Abstract
Purpose:
Niosomes play an increasingly important role in drug delivery as they can reduce toxicity and modify pharmacokinetic and bio-availability. Topically applied niosomes can increase the residence time of drugs in the stratum corneum and epidermis, while reducing the systemic absorption of the drug. It can act as drug containing reservoirs and the modification of the vesicular compositions or surface properties can adjust the drug release rate and the affinity for the target site. Ketoconazole is a broad spectrum Imidazole derivative useful in the treatment of superficial and systemic fungal infections.
Materials and Methods:
In order to improve the low skin penetration and to minimize the side effects associated with topical conventional drug administration, Ketoconazole niosomes were prepared by a thin film hydration method using different ratios of non-ionic surfactants (Span 40, 60 and Tween 60) along with cholesterol (CHO). The formulations were evaluated for size, shape, entrapment efficiency and in vitro drug release.
Results:
Niosomes appeared spherical in shape and size range was found to be 4.86 ± 1.24-7.38 ± 3.64 μm. The entrapment efficiency was found in the range of 55.14 ± 2.29-78.63 ± 0.91% and in vitro drug release in the range of 46.63 ± 0.95-72.37 ± 0.59% in 24 h. Ketoconazole niosomes formulated with Span 60 and CHO in the ratio of 1:0.2 were found to be promising and were incorporated into 1% Carbopol gel. The formulated gel was evaluated for various physicochemical parameters and antifungal activity. The in vitro drug release study was carried out using phosphate buffer saline pH 7.4 and was found to be 36.18 ± 1.50% in 12 h.
Conclusion:
Gel formulation containing niosomes loaded with Ketoconazole showed prolonged action than formulations containing Ketoconazole in non-niosomal form and it can be developed successfully to improve the antifungal activity.
Keywords: Ketoconazole, in vitro drug release studies, niosomes, skin permeation
INTRODUCTION
Several antifungal agents are available in the market in different topical preparations (e.g., creams, ointments, and powders for the purpose of local dermatological therapy). One of the antifungal agents is Ketoconazole, a substituted Imidazole; it is having broad spectrum activity against systemic and superficial mycoses. It is readily but incompletely absorbed after oral dosing and it varies among individuals.[1] Common side effects associated with Ketoconazole therapy include mild burning at the application site, severe allergic reactions, blisters, irritation, pain or redness.
Topical drug administration is a localized drug delivery system anywhere in the body through ophthalmic, rectal, vaginal and skin as topical routes. However, the major barrier of the skin is the stratum corneum, the top layer of the epidermis. Low molecular weight (≤500 Da), lipophilicity, and effectiveness at a low dosage are the ideal characteristics of the drugs for transdermal delivery.[2] Thus, the therapeutic effectiveness of the existing drugs is improved by formulating them in an advantageous way. Transdermal drug delivery system has recently been developed, aiming to achieve the objective of systemic medication through topical application to the intact skin surface. The skin acts as a major target as well as a principle barrier for transdermal drug delivery.
In the past few decades, considerable attention has been focused on the development of a new drug delivery system. To increase permeability, chemical and physical approaches have been examined to lower stratum corneum barrier properties. These approaches include tape stripping, iontophoresis, electroporation and vascular systems, such as liposomes and niosomes. Among these approaches, liposomes and niosomes are widely used to enhance drug permeation across the skin in cosmetic and dermatologic fields.[3] Vesicles can play a major role in modeling biological membranes, and in the transportation and targeting of active agents. Different types of pharmaceutical carriers, e.g., Particulate, polymeric, macromolecular or cellular carrier is present. Particulate type carrier, also known as the colloidal carrier system, includes lipid particles, microspheres, nanoparticles, polymeric micelles and vesicular systems.[4] Niosomes have also been widely studied as drug carriers for controlled and targeted delivery. Niosomes behave in-vivo like liposomes, prolonging the circulation of the entrapped drug to alter its organ distribution and metabolic stability or prolonging contact time of the drug with the applied tissues.
