Abstract
Nicotine, a large constituent of cigarette smoke, is associated with an increased risk of lung cancer, but the data supporting this relationship are inconsistent. Here, we found that nicotine treatment not only induced emphysema but also increased both lung tumor multiplicity and volume in 4-nitrosamino-1-(3-pyridyl)-1-butanone (NNK)-initiated lung cancer in A/J mice. This tumor-promoting effect of nicotine was accompanied by significant reductions in survival probability and lung Sirtuin 1 (SIRT1) expression, which has been proposed as a tumor suppressor. The decreased level of SIRT1 was associated with increased levels of AKT phosphorylation and interleukin (il)-6 mRNA but decreased tumor suppressor p53 and retinoic acid receptor (RAR)-β mRNA levels in the lungs. Using this mouse model, we then determined whether β-cryptoxanthin (BCX), a xanthophyll that is strongly associated with a reduced risk of lung cancer in several cohort studies, can inhibit nicotine-induced emphysema and lung tumorigenesis. We found that BCX supplementation at two different doses was associated with reductions of the nicotine-promoted lung tumor multiplicity and volume, as well as emphysema in mice treated with both NNK and nicotine. Moreover, BCX supplementation restored the nicotine-suppressed expression of lung SIRT1, p53, and RAR-β to that of the control group, increased survival probability; and decreased the levels of lung il-6 mRNA and phosphorylation of AKT. The present study indicates that BCX is a preventive agent against emphysema and lung cancer with SIRT1 as a potential target. In addition, our study establishes a relevant animal lung cancer model for studying tumor growth within emphysematous microenvironments.
Keywords: nicotine, β-cryptoxanthin, NNK, lung cancer, emphysema, SIRT1
Introduction
Despite anti-smoking campaigns and efforts toward prevention and treatment, lung cancer accounts for 14% of all new cancers. Approximately 80-90% of lung cancers occur in smokers (1). Smoking is the leading cause of chronic obstructive pulmonary disease (COPD), which exists in two forms: chronic bronchitis and emphysema (2). Emphysema, a permanent enlargement of the lung airspaces that is accompanied by alveolar wall destruction, is associated with a 3- to 4-fold increased lung cancer risk (3).
More than 60 carcinogens present in cigarette smoke are accountable for lung carcinogenesis (1, 4). Nicotine, a non-carcinogenic constituent of tobacco and cigarette-smoke, is responsible for smoking addiction (5). Smokers who switch from smoking to using smokeless-tobacco-products containing nicotine, have a higher risk of lung cancer than those who stopped smoking completely (5). Previous animal studies demonstrated that intraperitoneal (i.p.) and oral administrations of nicotine promotes lung tumorigenesis in female A/J mice (6) and in the mouse Lewis lung tumor models (7), respectively. However, two studies reported that nicotine in drinking water does not promote lung tumorigenesis in female A/J mice (8) or the AB6F1 mouse models (9). The discrepancy may be due to strain variability and gender as well as dosage, metabolism, and route of administration of nicotine. Although emphysema develops in the offspring of rats exposed to nicotine during gestation and lactation (10), no study had addressed lung tumor development within emphysematous microenvironments.
Sirtuin 1 (SIRT1), the mammalian ortholog of yeast sir2, is a NAD+-dependent deacetylase that has been implicated in various biological processes, such as metabolism, inflammation, immune function, and apoptosis (11). SIRT1 displaces acetyl groups from histones and proteins (e.g., forkhead box class O, nuclear factor κB, p300, and the DNA repair factors Ku70, among others) (11). Smoke exposure induces emphysema in heterozygous sirt1+/− mice (12). Moreover, SIRT1 levels are reduced in smokers and COPD patients (13). Although modulating SIRT1 with activators is an attractive therapeutic strategy in COPD/emphysema (12), SIRT1 roles have not been explored in lung tumorigenesis, particularly during the promotion and progression phases. SIRT1 was originally identified as a tumor promoter (11). However, studies using genetically modified sirt1 mice have consistently supported that a tumor suppressive role of SIRT1 in a wide range of cancers (11).
No dietary components have been shown to prevent the progression of emphysema and lung cancer. The beneficial effect of carotenoid-rich fruits and vegetables against lung cancer risk has been reported in epidemiological studies. Most research has focused on the pro-vitamin A carotenoid β-carotene because of the association between high levels of both vitamin A and β-carotene, and lower cancer risk. Nevertheless, intervention studies using β-carotene have led to disappointing results (14). Recently, β-cryptoxanthin (BCX), an oxygenated carotenoid (xanthophyll) with pro-vitamin A activity found at high levels in citrus fruits, pumpkins, red peppers, and papayas, was identified to be the only carotenoid for which intake was inversely associated with lung cancer risk in a pooled analysis of seven prospective cohort studies (15). This intake of BCX produced a protective association among current smokers but not among past smokers or never smokers (15). Furthermore, a meta-analysis reported that BCX intake is associated with a lower lung cancer risk in current smokers (16). We previously reported that BCX treatment reduces the growth of immortalized bronchial epithelial and non-small cell lung cancer cells, induces retinoic acid receptor (RAR)-β levels, and increases the transactivation activity of the retinoic acid-response element-dependent RAR-β promoter (17). Recently, we demonstrated that BCX supplementation reduced cigarette-smoke-induced lung inflammation and pre-cancerous lesions (e.g., squamous metaplasia), as well as suppressed cigarette-smoke-activated nuclear factor κB and tumor necrosis factor-α protein expression in ferrets (18). However, this ferret study could not address the efficacy of BCX supplementation on lung tumor formation and promotion. Furthermore, delineating the potential mechanism(s) of BCX against lung tumorigenesis is needed.
In the present study, we first investigated whether nicotine exposure decreased lung SIRT1 expression and promoted lung tumorigenesis. Further, we determined the effects of BCX supplementation on this nicotine-promoted tumorigenesis and emphysema.
Materials and Methods
Animals and experimental groups
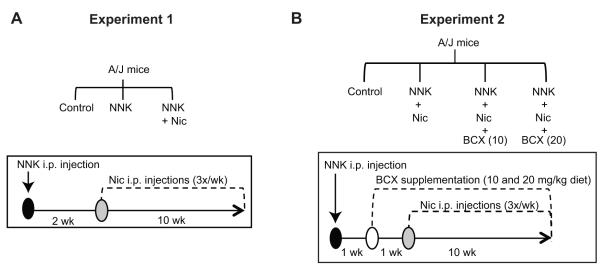
Male A/J mice (5-6 weeks-old) were purchased from the Jackson Laboratory. To investigate whether nicotine promoted both emphysema and lung tumorigenesis, we conducted Experiment 1 (Fig. 1A): Mice were divided into 3 groups with 16 mice per group: (i) the control group received sham injections, (ii) the NNK group received a single intraperitoneal (i.p.) NNK injection (100 mg/kg body weight [bw]), and (iii) the NNK + Nic group received a single i.p. NNK injection (100 mg/kg bw) and i.p. nicotine injections (1 mg/kg bw) three times weekly for 10 weeks. No statistically significant differences were detected among the means body weight of all groups at the beginning of the study (Supplementary Table 1).
