Abstract
Failure to thrive is common in infants with hypoplastic left heart syndrome and its variants and those with poor growth may be at risk for worse surgical and neurodevelopmental outcomes. The etiology of growth failure in this population is multifactorial and complex, but may be impacted by nutritional intervention. There are no consensus guidelines outlining best practices for nutritional monitoring and intervention in this group of infants. The Feeding Work Group of the National Pediatric Cardiology Quality Improvement Collaborative performed a literature review and assessment of best nutrition practices from centers participating in the collaborative in order to provide nutritional recommendations and levels of evidence for those caring for infants with single ventricle physiology.
Keywords: Nutrition, Hypoplastic Left Heart Syndrome, Feeding Guidelines, Enteral Nutrition, Parenteral Nutrition
Introduction
Despite improving surgical outcomes following Norwood palliation for hypoplastic left heart syndrome (HLHS) and its variants, there has not been commensurate improvement in interstage mortality and long-term morbidities.1-4 The first interstage period, the time between hospital discharge following the Norwood procedure and presentation for superior cavopulmonary connection (SCPC), continues to be significantly high risk, with some centers reporting interstage mortality rates as high as 22%.4-6 Through the growing experience of many centers focused on reducing interstage mortality, normal infant growth has been recognized as an important sign of health which is associated with improved operative and long-term outcomes.7,8 Despite the high prevalence of growth failure in this population and the unique challenges patients with single ventricle physiology face related to feeding and growth, there are no consensus guidelines available which address this issue. Therefore, the Feeding Work Group (FWG) of The National Pediatric Cardiology Quality Improvement Collaborative (NPC-QIC) was formed to develop consensus recommendations for feeding and nutrition in infants with single ventricle heart disease through a review of the literature and survey of participating centers.
Growth failure occurs when the rate of growth is below expectations based on age and gender.9 This is traditionally defined as weight-for-age decreasing across two major percentile channels from a previously established growth pattern or weight-for-length <80% of ideal body weight.10 Most children who experience growth failure as infants have adequate catch-up growth by the time they reach school years.11 However, several studies have shown that children with early growth failure may experience long-term problems, including short stature, poor arithmetic performance, attention problems, aggressive behavior, and poor overall emotional, social, and cognitive development.8,12,13 In addition, poor nutrition is associated with worse outcomes in children undergoing surgery and those with critical illness.14-16 Growth failure is common in infants with congenital heart disease (CHD) and is especially prevalent in infants with single ventricle physiology, including those with HLHS.5,17-19 Factors contributing to growth failure in single ventricle infants include inadequate calorie intake, high metabolic demands, gastrointestinal pathology, and genetic and extracardiac abnormalities.20,21 Despite the importance of weight gain, advancement to nutrition goals is often slow in the perioperative period and is often hampered by concerns for poor systemic output, the need for inotropic support, limitations of fluid intake, the risk of necrotizing enterocolitis (NEC), and frequent interruptions in nutrient delivery.18,22 Following the immediate perioperative period and during the interstage period, many children continue to experience suboptimal growth.23 Nutrition protocols in this population are largely based on single center experience and are influenced by hospital culture as well as care provider opinion.22,24
The NPC-QIC is the first multicenter quality improvement collaborative within pediatric cardiology and was organized under the leadership of the Joint Council on Congenital Heart Disease.25 The NPC-QIC leadership defined the aim of the initial collaborative project “To reduce mortality and improve the quality of life of infants with HLHS during the interstage period between discharge from the Norwood and admission for the SCPC.”25 The collaborative is a nationwide network currently comprising 57 pediatric cardiology centers in North America who contribute to an HLHS registry. The formation of this collaborative has allowed teams caring for patients with HLHS to work together to come up with new solutions to problems commonly seen caring for this population of patients. One of the primary drivers of reduction in mortality is improvement in growth in this high-risk population. The FWG of the NPC-QIC has focused on identifying best nutritional practices in order to improve growth in this high-risk population. In the following guidelines, we identified areas of relative consensus and controversy, and outlined the existing levels of evidence supporting best feeding and nutrition practices during the pre-Norwood, post-Norwood, and interstage phases.
Methods
Etiology of the NPC-QIC FWG
In order to identify best nutritional practices in infants with HLHS, the FWG of the NPC-QIC was developed. NPC-QIC leadership sought volunteers from centers participating in the collaborative to form the FWG, ultimately comprised of a multidisciplinary team of dietitians, physicians, and nurse practitioners from 10 centers caring for children with CHD. The FWG conducted a literature review and assessment of best nutrition practices from centers participating in the collaborative with the objective of developing nutritional guidelines for those caring for infants with single ventricle physiology.
Data Collection/Literature Review
The FWG met via several conference calls over 3 months to answer the following question: “What is the most effective way to feed infants with HLHS and its variants, to achieve improved growth and survival with minimal complications?”
This question was approached using two different methodologies: First, an in-depth literature search was performed using several databases: PubMed, Scopus, and CINAHL. We searched for any publication <10 years old using the following search terms: for PubMed: “heart diseases/congenital, infant, child or adolescent” or “infant food,” or “infant nutrition disorders” or “nutritional requirements”; for Scopus: “congenital heart disease” and “nutrition”; and for CINAHL: “heart defects, congenital” and “child nutritional physiology” or “nutrition” or “nutritional assessment” or “nutritional requirements” or “nutritional status,” or “nutritional support.” The literature search results were reviewed and articles that did not pertain to feeding algorithms, guidelines, or protocols were eliminated. Second, a collection and assessment of current feeding practices among centers participating in the NPC-QIC were performed. Unpublished individual institution guidelines and policies related to feeding infants with CHD were contributed by various members of the collaborative and reviewed by the FWG.
Levels of Evidence and Grading of Feeding Recommendations (Table 1)
Table 1.
Grading System for Levels of Evidence and Feeding Recommendations
| Grading of feeding recommendations | |
| “Strongly recommend” | There is consensus that benefits clearly outweigh risks |
| “Recommend” | There is consensus that benefits outweigh risks |
| “No recommendation made” | There is no consensus |
| Levels of evidence* | |
| I | Large, randomized control trials with clear-cut results |
| II | Small, randomized trials with uncertain results |
| III | Nonrandomized cohort with contemporaneous controls |
| IV | Nonrandomized cohort with historical controls |
| V | Case series, uncontrolled studies, and expert opinion |
Adapted from Dellinger RP.
Table 1 describes the grading scheme used to evaluate the level of evidence of articles considered in this review. The system used was based on the modified Delphi methodology, as published by Dellinger et al.26 This was chosen as it has been used in the most recent American Society for Parenteral and Enteral Nutrition pediatric guidelines, and is commonly used in the Critical Care literature.8 All investigational articles were reviewed by two FWG members and assigned a grade of level of evidence.
