Abstract
The dorsomedial hypothalamus (DMH) has long been implicated in feeding behavior and thermogenesis. The DMH contains orexigenic neuropeptide Y (NPY) neurons, but the role of these neurons in the control of energy homeostasis is not well understood. NPY expression in the DMH is low under normal conditions in adult rodents, but is significantly increased during chronic hyperphagic conditions such as lactation and diet-induced obesity (DIO). To better understand the role of DMH-NPY neurons, we characterized the efferent projections of DMH-NPY neurons using the anterograde tracer biotinylated dextran amine (BDA) in lactating rats and DIO mice. In both models, BDA and NPY co-labeled fibers were mainly limited to the hypothalamus including the paraventricular nucleus of the hypothalamus (PVH), lateral hypothalamus/perifornical area (LH/PFA), and anteroventral periventricular nucleus (AVPV). Specifically in lactating rats, BDA and NPY co-labeled axonal swellings were in close apposition to CART expressing neurons in the PVH and AVPV. Although the DMH neurons project to the rostral raphe pallidus (rRPa) these projections did not contain NPY immunoreactivity in either the lactating rat or DIO mouse. Instead, the majority of BDA-labeled fibers in the rRPa were orexin positive. Furthermore, DMH-NPY projections were not observed within the nucleus of the solitary tract (NTS), another brainstem site critical for the regulation of sympathetic outflow. The present data suggest that NPY expression in the DMH during chronic hyperphagic conditions plays important roles in feeding behavior and thermogenesis by modulating neuronal functions within the hypothalamus, but not in the brainstem.
Keywords: hypothalamus, lactation, obesity, food intake, thermogenesis
INTRODUCTION
Neuropeptide Y (NPY) is a potent orexigenic neuropeptide and is widely expressed in the brain (Beck, 2006; Chee and Colmers, 2008). In the hypothalamus, NPY expressing neurons are primarily localized in the arcuate nucleus of the hypothalamus (ARH), a key site for the integration of hormonal and nutritional signals from the periphery (Gehlert et al., 1987; Morris, 1989). NPY neurons are also located in the dorsomedial hypothalamus (DMH), another important component of the hypothalamic neurocircuitry involved in ingestive behavior and thermoregulation (Bellinger and Bernardis, 2002; Dimicco and Zaretsky, 2007). Lesion studies in rats demonstrated that the DMH is critical for maintaining normal food intake and body weight (Bellinger and Bernardis, 2002; Bellinger et al., 1979; Bellinger et al., 1986), and that these lesioned animals are resistant to diet-induced obesity (DIO) (Bernardis and Bellinger, 1986; Bernardis and Bellinger, 1991). DMH neurons also regulate brown adipose tissue (BAT) activity and temperature via direct projections to the rostral raphe pallidus (rRPa), an important thermoregulatory site in the brainstem (Cano et al., 2003; Cao et al., 2004; Cao and Morrison, 2006; Nakamura et al., 2005; Oldfield et al., 2002; Yoshida et al., 2009). Several recent studies have also emphasized the importance of DMH neurons in the regulation of energy homeostasis (Chao et al., 2011; Enriori et al., 2011; Tupone et al., 2011; Yang et al., 2009; Zhang et al., 2011). In particular, NPY neurons in the DMH have been associated with hyperphagia and suppression of BAT thermogenic capacity in several rodent models (Chao et al., 2011; Chen et al., 2004; Guan et al., 1998a; Kesterson et al., 1997; Yang et al., 2009).
There are some key species differences in the expression of NPY within the DMH. NPY is constitutively expressed in the compact subdivision of the DMH (DMHc) in intact rats, while no expression is detected in the DMHc in intact mice (Bi et al., 2003; Guan et al., 1998a; Li et al., 1998a). However, both rats and mice exhibit a transient induction of NPY in a separate population of neurons in the non-compact subdivision (DMHnc) during specific physiological conditions, including during early postnatal development, lactation, and obesity (Grove et al., 2001; Grove and Smith, 2003; Guan et al., 1998a; Guan et al., 1998b; Kesterson et al., 1997; Li et al., 1998a; Smith and Grove, 2002). The common link between these conditions is hyperphagia; however, the hormonal or neuronal signals inducing DMH-NPY expression is not known.
Lactation is a state of negative energy balance due to the energy drain from suckling and milk production. To compensate for this energy drain, lactating animals exhibit several adaptive physiological responses, including hyperphagia, energy conservation by reducing heat production, and suppression of cyclic reproductive function (Brogan et al., 1999; Wade and Schneider, 1992; Wade et al., 1996). Hyperphagic behavior during lactation is supported by an increase in NPY and agouti-related protein (AgRP) mRNA expression in the ARH (Li et al., 1998a; Li et al., 1998b). In addition, NPY mRNA expression is increased in the DMHnc, which is also believed to play a crucial role in the physiological adaptations occurring during lactation (Chen and Smith, 2004; Chen et al., 2004; Smith and Grove, 2002; Smith et al., 2010).
NPY mRNA induction in the DMH is also reported in several obese mouse models including diet-induced obese, lethal yellow (Ay), MC4-R knockout (MC4-RKO), brown adipose tissue deficient (UCP-DTA) , and tubby mice (Guan et al., 1998a; Guan et al., 1998b; Kesterson et al., 1997; Tritos et al., 1998). Guan et al. first reported that NPY mRNA expression is induced in the DMH after 24 weeks of high fat diet treatment in mice (Guan et al., 1998a), while NPY expression in the ARH is noticeably decreased, suggesting that DMH-NPY induction plays a major role in hyperphagic behavior observed in obese mice.
The DMH neurons project to many hypothalamic areas, including the paraventricular nucleus (PVH) and lateral hypothalamus (LH) (Thompson and Swanson, 1998), where hypothalamic and brainstem signals are integrated to modulate feeding behavior and sympathetic outflow (Bai et al., 1985; Broberger et al., 1998; Legradi and Lechan, 1999; Li et al., 1998b). Although DMH-NPY mRNA induction is closely linked to hyperphagic behavior, there is little evidence of a functional connection between DMH-NPY neurons and these feeding regulatory sites in the brain. To better understand the role of DMH-NPY induction in chronic hyperphagic models, we injected the anterograde tracer, biotinylated dextran amine (BDA), into the DMH to identify the potential targets of DMH-NPY projections in the hypothalamus and brainstem of lactating rats and DIO mice.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee. Rat studies: Pregnant or cycling female Wistar rats (220g) were purchased from Simonsen Laboratories (Gilroy, CA). The animals were housed individually and maintained under a 12 hr light/dark cycle (lights on at 7:00 A.M.) and constant temperature (23 ± 2°C). Food and water were provided ad libitum. The pregnant rats were checked for the birth of the pups every morning; the day of delivery was considered day 0 postpartum, and litters were adjusted to eight pups on day 2. Mouse studies: 4 week-old C57BL/6 male mice were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA). To generate DIO mice, 5 week-old C57BL/6 mice were fed a 60% high fat diet (Research Diets, NJ, USA, Cat# D12492) or normal chow diet (Purina lab chow #5001) for 20 weeks. Five mice were group-housed in the same cage and maintained under a 12 hr light/dark cycle (lights on at 7:00 A.M.) and constant temperature (23 ± 2°C). Food and water were provided ad libitum.
Stereotaxic surgery for BDA injection
Lactating rats
On postpartum day 6 or 7, the lactating rats were separated from the pups and anesthetized with 3% isoflurane. The rats (both lactating and cycling) were placed in a stereotaxic apparatus and maintained under 2-2.5% isoflurane mixed with oxygen for the entire duration of the surgery. A small hole was drilled into the skull under aseptic conditions. A glass micropipette (20μm tip diameter) connected to an air pressure injector system was positioned via the stereotaxic manipulator. 60nls of the anterograde tracer biotin dextran amine (BDA; 5% in water; Molecular Probes, Eugene, OR; 10,000 MW; cat. # D1956)(Reiner et al., 2000) was inserted into the area surrounding the compact zone of DMH (coordinates: 3.3 mm caudal, 0.5 mm lateral to bregma, and 8.4 mm ventral to the dura), according to the atlas of Paxinos and Watson. This is the area containing a high density of suckling-activated NPY neurons. As a comparison to lactating rats (with NPY expression in both DMHnc and DMHc), we also investigated DMH projections in virgin rats (which have expression only in the DMHc). The virgin rats were studied during random stages of the estrous cycle. BDA injections were targeted to the NPY neurons in the DMHc (coordinates: 3.3 mm caudal, 0.4 mm lateral to bregma, and 8.4 mm ventral to the dura). After injection, the micropipette was removed and the incision was closed with surgical staples. Rats were monitored during the recovery period, and the pups were returned to the dams immediately after the recovery. The rats were monitored for normal food intake and weight recovery for 7 days before sacrificing for the following experiments.
Male DIO mice
On the day of surgery, DIO or chow diet (CD) mice were anesthetized with tribromoethanol (20 mg/kg body weight, i.p.). The mice were placed in a stereotaxic apparatus and a small hole was drilled into the skull under aseptic conditions. A glass micropipette (20 μm tip diameter) connected to an air pressure injector system was positioned via the stereotaxic manipulator. 40 nl of BDA was injected into the area surrounding the compact zone of DMH [coordinates: 1.2 mm caudal, 0.2 mm lateral to bregma, and 5.15 mm ventral to the dura, according to the mouse brain atlas of Paxinos and Franklin (second edition, 2001)]. After injection, the micropipette was removed and the incision was closed with surgical staples. The mice were monitored for normal food intake and weight recovery for 3 days before sacrificing for the following experiments.
Tissue preparation
The animals were anesthetized with tribromoethanol (20 mg/100g body weight, i.p.) and perfused transcardially with 150 ml of 0.9 % in saline, followed by 300 ml of 4% - paraformaldehyde, pH 7.4. The brain was removed and post-fixed overnight in 4% paraformaldehyde. The following day, the brains were transferred and stored in 25% sucrose solution containing 0.05 M potassium PBS (KPBS; pH 7.4) until frozen in dry ice. The frozen brains were cut on a sliding microtome in 25 μm sections and collected and stored in cryoprotectant at −20°C until use.