Figure 1. Study design and experiment schemes.
The study design (top) and experiment scheme (bottom) of Experiment 1, which tested the effects of nicotine on promoting lung tumor and emphysema development (A), and Experiment 2, which tested the effect of BCX supplementation on the promotion of lung tumor and emphysema development (B) are shown. Abbreviations: BCX (10): β-cryptoxanthin at 10 mg/kg diet; BCX (20): β-cryptoxanthin at 20 mg/kg diet; C: control; i.p.: intraperitoneal; Nic: nicotine; NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; wk: week(s).
To determine whether BCX protects against nicotine-promoted emphysema and lung tumorigenesis, we performed Experiment 2 (Fig. 1B): Mice were divided into 4 groups with 16 mice per group: (i) the control group received sham injections, (ii) the NNK + Nic group received a single i.p. NNK injection (100 mg/kg bw) and i.p. nicotine injections (1 mg/kg bw) three times weekly for 10 weeks, (iii) NNK + Nic + BCX (10) group received a single i.p. NNK injection (100 mg/kg bw), i.p. nicotine injections (1 mg/kg bw) three times weekly for 10 weeks, and a diet supplemented with BCX (10 mg/kg diet), and (iv) NNK + Nic + BCX (20) group received a single i.p. NNK injection (100 mg/kg bw), i.p. nicotine injections (1 mg/kg bw) three times weekly for 10 weeks, and a diet supplemented with BCX (20 mg/kg diet).
The body weights of the mice were recorded weekly. After the experimental periods, the mice were terminally exsanguinated under deep isoflurane anesthesia. The studies were conducted with the approval of the Animal Care and Use Committee at the Human Nutrition Research Center on Aging at Tufts University.
Carcinogen and nicotine treatment
The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (>98% purity, Toronto Research Chemicals) was injected i.p. (100 mg/kg bw) once into mice to induce lung tumors, as previously described (1). Nicotine (N3876) (Sigma-Aldrich) was injected i.p. into mice three times weekly at 1 mg/kg bw (Fig 1A and 1B). This dose of nicotine (a weekly dose of 3 mg/kg bw) corresponds to a daily dose of 0.428 ≈ 0.43 mg/kg bw. Because a 60-kg person absorbs approximately 1 mg of nicotine (0.017 mg nicotine/kg bw) from smoking one cigarette (19), the dose of nicotine in this present animal study was equivalent to smoking 25 cigarettes per day (0.017 mg nicotine/kg bw x 25 cigarettes = 0.425 ≈ 0.43 mg nicotine/kg bw) in humans, which is the mean level of cigarette consumption in the U.S. (20).
β-Cryptoxanthin treatment
β-Cryptoxanthin (BCX) (> 99% purity, BASF) was in powder form and directly mixed with AIN-93M semi-purified diet powder (Dyets Inc.) at concentrations of 10 and 20 mg/kg diet (equivalent to 2 and 4 mg/kg bw daily, respectively, for a 25-g mouse that consumes 5 g daily). The absorption of carotenoids in rodents is approximately 1/7th that in humans (a daily supplementation of 20 mg beta-carotene resulted in serum concentrations of β-carotene of 3000 μg/L in humans (21) and of ~416 μg/L [range, 134-698 μg/L] in mice (22, 23)). Moreover, we found that a daily supplementation of 10 mg BCX/kg diet resulted in a serum concentration of BCX of 9.9 μg/L in A/J mice (unpublished data) and the absorption of BCX in humans is approximately 8% of what is consumed (a diet consumption of 1300 μg BCX resulted in a serum concentration of 113 μg/L) (24). Therefore, the low dose of BCX (10 mg/kg diet) in this mouse study was equivalent to daily human consumption of 0.87 mg (9.9 μg/L x 7 x [100% / 8%] = 866.25 μg = 0.87 mg) and the high dose of BCX (20 mg/kg diet) in this study was equivalent to daily human consumption of approximately 1.74 mg of BCX. These doses can be obtained from dietary citrus fruits, such as by consuming 3-5 raw tangerines daily (25); thus, the doses of BCX supplementation used here are within the physiological range.
Quantification of lung lesions
Lung tumor lesions were quantified by determining the incidence and multiplicity of the pulmonary surface tumors on the day of euthanasia as previously described (26). Lung tumor multiplicity was used as an indicator of carcinogenicity as recommended (27). The diameter of each tumor was calculated using a caliper. The volume of each lung surface tumor was calculated based on the assumption that the tumor was spherical (volume = 4/3πr3, where r = diameter/2) (28). The upper right lungs were perfused with 10% buffered formaldehyde (Fisher Scientific) using standardized protocol by researcher blinded to the treatment group as previously described (26, 29). After the fixation for 24 hours, the lung tissues were transferred to 70% ethanol for a routine histological processing. Hematoxylin and eosin (H&E)-stained lung sections were microscopically examined to confirm the presence of lung tumors and emphysema that was based on the histological pattern of enlarged alveolar spaces with club-shaped septa distal to the terminal bronchioles in the H&E-stained lung sections. As previously suggested, a morphological evaluation should be conducted for biological studies on the pathogenesis of emphysema (30). These pathological evaluations on lung tumors and emphysema were conducted by researchers blinded to the treatment groups.
Quantification of emphysema
The degree of emphysema was assessed under the microscope by comparing the airspace enlargement (alveolar space area, mean linear intercept (Lm)) of the H&E stained lung sections in a blinded manner. The Lm was measured by placing a 0.5 × 0.5 μ-pixel grid over each field (10 field/mice). The Lm is calculated by dividing the total length of each line of the grid by the number of alveolar intercepts (31).
Protein isolation and Western blotting
Whole-cell lysates of lung tissues were prepared as previously described (26). The whole-cell lysate samples were collected and either kept at −80°C or used for Western blotting as previously described (26). The following antibodies were used for Western blotting: SIRT1, cyclin D1, and p50 (Santa Cruz Biotechnology, Inc.); phosphorylated AKT (Ser473) and total AKT (Cell Signaling Technology); and GAPDH (Millipore). All of the antibodies were used according to the manufacturers’ protocols.
