Abstract
Purpose
Longitudinal testing plays a key role in glaucoma management. Variability between visits hampers the ability to monitor progression. It has previously been shown that average intraocular pressure (IOP) exhibits seasonal fluctuations. This study examines whether visual field sensitivity also exhibits seasonal fluctuations, and seeks to determine whether such fluctuations are correlated to seasonal IOP effects.
Design
Comparative Case Series.
Participants
33873 visits by 1636 participants enrolled in the Ocular Hypertension Treatment Study. Participants were split into six geographic zones according to the prevailing climate in their location.
Testing
At each visit, standard automated perimetry was conducted on each eye, and IOP was measured.
Main Outcome Measures
Mixed effects regression models were formed to look for sinusoidal periodic effects on the change in perimetric Mean Deviation since the last visit (ΔMD), and also on IOP, both overall and within each zone.
Results
When all the data were included, a significant seasonal effect on ΔMD was found with magnitude 0.06dB, peaking in February, (p<0.001). Five of the six geographic zones exhibited significant seasonal effects on ΔMD, peaking between January and April, with magnitudes ranging from 0.04dB (p=0.049) to 0.21dB (p<0.001). Zones with greater climactic variation showed larger seasonal effects on ΔMD. All six zones exhibited a seasonal effect on IOP, peaking in January or February, with magnitudes ranging from 0.14mmHg to 0.39mmHg (p≤0.02 in all cases). However, there was no evidence of a significant association between the magnitudes or dates of peaks of the two seasonal effects.
Conclusions
MD was significantly higher in winter than summer. There is no evidence of an association with seasonal IOP fluctuations. The cause of the seasonal effect on visual field sensitivity is unknown. These findings may help shed light on the glaucomatous disease process, as well as aid efforts to reduce test-retest variability.
Introduction
Glaucoma is one of the most common causes of visual disability and blindness. However, assessing visual disability in glaucoma is difficult because there is considerable between-subject and within-subject variability in functional testing, both in cross-sectional test results and in the longitudinal profile of progression over time.1-8 This variability is clinically problematic, hindering the ability to implement optimal management strategies in a timely manner. Furthermore, it hampers clinical trials because it increases both the sample size and the series duration required before meaningful results can be generated. Variability also obscures potential contributory factors, with the consequence that the causes and mechanisms of glaucoma are still poorly understood. Therefore, understanding and reducing this variability is a key aim of glaucoma research.
Elevated intraocular pressure (IOP) and increased age are recognized as major risk factors for glaucomatous damage and progression. IOP is the major modifiable risk factor, and reducing IOP has been shown to reduce the risk of disease progression by studies including the recent Ocular Hypertension Treatment Study (OHTS).9, 10 However, IOP is also variable. Diurnal variations cause IOP to be higher at certain times of day.11-13 More relevantly to this study, IOP has also been reported to vary through the year, being higher in the winter than in the summer both in normotensive14, 15 and ocular hypertensive subjects.11
This study uses data from the OHTS to determine whether similar cyclic annual fluctuations affect visual field (VF) sensitivity. Such an effect has recently been described by Montolio et al, using data from the Groningen Longitudinal Glaucoma Study.16 It could be responsible for some of the variability observed in longitudinal studies in patients with ocular hypertension and/or early glaucoma. Accounting for such seasonal variations, either in the study design or by appropriate post-processing of the data, could then reduce variability with the concomitant benefits outlined above. Additionally, determination of the causes of any seasonal effects could help elucidate the glaucomatous disease process.
Methods
Baseline data and design of the OHTS have been described elsewhere.17 All OHTS participants signed a statement of informed consent prior to study entry after having the risks and benefits of participation explained to them. The institutional review boards at all participating clinical sites approved their respective informed consent statements and procedures, and only de-identified data were used for this analysis.