Practices that had a scientific basis in the literature and those that were consistent among sites were used to form the foundation for the nutrition recommendations. Practices that were variable among sites were discussed at length and only added to the feeding program once a consensus was reached. Nutrition recommendations were divided into three separate time periods: (1) Pre-Norwood procedure recommendations; (2) Immediate post-Norwood procedure recommendations; and (3) Interstage recommendations. The review of the literature also allowed us to identify gaps in our knowledge in this area, giving us a road map for future research. Recommendations found within the below guidelines were assigned a grade as described in Table 1, where those with clear consensus among the FWG members and the benefits clearly outweighed the risks was assigned “strongly recommended.”
Results/Recommended Feeding Guidelines
Our initial literature search identified 71 articles eligible for review. After initial screening, 26 articles were eliminated, leaving 45 available for further review and grading by the work group. Of these, 13 articles were specifically related to feeding infants with single ventricle physiology, while 32 articles were related to the care of infants with CHD in general. Figures 1-6 represent the consensus recommendations of the FWG following literature review and assessment of best practices among the centers participating in the NPC-QIC.
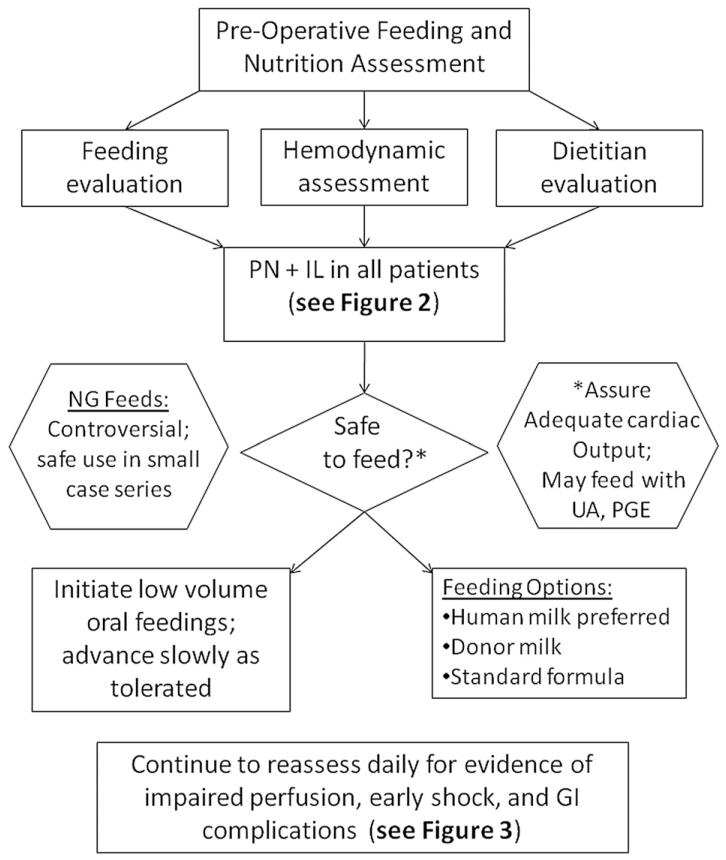
Figure 1.
Preoperative enteral feeding (EN).8,12,18,22,27-47 Benefits of preoperative enteral nutrition may include: development of normal feeding patterns, prevention of bacterial translocation, and promotion of immunologic and gut mucosal health. Oral feeding should be attempted only in the stable patient. PN, total parenteral nutrition; IL, intravenous lipid solution; NG, nasogastric; UA, umbilical arterial catheter; PGE prostaglandin infusion; GI, gastrointestinal.
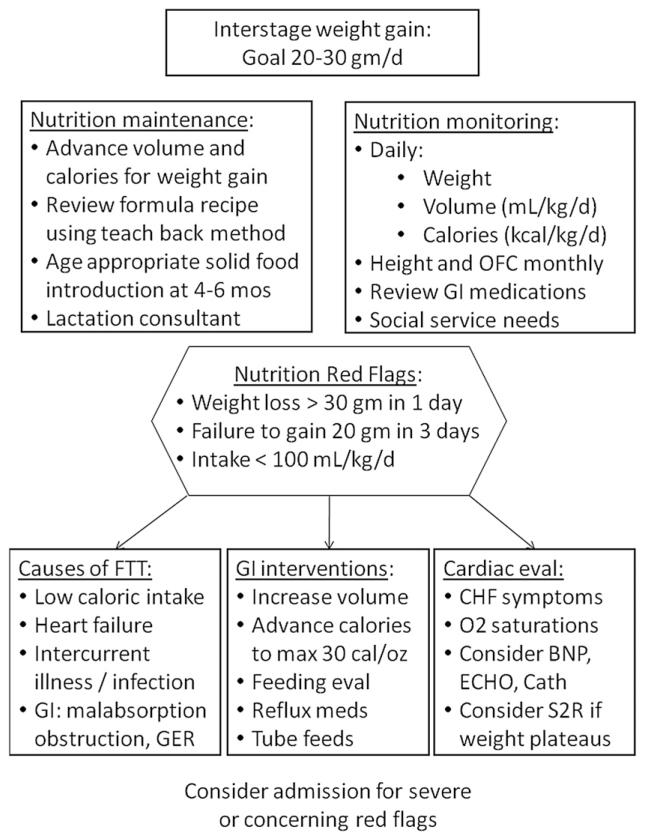
Figure 6.
Interstage feeding program.8,17-19,22-24,29,31,32,35,46,54,56-60 Goal: Achieve normal infant growth during the interstage period. OFC, occipitofrontal head circumference; GI, gastrointestinal; FTT, failure to thrive; GER, gastroesophageal reflux; CHF, congestive heart failure; BNP, brain natriuretic peptide; ECHO, echocardiogram; cath, cardiac catheterization; S2R, stage two reconstruction.
Preoperative Enteral Feeding Guideline (Figure 1)
The decision to feed enterally in the pre-Norwood period remains controversial. While case reports and case series of successful preoperative feeding exist, the risk of impaired systemic perfusion and potential gastrointestinal complications such as NEC may outweigh any potential benefit of achieving full enteral nutrition (EN) preoperatively. Practitioners are often averse to initiate and advance early EN due to the risk of NEC in this tenuous population.27-30 However, several studies among other population groups have demonstrated that early EN decreases infection rates, decreases length of stay, improves wound healing, and shortens the duration of mechanical ventilatory support.8,61-64 Benefits to early feeding may include the initiation of normal feeding patterns and mechanics, immunologic benefits, and prevention of bacterial translocation.8,61-64 Enteral feeding in the patient with unstable ductal-dependent systemic blood flow may result in gastrointestinal complications.17,28,29,34,53
Below we outline the level of evidence and rationale supporting the consensus guidelines for preoperative feeding found in figure 1. Our literature review revealed significant gaps in the understanding of the potential benefits of preoperative feeding, and their impact on outcomes.