RNA extraction and quantitative real-time PCR (qRT-PCR)
An RNeasy kit (Qiagen) was used to extract RNA according to the manufacturer’s protocol and as previously described (26). cDNA was prepared from the RNA samples using M-MLV reverse transcriptase (Invitrogen) and an automated thermal cycler PTC-200 (MJ Research). Real-time PCR was performed using FastStart Universal SYBR Green Master (ROX) (Roche). The relative gene expression was determined using the 2−ΔΔCT method. Primer sequences are listed in Supplementary Table 2.
High-performance liquid chromatography (HPLC)
The livers samples for HPLC analysis were prepared as previously described (32). A gradient reverse-phase HPLC system consisting of a Waters 2695 separation module and a Waters 2998 photodiode array detector was used for the detection of BCX, retinol, and retinyl palmitate. Briefly, BCX, retinol, and retinyl palmitate were analyzed on a reverse-phase C18 column (4.6 × 250 mm, 5 μM) (Vydac 201TP54, Grace Discovery Sciences, Inc.) with a flow rate of 1.00 mL/min, and quantified relative to an internal standard by determining the peak areas against known amounts of standards.
Statistical analyses
The mean values for each group were compared using a t-test or a one-way ANOVA with Tukey’s Honestly Significant Difference (HSD) post-hoc procedure applied for comparisons across multiple groups. The Statistical Analysis System (SAS® version 9.2) PROC LIFETEST, a nonparametric procedure for estimating survival rates, was used for the survival analysis. Kaplan-Meier survival curves were constructed to compare the survival of mice across the treatment groups using the log-rank test. All analyses were performed using SAS®. All measurements are expressed as the mean ± the standard error of the mean (sem) or as otherwise indicated. Differences were considered significant if p < 0.05.
Results
Nicotine decreases survival probability but not body weight
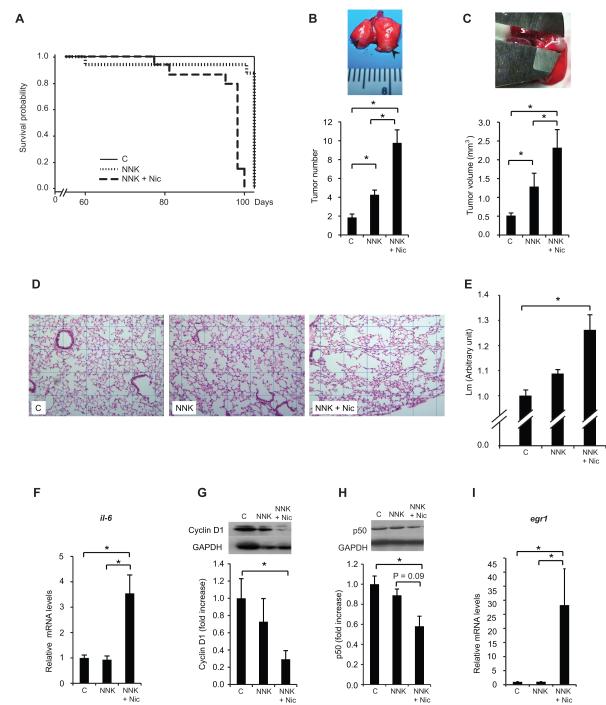
At the time of sacrifice, the mean body weight of the NNK group was lower than the control group (Supplementary Table 1). The mean body weight of the NNK + Nic group was not different than that of the NNK group. The NNK + Nic group exhibited decreased survival probability compared to the control and NNK groups (NNK + Nic group compared to control or NNK group [p < 0.01]; log-rank test). The NNK treatment alone did not alter the survival probability as compared to the control group (p = 0.94, log-rank test) (Fig. 2A).
Figure 2. Nicotine decreases survival probability, lung tumor multiplicity and size, and emphysema development.
The survival curves for the three treatment groups in Experiment 1 were analyzed using the SAS® PROC LIFETEST. The results of the log-rank test for the control, NNK, and NNK + Nic groups (p < 0.01) are shown in (A). The quantification of the lung tumor multiplicity is shown in (B). Inset: lung surface tumors (arrows). The number of lung surface tumors and the quantification of the lung tumor volume (mm3) are shown in (C). Inset: the measurement of the lung tumor diameter with a caliper. Representative images of the hematoxylin and eosin (H&E)-stained slides (grid: 0.5 × 0.5 μ-pixels) are shown in (D).The degree of emphysema was quantified by calculating the mean linear intercept (Lm) using 10 fields per animal (E). The lung il-6 mRNA levels are shown in (F). The lung cyclin D1 protein levels are shown in (G). Inset: a representative image of the cyclin D1 and GAPDH proteins. The lung p50 protein levels are shown in (H). Inset: a representative image of the p50 and GAPDH proteins. The lung egr1 mRNA levels are shown in (I). The proteins were detected using Western blotting. The intensity of the bands was quantified densitometry and was normalized to GAPDH. The mRNA levels were measured by qRT-PCR and normalized to β-actin mRNA levels. The columns represent the mean ± sem. *p < 0.05. Abbreviations: C: control; Nic: nicotine; NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; il-6: interleukin-6; egr1: early growth response 1.
Nicotine promotes NNK-induced lung tumor multiplicity and volume
The mean multiplicity of spontaneous tumors (due to Kras mutations (33)) in the control group was 1.86 ± 0.36 (Fig. 2B). The mean lung tumor multiplicity of the NNK group (4.3 ± 0.5) was twice as that of the control group (p < 0.01) (Fig 2B). The lung tumor volume of the NNK group (1.29 mm3 ± 0.07) was 2.5-fold higher than that of the control group (0.52 mm3 ± 0.35) (Fig. 2C). Moreover, the mean lung tumor multiplicity of the NNK + Nic group (9.7 ± 1.7) was 2.5-fold greater than the NNK group (p < 0.01) (Fig. 2B), and the lung tumor volume of the NNK + Nic group (2.32 mm3 ± 0.48) was 79% larger than the NNK group (p < 0.01) (Fig 2C).
Nicotine induces emphysema development
The incidence of emphysema in the NNK + Nic group (90%) was greater than that in the control and NNK groups (p < 0.01; Fisher’s exact test). The Lm of the NNK + Nic group was greater than the NNK group and the control group (p < 0.01) (Fig. 2D). The Lm for the NNK group did not differ from that of the control group (p = 0.13) (Fig. 2D).
The mRNA level of lung interleukin 6 (il-6) mRNA in the NNK + Nic group was 3.5-fold greater than that in the NNK group (p < 0.01) (Fig. 2E). The il-6 mRNA level in the NNK group was not different from the control group (p > 0.05).
The level of cyclin D1 in the NNK + Nic group was decreased, by 30%, as compared to the control group (p < 0.05) (Fig. 2F). NNK treatment was not associated with a decreased cyclin D1 protein levels (NNK group compared to control group [p = 0.65]).