All participants enrolled in the OHTS were required to have at least two reliable achromatic automated visual fields (Humphrey Visual Field Analyzer Pattern 30-2, Carl Zeiss Meditec, Inc., Dublin, CA) that were within normal limits during the qualifying period. Reliable fields were defined as having false positives, false negatives and fixation losses all < 33% when testing was performed using the Full Threshold algorithm; or false positives < 15%, false negatives and fixation losses < 33% when testing was performed using the Swedish Interactive Threshold Algorithm (SITA). The OHTS analysis dataset available for this study contained all VF tests, IOP measurements and endpoint determinations in the OHTS database as of March 2009. Testing was carried out approximately once every six months for each participant. Follow-up VF tests that did not meet the reliability criteria above were excluded.18, 19
For each visit, the following information was taken from the database:
De-identified patient ID
Site ID
Month of testing
Mean Deviation (MD) of each eye
Change in MD from previous visit for each eye (ΔMD)
IOP for each eye
For the primary analysis, ΔMD (the change in sensitivity since the participant’s visit approximately six months previously) was analyzed instead of MD. This removes the confounding effect that some participants had greater amounts of damage than others. Note that while the reliability criteria for Full Threshold and SITA testing differed, it was assumed that Mean Deviation (MD) values from the two algorithms (SITA and Full Threshold) were equivalent.18
The study sites were split into six geographical zones of the USA according to prevailing climate: Atlantic Seaboard (including the region from New York to Washington DC), Central (the Southern portion of the Midwest), North (the Northern portion of the Midwest, including the Great Lakes region), Pacific Northwest (Oregon and Washington states), Southeast (extending from Florida to Texas), and West (including California). Within each of these zones, random effects models were formed to look for seasonal effects on each of ΔMD and IOP, assuming a sinusoidal pattern through the year. Specifically, the model used was:
where Month was coded as 1 (representing January) through 12 (representing December). The residuals ε2 were minimized to determine the optimal values of Offset (giving the month at which any seasonal trend in ΔMD reaches its maximum) and α (giving the magnitude of any seasonal effect). A finding that α was significantly different from zero was taken as evidence for a seasonal effect on ΔMD. Similar models were created for IOP.
As this is a large retrospective analysis, the dataset is by nature heterogeneous. It contains participants with various confounding conditions, such as systemic diseases and medications, differing refractive status, and differing lens transparencies. To ensure that these factors did not affect the conclusions, the main analysis was repeated restricted to participants below the age of 60 at study entry, since these should comprise a more homogeneous cohort with fewer confounding pathologies.
Results
Table 1 shows the characteristics of the dataset within each geographical zone. In total, there were 1636 participants. Some participants moved location during the study period, and so are included in the datasets for more than one zone. However, each zone was analyzed separately including only those visits that took place when the participant was within that zone, and so any movement of participants during they study should not affect the zonal results.
Table 1.
Characteristics of the dataset within each geographical zone.
| Zone | Subjects | Visits | IOP IOP (mmHg) | MD (dB) | ||
|---|---|---|---|---|---|---|
| Mean | 95% CI A | Mean | 95% CI A | |||
| Atlantic | 397 | 7798 | 20.0 | 10.8, 29.3 | −0.31 | −4.37, 3.75 |
| Central | 291 | 5795 | 20.7 | 12.1, 29.3 | −0.49 | −5.20, 4.23 |
| North | 213 | 4402 | 19.7 | 11.8, 27.7 | −0.21 | −4.19, 3.78 |
| Pacific Northwest | 117 | 2239 | 19.7 | 12.5, 27.0 | 0.02 | −3.37, 3.40 |
| Southeast | 269 | 5510 | 19.0 | 10.2, 27.8 | −0.32 | −4.57, 3.92 |
| West | 401 | 8129 | 19.6 | 11.4, 27.8 | −0.05 | −4.29, 4.19 |
The 95% confidence intervals for Intraocular Pressure (IOP) and perimetric Mean Deviation (MD) are based on an assumption of normality.