Antibodies
All primary antibodies (Table 1) were titrated to give optimum signal with minimal background. For detection of NPY immunoreactivity, a sheep polyclonal NPY antibody (Chemicon, Temecula, CA; Cat. #1583), raised against synthetic NPY peptide conjugated to bovine thyroglobulin, was used. This antibody was shown by competitive radioimmunoassay to exhibit ≈36% and 0.05% crossreactivity for Peptide YY (PYY) and bovine pancreatic polypeptide (PP), respectively, and staining was completely abolished following preabsorption with the immunogen (manufacturer’s technical information). To determine whether the NPY antibody crossreacted with PYY or other antigens in our preparation, we compared antibody staining between an NPY knockout mouse and a wildtype mouse. No staining was seen in the forebrain or brainstem when the antibody was used in the NPY knockout mouse (not shown), suggesting that this antibody is specific for NPY and does not crossreact with PYY in our preparation.
Table 1.
Primary antibodies
| Antigen | Immunogen | Host | Dilution | Company | Catalog No. |
|---|---|---|---|---|---|
| NPY | Synthetic NPY peptide conjugated to bovine thyroglobulin |
sheep | 1:3000 | Chemicon | AB1583 |
| NPY | Neuropeptide Y coupled to bovine thyroglobulin |
rabbit | 1:1000 | ImmunoStar | 22940 |
| CART | CART peptide 55-102 | rabbit | 1:5000 | Phoenix Pharmaceuticals |
H-003-62 |
| Orexin | carboxy terminus (amino acids 48-66) of human orexin A |
goat | 1:2000 | Santa Cruz Biotechnology |
SC-8070, C-19 |
| MCH | The full-length (19 amino acid) MCH peptide |
rabbit | 1:1000 | Phoenix Pharmaceuticals |
H-070-47 |
| GnRH | Mammalian GnRH decapeptide |
mouse | 1:5000 | Dr. Henryk Urbanski, ONPRC |
HU11B, Lot 4 |
| TPH | A recombinant rabbit TPH |
mouse | 1:1000 | Sigma | T0678, clone WH-3 |
Cocaine- and amphetamine-regulated transcript (CART) immunoreactivity was detected using a well-characterized rabbit polyclonal CART antibody (Phoenix Pharmaceuticals, Burlingame, CA; Cat.# H-003-62) raised against CART peptide 55-102. This antibody was shown to exhibit 100% crossreactivity with human, rat, and mouse CART and 0% crossreactivity with NPY, α-MSH, orexin A and B by competitive radioimmunoassay (manufacturer’s technical information). The antiserum stains a single band of 5-kD molecular weight on Western blot (manufacturer’s technical information) and preabsorption of this antiserum with the CART (55–102) peptide fragments eliminated immunostaining of neurons and fibers in the brain of rats (Dun et al., 2000a; Dun et al., 2000b; Dun et al., 2000c).
The orexin antibody used in the present study was a goat polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA; Cat. #sc-8070, C-19) raised against a peptide mapping at the carboxy terminus of human orexin A (residues 48–66 of the orexin precursor, identical to corresponding mouse sequence; manufacturer’s technical information). The specificity of this antibody has previously been verified by preabsorption with orexin, which abolished all staining (Florenzano et al., 2006) and by Western blotting which shows specificity for rat, mouse, and human orexin A (manufacturer’s technical information). The antibody was also shown to label hypothalamic neurons in the lateral hypothalamus, perifornical region, and dorsomedial nucleus (Florenzano et al., 2006), consistent with the known pattern of orexin neurons (Sakurai et al., 1998). We confirmed the expected pattern of orexin staining in our preparation and that preabsorption of the antibody with orexin A (Phoenix Pharmaceuticals) abolished all staining in both the forebrain and the brainstem.
For gonadotropin releasing hormone (GnRH) immunoreactivity, we used a monoclonal mouse anti-GnRH (HU4H) directed against the mammalian GnRH decapeptide. This antibody has been extensively characterized (Urbanski, 1991). Immunocytochemical labeling of brain sections using HU4H as a primary antibody produced intense and highly specific staining of neuronal cell bodies and fibers; preabsorption of the primary antibody with excess GnRH or substitution with a non-GnRH monoclonal antibody eliminated the specific staining.
The anti-tryptophan hydroxylase (TPH) mouse monoclonal antibody (Sigma, St. Louis, MO ; Cat. # T0678, clone WH-3 ) was a purified immunoglobulin raised against a recombinant rabbit TPH and was generated from a WH-3 hybridoma. This antibody reacted specifically with TPH in immunoblotting assays, with a single band of the correct molecular weight of 55 kDa (manufacturer’s technical information). Western blotting of this antibody was also shown in human brains (Kish et al., 2008). T0678 has also been used in double-labeling experiments of brainstem serotonergic neurons by a number of investigators (Burman et al., 2003; Liu and Wong-Riley, 2010).
Immunohistochemistry for BDA and NPY
To visualize the BDA injection site, the sections containing the DMH were washed in 0.05 M potassium PBS (KPBS) and incubated in 1:200 Streptavidin Alexa Fluor 568 conjugate (Invitrogen; Cat # S11226) in KPBS for 4 hrs. The sections were then rinsed in KPBS and incubated in Hoechst nuclear staining solution (Molecular probes, Cat# H1398) for 5min at room temperature to identify the morphological limits of the DMH. BDA injection sites were determined under fluorescent microscopy.
To visualize BDA and NPY co-localized fibers, a one in six series of 25 μm sections were rinsed in KPBS (pH 7.4), followed by blocking solution containing 0.4% Triton-X and 2% normal donkey serum in KPBS (KPBS-TX-NDS) for 30 min at room temperature. Sections were then incubated in 1:3000 sheep anti NPY antibody (Chemicon) in KPBS-TX-NDS for 48 hr at 4° C. After incubation, the tissue was rinsed in KPBS and incubated in 1:1000 Alexa Fluor 488 donkey anti-sheep (Invitrogen ; Cat# A11015) and 1:200 Streptavidin Alexa Fluor 568 in KPBS containing 0.4% Triton-X (KPBS-TX) for 4 hr at room temperature. The tissue sections were then mounted on gelatin-coated glass slides and coverslipped with SlowFade Gold antifade reagent (Invitrogen; Cat# S36936).
Triple-label Immunohistochemistry
PVH
To visualize BDA/NPY co-localized axonal swellings in relation to CART neurons, tissue sections were incubated in NPY antibody (described above) and 1:5000 rabbit anti CART (Phoenix pharmaceuticals) in KPBS-TX-NDS for 48 hrs at 4° C. The tissues were then rinsed and incubated in 1:1000 Alexa Fluor 488 donkey anti-rabbit (Invitrogen; Cat# A21206,) 1:200 Alexa Fluor 568 Streptavidin for BDA detection and 1:1000 Dylight 649 donkey anti-sheep (Jackson ImmunoResearch; Cat# 713-496-147) for 4 hrs at room temperature. After rinsing in KPBS, the tissue sections were mounted on gelatin-coated glass slides and coverslipped with SlowFade Gold antifade reagent.
AVPV
To visualize BDA/NPY fibers with GnRH neurons, tissue sections were incubated in 1:5000 mouse anti GnRH (HU4H, Dr. Henryk Urbanski, OHSU, OR) and NPY antibody containing KPBS-TX-NDS solution for 48 hrs. GnRH, BDA and NPY were visualized with 1:1000 Alexa Fluor 488 donkey anti-mouse, 1:200 Alexa Fluor 568 Streptavidin and 1:1000 Dylight 649 donkey anti-sheep. BDA, NPY, and CART triple label immunostaining was performed as described in the as described above (PVH section).
rRPa
Tissue sections were incubated with 1:2000 goat anti orexin antibody (Santa Cruz Biotechnology) for 48 hrs and BDA/orexin colocalized fibers were visualized by 1:1000 Alexa Fluor 488 donkey anti goat and 1:200 Alexa fluor 568 streptavidin treatment. For triple labeling of BDA, orexin and TPH, tissue sections were incubated in 1:2000 goat anti orexin antibody and 1:1000 mouse anti TPH (Sigma) KPBS-TX-NDS solution. BDA, orexin, and TPH were visualized with 1:1000 Alexa Fluor 488 donkey anti-mouse, 1:200 Alexa Fluor 568 Streptavidin and 1:1000 Cy5 donkey anti-goat (Jackson ImmunoResearch; Cat# 705-176-147) respectively.
Confocal microscopy
Confocal laser microscopy was used to analyze the double- and triple-label IF images for BDA/NPY colocalization and close appositions as described by our laboratory (Campbell et al., 2003; Glavas et al., 2008). The TSC SP confocal system (Leica Corp., Germany) was used to scan the images, consisting of a RBE inverted microscope, an Ar laser-producing light at 488 nm (for visualization of FITC), a Kr laser-producing light at 568 nm (for visualizing TRITC), and a HeNe laser-producing light at 647 nm (for visualization of Cy5). Various objectives (25X, numerical aperture 0.75 and 40X, numerical aperture 1.25) were used to scan and capture images. In order to detect BDA/NPY co-localization, each brain area (two medial sections per animal) was divided into smaller areas using 2x optical zoom at 40X magnification. Each image was scanned at 1μm intervals. For each experiment, fluorophore signals were checked individually for bleed-through to the apposing detector. Bleed-through was eliminated by adjusting laser intensity and the width of the detector window. To assess colocalization of BDA/NPY signals in close apposition to a cell body, a sequential series of optical sections, 0.5 μm intervals along the z-axis of the tissue section, were scanned for each fluorescent signal. The signals were obtained for each fluorophore on one series of optical sections and stored separately as a series of 512 × 512 pixel images. The stacks of individual optical slices were analyzed using ImageJ software (NIH, Bethesda, MA) to determine co-localization and close appositions. The confocal images are presented as projections of stacks of optical images or as individual slices, as indicated. The brightness and contrast of the images were adjusted in Photoshop to match microscope visualization (Adobe Systems Inc., San Jose, CA).
