are in accord with the lack of objective responses, the limited sample size precludes drawing definitive conclusions from these data. The possibility that vorinostat was unable to recapitulate its known in vitro actions appears less likely in view of previous evidence of activity against its biological targets in vivo (45), and the observation that up-regulation of p21CIP1, a major vorinostat target (46), occurred in multiple samples. However, dose-dependent effects of vorinostat cannot be excluded (18, 21). In addition, it has previously been suggested that yet to be defined plasma or microenvironmental factors may limit the impact of alvocidib on bone marrow cells in vivo (34). Additional studies will be required to distinguish between these possibilities.
An alternative explanation for these findings is that sustained plasma alvocidib concentrations were insufficient to exert anticipated biologic effects, or alternatively, plasma binding may have substantially reduced effective alvocidib concentrations, as previously observed in the case of other targeted agents (47, 48). To address this issue, U937 cells were exposed to vorinostat in the presence of pre-treatment and 24-h post-treatment plasma, after which p21CIP1, Mcl-1, and p-RNA Pol II expression were monitored. Interestingly, post-treatment plasma down-regulated these proteins in vorinostat-treated U937 cells for 68.4–84.2% of samples, but analogous changes in bone marrow bone marrow blasts following administration of alvocidib and vorinostat occurred relatively rarely (14.3–42.9%). Disparate responses were also noted in several instances in which plasma samples and bone marrow mononuclear cells from the same patients were evaluated. The discordance between these ex vivo actions and effects on bone marrow mononuclear cells may also stem from yet to be defined bone marrow microenvironmental factors (34), as alluded to above. Alternatively, they may reflect disparate responses of continuously cultured (e.g., U937) cells versus primary AML blasts. Finally, the presence of other non-CDK target proteins (40 kDa and 120 kDa) with high affinities to alvocidib in tumor cells cannot be excluded.
In summary, the present results demonstrate that a regimen combining alvocidib and vorinostat in patients with refractory AML is tolerable at pharmacologically relevant doses, although clear evidence of activity could not be demonstrated in this heavily pretreated patient population. Moreover, post-alvocidib plasma samples recapitulated the in vitro effects of exogenously administered alvocidib in a subset of specimens assayed. On the other hand, in vivo administration of alvocidib failed to down-regulate key targets (i.e., p21CIP1, Mcl-1, and p-RNA Pol II) in most patient-derived bone marrow cells, raising the possibility that this phenomenon might contribute to the minimal activity of this regimen observed in the present study. Elucidation of the factors responsible for this discordance may assist in future efforts designed to improve the anti-leukemic activity of alvocidib and possibly other CDK inhibitors. Finally, while the very limited activity observed, mainly in patients with relapsed/refractory AML, may reduce enthusiasm for successor phase II trials, it is conceivable that the anti-leukemic activity of this strategy may be improved by the addition of other agents (e.g., ara-C), or the use of newer and potentially more effective CDK inhibitors e.g., SCH727965 (49). Preclinical studies designed to test these possibilities are currently underway. There are currently no plans to proceed with a phase II clinical trial with this particular combination in patients with relapsed or refractory leukemia.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
This phase I study defines the maximum tolerated dose (MTD) of alvocidib that can be administered in combination with vorinostat in patients with relapsed, refractory, or poor prognosis acute leukemia, or refractory anemia with excess blasts-2 (RAEB-2). Several heavily pretreated patients exhibited disease stabilization although there were no objective responses. Plasma obtained from a subset of patients the day after alvocidib was administered blocked certain histone deacetylase inhibitor (HDACI)-associated actions (e.g., p21CIP1 induction) in leukemia cells that were exposed to vorinostat ex vivo. However, this and other pharmacodynamic effects were detected infrequently in bone marrow blasts obtained from patients who were treated with the 2 agents. These findings highlight the importance of identifying the factors that prevent targeted anti-leukemic agents from recapitulating their in vitro actions in bone marrow blasts following in vivo administration, and raise the possibility that circumventing this problem might improve the anti-leukemic activity of such regimens.