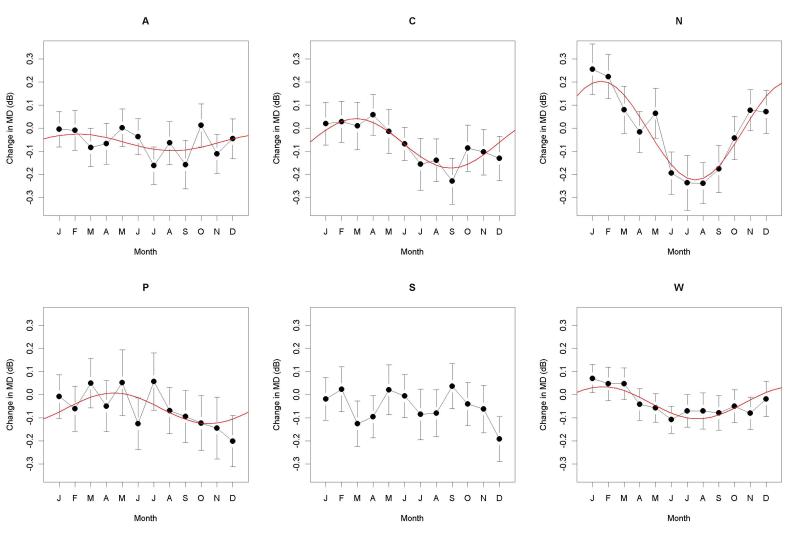
When all the data were included, a significant seasonal effect on ΔMD was found. This had magnitude 0.06dB (α in the equation given in the Methods section), peaking in February, with p<0.001. Table 2 shows the magnitude of seasonal effect on ΔMD for each zone, its peak (the date at which it reaches its maximum, from Offset in the equation in the Methods section), and its significance level. A significant seasonal effect was found for ΔMD for all zones except the Southeast, reaching a peak sometime between January and April. The seasonal effect was most pronounced in the North zone, where MDs in the winter were 0.21dB higher than the same participant’s MD at their previous visit. Figure 1 shows the mean ΔMD in each month, together with the 95% confidence interval for this mean, and the fitted sinusoidal seasonal effect whenever this was significant with p<5%. Note that the difference between the maximum and minimum values of these fitted sinusoidal curves will be double the amplitudes reported in Table 2. When the analysis was performed using MD instead of ΔMD as the outcome variable, the results were almost identical (not shown).
Table 2.
The seasonal effects in each defined geographical zone on ΔMD, the change in Mean Deviation since the previous visit.
| Zone | Magnitude of seasonal effect (dB) |
Date of peak | Statistical significance of magnitude (p) |
|---|---|---|---|
| Atlantic | 0.04 | 18 Feb | 0.049 |
| Central | 0.11 | 12 Mar | <0.001 |
| North | 0.21 | 1 Feb | <0.001 |
| Pacific Northwest |
0.07 | 29 Apr | 0.005 |
| Southeast | 0.02 | 10 Jul | 0.364 |
| West | 0.07 | 4 Feb | <0.001 |
Figure 1.
The change in Mean Deviation (MD) since the previous visit, ΔMD, for visual fields measured during each month (January, February, March, April, May, June, July, August, September, October, November, December) for each of the six geographical zones (Atlantic, Central, North, Pacific Northwest, Southeast, and West). For each month, the black circle represents the mean, with vertical lines extending to the 95% confidence interval for the mean. If a significant seasonal effect was found in that zone, it is shown by a red line.
In this study, ΔMD was used as the outcome variable, rather than MD. This removes one source of variability and hence makes the seasonal effect more apparent, since it removes artefactual differences in the populations tested each month. For example, the participants tested in August in the Pacific Northwest zone had an average MD of 0.25dB, higher than those tested in July (0.01dB) or September (−0.13dB). Significant seasonal effects were still found in the same five zones when using MD as the outcome variable. A further alternative analysis was performed that adjusted for disease progression, adjusting ΔMD by the amount of change that would be expected over that time period based on the trend of MD over time. Again, this did not appreciably change the results; the magnitude of the seasonal effect was within 0.03dB/yr of the values reported in Table 2 in all cases.
To ensure that heterogeneity of the dataset did not materially affect the results, the analysis was repeated using ΔMD as the outcome variable, but restricting the analysis to the 940 participants below the age of 60 at study entry. As seen in Table 3, the seasonal effect was essentially unchanged in this subset of the dataset.
Table 3.
The seasonal effects in each defined geographical zone on ΔMD, the change in Mean Deviation since the previous visit, restricted to participants below 60 years old at study entry.