RESULTS
BDA labeling of DMH projections in rats and mice
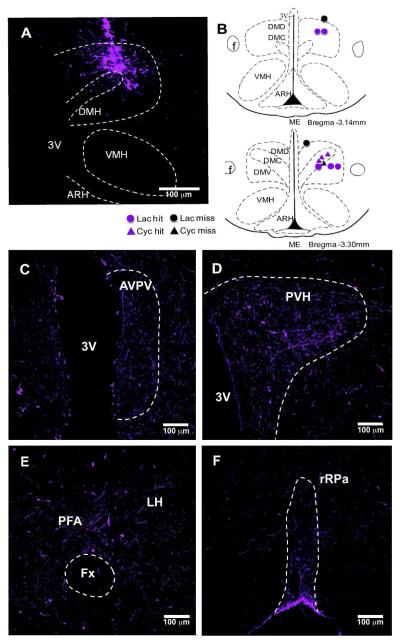
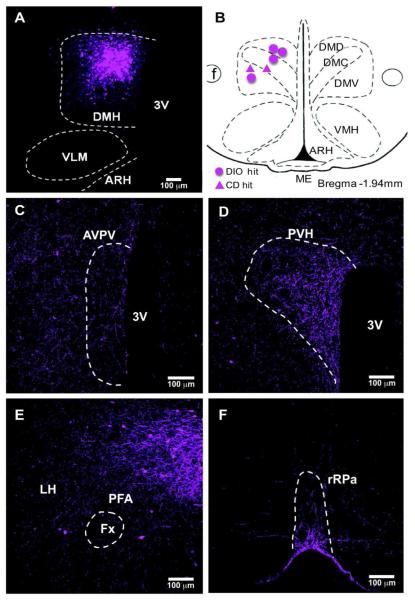
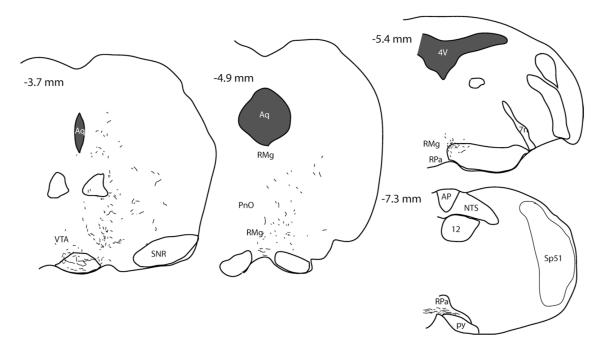
In order to trace DMH-NPY neuronal projections, BDA was stereotaxically injected into the DMH in lactating and cycling rats. DMH neuronal projections labeled with BDA closely resembled the pattern of PHA-L labeled DMH projections in the rat reported by Thompson and Swanson (1998). The BDA-labeled fiber projections were mainly restricted to the hypothalamus, with limited projections to the brainstem. The fiber projection pattern was indistinguishable between lactating and cycling rats. We also injected BDA into the DMH of male mice fed with either high fat diet (DIO) or normal chow diet (CD) for 20 weeks. BDA-labeled fiber projections in mice were similar to DMH projections in rats. As illustrated in Fig 1 A,B (rats), and Fig 2 A,B (mice), BDA injection sites were included in the areas where the most NPY induction is observed during lactation and DIO condition. In the present study, all the BDA injections within the DMH border also showed a similar fiber distribution in major target areas, including the anteroventral periventricular nucleus (AVPV), PVH, and LH in the hypothalamus, and the rRP in the brainstem as illustrated in Fig.1 C-F (rats) and Fig. 2 C-F (mice). In the ascending pathways, most BDA labeled fibers are found in the PVH, perifornical area (PFA) in the LH, and preoptic areas including parastrial nucleus (PS), lateral preoptic (LPO) and AVPV. Caudal to the DMH, numerous BDA labeled fibers were also detected in supramammillary nucleus (SUM), ventral tegmental area (VTA), ventrolateral periaqueductal grey (vlPAG), barrington’s nucleus (BA), locus ceoruleous (LC) and lateral parabrachial nucleus (LPB). A small number of BDA labeled fibers were also observed in caudal brainstem areas including the rRPa, raphe magnus (RM), nucleus of the solitary tract (NTS), and ventral lateral medulla (VLM).
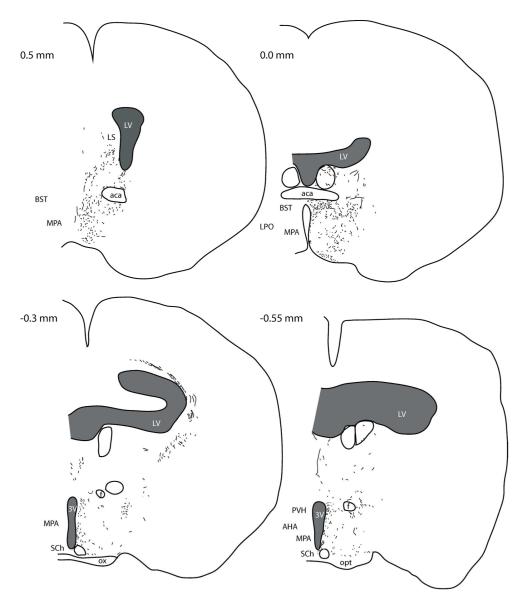
Figure 1. BDA injection in the DMH of lactating rats.
(A) Injection site in the DMH in 10x confocal image. BDA (magenta) was visualized 7 days after injection using streptavidin amplification method. (B) The mapping of BDA injection sites in rostral (upper) and caudal (lower) DMH. Lactating rats (circle) and cycling rats (triangles) hit and miss cases are illustrated. BDA fiber distribution in the AVPV (C), PVH (D), LH/PFA(E), and rRPa (F) are illustrated in 20x confocal images.
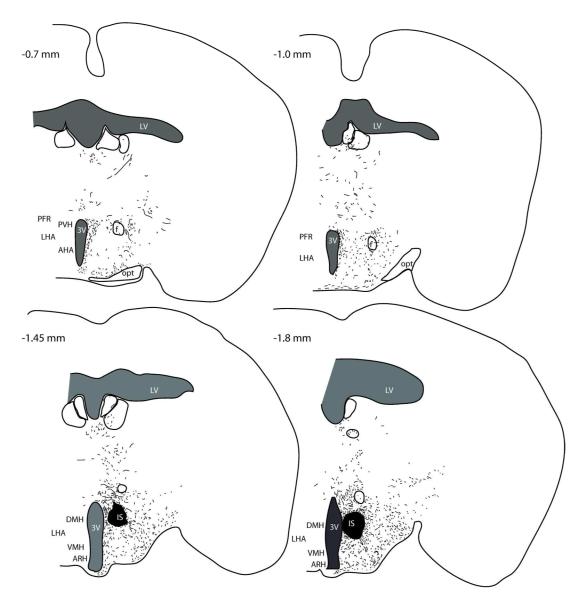
Figure 2. BDA injection in the DMH of DIO mice.
(A) Injection site in the DMH in 10x confocal image. BDA (magenta) was visualized 3 days after injection. (B) The mapping of BDA injection sites. The circles indicate the hit cases in DIO group and triangles indicate CD mice. BDA labeled fibers were mainly distributed in the AVPV (C), PVH (D), LH (E) in the hypothalamus, and rRPa (F) in the brainstem, as illustrated in 20x confocal images.
DMH-NPY neurons project to the PVH in lactating rats
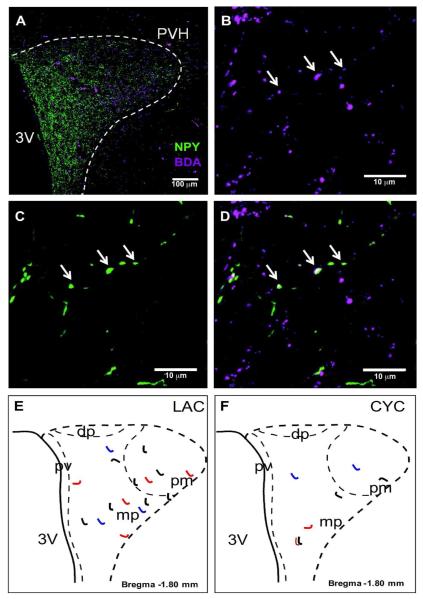
To further characterize DMH-NPY projections to the PVH, two medial PVH sections per animal were scanned at 1μm intervals using a confocal laser microscope to identify BDA/NPY labeled fibers (Fig. 3A). BDA/NPY co-localized fibers (total 8-10 fibers/animal) were detected in the PVH (Fig. 3B-D), primarily located in the medial parvicellular and posterior magnocellular areas in lactating rats (n=4) (Fig. 3E). In lactating animals, BDA labeled NPY neurons in both compact (DMHc) and non-compact (DMHnc) subdivision. No BDA/NPY co-localized fibers were detected in the PVH when the BDA was injected into the DMH border or slightly outside of the DMH in lactating rats. While cycling female rats express a low level of NPY mRNA in the DMHc with no expression within the DMHnc, the pattern of BDA/NPY co-localized fibers (total 2-4/ animal, n=3) within the PVH was similar to that observed in the lactating rat (Fig. 3F).
Figure 3. DMH-NPY projections to the PVH in lactating rats.
(A) 20x confocal image showing BDA (magenta) and NPY (green) fiber distribution in the PVH. (B) BDA fiber distribution in 40x confocal image. Arrows indicate BDA/ NPY co-localized fiber. (C) NPY fiber distribution in 40x confocal image. Arrows indicate BDA/NPY co-localized fiber. (D) Overlay of 40x confocal images showing BDA/NPY co-localized fiber. Schematic drawings show the distribution of BDA fibers co-localized with NPY in the PVH of lactating (E) and cycling (F) rat. Projections from 3 different cases with injections limited to the DMH are represented in different colors (red, blue and black).
CART-expressing neurons are located in the parvicellular area where it is co-localized with thyrotropin-releasing hormone (TRH) neurons involved in the regulation of energy homeostasis by the activation of the hypothalamic-pituitary-thyroid axis (Elias et al., 2001; Lechan and Fekete, 2006; Silva, 1995). To determine the spatial relationship between DMH-NPY projections and CART-immunoreactive (ir) neurons in the PVH single optical slice analysis with a confocal microscope was used. Triple-label immunostaining for BDA/NPY/CART revealed that DMH-BDA/NPY axonal swellings are in close apposition (within 0.5 μm) to PVH -CART-ir neurons in lactating rats (Fig. 4 A-G).