Niosomes (non-ionic surfactant vesicles) are microscopic lamellar structures obtained on an admixture of non-Ionic surfactant of the alkyl or dialkyl polyglycerol ether class and cholesterol (CHO) with subsequent hydration in aqueous media.[5] Niosomes attracts much attention because of its advantages in many aspects, such as chemical stability, high purity, content uniformity, low cost, convenient storage of non-ionic surfactants, and large numbers of surfactants available for the design of niosomes.[6] Niosomes are promising vehicle for drug delivery. The encapsulation of drugs in niosomes can minimize drug degradation and inactivation after administration; prevent undesirable side effects, increase drug bioavilability and targeting to the pathological area.[7] Surfactants also act as penetration enhancers as they can remove the mucus layer and break functional complexes. The non-ionic surfactants form a closed bilayer vesicle in aqueous media based on its amphiphilic nature using some energy, for instance, heat and physical agitation to form this structure. Their effectiveness is strongly dependent on their physiological properties, such as composition, size, charge, lamellarity and application conditions.[4] Niosomes as delivery devices have also been studied with anticancer, anti-tubercular, anti-leishmanial, anti-inflammatory, hormonal drugs and oral vaccine.[8]
Hence, in the present investigation, an attempt is made to develop and characterize niosomal gel formulation of Ketoconazole. Ketoconazole is selected as a drug candidate for the present study to treat fungal infections of the skin more efficiently.
MATERIALS AND METHODS
Materials
Ketoconazole was gifted by Alkem Pharmaceuticals, Mumbai. Span 40, Span 60, Tween 60 was purchased from SD Fine Chemicals Ltd., Mumbai. CHO was purchased from Loba Chemie Pvt Ltd., Mumbai. Carbopol 934 was purchased from Himedia, Mumbai. Disodium hydrogen orthophosphate and Potassium dihydrogen orthophosphate were purchased from SD Fine Chemicals Ltd., Mumbai. Chloroform, Methanol, Glycerol and Triethanolamine were purchased from SD Fine Chemicals Ltd., Mumbai. All other materials were of analytical grade.
Method of preparation of niosomes
Niosomes were prepared by a thin film hydration method using a lipid mixture consisting of surfactant (span 40, span 60 and tween 60) and CHO, at different specified ratios as given in Table 1. Surfactant, CHO and drug were dissolved in 10 ml of chloroform. The lipid mixture was then transferred to a 100 ml round bottom flask, and the solvent was evaporated under reduced pressure at a temperature of 55-65°C, using a rotary flash evaporator until the formation of a thin lipid film. The formed film was hydrated with 20 ml of Phosphate buffer saline pH 7.4. The hydration was continued for 1 h, while the flask was kept rotating at 55-65°C in the rotary evaporator. The hydrated niosomes were sonicated for 20 min using a bath sonicator to obtain niosomal dispersion containing both free and entrapped drugs of varying size.[9,10]
Table 1.
Formulations of Ketoconazole niosomes
Evaluation of Ketoconazole niosomes
Morphological characterization
The vesicle formation was confirmed by optical microscopy in 45× resolution. The niosomal suspension placed over a glass slide and fixed over by drying at room temperature, the dry thin film of niosomal suspension observed in the formation of vesicles. The microphotography of the niosomes also obtained from the microscope by using a digital camera[11] [Figure 1]. The detailed surface characteristic of the selected Ketoconazole niosomes formulation was observed using a scanning electron microscope.
Figure 1.
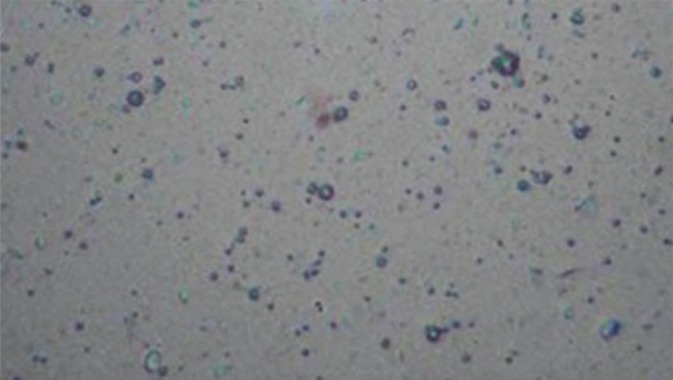
Microphotograph of niosomes
Fourier transform infrared spectroscopy study
Infrared spectrum of Ketoconazole, excipients and formulation (TNS4) was determined using the potassium bromide dispersion method.