| Zone | Magnitude of seasonal effect (dB) |
Date of peak | Statistical significance of magnitude (p) |
|---|---|---|---|
| Atlantic | 0.07 | 15 Feb | 0.001 |
| Central | 0.10 | 10 Mar | <0.001 |
| North | 0.23 | 3 Feb | <0.001 |
| Pacific Northwest |
0.05 | 5 May | 0.055 |
| Southeast | 0.03 | 13 Apr | 0.164 |
| West | 0.06 | 3 Feb | <0.001 |
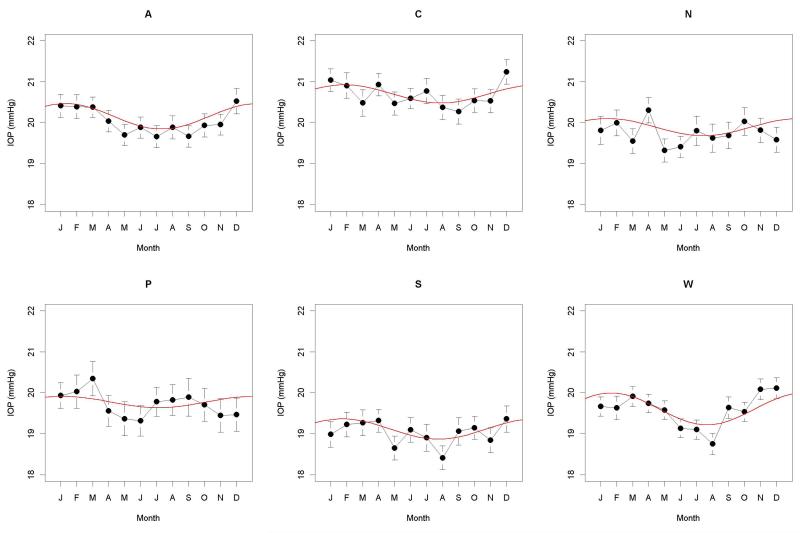
A significant seasonal effect on IOP was found when using all the data together, with magnitude 0.27mmHg, peaking at the start of February (p<0.001). Table 4 and Figure 2 show the seasonal effects for IOP within each geographic zone. A significant seasonal effect was found for all six zones, reaching a peak in January or February. However, there was no evidence of any causal relation between the seasonal peaks in IOP and visual field sensitivity. Between the different geographic zones, a greater amplitude of the seasonal effect on IOP did not correlate with a greater amplitude of the effect on ΔMD; the Spearman correlation between these six pairs of amplitudes was −0.31, not significant with p=0.564. The correlation between the dates of their peaks (under the assumption that all the peaks fall within a single calendar year) was 0.14, also not significant with p=0.803. When IOP was used as an additional predictor of ΔMD in the random effects model, the seasonal effect was still significant in all five of the zones for which a significant effect had previously been detected; p=0.038 for the Atlantic zone, p=0.001 for the Pacific Northwest zone, and p<0.001 for each of the Central, North and West zones.
Table 4.
The seasonal effects on Intraocular Pressure (IOP) in each defined geographical zone.
| Zone | Magnitude of seasonal effect (dB) |
Date of peak | Statistical significance of magnitude (p) |
|---|---|---|---|
| Atlantic | 0.31 | 25 Jan | <0.001 |
| Central | 0.22 | 10 Feb | <0.001 |
| North | 0.21 | 28 Jan | <0.001 |
| Pacific Northwest |
0.14 | 19 Jan | 0.023 |
| Southeast | 0.25 | 6 Feb | <0.001 |
| West | 0.39 | 4 Feb | <0.001 |
Figure 2.
Intraocular Pressure (IOP) measured during each month (January, February, March, April, May, June, July, August, September, October, November, December), for each of the six geographical zones (Atlantic, Central, North, Pacific Northwest, Southeast, and West). For each month, the black circle represents the mean, with vertical lines extending to the 95% confidence interval for the mean. If a significant seasonal effect was found in that zone, it is shown by a red line.
As seen in Table 5, regional differences in the magnitude of the seasonal effect on sensitivity correspond to some extent with regional differences in the magnitude of climatic seasonal variations. The Northern zone has the largest climactic variations through the year, with mean temperatures ranging from nearly 30°C in summer to below freezing in winter, and from around 10 hours of sunlight per day in summer to below 4 hours per day in winter. This is also the zone that showed the largest seasonal effect on visual field sensitivity. By contrast, the Southeast zone has less extreme climatic variation throughout the year, and showed smaller seasonal effects on sensitivity.
Table 5.
Seasonal effects on climate and on perimetric sensitivity within each geographic zone.
| Seasonal Variation in: | |||
|---|---|---|---|
| Zone | Temperature (°C) | Sunlight (Hours per day) A | ΔMD (dB) B |
| North | 30 | 7 | 0.22 |
| Central | 27 | 7 | 0.10 |
| West | 12 | 6 | 0.07 |
| Pacific Northwest | 19 | 8 | 0.06 |
| Atlantic | 26 | 5 | 0.03 |
| Southeast | 15 | 5 | 0.02 |
Taken as the maximum monthly mean minus the minimum monthly mean, averaged over all test sites within that zone.