Acknowledgments
The authors thank Sarah Kolla, PhD for assistance with the pharmacodynamic studies and Kevin T. Hogan, PhD for editorial assistance with the manuscript. The authors would also like to acknowledge Dr Igor Delgado-Espinoza in memoriam.
GRANT SUPPORT
R21 CA115260, R01 CA93738, P30 CA016059, M01 RR00065, U01 CA69912, 23XS026, P30 CA15083, and P30 CA60516
Footnotes
Trial Registration ID: NCT00278330
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed
References
- 1.Roboz GJ, Giles FJ, List AF, Cortes JE, Carlin R, Kowalski M, et al. Phase 1 study of PTK787/ZK 222584, a small molecule tyrosine kinase receptor inhibitor, for the treatment of acute myeloid leukemia and myelodysplastic syndrome. Leukemia. 2006;20:952–7. doi: 10.1038/sj.leu.2404213. [DOI] [PubMed] [Google Scholar]
- 2.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 3.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–84. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 4.Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–15. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008;111:1060–6. doi: 10.1182/blood-2007-06-098061. [DOI] [PubMed] [Google Scholar]
- 6.Senderowicz AM. Flavopiridol: the first cyclin-dependent kinase inhibitor in human clinical trials. Invest New Drugs. 1999;17:313–20. doi: 10.1023/a:1006353008903. [DOI] [PubMed] [Google Scholar]
- 7.Carlson B, Lahusen T, Singh S, Loaiza-Perez A, Worland PJ, Pestell R, et al. Down-regulation of cyclin D1 by transcriptional repression in MCF-7 human breast carcinoma cells induced by flavopiridol. Cancer Res. 1999;59:4634–41. [PubMed] [Google Scholar]
- 8.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;276:31793–9. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 9.Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996;56:2973–8. [PubMed] [Google Scholar]
- 10.Parker BW, Kaur G, Nieves-Neira W, Taimi M, Kohlhagen G, Shimizu T, et al. Early induction of apoptosis in hematopoietic cell lines after exposure to flavopiridol. Blood. 1998;91:458–65. [PubMed] [Google Scholar]
- 11.Cartee L, Wang Z, Decker RH, Chellappan SP, Fusaro G, Hirsch KG, et al. The cyclin-dependent kinase inhibitor (CDKI) flavopiridol disrupts phorbol 12-myristate 13-acetate-induced differentiation and CDKI expression while enhancing apoptosis in human myeloid leukemia cells. Cancer Res. 2001;61:2583–91. [PubMed] [Google Scholar]
- 12.Rosato RR, Almenara JA, Cartee L, Betts V, Chellappan SP, Grant S. The cyclin-dependent kinase inhibitor flavopiridol disrupts sodium butyrate-induced p21WAF1/CIP1 expression and maturation while reciprocally potentiating apoptosis in human leukemia cells. Mol Cancer Ther. 2002;1:253–66. [PubMed] [Google Scholar]
- 13.Ma Y, Cress WD, Haura EB. Flavopiridol-induced apoptosis is mediated through up-regulation of E2F1 and repression of Mcl-1. Mol Cancer Ther. 2003;2:73–81. [PubMed] [Google Scholar]
- 14.Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8:3527–38. [PubMed] [Google Scholar]
- 15.Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000;96:393–7. [PubMed] [Google Scholar]
- 16.Decker RH, Dai Y, Grant S. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in human leukemia cells (U937) through the mitochondrial rather than the receptor-mediated pathway. Cell Death Differ. 2001;8:715–24. doi: 10.1038/sj.cdd.4400868. [DOI] [PubMed] [Google Scholar]
- 17.Motwani M, Delohery TM, Schwartz GK. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin Cancer Res. 1999;5:1876–83. [PubMed] [Google Scholar]
- 18.Almenara J, Rosato R, Grant S. Synergistic induction of mitochondrial damage and apoptosis in human leukemia cells by flavopiridol and the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) Leukemia. 2002;16:1331–43. doi: 10.1038/sj.leu.2402535. [DOI] [PubMed] [Google Scholar]