1. Total parenteral nutrition (PN) should be initiated early and advanced to full calorie and protein goals; “Strongly Recommend”; Level of evidence: 2. The Norwood hospitalization and early perioperative period is associated with a significant decrease in weight-for-age z-score (WAZ) and failure to thrive for most patients.17,18,31-33 In addition, many patients will not be considered candidates for full enteral feeding due to local practice or patient condition.34,35 This patient population is at high risk for malnourishment.17,18,22,31 Therefore, it is reasonable to start PN early.
2. Enteral feeding is safe in hemodynamically stable patients under appropriate level of monitoring. “Recommend”; Level of evidence: 2. The use of preoperative enteral feeding varies considerably by center.28,29,36 While rigorous assessment of the safety of this practice is lacking, there is a growing number of observational studies documenting this practice.37,38 However, the benefits of early enteral feeding are extrapolated from other patient populations: no study has assessed the benefit of enteral feeding to any degree in this patient population.36,37 In addition, there are multiple case reports, case series, and observational studies documenting a high incidence of gastrointestinal complications and systemic perfusion abnormalities in this patient population.27,32,34,39,40 While risk factor analysis has failed to reveal a single modifiable cause for NEC, it has become clear that implementation of standard feeding practice alone can reduce the incidence of NEC.8,30,41-43 In addition, there is a growing body of literature that indicates that with appropriate vigilance and intensive monitoring, enteral feeding can be achieved safely.37,38,43-45 While in some patients the risk of impaired systemic perfusion may outweigh the potential benefits of advancing to full enteral feeding preoperatively, it is reasonable to assess safety and initiate preoperative feeds on an individual patient basis.
3. The use of nasogastric tube feeding may be utilized to deliver enteral feeding. “No Recommendation”; Level of evidence: 3. The use of nasogastric feeds in order to advance the preoperative Norwood patient to full enteral feeding preoperatively has been documented in the literature; however, this practice lacks rigorous evaluation of safety.36-38 Feeding intolerance is an important clinical sign of poor systemic perfusion in these patients, and some authors and experts argue that the deterioration in oral feeding behavior can be an early yet subtle sign of impending shock and/or NEC. However, this practice is currently in use, reflecting the wide variation in preoperative management, and is therefore included as an option in this protocol. This practice is not universally accepted.28,29,46
4. Patients with an umbilical arterial catheter may be fed enterally: “Strongly Recommend”; Level of evidence: 4. While many practitioners withhold feeds in this scenario due to concerns of arterial catheters compromising blood flow to the splanchnic bed, there is limited evidence to support this.29 In addition, there are a number of case series and observational studies where feeding with umbilical catheters in place has not resulted in increased adverse outcomes.27,38 Given the limited evidence in either direction and lack of a controlled study, it is reasonable to feed in this scenario if consistent with local practice.
5. Patients may be fed while on prostaglandin infusion: “Strongly Recommend”; Level of evidence: 3. Prostaglandin infusions are cited as an indication to withhold enteral feeding due to concerns for development of gastrointestinal complications related to shunting from the systemic to pulmonary circulation across a patent ductus arteriosus.28,36 In addition, PGE may result in systemic vasodilatation, respiratory depression, edema, and increased secretions over time. However, safe enteral feeding has been documented in this scenario in a number of case series and observational studies, and is reasonable to attempt.37,38
6. Human milk is the preferred option for initiation of enteral feeds: “Recommend”; Level of evidence: 3. The nutritional and immunologic benefits to human milk are numerous.47 Human milk has a high biologic value of protein content and is easily absorbed by the infant gut. Protocols have been previously published for feeding single ventricle infants recommending a preference for rehydralyte or elemental formula for the single ventricle population, although no concrete evidence for this practice exists.29 To date, there has been no study comparing the benefits of breast milk vs. infant formula in this population; the authors have based this recommendation on extrapolation from the preterm infant population.47 When human milk is unavailable, it is reasonable to initiate standard infant formula as there is no evidence supporting deviation from this practice.28 While there may be benefits to breastfeeding, the practice is limited by physiologic instability and reliance on intravenous access for prostaglandin delivery.
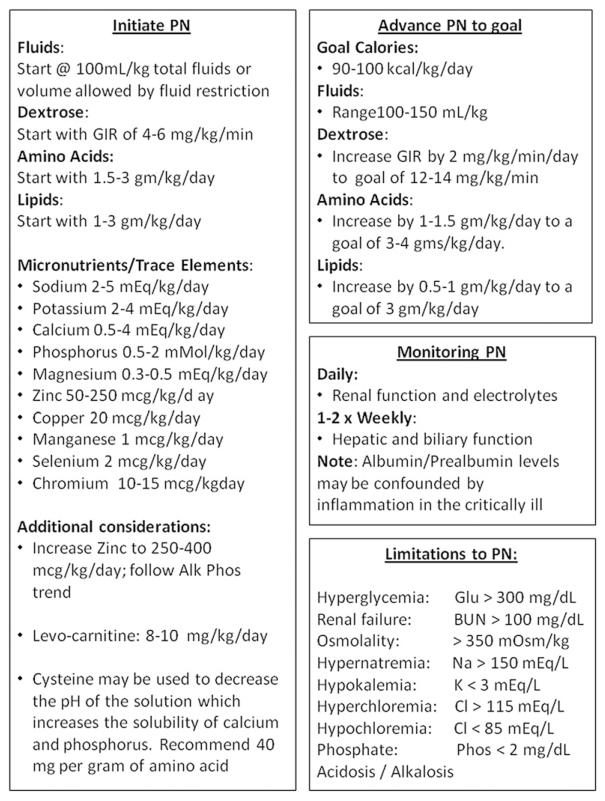
Parenteral Nutrition Guideline (Figure 2)
Figure 2.
Total parenteral nutrition (PN).35,48-51 Goal: PN should be initiated in all single ventricle patients preoperatively, and as soon as feasible postoperatively to minimize nutritional deficiencies. GIR, glucose infusion rate.
It should be anticipated that all patients will require PN in the pre-Norwood and early postoperative period in order to deliver adequate nutrition during this critical time. Studies have demonstrated an acute decline in WAZ and growth velocity in the early postoperative time period.7,17-19,23 Li and colleagues found a surprisingly high energy deficit in the early postoperative period following the Norwood operation.65 Neo-nates were found to be hypermetabolic and did not reach a positive energy balance until postoperative day number 3 despite the use of standard regimens of PN. It should be noted that PN is costly, and initiation should be carefully considered for cost-effectiveness and ability to achieve proper electrolyte and fluid balance. Later, we outline current level of evidence and rationale supporting the consensus guidelines for PN in Figure 2. These recommendations are extrapolated for the most part from general pediatric intensive care nutrition guidelines.48,49,65 More investigation is needed into the calorie and macronutrient needs of this high-risk population during the perioperative period.49
1. Total initial calorie goals for PN: 90–100 cal/kg/day.35 “Recommend”; Level of Evidence: 5. Other guidelines for PN support in children recommend providing resting energy expenditure with a stress factor (i.e., 55 cal/kg/day × 1.2–1.4).48 The thermogenic effect of food is negated when receiving PN support; therefore, energy needs are approximately 10% less than EN requirements.35
2. Total fluid should begin at 100–120 mL/kg/day and be adjusted daily in the postoperative period49: “Recommend”; Level of Evidence: 5. Fluid management should be discussed daily with the healthcare team and PN should be altered accordingly as other fluid sources are weaned. PN should be withheld until it can provide approximately 30–40 mL/kg or more of total fluid intake; at lower volumes, it is not cost-effective and may result in inappropriate concentrations of its constituents.