Morphology of formulated niosomal vesicles
The niosomal dispersion after hydration was stored under 4°C for congealing and a drop of dispersion was viewed under an optical microscope to observe the shape and lamellar nature of the vesicle.
Entrapment efficiency
Entrapment efficiency of niosomes was determined by exhaustive dialysis method. The measured quantity of niosomal suspension was taken into a dialysis tube to which dialysis membrane was securely attached on one side. The dialysis tube was suspended in 100 ml PBS pH 7.4 containing 10% v/v methanol, which was stirred on a magnetic stirrer. The un-entrapped drug was separated from the niosomal suspension into the medium through the membrane. At every hour, entire medium (100 ml) was replaced with fresh medium (for about 6-7 h) until the absorbance reached a constant reading indicating no drug is available in an un-entrapped form. Inclusion of CHO in niosomes increases its hydrodynamic diameter and entrapment efficiency. It stabilizes niosomes and decreases the permeability of vesicles to entrapped solute preventing leakage. An increase in CHO content of the bilayers resulted in a decrease in the release rate of encapsulated material and therefore, an increase of the rigidity of the bilayers obtained. Presence of charge tends to increase the inter lamellar distance between successive bilayers and leads to greater overall entrapped volume.
The withdrawn samples were analyzed at 225 nm using a UV spectrophotometer. Amount of entrapped drug was obtained by subtracting amounts of un-entrapped drug from the total drug incorporated.[12]
In-vitro drug release study
The release of Ketoconazole from niosomal formulations were determined using membrane diffusion technique. The niosomes left after removal of un-entrapped drug were dialyzed into a beaker containing 100 ml of PBS pH 7.4 containing 10% v/v methanol (to maintain sink condition), which acted as receptor compartment. The temperature of the receptor medium was maintained at 37 ± 0.5°C and agitated using a magnetic stirrer. Aliquots of 5 ml sample were withdrawn periodically and after each withdrawal, same volume of the medium was replaced. The collected samples were analyzed using a UV spectrophotometer at 225 nm.[13,14] The tests were carried out in triplicate.
Formulation of niosomes entrapped Ketoconazole gel
Promising niosomal suspension (formulation prepared by thin hydration film method containing span 60 as surfactant (TNS4) Formulations of niosomes prepared using span 60 containing Ketoconazole equivalent to 2 % w/w was incorporated into the gel base composed of Carbopol 934 (150 mg), glycerol (250 mg) Triethanolamine (quantity sufficient) and distilled water up to 15 g.[15,16]
EVALUATION OF NIOSOMAL GEL
Physical appearance
The prepared gel was examined for clarity, color, homogeneity and the presence of foreign particles.
pH
2.5 g of gel were accurately weighed and dispersed in 25 ml of distilled water. The pH of the dispersion was measured by using a digital pH meter.
Rheological study
Viscosity measurement: Viscosity was determined by Brookfield programmable DV III ultra viscometer. In the present study, spindle no. CP 52 with an optimum speed of 0.01 rpm was used to measure the viscosity of the preparation.
Content uniformity
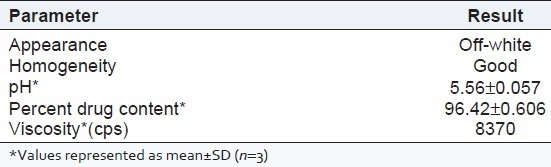
The drug content of the prepared gel was carried out by dissolving accurately weighed quantity of gel equivalent to 10 mg of the drug in 100 ml volumetric flask and volume was made up to 100 ml with methanol. The content was filtered through Whatman filter paper No. 41. 5 ml of above solution was taken into a 25 ml volumetric flask and volume was made up to mark with methanol. The content of Ketoconazole was determined at 243 nm against blank by using the Shimadzu UV/visible spectrophotometer. The drug content was determined from the calibration curve of Ketoconazole. The tests were carried out in triplicate results are given in Table 2.
Table 2.