We observed a decreased p50 protein in the NNK + Nic group compared to control (Fig. 2G). However, this decrease was not statistically significant compared to the NNK group (p = 0.09) (Fig. 2G).
There was a 28-fold increase in the egr1 mRNA level in the NNK + Nic group compared to the NNK or control group (Fig. 2H). Treatment of NNK did not affect the p50 protein and egr1 mRNA levels (NNK group compared to control group [p > 0.05]) (Fig. 2G and 2H).
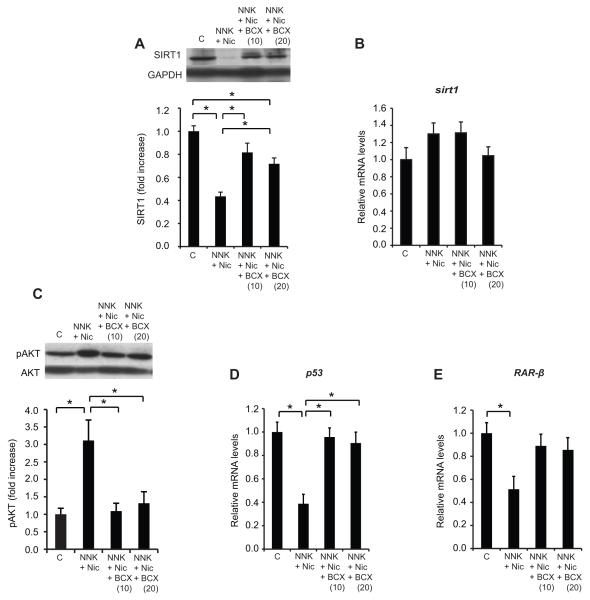
Nicotine decreases the SIRT1 protein levels in the lungs
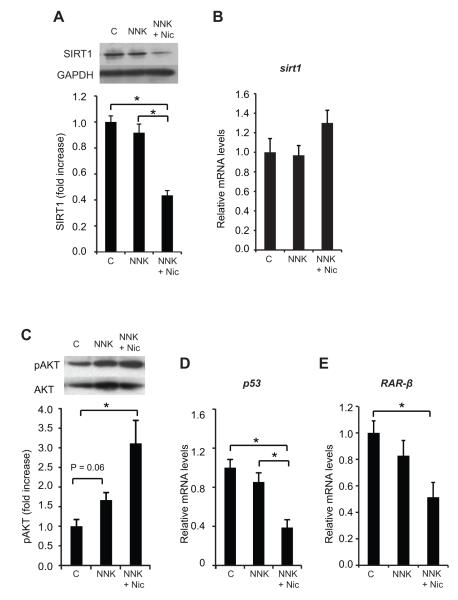
The lung levels of the SIRT1 protein in the NNK + Nic group was decreased, by 52%, compared to the NNK group (p < 0.01), and by 56%, compared to the control group (p < 0.01) (Fig.3A). The SIRT1 protein level in the NNK group did not differ from the control group (p > 0.05) (Fig. 3A). Sirt1 mRNA levels in the lungs were similar across all groups (Fig. 3B).
Figure 3. Nicotine decreases the level of SIRT1 protein and the transcription of tumor suppressor genes, and increases AKT phosphorylation.
The lung SIRT1 protein levels are shown in (A). Inset: a representative image of lung SIRT1 and GAPDH proteins. The lung sirt1 mRNA levels are shown in (B). The quantification of AKT phosphorylation on Ser473 is shown in (C). Inset: a representative image of phosphorylated AKT (pAKT) and total AKT. The mRNA levels of the p53 and RAR-β are shown in (D) and (E), respectively. The proteins were detected using Western blotting. The intensity of the bands was quantified using densitometry and normalized to GAPDH or total AKT. The mRNA levels were measured with qRT-PCR and normalized to β-actin mRNA levels. The columns represent the mean ± sem. *p < 0.05. Abbreviations: C: control; Nic: nicotine; NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; RAR-β: retinoic acid receptor-β, SIRT1: sirtuin 1.
Nicotine enhances AKT phosphorylation and decreases the mRNA levels of both p53 and retinoic acid receptor (RAR)-β
There was an increase in the lung AKT phosphorylation level in the NNK + Nic group compared to the control group (p < 0.01) (Fig. 3C). The lung mRNA level of p53 was reduced 61% in the NNK + Nic group as compared to the control and NNK groups (p < 0.01) (Fig. 3D). NNK treatment did not alter the lung p53 mRNA levels compared to the control group (Fig. 2E). A decrease (49%) in the lung RAR-β mRNA levels was observed in the NNK + Nic group compared to the control group (p < 0.01) (Fig. 3E). Nonetheless, the lung RAR-β mRNA levels in the NNK group did not differ from the control group (Fig. 3E).
BCX supplementation maintains body weights, increases survival probability and BCX accumulation in the liver
At the time of sacrifice, the means body weight of the BCX-supplemented groups were not different than the control group (Supplementary Table 1). Due to limited lung samples, we examined the livers for BCX accumulation. We did not detect BCX in the livers of mice without BCX supplementation (Table 1). BCX supplementation at 10 and 20 mg/kg diet dose-dependently increased the concentration of liver BCX (p < 0.01) (Table 1). We observed no significant differences in the hepatic retinol concentration across the groups (Table 1). However, the retinyl ester concentration was increased in the livers of mice supplemented with the high dose of BCX as compared to the control group (Table 1).
Table 1.
Concentrations of β-cryptoxanthin (BCX) and retinoids in the liver after 11 weeks of BCX supplementation
| Liver (nmol/g) |
|||
|---|---|---|---|
| Treatment group | BCX | Retinol | Retinyl palmitate |
| Control | ND | 12.51 ± 1.28 | 1240 ± 46 |
| NNK + Nic | ND | 14.20 ± 2.19 | 1457 ± 86 |
| NNK + Nic + BCX (10 mg/kg diet) | 0.25 ± 0.03 | 13.45 ± 1.24 | 1626 ± 47 |
| NNK + Nic + BCX (20 mg/kg diet) | 0.51 ± 0.04* | 18.95 ± 1.56† | 2017 ± 141†‡ |
Values are expressed as the mean ± sem; n= 12-14. The statistical analyses consisted of an ANOVA followed by post-hoc testing using Tukey’s HSD, unless otherwise indicated.
p < 0.05 compared to NNK+ Nic + BCX (10 mg/kg diet) using Student’s t-test.
p < 0.05 compared to the control.
p < 0.05 compared to NNK + Nic.
Abbreviations: BCX: β-cryptoxanthin; ND: not detected; Nic: nicotine; NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; sem: standard error of the mean.