The magnitude of the seasonal effect on the change in perimetric Mean Deviation since the previous visit (ΔMD), taken from Table 2. Zones are ordered according to the seasonal effect on ΔMD.
Discussion
The results above agree with previous studies which found a seasonal effect on IOP.11, 14, 15 As in those studies, we found that IOP was higher during the Northern winter than the summer, reaching a peak in January or February. The magnitude of this seasonal variation in each geographic zone ranged from 0.28mmHg to 0.78mmHg (double the amplitudes given in Table 4). This is considerably smaller than the magnitudes of the seasonal variations in normal individuals previously reported, whereby IOP was typically found to be 1 – 3 mmHg lower in summer than winter.14, 15 This may be because the IOP of participants in the OHTS was controlled by medications, either for the second half of the study (for those initially randomized to the observation group, since all such participants were offered IOP-lowering medication at the conclusion of phase I of the OHTS), or for the entire study period (for those participants initially randomized to treatment). Medications have previously been shown to reduce the magnitude of fluctuations in IOP.12, 20, 21 Indeed, when only data from the observation group within the first five years of the study were used (the period for which they did not receive medication), the seasonal effect on IOP was generally larger, with IOP being 0.9mmHg higher in winter than in summer for three of the zones. The physiological mechanism responsible for this annual fluctuation remains unclear. Qureshi et al11 hypothesized that the annual variation in IOP may be caused by changes in the amount of certain chemicals secreted by the pineal gland, which is affected by the daily total amount of light entering the eyes. Melatonin (or one of the other similar substances) secreted by the pineal gland affects the anterior pituitary gland, resulting in an increase in the secretion of progesterone and estrogen, which have been reported to reduce IOP values by increasing outflow.22, 23 By this hypothesis, during the summer months increased light levels reduce the amount secreted from the pineal gland, causing IOP to be reduced.
The seasonal effect on visual field sensitivity, however, has not yet been fully characterized or explained. The magnitude of the effect is small, which may explain why it has not previously been noted. It is unlikely to be apparent when looking at a series of visual fields from an individual. Crucially, it is sufficiently small that it does not bring the conclusions of clinical trials such as OHTS into question. However, in a large enough dataset its existence becomes apparent.
A similar seasonal effect on sensitivity was recently described by Montolio et al.16 Their study relied on less frequent testing and a much smaller sample size, with all subjects being from a single clinic in Groningen in the Netherlands. Our study goes further than theirs. The size of the OHTS dataset allows us to give a much better characterization of the seasonal effect on a monthly basis, whereas they split test dates into one of four seasons. Our study is also enhanced by the regularity of testing within OHTS. Subjects in their study were initially tested annually, which would not allow a seasonal effect to become apparent. More frequent testing occurred only if requested by their physician, normally due to suspected progression. Additionally, the different test sites used in OHTS revealed geographic differences in the magnitude of the seasonal effect (which was not possible in their study). It is certainly interesting to see that they found a very similar seasonal effect to our study, with a peak occurring at the same time of year and of similar magnitude (they found that sensitivities were 0.2dB lower in summer than in the winter/spring).
Accounting for this seasonal effect could reduce variability, resulting in earlier and more accurate detection of progression. The maximum amplitude of the seasonal effect on sensitivity was seen in the North region, where an MD measured in January would be, on average, 0.21dB higher than the same patient’s MD in July (the amplitude of the sinusoidal effect shown in Table 2). 10,000 simulated patients were created with a rate of change of MD of −0.26dB/yr (the mean for participants reaching a primary open angle glaucoma endpoint in this dataset),18 undergoing twice annual testing as in OHTS, with the MD starting at 0dB and having standard deviation 0.32dB (based on the pointwise variability having standard deviation 2.32dB when sensitivity is 30dB6). To detect progression (using a criterion of the rate of change being worse than −0.1dB/yr and significant at the p<5% level) took on average 7.5 simulated fields. Adding a seasonal effect equal to that observed in the North region increased this time to detect change to 8.0 simulated fields, a significant increase with p<0.001 (Wilcox matched pairs non-parametric test). The seasonal effect increased the time to detect change by at least one field (i.e. by at least six months) in 4021 out of the 10000 simulated series. This simulation represents a “worst-case scenario”, using the maximum seasonal effect, and so it is likely that accounting for seasonal effects would make a smaller clinical difference than this. However, it is indicative of the potential benefits, and even a much smaller effect than this could significantly affect clinical trials.