Figure 4. DMH-NPY projections in close apposition with CART neurons in the PVH of lactating rats.
(A) 20x confocal image showing triple label immunostaining for NPY (green), BDA (red), and CART (blue) in the PVH of a lactating rat. The arrow indicates a fiber containing NPY and BDA near a CART cell body. (B) 40x confocal image showing BDA-containing axonal swelling (white arrow) in close apposition to a CART cell (yellow arrowhead). (C) 40x confocal image showing NPY-containing axonal swelling in close apposition to a CART cell. (D) Overlay image for BDA and NPY co-localized fiber in close apposition to a CART cell. (E-G) Stacks of optical slices (3 μm total at 0.5μm increments) of a CART cell body in close contact with BDA and NPY-containing axonal swelling. Z-coordinates are indicated in single optical slices relative to a reference point, z = 0 μm.
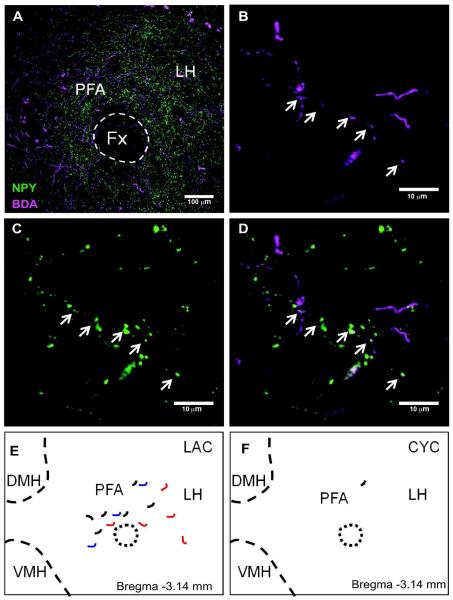
DMH-NPY neurons project to the LH in lactating rats
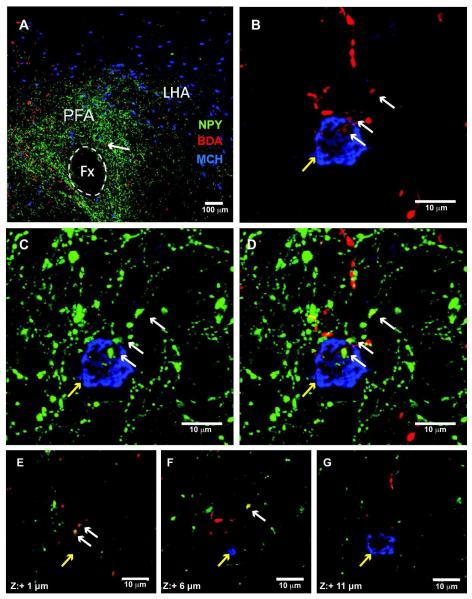
Using the same confocal analysis method (2 sections/animal), BDA/NPY co-localized fibers (total 5-6/animal) were observed in the LH of lactating rats (n=4), mainly concentrated in the perifonical area (Fig. 5). While BDA/NPY fibers were readily detected in all of the lactating rats, the occurrence of co-labeled fibers in the virgin cycling rats were scarce (total 0-2/animal, n=3). Furthermore, while these BDA/NPY projections were readily evident in areas containing MCH (Fig. 6) and Orexin (not shown) immunoreactive fibers, we observed no evidence of close appositions.
Figure 5. DMH-NPY projections to the LH in lactating rats.
(A) 20x confocal image showing BDA (magenta) and NPY (green) fiber distribution in the LH. (B) BDA fiber distribution in 40x confocal image. Arrows indicate the co-localized fiber. (C) NPY fiber distribution in 40x confocal image. (D) Overlay 40x confocal image showing BDA/NPY co-localized fiber indicated by arrows. Schematic drawing showing the distribution of BDA fibers co-localized with NPY in the LH of lactating (E) and cycling (F) rats. Projections from 3 different cases with injections limited to the DMH are represented in different colors (red, blue and black).
Figure 6. DMH-NPY projections do not make close apposition to MCH neurons in the LHA of the lactating rat.
A) 20x confocal image showing triple label immunostaining for NPY (green), BDA (red), and MCH (blue) in the LHA of a lactating rat. The arrow indicates a fiber containing NPY and BDA near a MCH cell body. (B) 40x confocal image showing BDA-containing axonal swelling (white arrow) in the vicinity of an MCH positive neuron (yellow arrowhead). (C) 40x confocal image showing NPY-containing axonal swelling in the vicinity of a MCH cell. (D) Overlay image for BDA and NPY co-localized fiber in the vicinity to a MCH cell. (E-G) Stacks of optical slices (5.0 μm increments) of a MCH cell body in near but not in close apposition to a BDA and NPY-containing axonal swelling. Z-coordinates are indicated in single optical slices relative to a reference point, z = 0 μm.
DMH-NPY neurons project to the AVPV in lactating rats
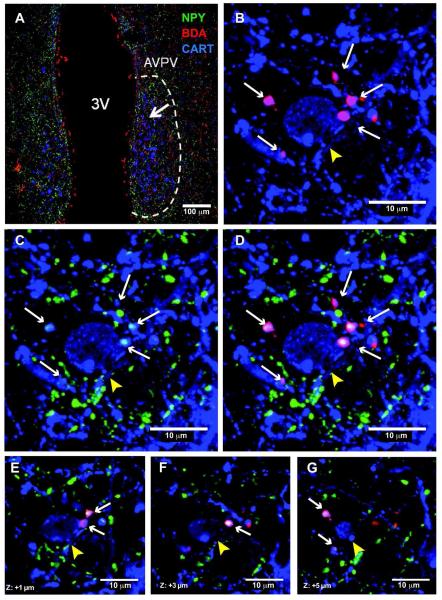
NPY is also one of the essential players in modulating reproductive function and NPY inhibits GnRH neuronal activity in lactation (Smith et al., 2010). Although BDA containing fibers were observed in close apposition to GnRH neurons, none of them contained NPY immunoreactivity (data not shown; n=4). However, the AVPV, which is known to have strong connections with the GnRH axis, did contain BDA/NPY co-localized fibers in lactating rats (Fig. 6). Furthermore, BDA/NPY containing axonal swellings were detected in close apposition to CART expressing neurons in the AVPV in lactating rats (Fig. 7). In contrast, BDA/NPY fibers were not present in the AVPV of cycling rats (n=3).
Figure 7. DMH-NPY fibers in close apposition to CART neurons in the AVPV of lactating rats.
(A) 20x confocal image showing triple label immunostaining for NPY (green), BDA (red), and CART (blue) in the AVPV of a lactating rat. The arrow indicates a fiber containing immunoreactivity for NPY and BDA near a CART neuron. (B) 40x confocal image showing BDA containing axonal swellings (white arrows) in close apposition to a CART neuron (yellow arrowhead). (C) 40x confocal image showing NPY-containing axonal swellings (white arrows) in close apposition to a CART neuron. (D) Overlay image for BDA/ NPY-containing axonal swellings in close apposition to a CART neuron. (E-G) Analysis of single optical slice (4 μm total, 0.5 μm increments) reveals a close apposition.
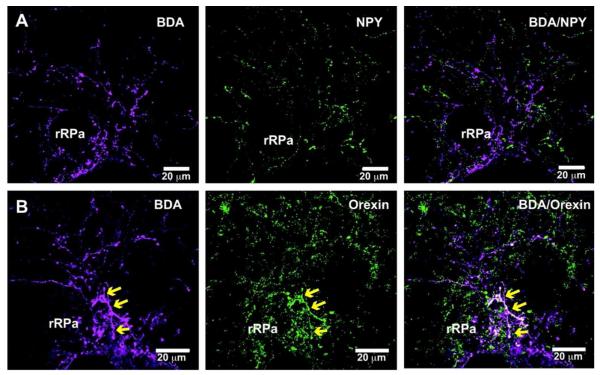
DMH-NPY neurons do not project to the rRPa in lactating rats
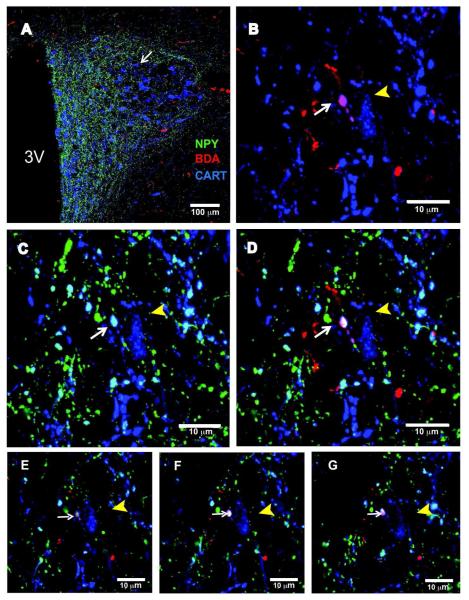
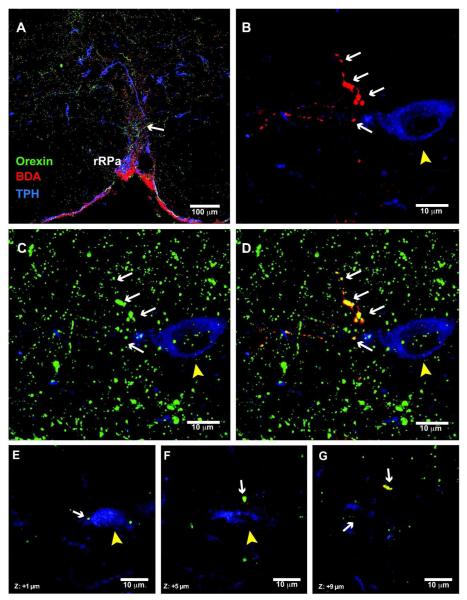
While DMH projections to the brainstem were sparse relative to the hypothalamus, we did detect significant BDA positive fibers in a few key areas in the regulation of sympathetic outflow. One such area with readily detectable BDA-labeled fibers was the rRPa. However, while NPY positive fibers were abundant within the rRPa, no BDA/NPY co-localization was observed in this region of either lactating or cycling rats (Fig. 8 A,B). Instead, many of the BDA-labeled fibers projecting to the rRPa were double-labeled for orexin (Fig. 9). Additionally, we found BDA /orexin co-localized axonal swellings in close apposition to the neurons expressing tryptophan hydroxylase (TPH), a serotonergic cell marker (Fig. 9 E-G). Furthermore, no BDA/NPY co-localized fibers were observed in other brainstem regions including the NTS, VLM and LPB.