- 19.Rosato RR, Almenara JA, Yu C, Grant S. Evidence of a functional role for p21WAF1/CIP1 down-regulation in synergistic antileukemic interactions between the histone deacetylase inhibitor sodium butyrate and flavopiridol. Mol Pharmacol. 2004;65:571–81. doi: 10.1124/mol.65.3.571. [DOI] [PubMed] [Google Scholar]
- 20.Gao N, Dai Y, Rahmani M, Dent P, Grant S. Contribution of disruption of the nuclear factor-kappaB pathway to induction of apoptosis in human leukemia cells by histone deacetylase inhibitors and flavopiridol. Mol Pharmacol. 2004;66:956–63. doi: 10.1124/mol.104.002014. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen DM, Schrump WD, Chen GA, Tsai W, Nguyen P, Trepel JB, et al. Abrogation of p21 expression by flavopiridol enhances depsipeptide-mediated apoptosis in malignant pleural mesothelioma cells. Clin Cancer Res. 2004;10:1813–25. doi: 10.1158/1078-0432.ccr-0901-3. [DOI] [PubMed] [Google Scholar]
- 22.Rosato RR, Almenara JA, Kolla SS, Maggio SC, Coe S, Gimenez MS, et al. Mechanism and functional role of XIAP and Mcl-1 down-regulation in flavopiridol/vorinostat antileukemic interactions. Mol Cancer Ther. 2007;6:692–702. doi: 10.1158/1535-7163.MCT-06-0562. [DOI] [PubMed] [Google Scholar]
- 23.Lin TS, Ruppert AS, Johnson AJ, Fischer B, Heerema NA, Andritsos LA, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–8. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96:3671–4. [PubMed] [Google Scholar]
- 25.Cheson BD, Cassileth PA, Head DR, Schiffer CA, Bennett JM, Bloomfield CD, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–9. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- 26.Schnier JB, Kaur G, Kaiser A, Stinson SF, Sausville EA, Gardner J, et al. Identification of cytosolic aldehyde dehydrogenase 1 from non-small cell lung carcinomas as a flavopiridol-binding protein. FEBS Lett. 1999;454:100–4. doi: 10.1016/s0014-5793(99)00773-5. [DOI] [PubMed] [Google Scholar]
- 27.Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, et al. Bioanalytical method validation--a revisit with a decade of progress. Pharm Res. 2000;17:1551–7. doi: 10.1023/a:1007669411738. [DOI] [PubMed] [Google Scholar]
- 28.Galanis E, Jaeckle KA, Maurer MJ, Reid JM, Ames MM, Hardwick JS, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27:2052–8. doi: 10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parise RA, Holleran JL, Beumer JH, Ramalingam S, Egorin MJ. A liquid chromatography-electrospray ionization tandem mass spectrometric assay for quantitation of the histone deacetylase inhibitor, vorinostat (suberoylanilide hydroxamicacid, SAHA), and its metabolites in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;840:108–15. doi: 10.1016/j.jchromb.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 30.Gabrielsson J, Weiner D. Pharmacokinetic--pharmacodynamic data analysis: concepts and applications. 2. Stockholm, Sweden: Apotekarsocieteten; 1997. [Google Scholar]
- 31.Lacey LF, Keene ON, Pritchard JF, Bye A. Common noncompartmental pharmacokinetic variables: are they normally or log-normally distributed? J Biopharm Stat. 1997;7:171–8. doi: 10.1080/10543409708835177. [DOI] [PubMed] [Google Scholar]
- 32.Miller RG. Jackknife - Review. Biometrika. 1974;61:1–15. [Google Scholar]
- 33.Rahmani M, Yu C, Reese E, Ahmed W, Hirsch K, Dent P, et al. Inhibition of PI-3 kinase sensitizes human leukemic cells to histone deacetylase inhibitor-mediated apoptosis through p44/42 MAP kinase inactivation and abrogation of p21(CIP1/WAF1) induction rather than AKT inhibition. Oncogene. 2003;22:6231–42. doi: 10.1038/sj.onc.1206646. [DOI] [PubMed] [Google Scholar]