The effect sizes reported here are small (although it could be hypothesized that the higher IOPs observed in winter would cause more rapid deterioration in MD, and so the magnitude of the true seasonal effect on MD is being underestimated due to this confound). Therefore the long-term significance of these findings will depend on the underlying cause. This cause is presently unknown, and suggestions are speculative. Plausible causes fall into two broad categories; a characteristic of perimetry and the testing process, or a characteristic of glaucomatous progression. The former category could be considered as a factor contributing to perimetric variability. One example of a seasonal difference that could, in theory, affect sensitivity would be changes in mood. However, it would be expected that a better mood would generally occur in the summer months in most regions of the USA, causing the participant to be more alert and hence causing the measured sensitivity to be higher, which is the opposite of the effect found in this study. The level of ambient light outside could affect the level of light adaptation, reducing sensitivity in the summer months when the eye is adapted to a higher light intensity. However, adaptation levels are assumed to have stabilized within a short time of entering the clinical site or testing room, in which case this would only affect sensitivity if testing were performed within a few minutes of entering the building (on the assumption that light levels inside the building are approximately constant year-round). It is also possible that sensitivity is decreased by increased recent exposure to light during the summer, as has been suggested for scotopic sensitivity.24,25 Whatever the exact cause, if the seasonal effect is indeed solely a characteristic of the testing, taking account of it could help reduce variability in longitudinal series of visual fields, aiding both clinical care and clinical trials.
It is possible that this study could have even more important consequences by revealing important information about the disease process. Our results are consistent with glaucoma progressing more rapidly during the summer months, even though IOP is lower. It has been suggested that increased light levels could increase apoptosis of retinal ganglion cells, especially when those cells are already stressed (as in ocular hypertension / glaucoma).26, 27 Even though the total amount of time spent in light will be similar throughout the year, sunlight is considerably more intense than artificial lighting. Therefore light of higher intensity enters the eyes during the summer, potentially increasing the likelihood of apoptosis, resulting in more rapid progression. Alternatively, a mechanism could be proposed in which the increased temperature during the summer months causes more rapid progression in damaged retinal ganglion cells, although this seems unlikely since core body temperature remains approximately constant year round.
Regional differences in the seasonal effect could provide clues as to the cause of the phenomenon. As seen in Table 5, larger seasonal effects on sensitivity were seen in the geographic zones with larger climactic seasonal variation. In particular, the Northern zone has a continental climate, being further from the ocean than the other testing sites, with very cold winters and warm summers. Correspondingly, sites in this zone showed the largest seasonal effect on ΔMD. By contrast, the Southeast zone has a subtropical climate, without the harsh winters experienced further north. Here, outdoor activities in the summer are limited by heat and humidity, and so there may be less seasonal difference in the number of hours per day spent in sunshine. Correspondingly, this is the sole geographic zone for which a significant seasonal effect on sensitivity was not detected. It can also be seen from Tables 2 and 4 that the peak of sensitivity in the Pacific Northwest zone occurred later in the year than in any of the other four zones where a significant seasonal effect was detected. This corresponds with the climate in the Pacific Northwest, where the warmest and sunniest weather typically occurs from the start of July through to the end of September (with cloudier weather the rest of the year), whereas most of the rest of the USA sees its warmest weather from June through August.
Only the month of testing was available in the dataset used for this analysis, rather than the exact date, in line with accepted methods for deidentifying data. Therefore the analysis assumed that all testing happened at the halfway point of the month (January 16th, etc). This means that the dates shown for the peak of the sinusoidal seasonal effect in Tables 2 and 4 are given to a greater precision than is justified by the input data. While this greater precision is informative with regards to comparisons between zones, the exact dates should be treated with caution.
In order to properly assess the magnitude of the seasonal effect, some parameterization of that effect is necessary. The fits presented in Figures 1 and 2, and summarized in Tables 2-4, assume that the seasonal effect is sinusoidal. However, there is no evidence that this functional form is optimal. The fact that a significant effect can still be detected using a potentially non-optimal parameterization could be considered evidence of the robustness of the main conclusions. Without assuming such a parameterization, a small seasonal effect is still apparent. The average MD was higher in the first three months of the year than in the months July through September in all six geographic zones, although this was only significant in the Northern region (difference 0.19dB, p=0.006 using a t-test).