3. The initiation and advancement of macronutrients for PN are described in detail in Figure 2.35 “Recommend”; Level of Evidence: 5. Initial dextrose should approximate a glucose infusion rate of 4–6 mg/kg/h. In cases of extreme hyperglycemia, advancement of dextrose may be delayed until blood sugar control is improved. Initiation of amino acids is recommended at 1.5–3 g/kg/day. Amino acids may be limited due to postoperative volume restrictions or in cases of renal impairment or azotemia. Initial lipids are recommended to start at 1–3 g/kg. Contraindications to initiating full lipid calories are rare but include elevated liver enzymes and triglyceride levels above 400 mg/dL.
4. Micronutrient recommendations for PN are also described in Figure 2. “Recommend”; Level of Evidence: 5. We also recommend that the practitioner consider additional zinc due to potential losses from chest tube drainage or open wounds.50 Zinc can be initiated at 250–400 mcg/kg/day and be increased to a total of 2000–4000 mcg/day. Carnitine is recommended in neonatal PN due to a potential deficit in endogenous stores in infancy, and should be initiated at 8–10 mg/kg/day.51 Carnitine supplementation is only beneficial if there is a deficiency, although supplementation in the presence of normal levels is benign.
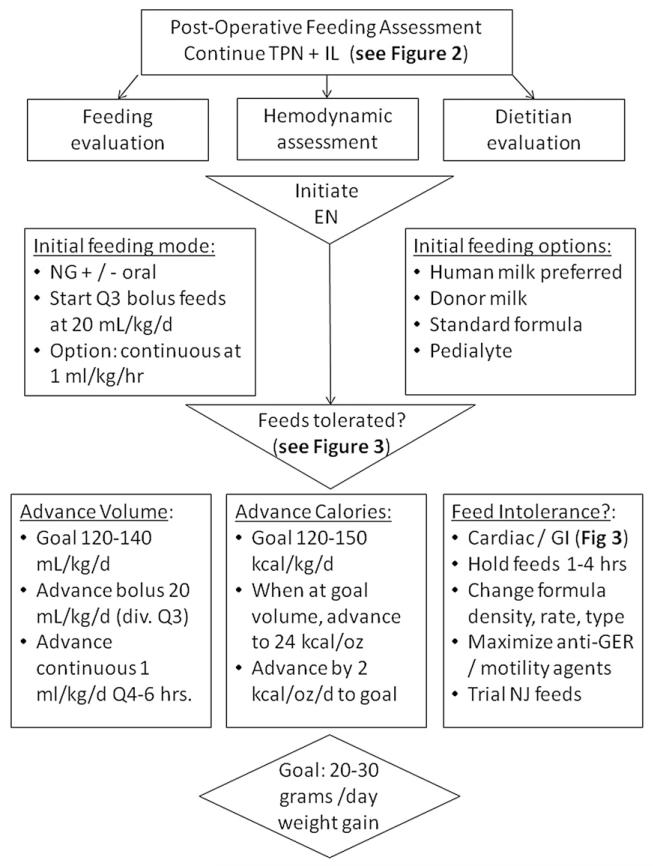
Postoperative Enteral Feeding Guideline (Figures 4 and 5)
Figure 4.
Postoperative enteral feeding (EN).8,17,18,23,24,27-30,34,35,46,52-56 Goal: Postoperative enteral nutrition should begin as soon as safe and feasible. PN, total parenteral nutrition; IL, intravenous lipid solution; NG, nasogastric; NJ nasojejunal.
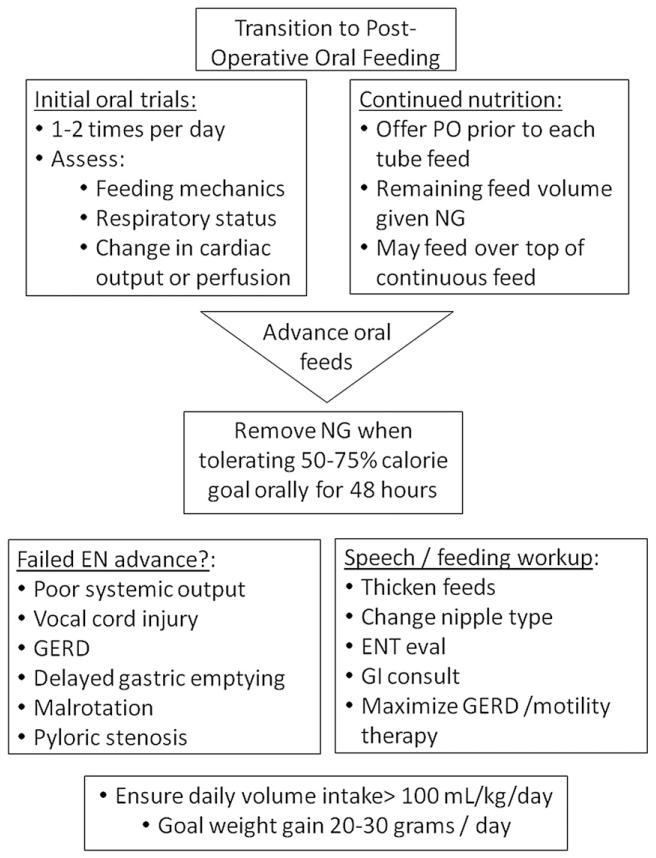
Figure 5.
Postoperative oral feeding.8,17,18,23,24,27-30,34,35,46,52-56 Goal: Postoperative oral feeding should begin as soon as is safe and feasible in order to assess potential complications and promote natural feeding behavior. PO, by mouth; NG, nasogastric; EN, enteral nutrition; GERD, gastroesophageal reflux disease; ENT, ear, nose, and throat specialist; GI, gastrointestinal.