Evaluation parameters of Ketoconazole niosomal gel (TNS4=Formulations of niosomes prepared using span 60)
In vitro drug diffusion study
The apparatus consists of a glass cylinder open at both ends. A dialysis membrane soaked in distilled water (24 h before use) is fixed to the one end of the cylinder with the aid of an adhesive. Gels equivalent to 10 mg of Ketoconazole is taken inside the cell (donor compartment) and the cell is immersed in a beaker containing 100 ml of PBS pH 7.4 containing 10% v/v methanol (to maintain sink condition), act as receptor compartment. The whole assembly is fixed in such a way that the lower end of the cell containing gel is just above the surface of the diffusion medium (1-2 mm deep) and the medium was agitated using a magnetic stirrer at the temperature 37 ± 0.5°C. Aliquots (5 ml) are withdrawn from the receptor compartment periodically and replaced with same volume with fresh buffer. The samples were analyzed by using UV-visible spectrophotometer at 225 nm.[16,17] The tests were carried out in triplicate.
In vitro antifungal activity
The antifungal efficacy studies were carried out to ascertain the biological activity of niosomal formulation, in comparison with plain Ketoconazole gel and marketed Ketoconazole ointment (Phytoral) against Aspergillus niger as the test microorganisms. This is determined by agar diffusion test employing ‘Cup plate’ technique. A layer of Sabouraud's dextrose agar media (20 ml) seeded with the test microorganisms were allowed to solidify in the petri dishes. Cups were made on the solidified agar layer with the help of sterile borer (5 mm). 0.5 ml of niosomal gel solution (1 μg of drug) is filled into one cup (i.e., hole) which is marked with ‘F’ and 0.5 ml of marketed gel solution (1 μg of drug) is filled into the second cup (i.e., hole) which is marked with ‘M’. Like wise the third. After keeping the petri dishes at room temperature for 1 h, the plates were incubated at 37 (C for 24 h. The zones of inhibition were measured around each cup.[18,19]
RESULTS AND DISCUSSION
In the present research work Ketoconazole niosomes were prepared using various non-ionic surfactants (Span 40, Span 60 and Tween 60) along with C HO in different proportions (1:0.2, 1.5:0.3, and 2:0.4) by the thin film hydration method. The prepared Ketoconazole niosomes were evaluated for various parameters like particle size, shape, entrapment efficiency and in vitro drug release. Finally, the promising formulation was selected and then it was incorporated into the gel for topical uses.
Vesicle formation
The hydrophilic-hydrophobic segments of non-ionic surfactants and a balance between them are of a paramount importance for the niosomal vesicle formation. The prepared vesicles were studied under ×45 magnification to observe the formation of vesicles. The niosomes were observed as spherical vesicles with smooth surface. Addition of CHO into these vesicular suspensions leads to a variation in size. The relationship observed between niosomes size and CHO content has been attributed to the decrease in surface energy with increasing hydrophobicity that results in smaller vesicles and also to the favourable interactions (hydrogen bonding or van der waal forces) between niosomal matrix and Ketoconazole. The diameter of the formulated niosomes was found to be in the range of 4.86 ± 1.24-7.38 ± 3.64 μm [Table 3]. Later, the surface morphology was observed using scanning electron microscope [Figure 2].
Table 3.
Evaluation of Ketoconazole niosomes
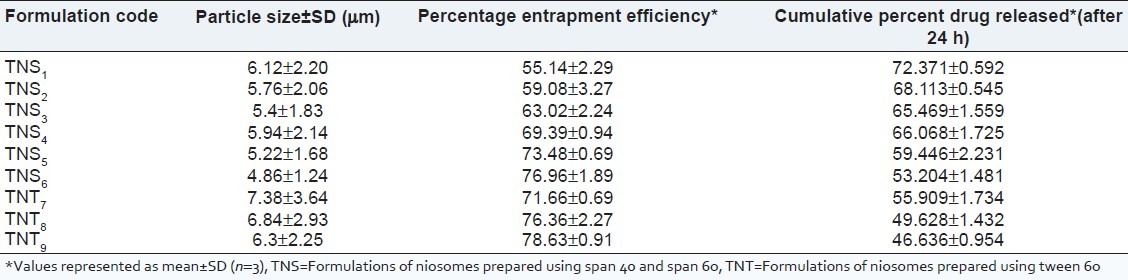
Figure 2.