Since this analysis was purely retrospective, data collection was not optimal for assessing the presence of a seasonal effect. Subjects were tested twice annually, and so were generally either tested every autumn and spring, or every summer and winter, rather than in all four seasons. The subjects varied in many factors that affect sensitivity measures by visual field testing and so could potentially affect the magnitude of a seasonal effect. These factors include age, ethnic origin, systemic diseases and medications, refractive status, lens transparency, and topical medication (approximately half of the subjects commenced IOP-lowering treatment during the study). These factors should not vary consistently and periodically between different times of year, and so they are not causing the seasonal effect, but may affect its magnitude. With all these sources of inter-subject variability in mind, it is impressive that a significant seasonal effect was still detectable, and that it is relatively consistent (in terms of the timing of the peak) across different geographically-based subsets of the dataset. A prospective study designed specifically to examine the issue of seasonality, using more regular testing, could be desirable to obtain improved estimates of the magnitude of the seasonal effect in different sub-populations, and so provide useful information regarding factors such as disease severity that affect this magnitude.
In summary, visual field sensitivity was found to be significantly higher in winter than in summer. The magnitude of this effect on sensitivity was greater in regions of the USA where seasonal variations in climate were greater. Even though IOP also exhibits seasonal variations, there was no evidence of a causal relation between the two. The cause of this seasonal effect on visual field sensitivity requires further investigation, as it may provide a means to reduce variability, and/or learn more about the pathophysiology of glaucoma.
Supplementary Material
Acknowledgments
Supported by the National Eye Institute, Bethesda, Maryland (grant nos. EY09341 [MOG], EY09307 [MAK]; Merck Research Laboratories, West Point, Pennsylvania (MAK); and unrestricted grants from Research to Prevent Blindness, Inc., New York, New York. The sponsors / funding organizations had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This material will be presented in part at the ARVO meeting in Fort Lauderdale, May 2012.
No conflicting relationship exists for any author.
References
- 1.Artes P, Hutchison D, Nicolela M, et al. Threshold and variability properties of matrix frequency-doubling technology and standard automated perimetry in glaucoma. Invest Ophthalmol Vis Sci. 2005;46:2451–7. doi: 10.1167/iovs.05-0135. [DOI] [PubMed] [Google Scholar]
- 2.Chauhan B, House P. Intratest variability in conventional and high-pass resolution perimetry. Ophthalmology. 1991;98:79–83. doi: 10.1016/s0161-6420(91)32337-6. [DOI] [PubMed] [Google Scholar]
- 3.Gardiner S, Demirel S, Johnson C. Modeling the sensitivity to variability relationship in perimetry. Vision Res. 2006;46:1732–45. doi: 10.1016/j.visres.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Heijl A, Lindgren G, Olsson J. Normal variability of static perimetric threshold values across the central visual field. Arch Ophthalmol. 1987;105:1544–9. doi: 10.1001/archopht.1987.01060110090039. [DOI] [PubMed] [Google Scholar]
- 5.Heijl A, Lindgren A, Lindgren G. Test-retest variability in glaucomatous visual fields. Am J Ophthalmol. 1989;108:130–5. doi: 10.1016/0002-9394(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 6.Henson D, Chaudry S, Artes P, et al. Response variability in the visual field: comparison of optic neuritis, glaucoma, ocular hypertension, and normal eyes. Invest Ophthalmol Vis Sci. 2000;41:417–21. [PubMed] [Google Scholar]
- 7.Spry P, Johnson C, McKendrick A, Turpin A. Variability components of standard automated perimetry and frequency-doubling technology perimetry. Invest Ophthalmol Vis Sci. 2001;42:1404–10. [PubMed] [Google Scholar]
- 8.Turpin A, McKendrick AM, Johnson CA, Vingrys AJ. Properties of perimetric threshold estimates from full threshold, ZEST, and SITA-like strategies, as determined by computer simulation. Invest Ophthalmol Vis Sci. 2003;44:4787–95. doi: 10.1167/iovs.03-0023. [DOI] [PubMed] [Google Scholar]