Figure 8. DMH-NPY neurons do not project to the rRPa.
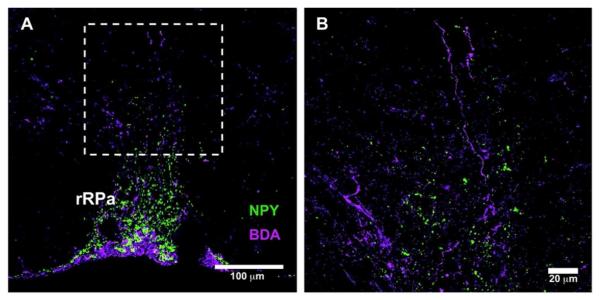
(A) 20x confocal image showing BDA (magenta) and NPY (green) fiber distribution in the rRPa of a lactating rat. (B) A magnified image of dotted area in (A) showing no co-localization for BDA and NPY fibers.
Figure 9. DMH-orexin neurons project to serotonin neurons in the rRPa in lactating rats.
(A) 20x confocal image showing triple label immunostaining for orexin (green), BDA (red), and TPH (blue) in the rRPa. The arrow indicates a fiber containing orexin and BDA near a TPH-expressing neuron. (B) 40x confocal image showing BDA-labeled axonal swellings (white arrows) in the vicinity of a TPH neuron (yellow arrowhead). (C) 40x confocal image showing orexin immunoreactivity in the same section. (D) Overlay image indicating colocalization of BDA and orexin immunoreactivities in the same axonal swellings in the vicinity of this TPH neuron. (E-G) Examples of single, confocal optical slices (1 μm thickness) illustrate colocalization of BDA and orexin immunoreactivities in axonal swellings that are in close apposition to a TPH-expressing neuron.
DMH-NPY neuronal projections in DIO mice
Since DMH projections have not been reported in the mouse we performed a characterization of DMH projections in lean and DIO mice. As is shown in Fig. 10, the afferent projections of the DMH, as represented by the presence of BDA fibers, is similar to that previously reported for the rat.
Figure 10.
Distribution of DMH projections in the male DIO mouse. Computer-assisted line drawings illustrate the distribution of BDA immunoreactive fibers in the mouse receiving a BDA injection into the DMH. The line drawings represent a single case with a BDA injection in the DMH of a DIO mouse. Brain levels are indicated in the upper left corner of each representative section (according to the mouse brain atlas Franklin and Paxinos, 1997). The injection site is indicated by the black shading labeled IS.
In contrast to the rat, NPY neurons are limited to the DMHnc in the DIO mouse. In spite of this, BDA/NPY projections were similar between male DIO mouse and the lactating rat. Most notably, BDA fibers containing NPY immunoreactivity were readily detectable in the parvicellular area of the PVH (Fig. 11) and PFA of the LH in DIO mice (two sections for each area, n=4). In the brainstem, consistent with the findings in lactating rats, BDA and NPY were not co-localized in the rRPa in DIO mice. Similar to the rat, BDA labeled fibers contained orexin immunoreactivity in the rRPa (Fig. 12). Importantly, no BDA/NPY co-localized fibers were observed in the same regions in CD mice (n=2), which do not have detectable NPY mRNA expression in the DMH.
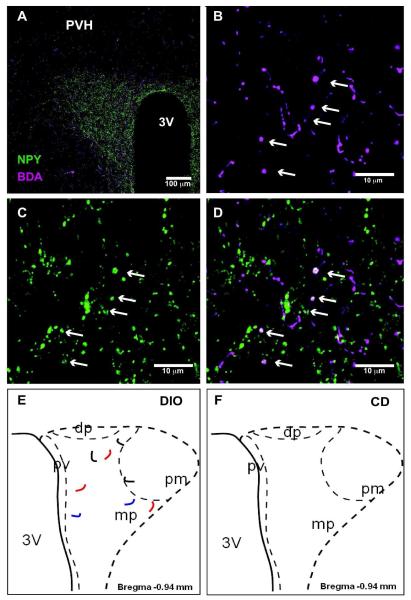
Figure 11. DMH-NPY projections to the PVH in DIO mice.
(A) 20x confocal image showing BDA (magenta) and NPY (green) immunostaining in the PVH. An arrow indicates the location of BDA/NPY co-localized fiber. (B) 40x confocal image showing BDA fibers in the PVH. (C) 40x confocal image showing NPY fibers in the PVH. (D) Overlay 40x confocal image showing BDA/NPY co-localized fiber in the PVH. Arrows indicate the location of co-localization. Schematic drawings show the distribution of BDA fibers co-localized with NPY in the PVH of a DIO mouse (E) and no BDA/NPY co-localized fibers in a CD mouse (F). Projections from 3 different cases with injections limited to the DMH are represented in different colors (red, blue and black).
Figure 12. DMH-NPY neurons do not project to the rRPa, but DMH-orexin neurons project to the rRPa in DIO mice.
(A) 20x confocal images showing BDA (magenta, left), NPY (green, middle) and BDA/NPY (right) fiber distribution in the rRPa in a DIO mouse. No BDA/NPY co-localization is observed. (B) 20x confocal images showing BDA (magenta, left), orexin (green, middle) and BDA/orexin (right) fiber distribution in the rRPa in a DIO mouse. Yellow arrows indicate BDA/orexin co-localization.
DISCUSSION
The present study characterizes the efferent projections of NPY expressing neurons in the DMH in chronic hyperphagic models, the lactating rat and DIO mouse, using BDA anterograde tracing method. The primary DMH-NPY projection targeted hypothalamic regions, i.e., PVH and LH, could be involved in the regulation of many physiological functions, including food intake and autonomic function. We also demonstrated interactions between DMH-NPY projections and CART neurons in the hypothalamic target areas. In contrast, DMH-NPY projections were not observed in the brainstem areas implicated in energy homeostasis, including the rRPa and NTS. Instead, many of the BDA fibers in the rRPa contained orexin immunoreactivity, consistent with a role for orexin neurons in the DMH in the regulation of BAT thermogenesis.
Technical considerations
NPY is expressed in two subregions of the DMH, the DMHc and DMHnc, depending on the species. NPY is constitutively expressed in the DMHc in intact rats. However, there is no evidence of DMHc-NPY expression in other species including the mouse and non-human primate, during development or in adults (Grayson et al., 2006), suggesting that DMHc-NPY expression is unique to rats. The absence of NPY expression in the DMHc suggests that DMHc-NPY neurons are not required for normal energy balance in mice. On the other hand, both rats and mice exhibit a transient expression of NPY in the DMHnc during postnatal development, lactation, and obesity, all of which are characterized by hyperphagia, suggesting that DMHnc-NPY neurons have similar functions in both species.
The DMH is closely surrounded by other hypothalamic regions including the PVH, LHA, VMH and ARH, therefore it is especially difficult to make accurate injections selectively into the DMH without diffusion of the tracer into neighboring regions. Thus, we injected a small amount of BDA tracer into the DMH to limit the spread, and specifically to avoid the spread to the ARH, which also contains NPY neurons. Since no BDA contamination was observed in the ARH, DMH-NPY neurons are likely to be the only sources of NPY fiber projections labeled with BDA. In spite of the small injection volumes, we were unable to distinguish between projections of the two subregions of the DMH since the tracer injections directed at the DMHnc diffused into the neighboring DMHc. In an attempt to distinguish between the DMHc and DMHnc projections, we instead used a combination of models: a) lactating rats that have NPY expression in both DMH subregions, b) virgin female rats that have NPY expression specifically in the DMHc, c) male DIO mice that have NPY expression specifically in the DMHnc, and d) male CD mice that have no NPY expression in the DMH.
Another limitation of these studies is that NPY cells in the DMHnc are scattered throughout the rostral to caudal extent of the nucleus. Therefore, it is likely that the amount of BDA volumes used in this study only labeled a small percentage of NPY neurons in the DMH in each animal. This sparse labeling greatly limits our ability to quantify the density of projections. Due to the technical limitations with the antibody staining, we were also unable to count the number of DMH-NPY neurons labeled with BDA. While we observed some differences in the projections between the models, it is not clear whether this is due to a true difference in projections or due to the number of NPY neurons labeled in each case. Despite these variations, all animals with injection sites within the borders of the DMH showed a similar BDA/NPY co-localized fiber projection pattern.
In the present study, a single optical slice analysis was used to characterize the spatial relationship between BDA/NPY-containing axonal swellings and CART neurons. Although we often detected a close apposition between them in the lactating animals, it is difficult to make any quantifiable conclusions from this observation due to the small number of DMH-NPY projections labeled with BDA. A close apposition within 0.5 μm distance suggests a potential synaptic contact, but it is also not possible from this technique to determine the precise anatomical structure of close appositions without electron microscopic analysis. However, previous studies which imaged close appositions of peptidergic fibers on a cell soma using confocal microscopy found evidence that the peptidergic appositions were indeed synaptic in nature (Kiyoshi et al., 1998; Takenoya et al., 2006). Furthermore, lack of synapse contacts does not rule out the possibility of volume transmission of NPY over considerable distances to reach the targets (Agnati et al., 2010).