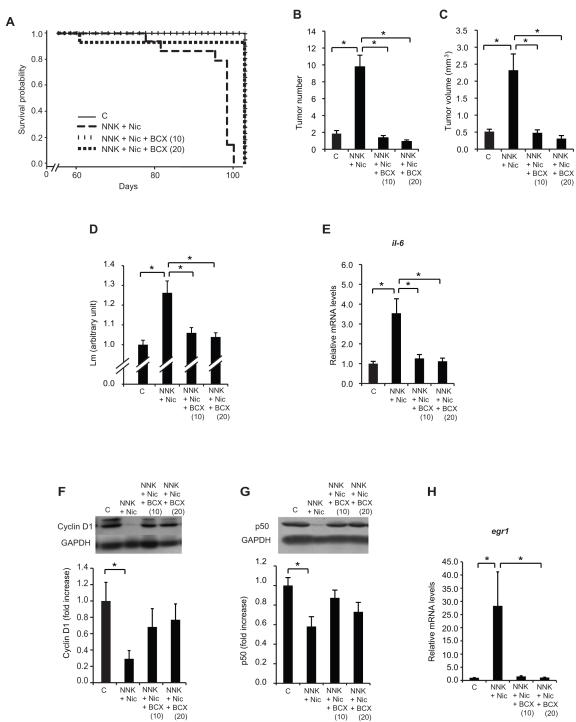
Both doses of BCX maintained the survival probability at the level of the control group (Fig. 4A) (NNK + Nic + BCX (10) compared to control group [p = 0.99] and NNK + Nic + BCX (20) compared to control group [p = 0.99]; log-rank tests). The two doses of BCX supplementation increased the survival probability of the mice compared to the NNK + Nic group (Fig. 4A) (NNK + Nic + BCX (10) group compared to NNK + Nic group [p < 0.01], and NNK + Nic + BCX (20) group compared to NNK + Nic group [p < 0.01]; log-rank tests).
Figure 4. BCX supplementation maintains survival probability, decreases lung tumor multiplicity and size, and prevents emphysema.
The survival curves for the three treatment groups in Experiment 2 were analyzed using SAS® PROC LIFETEST. The log-rank test results for the control, NNK + Nic, NNK + Nic + BCX (10), and NNK + Nic + BCX (20) groups (P < 0.01) are shown in (A). The quantification of the lung tumor multiplicity is shown in (B). The number of lung suface tumors and the quantification of the lung tumor volume (mm3) are shown in (C). Effects of BCX supplementation on nicotine-induced emphysema and lung inflammation. The degree of emphysema was quantified by calculating the mean linear intercept (Lm) using 10 fields per animal (D). The lung il-6 mRNA levels are shown in (E). The lung cyclin D1 protein levels are shown in (F). Inset: a representative image of the cyclin D1 and GAPDH proteins. The lung p50 protein levels are shown in (G). Inset: a representative image of the p50 and GAPDH proteins. The lung egr1 mRNA levels are shown in (H). The proteins were detected using Western blotting. The intensity of the bands was quantified using a densitometry and normalized to GAPDH levels. The mRNA levels were measured using qRT-PCR and normalized to β-actin mRNA levels. The columns represent the mean ± sem. *p < 0.05. Abbreviations: BCX (10): β-cryptoxanthin at 10 mg/kg diet; BCX (20): β-cryptoxanthin at 20 mg/kg diet; C: control; Nic: nicotine; NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; il-6: interleukin-6; egr1: early growth response 1.
BCX inhibits the nicotine-promoted lung tumor multiplicity/volume and emphysema
The supplementation of BCX at 10 and 20 mg/kg diet decreased the lung tumor multiplicity by 86% and 91%, respectively, compared to the NNK + Nic group (Fig. 4B). The lung tumor volumes of the mice supplemented with 10 and 20 mg BCX/kg diet were 79% and 87% smaller, respectively, compared to the NNK + Nic group (Fig. 4C). The incidences of emphysema in the NNK + Nic + BCX (10) (17%) and NNK + Nic + BCX (20) (17%) groups were lower than the NNK + Nic group (90%) (p < 0.01). Moreover, the Lm of the lungs of the NNK + Nic + BCX (10) and NNK + Nic + BCX (20) groups were lower than the NNK + Nic group (p < 0.01) (Fig. 4D).
The 10 and 20 mg/kg diet of BCX-supplemented groups had 64% and 68% decreases, respectively, of il-6 mRNA level compared to the NNK + Nic group (p < 0.05) (Fig. 4E). The levels of cyclin D1 and p50 proteins in the NNK + Nic were lower but the levels of egr1 mRNA were higher than that of the control group (p < 0.05) (Fig.4F, 4G. and 4H). There were no differences in the levels of cyclin D1 protein, p50 protein, and egr1 mRNA between the BCX-supplemented groups and the control group (Fig. 4F, 4G, and 4H).
BCX restores the SIRT1 protein levels
The 10 and 20 mg/kg diet of BCX-supplemented groups had increased levels of the SIRT1 protein, by 91% and 67%, respectively, compared to the NNK + Nic group (Fig. 5A) (p < 0.01). However, sirt1 mRNA levels in the lungs were similar across all groups (Fig. 5B).
Figure 5. BCX restores the SIRT1 protein level and the transcription of tumor suppressor genes and decreases the phosphorylation of AKT.
The lung SIRT1 protein levels are shown in (A). Inset: a representative image of the SIRT1 and GAPDH proteins. The lung sirt1 mRNA levels are shown in (B). The quantification of AKT phosphorylation on Ser473 is shown in (C). Inset: a representative image of phosphorylated AKT (pAKT) and total AKT. The mRNA levels of p53 and RAR-β are shown in (D) and (E), respectively. The proteins were detected using Western blotting. The intensity of the bands was quantified using densitometry and normalized to GAPDH or total AKT levels. The mRNA levels were measured using qRT-PCR and were normalized to β-actin mRNA levels. The columns represent the mean ± sem. *p < 0.05. Abbreviations: BCX (10): β-cryptoxanthin at 10 mg/kg diet; BCX (20): β-cryptoxanthin at 20 mg/kg diet; C: control; Nic: nicotine; NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; RAR-β: retinoic acid receptor-β, SIRT1: sirtuin 1.
BCX supplementation decreases AKT phosphorylation and induces the mRNA levels of both p53 and RAR-β
The 10 and 20 mg/kg diet of BCX-supplemented groups had decreases of phosphorylated AKT levels, by 65% and 58%, respectively, as compared to the NNK + Nic group (Fig. 5C). There were restored lung levels of tumor suppressor p53 and RAR-β mRNA in the BCX-supplemented groups to their normal levels, as observed in the control group (Fig. 5D and Fig. 5E).