- 9.Kass MA, Heuer DK, Higginbotham EJ, et al. Ocular Hypertension Treatment Study Group The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 10.Kass MA, Gordon MO, Gao F, et al. Ocular Hypertension Treatment Study Group Delaying treatment of ocular hypertension: the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2010;128:276–87. doi: 10.1001/archophthalmol.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi IA, Xiao RX, Yang BH, et al. Seasonal and diurnal variations of ocular pressure in ocular hypertensive subjects in Pakistan. Singapore Med J. 1999;40:345–8. [PubMed] [Google Scholar]
- 12.David R, Zangwill L, Briscoe D, et al. Diurnal intraocular pressure variations: an analysis of 690 diurnal curves. Br J Ophthalmol. 1992;76:280–3. doi: 10.1136/bjo.76.5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeimer RC, Wilensky JT, Gieser DK. Presence and rapid decline of early morning intraocular pressure peaks in glaucoma patients. Ophthalmology. 1990;97:547–50. doi: 10.1016/s0161-6420(90)32543-5. [DOI] [PubMed] [Google Scholar]
- 14.Bengtsson B. Some factors affecting the distribution of intraocular pressures in a population. Acta Ophthalmol (Copenh) 1972;50:33–46. doi: 10.1111/j.1755-3768.1972.tb05639.x. [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal M, Blumenthal R, Peritz E, Best M. Seasonal variation in intraocular pressure. Am J Ophthalmol. 1970;69:608–10. doi: 10.1016/0002-9394(70)91628-4. [DOI] [PubMed] [Google Scholar]
- 16.Junoy Montolio FG, Wesselink C, Gordijn M, Jansonius NM. Factors that influence standard automated perimetry test results in glaucoma: test reliability, technician experience, time of day and season. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.12-10268. In press. [DOI] [PubMed] [Google Scholar]
- 17.Gordon MO, Kass MA, Ocular Hypertension Treatment Study Group The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–83. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 18.Demirel S, De Moraes CG, Gardiner SK, et al. Ocular Hypertension Treatment Study Group The rate of visual field change in the Ocular Hypertension Treatment Study. Invest Ophthalmol Vis Sci. 2012;53:224–7. doi: 10.1167/iovs.10-7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Moraes CG, Demirel S, Gardiner SK, et al. Ocular Hypertension Treatment Study Group Effect of treatment on the rate of visual field change in the Ocular Hypertension Treatment Study observation group. Invest Ophthalmol Vis Sci. 2012;53:1704–9. doi: 10.1167/iovs.11-8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sit AJ, Asrani S. Effects of medications and surgery on intraocular pressure fluctuation. Surv Ophthalmol. 2008;53(suppl):S45–55. doi: 10.1016/j.survophthal.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Varma R, Hwang LJ, Grunden JW, Bean GW. Using diurnal intraocular pressure fluctuation to assess the efficacy of fixed-combination latanoprost/timolol versus latanoprost or timolol monotherapy. Br J Ophthalmol. 2010;94:80–4. doi: 10.1136/bjo.2009.162107. [DOI] [PubMed] [Google Scholar]
- 22.Treister G, Mannor S. Intraocular pressure and outflow facility: effect of estrogen and combined estrogen-progestin treatment in normal human eyes. Arch Ophthalmol. 1970;83:311–8. doi: 10.1001/archopht.1970.00990030313008. [DOI] [PubMed] [Google Scholar]
- 23.Paterson GD, Miller SJ. Hormonal influence in simple glaucoma: a preliminary report. Br J Ophthalmol. 1963;47:129–37. doi: 10.1136/bjo.47.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweeney EJ, Kinney JA, Ryan A. Seasonal changes in scotopic sensitivity. J Opt Soc Am. 1960;50:237–40. doi: 10.1364/josa.50.000237. [DOI] [PubMed] [Google Scholar]
- 25.Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood JP, Lascaratos G, Bron AJ, Osborne NN. [Accessed September 19, 2012];The influence of visible light exposure on cultured RGC-5 cells. Mol Vis [serial online] 2007 11:334–44. Available at: http://www.molvis.org/molvis/v14/a42/ [PMC free article] [PubMed] [Google Scholar]
- 27.Osborne NN, Li GY, Ji D, et al. Light affects mitochondria to cause apoptosis to cultured cells: possible relevance to ganglion cell death in certain optic neuropathies. J Neurochem. 2008;105:2013–28. doi: 10.1111/j.1471-4159.2008.05320.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.