Practice varies as to the timing and mode of post-operative enteral feeding, which may contribute to consistently poor growth patterns throughout the hospital course reported in the literature.17,18,22,54 Early initiation of postoperative feeds may be delayed for many reasons, including low cardiac output, high inotrope requirement, prolonged intubation or respiratory insufficiency, excessive chest tube drainage, or gastrointestinal complications. During this time, PN is necessary to supplement the infants nutritional demands.8,34,35,55 Once feeds are initiated, advancement may be hindered by ongoing concerns for cardiac and respiratory insufficiency, feeding intolerance, and vocal cord dysfunction. The initiation of a postoperative feeding protocol may ameliorate these challenges in postoperative feeding and growth. Several studies have shown a benefit to the initiation of a postoperative feeding protocol in this population, including a decreased length of hospital stay, decreased incidence of NEC, shorter time to achieve goals of caloric intake, and reduced requirement for parental nutrition.27-30
Below we outline current level of evidence and rationale supporting the consensus guidelines for postoperative enteral feeding guidelines found in Figures 4 and 5. The early postoperative period has received perhaps the least attention from past studies on the topic, and we have identified significant knowledge gaps in this area. While a significant decline in WAZ between Norwood surgery and hospital discharge has been shown universally demonstrated in past studies, the factors responsible are poorly understood. To date, no study has rigorously assessed modifiable risk factors for poor postoperative growth.
1. EN should be initiated as soon as the patient is hemodynamically stable.8,28,29,34,35,52,53 “Strongly Recommend”; Level of Evidence: 2. Figure 4 provides an algorithm for initiation and advancement of EN. A trophic rate of 20 mL/kg/day or 1–2 mL/kg/h is recommended.17,28,29 Human milk is recommended; however, standard infant formula is an acceptable alternative.47 PN should continue during enteral feed advancement to ensure adequate caloric intake.8,34,35 There is no clear evidence to support one method of enteral tube feeding (intermittent vs. continuous) over another, but it has been shown that using continuous feeding requires more time to reach goal feeds.23,24 For this reason, coupled with the fact that intermittent feeds are more physiologic, intermittent feeds should be considered when possible. Feeds should be advanced 20 mL/kg/day to a goal of 120–140 mL/kg/day and 120–150 kcal/kg/day.34,46,55 When continuous feeding goals are initially used, transition to bolus feeds by first turning off the continuous enteral infusion for 2 hours and then providing 3 hours of volume over 1 hour.17,29 If the patient tolerates this transition, then feedings can be continued over 1 hour, eight times per day. The time in which each bolus feed is running may then be gradually decreased from 60 minutes down to 20–30 minutes per feed or run by gravity.
2. Oral feeds should be initiated following feeding evaluation. “Strongly Recommend”; Level of Evidence: 2. Figure 5 depicts guidelines for oral feeding. Due to the high incidence of vocal cord injury and feeding dysfunction, a feeding evaluation should be conducted on all infants postoperatively to evaluate oral-motor coordination and aspiration risk.17,46,54,56 This can be performed per institution preference either by bedside nurses or ancillary staff such as speech or occupational therapists. Once the infant is deemed safe for oral feeding, trial feeds should be attempted prior to each NG feed or on top of continuous drip feeds.17,46 During initial oral feedings, infants should continually be evaluated for evidence of low cardiac output and respiratory compromise. Once the infant is able to take ≥50–75% of their caloric goal orally for 48 hours, we recommend removing the enteral feeding tube and attempting a complete oral feeding regimen.46 If the infant struggles with oral feedings, consider ongoing therapies from a speech therapist and/or a video fluoroscopic swallow study and exam by an otolaryngologist.17,46 Once adequate growth is established, breastfeeding may be considered on an individual basis with close monitoring of physiologic stability and demonstration of ongoing normal infant weight gain. Often, supplemental bottle feeds with a higher caloric density are required to achieve optimal growth in this population.24,46,53
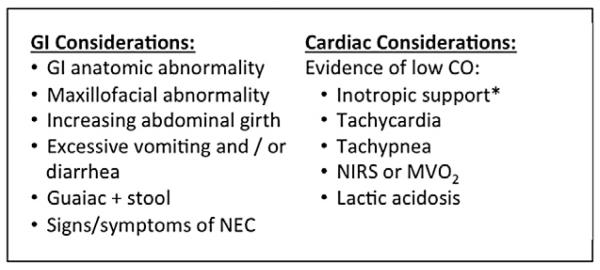
3. During feeding advance, close monitoring for gastrointestinal and cardiac complications is required.17,27-30,34 “Strongly Recommend”; Level of Evidence: 2. Figure 3 lists both gastrointestinal and cardiac symptoms to continuously evaluate. These signs and symptoms may indicate marginal cardiac output, limited respiratory reserve, the presence of an intercurrent illness, or specific feeding complications. Once significant cardiac pathology has been ruled out, the practitioner may consider several interventions to reduce feeding complications. These may include decreasing caloric density, decreasing volume, maximizing antireflux medications, or changing formula type.17,18,24,28,29,46,53 Prophylactic use of anti reflux medication has not been shown to be efficacious in all patients but may be beneficial on an individual basis. Other antireflux precautions include elevating the head of the bed, smaller frequent feedings, and upright positioning of the patient.24 Nasojejunal feedings may also be considered if there is continued feeding intolerance.8,52 Motility agents could also be considered if feeding intolerance persists.
Figure 3.
Considerations/possible contraindications to enteral feeding (EN).27-30,39,46,52 *Implications of inotropic support may differ during various phases of the hospital course. In the preoperative period, the need for inotropic support implies unstable systemic circulation, while in the patient with stabilized postoperative physiology, it is appropriate to enterally feed with evidence of good systemic output while weaning inotropic support. CO, cardiac output; AVO2, arteriovenous oxygen difference; NIRS, near-infrared spectroscopy; MVO2, mixed venous oxygen saturation; NEC, necrotizing enterocolitis.
Interstage Feeding Guideline (Figure 6)
Continued assessment of growth and nutrition is critical for the infant following discharge from Norwood palliation and through presentation for SCPC. Poor growth velocity during this high-risk interstage period is common and has been associated with worse outcomes.17,18,23,31 However, there is recent evidence from the NPC-QIC registry and others demonstrating tremendous variability in infant growth between programs, with some documenting that normal infant growth can be achieved and that normal growth may be associated with an interstage monitoring program.32,46,56
Below outline current level of evidence and rationale supporting the consensus guidelines for preoperative feeding in Figure 6. Several gaps in knowledge regarding interstage growth were identified. While there is mixed evidence in the literature regarding the efficacy and safety of the use of nasogastric or gastrostomy tubes in promoting interstage growth, these systems may be effective in individual patients. There continues to be a lack of understanding regarding the physiology leading to poor interstage growth in these infants. A better understanding of the etiology of their growth problems will ultimately allow us to tailor interventions to individual patients.
1. Close monitoring of weight changes in the interstage period is critical to identifying growth faltering early and allowing for intervention “Strongly Recommend”;24,46,56-59 Level of Evidence: 4. “Red flags” for inappropriate weight change during the interstage period should trigger evaluation by the interstage home monitoring team and/or primary cardiologist, and are listed in Figure 6.24,56-60 Interventions following identification of a red flag may include outpatient diet modification, or hospital admission with nutritional assessment as well as medical assessment for infection, gastrointestinal pathology, or cardiac workup.