The PVH is the major target for DMH-NPY neurons
According to a previous report (Swanson and Kuypers, 1980), DMH neurons project heavily to the parvicellular region of the PVH, which contains neurons that project to sympathetic and parasympathetic preganglionic nuclei, as well as to the median eminence and posterior pituitary (Engelmann et al., 2004; Hallbeck et al., 2001; Sawchenko and Swanson, 1982). In the present study, BDA fibers containing NPY immunoreactivity were observed in the parvicellular and, although less dense, the magnocellular areas of the PVH in lactating and virgin rats as well as male DIO mice. Because we observed DMH-NPY projections to the PVH in both virgin (which express NPY only in the DMHc) and lactating rats (which express NPY in both the DMHnc and DMHc) we have to conclude that both subregions of the DMH contribute to the NPY projections to this area. However, because of the limitations of this technique, it is not possible to determine the relative contribution of the two subregions of NPY neurons in the DMH.
In the parvicellular area of the PVH, TRH neurons are one of the important players in the regulation of food intake and autonomic function (Lechan and Fekete, 2006). In the fasting condition, increased NPY inputs from the ARH inhibit TRH production and stimulate food intake (Fekete and Lechan, 2007). Despite high levels of NPY expression in the ARH, lactation is associated with increased TRH expression in the PVH, which stimulates prolactin secretion (Sanchez et al., 2007). In addition to its main role in milk production, prolactin has an orexigenic effect and is involved in the hyperphagia associated with lactation (Chen and Smith, 2004; Woodside, 2007). CART is also expressed in the parvicellular area (Elias et al., 2001; Fekete et al., 2000), and is linked to the inhibition of TRH-induced prolactin release from the anterior pituitary gland (Fekete and Lechan, 2006). Therefore, an inhibition of CART expression controlled by DMH-NPY neurons could potentially contribute to the increase in prolactin secretion and hyperphagia during lactation. In support of this hypothesis, we demonstrated that DMH-NPY fibers are in close apposition to CART expressing neurons in the PVH in lactating rats.
CART expressing terminals in the PVH are also implicated in the regulation of BAT thermogenesis (Kong et al., 2003; Lechan and Fekete, 2006). I.c.v. injection of CART up-regulates BAT uncoupling protein-1 (UCP-1) mRNA in rats (Wang et al., 2000) and CART mRNA expression in the PVH is increased during cold exposure in lactating rats (Sanchez et al., 2007). Therefore, increased DMH-NPY inputs to suppress CART neurons may also contribute to the suppression of BAT activity during lactation. Additionally, NPY inputs to PVH could reduce BAT activity by acting presynaptically to reduce GABA release (Melnick et al., 2007) onto BAT sympathoinhibitory neurons in the PVH (Madden and Morrison, 2009).
The LH is a potential mediator of hyperphagia
Although NPY in the PVH coordinates various aspects of energy metabolism in addition to controlling appetite, NPY in the LH is a potent stimulator of ingestive behavior but may have less of a direct impact on other metabolic parameters (Currie and Coscina, 1995). DMH-NPY neurons project to the PFA of the LH in both lactating rats and DIO mice, suggesting that NPY neurons in the DMHnc are the main sources of DMH-LH projections and may contribute to the hyperphagic behavior. In support of this, BDA/NPY co-localized fibers from the DMHc were scarce in the LH in cycling rats.
The NPY effect on food intake may be mediated by the orexigenic neuropeptides, melanin concentrating hormone (MCH) and orexin, in the LH. Expression of MCH and orexin is significantly increased in the LH during lactation (Sun et al., 2003; Sun et al., 2004), suggesting a potential role in the hyperphagia. However, whether NPY plays any role in increasing MCH and orexin expression during lactation is unknown. Although some studies have suggested direct actions of NPY on these neurons (Broberger et al., 1998; Horvath et al., 1999), more recent studies have indicated that this may be indirect through modulation of presynaptic inputs into the MCH and orexin neurons. NPY Y1R is not expressed in MCH and orexin neurons, but it was detected in nitric oxide synthesizing (NOS) neurons, which also stimulate food intake (Fetissov et al., 2003; Morley and Flood, 1991). This is supported by the finding that NPY injection into the LH did not significantly stimulate c-Fos in either MCH or orexin neurons, although c-Fos expression was observed in a population of neurons with unknown phenotype (Campbell et al., 2003). Therefore, the effect of NPY on food intake may be mediated by direct actions other unidentified cell types in the LH. Indeed, in the current study, while BDA-NPY fibers were observed in the vicinity of MCH and orexin neurons, close appositions, as determined by confocal microscopy, were not detected.
Potential role of DMH-NPY neurons in suppression of reproduction
The increase in hypothalamic NPY activity during lactation may be a key element in linking changes in food intake to the suppression of GnRH neuronal activity. NPY fibers from the ARH make direct contacts with GnRH neurons and act as an inhibitory signal to GnRH/LH secretion during lactation where estrogen levels are very low and NPY is chronically elevated (Smith et al., 2010). The NPY effect on GnRH neurons is directly mediated by NPY Y5 receptors and/or an indirect modulation via NPY Y1 receptors (Campbell et al., 2001; Li et al., 1999). In the present study, we failed to observe DMH-NPY terminals derived from the DMH on GnRH cell bodies. Therefore, it is likely that if DMH-NPY neurons modulate GnRH neuronal function, they do so indirectly through modulation of other neuronal populations that have direct contacts on GnRH neurons.
One possible mechanism by which DMH-NPY neurons may indirectly modulate GnRH neuronal activity is through their projections to the AVPV. AVPV-CART neurons send projections to the area where GnRH neurons are located and CART fibers make close contacts with GnRH neurons (Leslie et al., 2001; Rondini et al., 2004). CART increases GnRH pulse amplitude in cycling females and decreases GnRH pulse intervals in prepubertal rats (Lebrethon et al., 2000; Parent et al., 2000). In the present study, we demonstrated that DMH-NPY fiber terminals are in close apposition to the CART neurons within the AVPV in lactating rats. Furthermore, CART neurons in the AVPV appear to be inhibited in lactating rats (unpublished observation). Therefore, our studies identify DMH-NPY neurons as candidates for inhibiting CART neurons during lactation that could result in suppressed GnRH activity.
Projections of DMH-orexin neurons involved in BAT thermoregulation
Since the DMH contains neurons that are synaptically linked to the sympathetic premotor neurons involved in BAT regulation (Bamshad et al., 1999; Cano et al., 2003; Oldfield et al., 2002), NPY neurons in the DMH might contribute to the alteration of BAT energy expenditure in lactation and obesity. One of the critical brainstem areas involved in the sympathetic regulation of BAT is the rRPa, which contains a dense NPY terminal input. However, our data showing an absence of BDA/NPY terminals in the rRPa in either the lactating rat or DIO mouse indicate that NPY-containing neurons in DMH do not contribute to the NPY input to rRPa. Instead, since NPY terminals in the rRPa also contain markers for catecholamine synthesis (dopamine β hydroxylase; DBH), they are likely derived from the brainstem noradrenergic neurons (unpublished observation). While the true source of the NPY terminals in the rRPa remains to be determined, it is clear that they do not originate from the DMH, since these terminals do not express the enzyme for catecholamine synthesis (tyrosine hydroxylase or DBH). Furthermore, the DMH neurons projecting to rRPa (Tupone et al., 2011) and those synaptically-connected to BAT (Cao et al, 2004) are located in the dorsomedial portion of the DMH and extend dorsally into the dorsal hypothalamic area. This area of the DMH contains few NPY-containing neurons and was not directly targeted by our BDA injections. On the other hand, orexin-containing neurons, while located primarily in the PFA region of the LH, do extend into the dorsolateral portion of the DMH, an area covered by the majority of our BDA injections. Thus, our finding that most of the BDA fibers within the rRPa were co-labeled with orexin is consistent with the demonstration that orexin-containing neurons project to rRPa (Tupone et al, 2011) and that orexin fibers are detected in close apposition with BAT-projecting neurons in the rRPa (Berthoud et al., 2005; Tupone et al., 2011; Zheng et al., 2005). Our demonstration that orexin-BDA fibers make close appositions to serotonin neurons may reveal another aspect of the regulation of BAT thermogenesis since serotonin neurons in this region are important for the regulation of BAT thermogenesis (Madden and Morrison, 2010; Tupone et al., 2011). Overall, our anatomical findings support the role of a direct orexinergic input to rRPa in the modulation of BAT thermogenesis (Date et al., 1999; Szekely et al., 2002; Tupone et al., 2011), but indicate that DMH NPY neurons do not regulate BAT energy expenditure via a direct projection to BAT sympathetic premotor neurons in the rRPa.
Functional significance and summary
NPY induction in the DMHnc in both rats and mice during hyperphagic conditions suggests a key role in the stimulation of food intake. In a recent study, AAV mediated RNA interference was used to specifically knock down DMH-NPY expression in DIO rats which reduced food intake and induced a protection from HFD -induced obesity (Chao et al., 2011), suggesting that NPY expression in the DMH may be partially responsible for the development of obesity. Additionally, these rats exhibited increased BAT sympathetic activity and as well as a remarkable transdifferentiation of subcutaneous WAT to a BAT-like phenotype. These findings strongly imply that DMH-NPY expression is involved in an inhibitory regulation of BAT thermogenesis as well as the stimulation of food intake. However, the mechanisms by which DMH-NPY neurons regulate feeding behavior and BAT thermogenesis are not well understood. In the current study, we identified DMH-NPY projections to the PVH and LH as potential pathways for the hyperphagia and BAT thermogenesis in lactation and diet-induced obesity. NPY neurons in the DMHc are clearly not the major orexigenic inputs to these hypothalamic targets in normal conditions. Our data suggest that hormonal/neural adaptations during physiological conditions requiring energy conservation activate additional NPY neuronal populations in the DMH and increase DMH-NPY inputs to the PVH and LH, contributing to the hyperphagia and reduced BAT thermogenesis. Although we provide evidence for possible interactions between DMH-NPY projections and CART neurons, additional studies are needed to confirm the functional connection between two neurons.
In summary, we characterized the distribution of DMH-NPY neuronal projections in the brain areas involved in the regulation of food intake and BAT thermogenesis in two chronic hyperphagic models, lactation and DIO. Comparison of DMH-NPY projections in these two models clearly suggests that DMH-NPY induction plays a critical role in promoting hyperphagia, reducing BAT thermogenesis, and suppressing reproduction via hypothalamic projections, but not the brainstem. The current findings provide a neuroanatomical framework to understand the potential roles and mechanism of DMH-NPY neurons.