Discussion
This study demonstrates that nicotine exposure not only increases lung tumor multiplicity and size but also induces emphysema in the A/J lung cancer mouse model. To our knowledge, this is the first model reporting lung tumor development within an emphysematous microenvironment. This study would provide a relevant animal model mimicking lung cancer patient with emphysema. The daily dose of nicotine was equal to the average cigarette consumption in the U.S. (23) or to the dose/serving in nicotine replacement therapy products, such as gums, inhalers and lozenges (34). This animal model would be useful in studying the molecular pathways leading to emphysema and lung tumorigenesis associated with smoking and smokeless tobacco products. This model would be valuable in examining potential chemopreventive agents against emphysema and lung cancer.
Using this model, we provided a verification of BCX as a chemopreventive agent that prevented both nicotine-promoted lung tumor and emphysema. The BCX doses were relevant to physiological levels in humans based on several observations. First, although mice have lower absorption of the intact carotenoids compared to humans (35); we detected a serum concentration of BCX of 9.9 μg/L in A/J mice supplemented with 10 mg BCX/kg diet (unpublished data), which is lower than that of the average U.S. population (77 μg/L) (36). Second, the mean liver BCX concentration in the mice supplemented with 10 mg BCX/kg diet (0.25 nmol/g) was lower than that in human livers (0.66 nmol/g) (37). Finally, the BCX doses used in this study were equivalent to daily human consumption of 0.87 and 1.74 mg BCX (see Materials and Methods). These doses are slightly higher than that in a cohort of Chinese men (38), in which 0.74 mg BCX/2000 kcal intake was associated with a lower lung cancer risk (38).
The protective role of SIRT1 against emphysema is supported by previous reports, in which SIRT1 maintains genomic integrity and protects against cigarette smoke-induced emphysema in mice (12). This study showed that the decreased SIRT1 levels were associated with nicotine-induced emphysema and lung tumorigenesis. Moreover, the restoration of SIRT1 protein levels to the normal levels was associated with the protective action of BCX against emphysema and lung tumorigenesis. We found that the down-regulation of SIRT1 in the nicotine-treated mice and the up-regulation of SIRT1 in the BCX-supplemented mice occurred without changes in sirt1 mRNA levels, suggesting a post-translational regulation of sirt1. The exact mechanism(s) how the SIRT1 protein levels are regulated by nicotine or BCX needs further investigation, which currently undergoes in our lab.
The BCX supplementation was associated with the induction of both the SIRT1 and p53 mRNA levels. This observation is in agreement with a previous study in that double heterozygous sirt1+/−; p53+/− mice had a higher incidence of tumors (76%) than heterozygous sirt1+/− and p53+/− mice (10% and 13%, respectively) (39). Moreover, the increased SIRT1 levels observed in the BCX-supplemented groups were associated with increased RAR-β mRNA. This observation can be explained by a previous observation in that SIRT1 co-activates RAR-β (40), which itself is capable to regulate its transcription (i.e., RAR-β mRNA) (41). Therefore, the induction of SIRT1 in the BCX-supplemented mice may explain the increased RAR-β transcription.
In this study, the reduced SIRT1 protein levels were associated with elevated il-6 mRNA. These findings are consistent with a previous study demonstrating that homozygous sirt1−/− mice exhibited increased lung levels of both IL-6 and TNF-α following exposure to fine air particulates (42). Although we could not obtain the data on IL-6 protein due to the limited mouse lung samples, a direct correlation between mRNA and protein levels of cytokines has been reported (43, 44). The increase of il-6 mRNA in the NNK + Nic group was also associated with an induction of egr1 mRNA. EGR1 has been implicated in emphysema as it regulates genes important for extracellular matrix remodeling and those encoding cytokines/chemokines (45). Both egr1 transcript and EGR1 protein levels were increased in patients with advanced emphysema (45), supporting our observation in which egr1 mRNA levels were increased in the NNK + Nic group that exhibited emphysema. We also found that the increased il-6 levels were associated with lower nuclear factor κB subunit p50 protein levels. This observation is inconsistent with the notion that an activation of nuclear factor κB increases the transcription of inflammatory cytokines (13). However, our data are in agreement with a previous study in which p50−/− knockout mice develop emphysema (46). Earlier reports have described that the p50 protein is inactive because it lacks a transactivation domain and that the formation of p50/p50 homodimer binds to co-repressor complexes that are capable in repressing the nuclear factor κB-dependent transcription of inflammatory genes (46).
The decreased cyclin D1 protein level in the NNK + Nic group was in contrast to what we expect in tumorigenesis since the NNK + Nic group had increased lung tumor volume. Increased cyclin D1 level is found in many human tumors, reflecting an increase in cell proliferation. However, we believe that this reduced cyclin D1 protein could be due to decreased capability of alveolar repair/regeneration in emphysema (47). For example, fibroblasts obtained from emphysematous lungs have a reduced proliferation rate (50-60% reduction) as compared to fibroblasts from normal human lungs (48). This notion is consistent with our observation in that cyclin D1 levels in the BCX-supplemented groups were restored to the control group.
We observed the highest AKT phosphorylation levels in the NNK + Nic group, which had the lowest survival probability compared to the NNK and control groups. This result is consistent with a previous report in which tumors of non-small cell lung cancer patients displaying increased AKT phosphorylation levels that were associated with lower survival compared to tumors displaying lower AKT phosphorylation levels (49). Furthermore, the increased SIRT1 levels observed in the BCX-supplemented groups were associated with lower AKT phosphorylation levels. This observation is supported by previous studies reporting an inverse association between SIRT1 and AKT phosphorylation (50); the loss of SIRT1 protein resulted in higher AKT phosphorylation levels in mouse embryonic fibroblasts (50).
Because BCX is a pro-vitamin A carotenoid, this study raises an important question whether its protective effects resulted from BCX as an intact molecule or from its metabolites. Our study suggests that the action of BCX against lung tumor and emphysema is independent of its metabolite vitamin A (retinol and retinoic acid) activity due to the following factors. First, unlike the pro-vitamin A carotenoid β-carotene, for which supplementation at 120 mg/kg diet resulted in no anti-tumor effects in NNK-treated male A/J mice (22), we show here that BCX supplementation at 10 and 20 mg/kg diet was effective. Second, we observed that in contrast to β-carotene, which has no effect or even detrimental effects on lung tumorigenesis at a high-dose β-carotene in a ferret model (51), BCX supplementation decreased cigarette smoke-induced lung inflammation and pre-cancerous lesions in the same ferret model (18). Third, treatment with the derivative of vitamin A all-trans retinoic acid (RA) at 0.5 to 2.5 mg/bw i.p. does not reverse cigarette-smoke-induced emphysema in A/J mice (52) and treatment with all-trans RA at 0.5 mg/bw i.p. does not alter cigarette smoke-induced emphysema in guinea pigs (53). Fourth, treatment with 9-cis RA (7.5, 15, and 30 mg 9-cis RA/kg diet) causes loss of body weight in NNK-treated A/J mice (29); yet, BCX did not. Finally, BCX itself affects the transcription activity of RAR (17, 54). BCX has a greater effect on the transcription activity of RAR than other carotenoids (i.e., astaxanthin, lutein, β-carotene, zeaxanthin, and lycopene) (54). Therefore, this study suggests a new avenue of research regarding the function of BCX independent of its metabolites.