2. When growth faltering is identified in the interstage period, changes in nutritional management should be made. “Strongly Recommend”; Level of Evidence: 2. In the absence of significant cardiac pathology and when oral intake is inadequate to support growth, nasogastric supplementation or placement of gastrostomy tube may be warranted.8,35,46,57,66 If there are no red flags present but nutrition is still considered inadequate, consider increasing volume of feeds or increasing calorie concentration to promote improved growth.24,46,56,60,66 We recommend increasing calorie concentration by 2–3 calories per ounce every 24 hours to a maximum concentration of 30 calories per ounce.29 Other interventions for feeding intolerance may include treating constipation, assessing for allergic disease (i.e., stool guaiac), or trialing semi-elemental or amino acid-based formula. In all patients, consider referring the patient for speech and occupational therapy to improve their oral-motor skills. Feeding specialists will be able to assess the infant for choking or gagging with feeds, weak cry, or stridor as well as evidence of respiratory distress with oral feedings.17,22,54,57
3. A registered dietitian should be involved in assessment and management of growth at each clinic visit and when nutritional concerns arise.46,58 “Strongly Recommend”; Level of Evidence: 4. Necessary components at each evaluation include: anthropometrics, daily weight change or change in anthropometric percentiles, review of feeding regimen and recipe mixing, calculated total daily volume and caloric intake, medication review, and review of any social service needs (i.e., home nursing, supplemental services like Women Infant Children or Early Intervention).8 Weight gain goals should be clearly communicated and parents or caregivers should demonstrate competency of new nutrition goals and recipe mixing by routine parent teach-back methods at each evaluation during the interstage period. Weight gain of >20 g per day during the interstage period should be communicated as the goal to achieve adequate growth.17-19,22,23,31,46
Limitations
These feeding guidelines are based upon the limited literature available on the subject as well as expert opinion of nutrition professionals who have attempted to balance current practice guidelines, published literature reviews on the topic, and potential benefits of nutrition support for infants with single ventricle physiology. These guidelines should not replace clinical judgment of the health practitioner caring for individual patients. Given the limitations of existing evidence to support individual practices supporting these guidelines, prospective validation is required. Specifically, further work to understand the gaps in knowledge in this area is vital.
Conclusions
Infants with HLHS are known to be at risk for poor nutrition and growth failure. There is little published information regarding successful nutrition interventions for infants with single ventricle physiology. Feeding protocols have been shown to maximize the benefits and minimize the risks of EN in the management of critically ill patients. The FWG reviewed the literature as well as current nutritional practices at several centers participating in the NPC-PIC in order to develop guidelines summarizing best nutritional practices for this high-risk group.
Footnotes
Author Contributions
Ms. Slicker and Mrs. Horsley coordinated and led the Feeding Working Group (FWG), supervising both the literature search as well as the compilation of recommendations. All authors contributed to the development and design of the feeding algorithms. Ms. Slicker, Dr. Hehir, Mrs. Horsley, and Dr. Anderson primarily wrote the manuscript. All authors were involved in critical revisions and final approval of the manuscript prior to submission.
Conflicts of interest: The authors note that they have no financial relationships relevant to this article to disclose.
References
- 1.Krasemann T, Fenge H, Kehl HG, et al. A decade of staged Norwood palliation in hypoplastic left heart syndrome in a midsized cardiosurgical center. Pediatr Cardiol. 2005;26:751–755. doi: 10.1007/s00246-005-0908-5. [DOI] [PubMed] [Google Scholar]
- 2.Ashburn DA, McCrindle BW, Tchervenkov CI, et al. Outcomes after the Norwood operation in neonates with critical aortic stenosis or aortic valve atresia. J Thorac Cardiovasc Surg. 2003;125:1070–1082. doi: 10.1067/mtc.2003.183. [DOI] [PubMed] [Google Scholar]
- 3.Tweddell JS, Hoffman GM, Mussatto KA, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106:I82–I89. [PubMed] [Google Scholar]
- 4.Ohye RG. Multi-institutional studies: lessons learned from the Pediatric Heart Network Single Ventricle Reconstruction Trial. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13:76–78. doi: 10.1053/j.pcsu.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Ghanayem NS, Tweddell JS, Hoffman GM, Mussatto K, Jaquiss RD. Optimal timing of the second stage of palliation for hypoplastic left heart syndrome facilitated through home monitoring, and the results of early cavopulmonary anastomosis. Cardiol Young. 2006;16(suppl 1):61–66. doi: 10.1017/S1047951105002349. [DOI] [PubMed] [Google Scholar]
- 6.Hehir DA, Dominguez TE, Ballweg JA, et al. Risk factors for interstage death after stage 1 reconstruction of hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg. 2008;136:94–99. doi: 10.1016/j.jtcvs.2007.12.012. 99 e1-99 e3. [DOI] [PubMed] [Google Scholar]
- 7.Anderson J. Poor interstage weight gain is common and adversely affects short-term outcome after the bidirectional glenn shunt. J Am Coll Cardiol. 2008;51:A83–A97. [Google Scholar]
- 8.Corkins M. The A.S.P.E.N. Pediatric Nutrition Support Core Curriculum. American Society for Parenteral and Enteral Nutrition; Silver Springs, MD: 2010. [Google Scholar]
- 9.Argyle J. Approaches to detecting growth faltering in infancy and childhood. Ann Hum Biol. 2003;30:499–519. doi: 10.1080/0301446032000112698. [DOI] [PubMed] [Google Scholar]
- 10.Block RW, Krebs NF. Failure to thrive as a manifestation of child neglect. Pediatrics. 2005;116:1234–1237. doi: 10.1542/peds.2005-2032. [DOI] [PubMed] [Google Scholar]
- 11.Rudolf MC, Logan S. What is the long term outcome for children who fail to thrive? A systematic review. Arch Dis Child. 2005;90:925–931. doi: 10.1136/adc.2004.050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black MM, Dubowitz H, Krishnakumar A, Starr RH., Jr Early intervention and recovery among children with failure to thrive: follow-up at age 8. Pediatrics. 2007;120:59–69. doi: 10.1542/peds.2006-1657. [DOI] [PubMed] [Google Scholar]