The dorsomedial hypothalamic nucleus (DMH) is known to be involved in many homeostatic processes, and to have broad projections throughout the hypothalamus and brainstem. In specific models of obesity and hyperphagia Neuropeptide Y neurons are activated within the DMH; however, the efferent projections of these neurons was unknown. In the current study we used anterograde tracers combined with histochemical analysis to demonstrate that NPY neurons in the DMH of both lactating rats and obese mice have a strong projections within the hypothalamus, with limited projections to the brainstem.
Acknowledgments
Supporting grant: RR000163, HD14643, DK082558
LIST OF ABBREVIATIONS
- 3V
Third ventricle
- ARH
Arcuate nucleus of the hypothalamus
- AVPV
Anteroventral paravntricular nucleus
- BA
Barrington’s nucleus
- DMH
Dorsomedial hypothalamus
- DMHc
Dorsomedial hypothalamus, compact part
- DMHd
Dorsomedial hypothalamus, dorsal part
- DMHnc
Dorsomedial hypothalamus, non-compact part
- DMHv
Dorsomedial hypothalamus, ventral part
- DP
Paraventricular nucleus, dorsal parvicellular part
- Fx
Fornix
- LC
Locus coeruleus
- LH
Lateral hypothalamus
- LPO
Lateral preoptic nucleus
- LPB
Lateral parabrachial nucleus
- LS
Lateral septal nucleus
- ME
Median eminence
- MP
Paraventricular nucleus, medial parvicellular part
- MPO
Median preoptic nucleus
- NTS
Nucleus of the solitary tract
- PAG
Periaqueductal grey
- PB
Parabrachial nucleus
- PFA
Perifornical area
- PM
Paraventricular nucleus, posterior magnicellular part
- POA
Preoptic area
- PS
Parastrial nucleus
- PV
Paraventricular nucleus, periventricular part
- PVH
Paraventricular nucleus
- RM
Raphe magnus
- rRPa
Rostral raphe pallidus
- SUM
Supramammillary nucleus
- VLM
Ventrolateral medulla
- VLPAG
Ventrolateral periaqueductal grey
- VMH
Ventromedial hypothalamus
- VTA
Ventral tegmental area
Footnotes
Conflict of Interest: None of the authors have conflicts of interest related to the research presented in this manuscript.
REFERENCES
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Rev. 2010;64(1):137–159. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Bai FL, Yamano M, Shiotani Y, Emson PC, Smith AD, Powell JF, Tohyama M. An arcuato-paraventricular and -dorsomedial hypothalamic neuropeptide Y-containing system which lacks noradrenaline in the rat. Brain Res. 1985;331(1):172–175. doi: 10.1016/0006-8993(85)90730-9. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276(6 Pt 2):R1569–1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Beck B. Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1159–1185. doi: 10.1098/rstb.2006.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76(3):431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL, Brooks S. The effect of dorsomedial hypothalamic nuclei lesions on body weight regulation. Neuroscience. 1979;4(5):659–665. doi: 10.1016/0306-4522(79)90142-8. [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Mendel VE, Bernardis LL, Castonguay TW. Meal patterns of rats with dorsomedial hypothalamic nuclei lesions or sham operations. Physiol Behav. 1986;36(4):693–698. doi: 10.1016/0031-9384(86)90356-2. [DOI] [PubMed] [Google Scholar]
- Bernardis LL, Bellinger LL. Effect of palatable diet on growth, caloric intake and endocrine-metabolic profile in weanling rats with dorsomedial hypothalamic lesions. Appetite. 1986;7(3):219–230. doi: 10.1016/s0195-6663(86)80027-7. [DOI] [PubMed] [Google Scholar]
- Bernardis LL, Bellinger LL. Brown (BAT) and white (WAT) adipose tissue in high-fat junk food (HFJF) and chow-fed rats with dorsomedial hypothalamic lesions (DMNL rats) Behav Brain Res. 1991;43(2):191–195. doi: 10.1016/s0166-4328(05)80070-1. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol. 2005;123(2):147–156. doi: 10.1007/s00418-005-0761-x. [DOI] [PubMed] [Google Scholar]
- Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1030–1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95(25):15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogan RS, Mitchell SE, Trayhurn P, Smith MS. Suppression of leptin during lactation: contribution of the suckling stimulus versus milk production. Endocrinology. 1999;140(6):2621–2627. doi: 10.1210/endo.140.6.6802. [DOI] [PubMed] [Google Scholar]
- Burman KJ, Ige AO, White JH, Marshall FH, Pangalos MN, Emson PC, Minson JB, Llewellyn-Smith IJ. GABAB receptor subunits, R1 and R2, in brainstem catecholamine and serotonin neurons. Brain Res. 2003;970(1-2):35–46. doi: 10.1016/s0006-8993(02)04269-5. [DOI] [PubMed] [Google Scholar]
- Campbell RE, ffrench-Mullen JM, Cowley MA, Smith MS, Grove KL. Hypothalamic circuitry of neuropeptide Y regulation of neuroendocrine function and food intake via the Y5 receptor subtype. Neuroendocrinology. 2001;74(2):106–119. doi: 10.1159/000054676. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Smith MS, Allen SE, Grayson BE, Ffrench-Mullen JM, Grove KL. Orexin neurons express a functional pancreatic polypeptide Y4 receptor. J Neurosci. 2003;23(4):1487–1497. doi: 10.1523/JNEUROSCI.23-04-01487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460(3):303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126(1):229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology. 2006;51(3):426–437. doi: 10.1016/j.neuropharm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011;13(5):573–583. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MJ, Colmers WF. Y eat? Nutrition. 2008;24(9):869–877. doi: 10.1016/j.nut.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Chen P, Smith MS. Regulation of hypothalamic neuropeptide Y messenger ribonucleic acid expression during lactation: role of prolactin. Endocrinology. 2004;145(2):823–829. doi: 10.1210/en.2003-1255. [DOI] [PubMed] [Google Scholar]
- Chen P, Williams SM, Grove KL, Smith MS. Melanocortin 4 receptor-mediated hyperphagia and activation of neuropeptide Y expression in the dorsomedial hypothalamus during lactation. J Neurosci. 2004;24(22):5091–5100. doi: 10.1523/JNEUROSCI.0588-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie PJ, Coscina DV. Dissociated feeding and hypothermic effects of neuropeptide Y in the paraventricular and perifornical hypothalamus. Peptides. 1995;16(4):599–604. doi: 10.1016/0196-9781(95)00020-k. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96(2):748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R47–63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Kwok EH, Yang J, Chang J. Cocaine- and amphetamine-regulated transcript-immunoreactivity in the rat sympatho-adrenal axis. Neurosci Lett. 2000a;283(2):97–100. doi: 10.1016/s0304-3940(00)00935-6. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Wong PY, Yang J, Chang J. Cocaine- and amphetamine-regulated transcript peptide in the rat epididymis: an immunohistochemical and electrophysiological study. Biol Reprod. 2000b;63(5):1518–1524. doi: 10.1095/biolreprod63.5.1518. [DOI] [PubMed] [Google Scholar]
- Dun SL, Chianca DA, Jr., Dun NJ, Yang J, Chang JK. Differential expression of cocaine- and amphetamine-regulated transcript-immunoreactivity in the rat spinal preganglionic nuclei. Neurosci Lett. 2000c;294(3):143–146. doi: 10.1016/s0304-3940(00)01575-5. [DOI] [PubMed] [Google Scholar]
- Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M, Saper CB, Elmquist JK. Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol. 2001;432(1):1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25(3-4):132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31(34):12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete C, Lechan RM. Neuroendocrine implications for the association between cocaine- and amphetamine regulated transcript (CART) and hypophysiotropic thyrotropin-releasing hormone (TRH) Peptides. 2006;27(8):2012–2018. doi: 10.1016/j.peptides.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Fekete C, Lechan RM. Negative feedback regulation of hypophysiotropic thyrotropin-releasing hormone (TRH) synthesizing neurons: role of neuronal afferents and type 2 deiodinase. Front Neuroendocrinol. 2007;28(2-3):97–114. doi: 10.1016/j.yfrne.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete C, Mihaly E, Luo LG, Kelly J, Clausen JT, Mao Q, Rand WM, Moss LG, Kuhar M, Emerson CH, Jackson IM, Lechan RM. Association of cocaine- and amphetamine-regulated transcript-immunoreactive elements with thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and its role in the regulation of the hypothalamic-pituitary-thyroid axis during fasting. J Neurosci. 2000;20(24):9224–9234. doi: 10.1523/JNEUROSCI.20-24-09224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetissov SO, Xu ZQ, Byrne LC, Hassani H, Ernfors P, Hokfelt T. Neuropeptide y targets in the hypothalamus: nitric oxide synthesizing neurones express Y1 receptor. J Neuroendocrinol. 2003;15(8):754–760. doi: 10.1046/j.1365-2826.2003.01051.x. [DOI] [PubMed] [Google Scholar]
- Florenzano F, Viscomi MT, Mercaldo V, Longone P, Bernardi G, Bagni C, Molinari M, Carrive P. P2X2R purinergic receptor subunit mRNA and protein are expressed by all hypothalamic hypocretin/orexin neurons. J Comp Neurol. 2006;498(1):58–67. doi: 10.1002/cne.21013. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Chronwall BM, Schafer MP, O’Donohue TL. Localization of neuropeptide Y messenger ribonucleic acid in rat and mouse brain by in situ hybridization. Synapse. 1987;1(1):25–31. doi: 10.1002/syn.890010106. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Grayson BE, Allen SE, Copp DR, Smith MS, Cowley MA, Grove KL. Characterization of brainstem peptide YY (PYY) neurons. J Comp Neurol. 2008;506(2):194–210. doi: 10.1002/cne.21543. [DOI] [PubMed] [Google Scholar]
- Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL. Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience. 2006;143(4):975–986. doi: 10.1016/j.neuroscience.2006.08.055. [DOI] [PubMed] [Google Scholar]
- Grove KL, Brogan RS, Smith MS. Novel expression of neuropeptide Y (NPY) mRNA in hypothalamic regions during development: region-specific effects of maternal deprivation on NPY and Agouti-related protein mRNA. Endocrinology. 2001;142(11):4771–4776. doi: 10.1210/endo.142.11.8498. [DOI] [PubMed] [Google Scholar]