Supplementary Material
Acknowledgements
We thank Dr. Hansgeorg Ernst (the Fine Chemicals and Biocatalysis Research, BASF, Ludwigshafen, Germany) for providing β-cryptoxanthin.
Funding: Supported by the NIH grant CA104932, and the U.S. Department of Agriculture grant 1950-51000-064S. Any opinions, findings, conclusions, and recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the sponsors.
Footnotes
Disclosure of Potential Conflict of Interest: None
References
- 1.Hecht SS, Kassie F, Hatsukami DK. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat Rev Cancer. 2009;9:476–88. doi: 10.1038/nrc2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007;87:1047–82. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Swensen SJ, Karabekmez LG, Marks RS, Stoddard SM, Jiang R, et al. Effect of emphysema on lung cancer risk in smokers: a computed tomography-based assessment. Cancer Prev Res. 2011;4:43–50. doi: 10.1158/1940-6207.CAPR-10-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 5.Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–41. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- 6.Davis R, Rizwani W, Banerjee S, Kovacs M, Haura E, Coppola D, et al. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PLoS One. 2009;4:e7524. doi: 10.1371/journal.pone.0007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke JP, Bitterman H. Nicotine and angiogenesis: a new paradigm for tobacco-related diseases. Ann Med. 2004;36:33–40. doi: 10.1080/07853890310017576. [DOI] [PubMed] [Google Scholar]
- 8.Murphy SE, von Weymarn LB, Schutten MM, Kassie F, Modiano JF. Chronic nicotine consumption does not influence 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis. Cancer Prev Res. 2011;4:1752–60. doi: 10.1158/1940-6207.CAPR-11-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maier CR, Hollander MC, Hobbs EA, Dogan I, Linnoila RI, Dennis PA. Nicotine does not enhance tumorigenesis in mutant K-ras-driven mouse models of lung cancer. Cancer Prev Res. 2011;4:1743–51. doi: 10.1158/1940-6207.CAPR-11-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maritz GS. Maternal nicotine exposure during gestation and lactation of rats induce microscopic emphysema in the offspring. Exp Lung Res. 2002;28:391–403. doi: 10.1080/01902140290092010. [DOI] [PubMed] [Google Scholar]
- 11.Fang Y, Nicholl MB. Sirtuin 1 in malignant transformation: friend or foe? Cancer Lett. 2011;306:10–4. doi: 10.1016/j.canlet.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest. 2012;122:2032–45. doi: 10.1172/JCI60132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–70. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayne ST, Ferrucci LM, Cartmel B. Lessons learned from randomized clinical trials of micronutrient supplementation for cancer prevention. Annu Rev Nutr. 2012;32:369–90. doi: 10.1146/annurev-nutr-071811-150659. [DOI] [PubMed] [Google Scholar]
- 15.Mannisto S, Smith-Warner SA, Spiegelman D, Albanes D, Anderson K, van den Brandt PA, et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol Biomarkers Prev. 2004;13:40–8. doi: 10.1158/1055-9965.epi-038-3. [DOI] [PubMed] [Google Scholar]
- 16.Gallicchio L, Boyd K, Matanoski G, Tao XG, Chen L, Lam TK, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr. 2008;88:372–83. doi: 10.1093/ajcn/88.2.372. [DOI] [PubMed] [Google Scholar]
- 17.Lian F, Hu KQ, Russell RM, Wang XD. Beta-cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor beta expression. Int J Cancer. 2006;119:2084–9. doi: 10.1002/ijc.22111. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Bronson RT, Russell RM, Wang XD. Beta-cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets. Cancer Prev Res. 2011;4:1255–66. doi: 10.1158/1940-6207.CAPR-10-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Lung Association [cited 2012 Aug 21];Trends in Tobacco Use [Internet] 2011 Jul; Available from: http://www.lung.org/finding-cures/our-research/trend-reports/Tobacco-Trend-Report.pdf. [Google Scholar]
- 21.ATBC group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 22.Goralczyk R, Bachmann H, Wertz K, Lenz B, Riss G, Buchwald Hunziker P, et al. beta-carotene-induced changes in RARbeta isoform mRNA expression patterns do not influence lung adenoma multiplicity in the NNK-initiated A/J mouse model. Nutr Cancer. 2006;54:252–62. doi: 10.1207/s15327914nc5402_12. [DOI] [PubMed] [Google Scholar]
- 23.Umegaki K, Aoshima M, Hirota S, Uramoto H, Esashi T. Simultaneous dietary supplementation of sodium cholate and beta-carotene markedly enhances accumulation of beta-carotene in mice. J Nutr. 1995;125:3081–6. doi: 10.1093/jn/125.12.3081. [DOI] [PubMed] [Google Scholar]
- 24.Breithaupt DE, Weller P, Wolters M, Hahn A. Plasma response to a single dose of dietary beta-cryptoxanthin esters from papaya (Carica papaya L.) or non-esterified beta-cryptoxanthin in adult human subjects: a comparative study. Br J Nutr. 2003;90:795–801. doi: 10.1079/bjn2003962. [DOI] [PubMed] [Google Scholar]
- 25.National Nutrient Database U.S. Department of Agriculture [cited 2012 Aug 21];Cryptoxanthin, beta (microgram) Content of Selected Food per Common Measure [Internet] Available from: https://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/SR24/nutrlist/sr24w334.pdf.