- 13.Dykman RA, Casey PH, Ackerman PT, McPherson WB. Behavioral and cognitive status in school-aged children with a history of failure to thrive during early childhood. Clin Pediatr (Phila) 2001;40:63–70. doi: 10.1177/000992280104000201. [DOI] [PubMed] [Google Scholar]
- 14.Leite HP, Fisberg M, de Carvalho WB, de Camargo Carvalho AC. Serum albumin and clinical outcome in pediatric cardiac surgery. Nutrition. 2005;21:553–558. doi: 10.1016/j.nut.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 15.de Luis DA, Izaola O, Cuellar L, et al. Nutritional assessment: predictive variables at hospital admission related with length of stay. Ann Nutr Metab. 2006;50:394–398. doi: 10.1159/000094362. [DOI] [PubMed] [Google Scholar]
- 16.Kudsk KA, Tolley EA, DeWitt RC, et al. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. JPEN J Parenter Enteral Nutr. 2003;27:1–9. doi: 10.1177/014860710302700101. [DOI] [PubMed] [Google Scholar]
- 17.Davis D, Davis S, Cotman K, et al. Feeding difficulties and growth delay in children with hypoplastic left heart syndrome versus d-transposition of the great arteries. Pediatr Cardiol. 2008;29:328–333. doi: 10.1007/s00246-007-9027-9. [DOI] [PubMed] [Google Scholar]
- 18.Kelleher DK, Laussen P, Teixeira-Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition. 2006;22:237–244. doi: 10.1016/j.nut.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Vogt KN, Manlhiot C, Van Arsdell G, Russell JL, Mital S, McCrindle BW. Somatic growth in children with single ventricle physiology impact of physiologic state. J Am Coll Cardiol. 2007;50:1876–1883. doi: 10.1016/j.jacc.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 20.Gingell RL, Hornung MG. Growth problems associated with congenital heart disease in infancy. In: Lebenthal E, editor. Textbook of Gastroenterology and Nutrition in Infancy. Raven Press; New York: 1989. pp. 639–649. [Google Scholar]
- 21.Hansen SR, Dorup I. Energy and nutrient intakes in congenital heart disease. Acta Paediatr. 1993;82:166–172. doi: 10.1111/j.1651-2227.1993.tb12632.x. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan C, Sachdeva R, Morrow WR, et al. Standardized management improves outcomes after the Norwood procedure. Congenit Heart Dis. 2009;4:329–337. doi: 10.1111/j.1747-0803.2009.00323.x. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JB, Beekman RH, 3rd, Eghtesady P, et al. Predictors of poor weight gain in infants with a single ventricle. J Pediatr. 2010;157:407–413. doi: 10.1016/j.jpeds.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Steltzer M, Rudd N, Pick B. Nutrition care for newborns with congenital heart disease. Clin Perinatol. 2005;32:1017–1030. xi. doi: 10.1016/j.clp.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Kugler JD, Beekman Iii RH, Rosenthal GL, et al. Development of a pediatric cardiology quality improvement collaborative: from inception to implementation. From the Joint Council on Congenital Heart Disease Quality Improvement Task Force. Congenit Heart Dis. 2009;4:318–328. doi: 10.1111/j.1747-0803.2009.00328.x. [DOI] [PubMed] [Google Scholar]
- 26.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 27.Jeffries HE, Wells WJ, Starnes VA, Wetzel RC, Moromisato DY. Gastrointestinal morbidity after Norwood palliation for hypoplastic left heart syndrome. Ann Thorac Surg. 2006;81:982–987. doi: 10.1016/j.athoracsur.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Braudis NJ, Curley MA, Beaupre K, et al. Enteral feeding algorithm for infants with hypoplastic left heart syndrome poststage I palliation. Pediatr Crit Care Med. 2009;10:460–466. doi: 10.1097/PCC.0b013e318198b167. [DOI] [PubMed] [Google Scholar]
- 29.del Castillo SL, McCulley ME, Khemani RG, et al. Reducing the incidence of necrotizing enterocolitis in neonates with hypoplastic left heart syndrome with the introduction of an enteral feed protocol. Pediatr Crit Care Med. 2010;11:373–377. doi: 10.1097/PCC.0b013e3181c01475. [DOI] [PubMed] [Google Scholar]
- 30.Weidmeier SE, Henry E, Baer VL, et al. Center differences in NEC within one health-care system may depend on feeding protocol. Am J Perinatol. 2008;25:5–11. doi: 10.1055/s-2007-995220. [DOI] [PubMed] [Google Scholar]
- 31.Anderson JB, Beekman RH, 3rd, Border WL, et al. Lower weight-for-age z score adversely affects hospital length of stay after the bidirectional Glenn procedure in 100 infants with a single ventricle. J Thorac Cardiovasc Surg. 2009;138:397–404. e1. doi: 10.1016/j.jtcvs.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 32.Williams RV, Victor Zak V, Ravishankar C, et al. Pediatric heart network investigators, factors affecting growth in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr. 2011;159:1017–1022.e2. doi: 10.1016/j.jpeds.2011.05.051. ISSN 0022-3476, 10.1016/j.jpeds. 2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medoff-Cooper B, Naim M, Torowicz D, Mott A. Feeding, growth, and nutrition in children with congenitally malformed hearts. Cardiol Young. 2010;20:149–153. doi: 10.1017/S1047951110001228. [DOI] [PubMed] [Google Scholar]
- 34.Hagau N, Culcitchi C. Nutritional support in children with congenital heart disease. Nutritional Therapy & Metabolism. 2010;28:172–184. [Google Scholar]
- 35.Koletzko B, Goulet O, Hunt J, et al. Guidelines on paediatric parenteral nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR) J Pediatr Gastroenterol Nutr. 2005;41(suppl 2):S1–S87. doi: 10.1097/01.mpg.0000181841.07090.f4. [DOI] [PubMed] [Google Scholar]
- 36.Johnson BA, Mussatto K, Uhing MR, Zimmerman H, Tweddell J, Ghanayem N. Variability in the preoperative management of infants with hypoplastic left heart syndrome. Pediatr Cardiol. 2008;29:515–520. doi: 10.1007/s00246-007-9022-1. [DOI] [PubMed] [Google Scholar]
- 37.Willis L, Thureen P, Kaufman J, Wymore E, Skillman H, da Cruz E. Enteral feeding in prostaglandin-dependent neonates: is it a safe practice? J Pediatr. 2008;153:867–869. doi: 10.1016/j.jpeds.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natarajan G, Reddy AS, Aggarwal S. Enteral feeding of neonates with congenital heart disease. Neonatology. 2010;98:330–336. doi: 10.1159/000285706. [DOI] [PubMed] [Google Scholar]
- 39.McElhinney D, Hedrick H, Bush D, et al. Necrotizing entercolitis in neonates with congential hert diesease: risk factors and outcomes. Pediatrics. 2000;106:1080–1087. doi: 10.1542/peds.106.5.1080. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan C, Jaquiss RD, Morrow WR, et al. Impact of staged palliation on somatic growth in patients with hypoplastic left heart syndrome. Congenit Heart Dis. 2010;5:546–551. doi: 10.1111/j.1747-0803.2010.00457.x. [DOI] [PubMed] [Google Scholar]