- 34.Karp JE, Smith BD, Resar LS, Greer JM, Blackford A, Zhao M, et al. Phase 1 and pharmacokinetic study of bolus-infusion flavopiridol followed by cytosine arabinoside and mitoxantrone for acute leukemias. Blood. 2011;117:3302–10. doi: 10.1182/blood-2010-09-310862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickson MA, Rathkopf DE, Carvajal RD, Grant S, Roberts JD, Reid JM, et al. A phase I pharmacokinetic study of pulse-dose vorinostat with flavopiridol in solid tumors. Invest New Drugs. 2011;29:1004–12. doi: 10.1007/s10637-010-9447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan AR, Headlee D, Messmann R, Sausville EA, Arbuck SG, Murgo AJ, et al. Phase I clinical and pharmacokinetic study of flavopiridol administered as a daily 1-hour infusion in patients with advanced neoplasms. J Clin Oncol. 2002;20:4074–82. doi: 10.1200/JCO.2002.01.043. [DOI] [PubMed] [Google Scholar]
- 38.Zhai S, Sausville EA, Senderowicz AM, Ando Y, Headlee D, Messmann RA, et al. Clinical pharmacology and pharmacogenetics of flavopiridol 1-h i.v. infusion in patients with refractory neoplasms. Anticancer Drugs. 2003;14:125–35. doi: 10.1097/00001813-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Phelps MA, Lin TS, Johnson AJ, Hurh E, Rozewski DM, Farley KL, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–45. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balliet RM, Chen G, Gallagher CJ, Dellinger RW, Sun D, Lazarus P. Characterization of UGTs active against SAHA and association between SAHA glucuronidation activity phenotype with UGT genotype. Cancer Res. 2009;69:2981–9. doi: 10.1158/0008-5472.CAN-08-4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramirez J, Iyer L, Journault K, Belanger P, Innocenti F, Ratain MJ, et al. In vitro characterization of hepatic flavopiridol metabolism using human liver microsomes and recombinant UGT enzymes. Pharm Res. 2002;19:588–94. doi: 10.1023/a:1015341726183. [DOI] [PubMed] [Google Scholar]
- 42.Shiozawa K, Nakanishi T, Tan M, Fang HB, Wang WC, Edelman MJ, et al. Preclinical studies of vorinostat (suberoylanilide hydroxamic acid) combined with cytosine arabinoside and etoposide for treatment of acute leukemias. Clin Cancer Res. 2009;15:1698–707. doi: 10.1158/1078-0432.CCR-08-1587. [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Keating MJ, Gandhi V, Plunkett W. Transcription inhibition by flavopiridol: mechanism of chronic lymphocytic leukemia cell death. Blood. 2005;106:2513–9. doi: 10.1182/blood-2005-04-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S, Fischer PM. Cyclin-dependent kinase 9: a key transcriptional regulator and potential drug target in oncology, virology and cardiology. Trends Pharmacol Sci. 2008;29:302–13. doi: 10.1016/j.tips.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Kelly WK, Richon VM, O’Connor O, Curley T, MacGregor-Curtelli B, Tong W, et al. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–88. [PubMed] [Google Scholar]
- 46.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A. 2000;97:10014–9. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sausville EA. Dragons ‘round the fleece again: STI571 versus alpha1 acid glycoprotein. J Natl Cancer Inst. 2000;92:1626–7. doi: 10.1093/jnci/92.20.1626. [DOI] [PubMed] [Google Scholar]
- 48.Vogler M, Furdas SD, Jung M, Kuwana T, Dyer MJ, Cohen GM. Diminished sensitivity of chronic lymphocytic leukemia cells to ABT-737 and ABT-263 due to albumin binding in blood. Clin Cancer Res. 2010;16:4217–25. doi: 10.1158/1078-0432.CCR-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2010;9:2344–53. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- 50.Badros A, Burger AM, Philip S, Niesvizky R, Kolla SS, Goloubeva O, et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009;15:5250–7. doi: 10.1158/1078-0432.CCR-08-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.