- 26.Mernitz H, Smith DE, Zhu AX, Wang XD. 9-cis-Retinoic acid inhibition of lung carcinogenesis in the A/J mouse model is accompanied by increased expression of RAR-beta but no change in cyclooxygenase-2. Cancer Lett. 2006;244:101–8. doi: 10.1016/j.canlet.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Witschi H. The complexities of an apparently simple lung tumor model: The A/J mouse. Exp Toxicol Pathol. 2005;57(Suppl 1):171–81. doi: 10.1016/j.etp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Li D, Graef GL, Yee JA, Yan L. Dietary supplementation with high-selenium soy protein reduces pulmonary metastasis of melanoma cells in mice. J Nutr. 2004;134:1536–40. doi: 10.1093/jn/134.6.1536. [DOI] [PubMed] [Google Scholar]
- 29.Mernitz H, Smith DE, Wood RJ, Russell RM, Wang XD. Inhibition of lung carcinogenesis by 1alpha,25-dihydroxyvitamin D3 and 9-cis retinoic acid in the A/J mouse model: evidence of retinoid mitigation of vitamin D toxicity. Int J Cancer. 2007;120:1402–9. doi: 10.1002/ijc.22462. [DOI] [PubMed] [Google Scholar]
- 30.Robbesom AA, Versteeg EM, Veerkamp JH, van Krieken JH, Bulten HJ, Smits HT, et al. Morphological quantification of emphysema in small human lung specimens: comparison of methods and relation with clinical data. Mod Pathol. 2003;16:1–7. doi: 10.1097/01.MP.0000043519.29370.C2. [DOI] [PubMed] [Google Scholar]
- 31.Bracke KR, D’Hulst AI, Maes T, Moerloose KB, Demedts IK, Lebecque S, et al. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol. 2006;177:4350–9. doi: 10.4049/jimmunol.177.7.4350. [DOI] [PubMed] [Google Scholar]
- 32.Liu C, Lian F, Smith DE, Russell RM, Wang XD. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res. 2003;63:3138–44. [PubMed] [Google Scholar]
- 33.Belinsky SA, Devereux TR, Foley JF, Maronpot RR, Anderson MW. Role of the alveolar type II cell in the development and progression of pulmonary tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the A/J mouse. Cancer Res. 1992;52:3164–73. [PubMed] [Google Scholar]
- 34.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 35.Lee CM, Boileau AC, Boileau TW, Williams AW, Swanson KS, Heintz KA, et al. Review of animal models in carotenoid research. J Nutr. 1999;129:2271–7. doi: 10.1093/jn/129.12.2271. [DOI] [PubMed] [Google Scholar]
- 36.Center of Disease Control and Prevention [cited 2012 Aug 21];Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population [Internet] 2012 Available from: http://www.cdc.gov/nutritionreport/pdf/Nutrition_Book_complete508_final.pdf#zoom=100. [Google Scholar]
- 37.Furr HC, Clark RM. Transport, uptake, and target tissue storage of carotenoids. In: Krinsky NI, Mayne ST, Sies H, editors. Carotenoids in Health and Disease. Marcel Dekker, Inc.; New York, NY: 2004. pp. 229–78. [Google Scholar]
- 38.Yuan JM, Stram DO, Arakawa K, Lee HP, Yu MC. Dietary cryptoxanthin and reduced risk of lung cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2003;12:890–8. [PubMed] [Google Scholar]
- 39.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–23. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–32. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Chiba H, Clifford J, Metzger D, Chambon P. Distinct retinoid X receptor-retinoic acid receptor heterodimers are differentially involved in the control of expression of retinoid target genes in F9 embryonal carcinoma cells. Mol Cell Biol. 1997;17:3013–20. doi: 10.1128/mcb.17.6.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Z, Liu MC, Liang M, Fu J. Sirt1 protects against thrombomodulin down-regulation and lung coagulation after particulate matter exposure. Blood. 2012;119:2422–9. doi: 10.1182/blood-2011-04-350413. [DOI] [PubMed] [Google Scholar]
- 43.Halappanavar S, Wu D, Williams A, Kuo B, Godschalk RW, Van Schooten FJ, et al. Pulmonary gene and microRNA expression changes in mice exposed to benzo(a)pyrene by oral gavage. Toxicology. 2011;285:133–41. doi: 10.1016/j.tox.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Nathe KE, Mancuso CJ, Parad R, Van Marter LJ, Martin CR, Stoler-Barak L, et al. Innate Immune Activation in Neonatal Tracheal Aspirates Suggests Endotoxin-Driven Inflammation. Pediatr Res. 2012;72:203–11. doi: 10.1038/pr.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, Yan SD, Zhu A, Zou YS, Williams M, Godman GC, et al. Expression of Egr-1 in late stage emphysema. Am J Pathol. 2000;157:1311–20. doi: 10.1016/S0002-9440(10)64646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajendrasozhan S, Chung S, Sundar IK, Yao H, Rahman I. Targeted disruption of NF-{kappa}B1 (p50) augments cigarette smoke-induced lung inflammation and emphysema in mice: a critical role of p50 in chromatin remodeling. Am J Physiol Lung Cell Mol Physiol. 2010;298:L197–209. doi: 10.1152/ajplung.00265.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plantier L, Rochette-Egly C, Goven D, Boutten A, Bonay M, Leseche G, et al. Dysregulation of elastin expression by fibroblasts in pulmonary emphysema: role of cellular retinoic acid binding protein 2. Thorax. 2008;63:1012–7. doi: 10.1136/thx.2007.093302. [DOI] [PubMed] [Google Scholar]
- 48.Nobukuni S, Watanabe K, Inoue J, Wen FQ, Tamaru N, Yoshida M. Cigarette smoke inhibits the growth of lung fibroblasts from patients with pulmonary emphysema. Respirology. 2002;7:217–23. doi: 10.1046/j.1440-1843.2002.00400.x. [DOI] [PubMed] [Google Scholar]
- 49.David O, Jett J, LeBeau H, Dy G, Hughes J, Friedman M, et al. Phospho-Akt overexpression in non-small cell lung cancer confers significant stage-independent survival disadvantage. Clin Cancer Res. 2004;10:6865–71. doi: 10.1158/1078-0432.CCR-04-0174. [DOI] [PubMed] [Google Scholar]
- 50.Ming M, Shea CR, Guo X, Li X, Soltani K, Han W, et al. Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C. Proc Natl Acad Sci U S A. 2010;107:22623–8. doi: 10.1073/pnas.1010377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell M. Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst. 1999;91:60–6. doi: 10.1093/jnci/91.1.60. [DOI] [PubMed] [Google Scholar]
- 52.March TH, Bowen LE, Finch GL, Nikula KJ, Wayne BJ, Hobbs CH. Effects of strain and treatment with inhaled aII-trans-retinoic acid on cigarette smoke-induced pulmonary emphysema in mice. COPD. 2005;2:289–302. [PubMed] [Google Scholar]
- 53.Meshi B, Vitalis TZ, Ionescu D, Elliott WM, Liu C, Wang XD, et al. Emphysematous lung destruction by cigarette smoke. The effects of latent adenoviral infection on the lung inflammatory response. Am J Respir Cell Mol Biol. 2002;26:52–7. doi: 10.1165/ajrcmb.26.1.4253. [DOI] [PubMed] [Google Scholar]
- 54.Matsumoto A, Mizukami H, Mizuno S, Umegaki K, Nishikawa J, Shudo K, et al. beta-Cryptoxanthin, a novel natural RAR ligand, induces ATP-binding cassette transporters in macrophages. Biochem Pharmacol. 2007;74:256–64. doi: 10.1016/j.bcp.2007.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.