- 41.Uauy RD, Fanaroff AA, Korones SB, Phillips EA, Phillips JB, Wright LL. Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1991;119:630–638. doi: 10.1016/s0022-3476(05)82418-7. [DOI] [PubMed] [Google Scholar]
- 42.Patole SK, de Klerk N. Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: a systematic review and meta-analysis of observational studies. Arch Dis Child Fetal Neonatal Ed. 2005;90:F147–F151. doi: 10.1136/adc.2004.059741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearl JM, Nelson DP, Schwartz SM, Manning PB. First-stage palliation for hypoplastic left heart syndrome in the twenty-first century. Ann Thorac Surg. 2002;73:331–339. doi: 10.1016/s0003-4975(01)02720-5. discussion 339-340. [DOI] [PubMed] [Google Scholar]
- 44.Jadcherla SR, Vijayapal AS, Leuthner S. Feeding abilities in neonates with congenital heart disease: a retrospective study. J Perinatol. 2009;29:112–118. doi: 10.1038/jp.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howley LW, Kaufman J, Wymore E, et al. Enteral feeding in neonates with prostaglandin-dependent congenital cardiac disease: international survey on current trends and variations in practice. Cardiol Young. 2012;22:121–127. doi: 10.1017/S1047951111001016. [DOI] [PubMed] [Google Scholar]
- 46.Hehir DA, Rudd N, Slicker J, et al. Normal Interstage growth after the Norwood operation associated with interstage home monitoring. Pediatr Cardiol. 2012 Apr 20; doi: 10.1007/s00246-012-0320-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thibeau S, D’Apolito K. Review of the relationships between maternal characteristics and preterm breastmilk immune components. Biol Res Nurs. 2012;14:207–216. doi: 10.1177/1099800411400064. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization Energy and protein requirements. Report of a joint FAO/WHO/UNU Expert Consultation. World Health Organ Tech Rep Ser. 1985;724:1–206. [PubMed] [Google Scholar]
- 49.Boineau FG, Lewy JE. Estimation of parenteral fluid requirements. Pediatr Clin North Am. 1990;37:449–459. doi: 10.1016/s0031-3955(16)36866-3. [DOI] [PubMed] [Google Scholar]
- 50.Domej W, Tilz GP, Demel U, et al. Sequential thoracentesis: minor change of zinc compared to other selected essential trace elements in the serum. Biol Trace Elem Res. 2002;87:29–43. doi: 10.1385/BTER:87:1-3:029. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt-Sommerfeld E, Penn D, Wolf H. Carnitine deficiency in premature infants receiving total parenteral nutrition: effect of L-carnitine supplementation. J Pediatr. 1983;102:931–935. doi: 10.1016/s0022-3476(83)80027-4. [DOI] [PubMed] [Google Scholar]
- 52.Golbus JR, Wojcik BM, Charpie JR, Hirsch JC. Feeding complications in hypoplastic left heart syndrome after the Norwood procedure: a systemic review of the literature. Pediatr Cardiol. 2011;32:539–552. doi: 10.1007/s00246-011-9907-x. [DOI] [PubMed] [Google Scholar]
- 53.Pillo-Blocka F, Adatia I, Sharieff W, et al. Rapid advancement to more concentrated formula in infants after surgery for congenital heart disease reduces duration of hospital stay: a randomized clinical trial. J Pediatr. 2004;145:761–766. doi: 10.1016/j.jpeds.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 54.Skinner ML, Halstead LA, Rubinstein CS, Atz AM, Andrews D, Bradley SM. Laryngopharyngeal dysfunction after the Norwood procedure. J Thorac Cardiovasc Surg. 2005;130:1293–1301. doi: 10.1016/j.jtcvs.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Schwalbe-Terilli CR, Hartman DH, Nagle ML, et al. Enteral feeding and caloric intake in neonates after cardiac surgery. Am J Crit Care. 2009;18:52–57. doi: 10.4037/ajcc2009405. [DOI] [PubMed] [Google Scholar]
- 56.St Pierre A, Khattra P, Johnson M, Cender L, Manzano S, Holsti L. Content validation of the infant malnutrition and feeding checklist for congenital heart disease: a tool to identify risk of malnutrition and feeding difficulties in infants with congenital heart disease. J Pediatr Nurs. 2010;25:367–374. doi: 10.1016/j.pedn.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Petit CJ, Fraser CD, Mattamal R, Slesnick TC, Cephus CE, Ocampo EC. The impact of a dedicated single-ventricle home-monitoring program on interstage somatic growth, interstage attrition, and 1-year survival. J Thorac Cardiovasc Surg. 2011;142:1358–1366. doi: 10.1016/j.jtcvs.2011.04.043. ISSN 0022-5223. [DOI] [PubMed] [Google Scholar]
- 58.Anderson JB, Iyer SB, Schidlow DN, et al. Variation in growth of infants with single ventricle. J Pediatr. 2012;161:16–21. doi: 10.1016/j.jpeds.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Anderson J, Iyer S, Williams R, et al. Variation in interstage weight gain between surgical centers in single ventricle infants: report from the National Pediatric Cardiology Quality Improvement Collaborative registry. Congenit Heart Dis. 2011;6:535–536. [Google Scholar]
- 60.Ghanayem NS, Hoffman GM, Mussatto KA, et al. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg. 2003;126:1367–1377. doi: 10.1016/s0022-5223(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 61.Briassoulis G, Zavras N, Hatzis T. Malnutrition, nutritional indices and early enteral feeding in critically ill children. Nutrition. 2001;17:548–557. doi: 10.1016/s0899-9007(01)00578-0. [DOI] [PubMed] [Google Scholar]
- 62.Petrillo-Albarano T, Pettignano R, Asfaw M, et al. Use of a feeding protocol to improve nutritional support though early, aggressive, enteral nutrition in the pediatric intensive care unit. Pediatr Crit Care Med. 2006;7:340–344. doi: 10.1097/01.PCC.0000225371.10446.8F. [DOI] [PubMed] [Google Scholar]
- 63.Barr J, Hecht M, Flavin KE, et al. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest. 2004;125:1446–1457. doi: 10.1378/chest.125.4.1446. [DOI] [PubMed] [Google Scholar]
- 64.Marik PE, Zaloga GT. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29:2264–2270. doi: 10.1097/00003246-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Zhang G, Herridge J, et al. Energy expenditure and caloric and protein intake in infants following the Norwood procedure. Pediatr Crit Care Med. 2008;9:55–61. doi: 10.1097/01.PCC.0000298756.82286.23. [DOI] [PubMed] [Google Scholar]
- 66.Schidlow DN, Anderson JB, Klitzner TS, et al. Variation in interstage outpatient care after the Norwood procedure: a report from the Joint Council on Congenital Heart Disease National Quality Improvement Collaborative. Congenit Heart Dis. 2011;6:98–107. doi: 10.1111/j.1747-0803.2011.00509.x. [DOI] [PubMed] [Google Scholar]