- Grove KL, Smith MS. Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav. 2003;79(1):47–63. doi: 10.1016/s0031-9384(03)00104-5. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Trumbauer M, Frazier E, Van der Ploeg LH, Chen H. Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet-induced obese mice. Neuroreport. 1998a;9(15):3415–3419. doi: 10.1097/00001756-199810260-00015. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Van der Ploeg LH. Evidence of altered hypothalamic pro-opiomelanocortin/ neuropeptide Y mRNA expression in tubby mice. Brain Res Mol Brain Res. 1998b;59(2):273–279. doi: 10.1016/s0169-328x(98)00150-8. [DOI] [PubMed] [Google Scholar]
- Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J Comp Neurol. 2001;433(2):222–238. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci. 1999;19(3):1072–1087. doi: 10.1523/JNEUROSCI.19-03-01072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesterson RA, Huszar D, Lynch CA, Simerly RB, Cone RD. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Mol Endocrinol. 1997;11(5):630–637. doi: 10.1210/mend.11.5.9921. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146(10):4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Tong J, Hornykiewicz O, Rajput A, Chang LJ, Guttman M, Furukawa Y. Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain. 2008;131(Pt 1):120–131. doi: 10.1093/brain/awm239. [DOI] [PubMed] [Google Scholar]
- Kiyoshi K, Kondoh M, Hirunagi K, Korf H. Confocal laser scanning and electron-microscopic analyses of the relationship between VIP-like and GnRH-like-immunoreactive neurons in the lateral septal-preoptic area of the pigeon. Cell Tissue Res. 1998;293(1):39–46. doi: 10.1007/s004410051096. [DOI] [PubMed] [Google Scholar]
- Kong WM, Stanley S, Gardiner J, Abbott C, Murphy K, Seth A, Connoley I, Ghatei M, Stephens D, Bloom S. A role for arcuate cocaine and amphetamine-regulated transcript in hyperphagia, thermogenesis, and cold adaptation. FASEB J. 2003;17(12):1688–1690. doi: 10.1096/fj.02-0805fje. [DOI] [PubMed] [Google Scholar]
- Lebrethon MC, Vandersmissen E, Gerard A, Parent AS, Bourguignon JP. Cocaine and amphetamine-regulated-transcript peptide mediation of leptin stimulatory effect on the rat gonadotropin-releasing hormone pulse generator in vitro. J Neuroendocrinol. 2000;12(5):383–385. doi: 10.1046/j.1365-2826.2000.00497.x. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res. 2006;153:209–235. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- Legradi G, Lechan RM. Agouti-related protein containing nerve terminals innervate thyrotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology. 1999;140(8):3643–3652. doi: 10.1210/endo.140.8.6935. [DOI] [PubMed] [Google Scholar]
- Leslie RA, Sanders SJ, Anderson SI, Schuhler S, Horan TL, Ebling FJ. Appositions between cocaine and amphetamine-related transcript- and gonadotropin releasing hormone-immunoreactive neurons in the hypothalamus of the Siberian hamster. Neurosci Lett. 2001;314(3):111–114. doi: 10.1016/s0304-3940(01)02291-1. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. The acute suckling stimulus induces expression of neuropeptide Y (NPY) in cells in the dorsomedial hypothalamus and increases NPY expression in the arcuate nucleus. Endocrinology. 1998a;139(4):1645–1652. doi: 10.1210/endo.139.4.5905. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. Neuropeptide Y (NPY) neurons in the arcuate nucleus (ARH) and dorsomedial nucleus (DMH), areas activated during lactation, project to the paraventricular nucleus of the hypothalamus (PVH) Regul Pept. 1998b;75-76:93–100. doi: 10.1016/s0167-0115(98)00057-3. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. Morphological evidence for direct interaction between arcuate nucleus neuropeptide Y (NPY) neurons and gonadotropin-releasing hormone neurons and the possible involvement of NPY Y1 receptors. Endocrinology. 1999;140(11):5382–5390. doi: 10.1210/endo.140.11.7093. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in tryptophan hydroxylase and serotonin transporter immunoreactivity in multiple brainstem nuclei of the rat: implications for a sensitive period. J Comp Neurol. 2010;518(7):1082–1097. doi: 10.1002/cne.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R831–843. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Endogenous activation of spinal 5-hydroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R776–783. doi: 10.1152/ajpregu.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick I, Pronchuk N, et al. Developmental switch in neuropeptide Y and melanocortin effects in the paraventricular nucleus of the hypothalamus. Neuron. 2007;56(6):1103–1115. doi: 10.1016/j.neuron.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Morley JE, Flood JF. Evidence that nitric oxide modulates food intake in mice. Life Sci. 1991;49(10):707–711. doi: 10.1016/0024-3205(91)90102-h. [DOI] [PubMed] [Google Scholar]
- Morris BJ. Neuronal localisation of neuropeptide Y gene expression in rat brain. J Comp Neurol. 1989;290(3):358–368. doi: 10.1002/cne.902900305. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 22(12):3137–46. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110(3):515–526. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- Parent AS, Lebrethon MC, Gerard A, Vandersmissen E, Bourguignon JP. Leptin effects on pulsatile gonadotropin releasing hormone secretion from the adult rat hypothalamus and interaction with cocaine and amphetamine regulated transcript peptide and neuropeptide Y. Regul Pept. 2000;92(1-3):17–24. doi: 10.1016/s0167-0115(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103(1):23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- Rondini TA, Baddini SP, Sousa LF, Bittencourt JC, Elias CF. Hypothalamic cocaine- and amphetamine-regulated transcript neurons project to areas expressing gonadotropin releasing hormone immunoreactivity and to the anteroventral periventricular nucleus in male and female rats. Neuroscience. 2004;125(3):735–748. doi: 10.1016/j.neuroscience.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(5) doi: 10.1016/s0092-8674(02)09256-5. 1 page following 696. [DOI] [PubMed] [Google Scholar]
- Sanchez E, Fekete C, Lechan RM, Joseph-Bravo P. Cocaine- and amphetamine-regulated transcript (CART) expression is differentially regulated in the hypothalamic paraventricular nucleus of lactating rats exposed to suckling or cold stimulation. Brain Res. 2007;1132(1):120–128. doi: 10.1016/j.brainres.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205(3):260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Silva JE. Thyroid hormone control of thermogenesis and energy balance. Thyroid. 1995;5(6):481–492. doi: 10.1089/thy.1995.5.481. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131(4):623–630. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Smith MS, Grove KL. Integration of the regulation of reproductive function and energy balance: lactation as a model. Front Neuroendocrinol. 2002;23(3):225–256. doi: 10.1016/s0091-3022(02)00002-x. [DOI] [PubMed] [Google Scholar]
- Smith MS, True C, Grove KL. The neuroendocrine basis of lactation-induced suppression of GnRH: role of kisspeptin and leptin. Brain Res. 2010;1364:139–152. doi: 10.1016/j.brainres.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Narita K, Murata T, Honda K, Higuchi T. Orexin-A immunoreactivity and prepro-orexin mRNA expression in hyperphagic rats induced by hypothalamic lesions and lactation. J Neuroendocrinol. 2003;15(1):51–60. doi: 10.1046/j.1365-2826.2003.00862.x. [DOI] [PubMed] [Google Scholar]
- Sun G, Tian Z, Murata T, Narita K, Honda K, Higuchi T. Central and peripheral immunoreactivity of melanin-concentrating hormone in hypothalamic obese and lactating rats. J Neuroendocrinol. 2004;16(1):79–83. doi: 10.1111/j.1365-2826.2004.01124.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194(3):555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Szekely M, Petervari E, Balasko M, Hernadi I, Uzsoki B. Effects of orexins on energy balance and thermoregulation. Regul Pept. 2002;104(1-3):47–53. doi: 10.1016/s0167-0115(01)00348-2. [DOI] [PubMed] [Google Scholar]
- Takenoya F, Guan JL, Kato M, Sakuma Y, Kintaka Y, Kitamura Y, Kitamura S, Okuda H, Takeuchi M, Kageyama H, Shioda S. Neural interaction between galanin-like peptide (GALP)- and luteinizing hormone-releasing hormone (LHRH)-containing neurons. Peptides. 2006;27(11):2885–2893. doi: 10.1016/j.peptides.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Brain Res Rev. 1998;27(2):89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Tritos NA, Elmquist JK, Mastaitis JW, Flier JS, Maratos-Flier E. Characterization of expression of hypothalamic appetite-regulating peptides in obese hyperleptinemic brown adipose tissue-deficient (uncoupling protein-promoter-driven diphtheria toxin A) mice. Endocrinology. 1998;139(11):4634–4641. doi: 10.1210/endo.139.11.6308. [DOI] [PubMed] [Google Scholar]
- True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23(1):52–64. doi: 10.1111/j.1365-2826.2010.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011;31(44):15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF. Monoclonal antibodies to luteinizing hormone-releasing hormone: production, characterization, and immunocytochemical application. Biol Reprod. 1991;44(4):681–686. doi: 10.1095/biolreprod44.4.681. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev. 1992;16(2):235–272. doi: 10.1016/s0149-7634(05)80183-6. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE, Li HY. Control of fertility by metabolic cues. Am J Physiol. 1996;270(1 Pt 1):E1–19. doi: 10.1152/ajpendo.1996.270.1.E1. [DOI] [PubMed] [Google Scholar]
- Wang C, Billington CJ, Levine AS, Kotz CM. Effect of CART in the hypothalamic paraventricular nucleus on feeding and uncoupling protein gene expression. Neuroreport. 2000;11(14):3251–3255. doi: 10.1097/00001756-200009280-00040. [DOI] [PubMed] [Google Scholar]
- Woodside B. Prolactin and the hyperphagia of lactation. Physiol Behav. 2007;91(4):375–382. doi: 10.1016/j.physbeh.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci. 2009;29(1):179–190. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29(38):11954–64. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31(5):1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol. 2005;485(2):127–142. doi: 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]