Scanning electron microscope image of niosomes
Effect of drug solubility on entrapment efficiency and particle size
It is well accepted that lipophilic drugs are preferentially up taken by niosomes compared to hydrophilic ones. The encapsulation of drug in nonionic surfactant vesicles usually increases particle size, probably by interaction of drug with surfactant head groups, increasing the charge and mutual repulsion of the surfactant bilayers. Lipophilic drug is expected to be located between the fatty acyl side chains of the bilayer membrane as the hydrocarbon chains provide a good solublizing environment for the drug molecules.
Sonication
Sonication is one of the most popular methods used for producing a lipid vesicle of known size. The principal effect of sonication is cavitation (bubble formation), which has been shown to be responsible for many physical effects of ultrasound on lipid membranes. Sonication for 20 min was done after preparation of niosomes.
Entrapment efficiency
Process variables like a vacuum, hydration medium, hydration time and speed of rotation of the flask are important to prepare niosomes. Improper selection of these parameters may result in improper hydration resulting in formation of fragile niosomes or drug leakage from niosomes. The rotational speed of the flask demonstrated discernible influence on the thickness and uniformity of the lipid film. The hydrating temperatures used must be above the gel liquid phase transition temperature of the system. The results reflect the effect of phase transition temperatures in terms of increased entrapment efficiency. Addition of CHO to the surfactant was required to form stable nonionic surfactant based vesicles. CHO almost always present in lipid vesicles as well as bio-membranes and influences a number of membrane properties such as ion permeability, aggregation, fusion process, elasticity, size and shape. Being amphipathic, CHO can insert itself into the bilayer membrane with its hydrophilic head oriented towards the aqueous surface and aliphatic chain line up parallel to the hydrocarbon chains in the center of the bilayer. It is known that CHO increases the chain order of the liquid-state bilayer and strengthen the non-polar tail of the non-ionic surfactant. An increase in CHO concentration, lead to an increase in the entrapment levels of Ketoconazole. The increase in the entrapment efficiency is attributed to the ability of CHO to cement the leaking space in the bilayer membranes, which in turn allow enhanced drug level in niosomes. Entrapment efficiency increases with an increase in surfactant concentration and more time is taken for maximum drug release. The entrapment efficiency was determined by separating the unentrapped drug using dialysis; it was in the range of 55.14 ± 2.29-78.63 ± 0.91% [Table 3].
In vitro release study
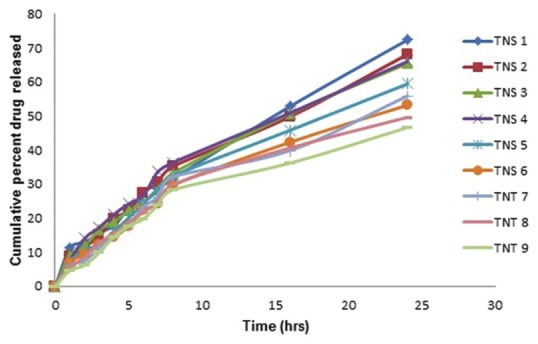
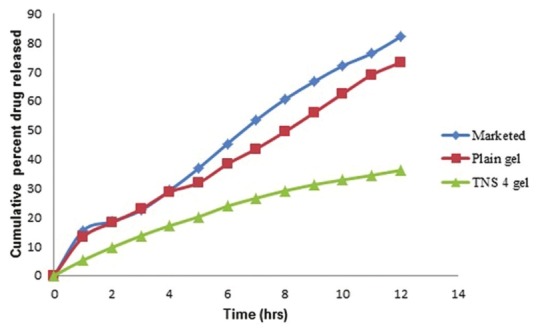
Since the drug is insoluble in water, it is needed to develop an appropriate medium that can provide sufficient solubility for the drug to maintain the required sink condition during diffusion studies. Initially, a PBS pH 7.4 was selected, which has shown a better stability of Ketoconazole. A medium with 10% methanol was considered to be sufficient for the diffusion study. The volume of the receptor medium used was 100 ml. The increase of CHO content resulted in a reduction of membrane permeability, which leads to lower drug elution from the vesicles. The in vitro drug release of the formulated niosomes was found to be in the range of 46.63 ± 0.95 to 72.37 ± 0.59% at the end of 24 h [Table 3]. For the characterization of the release kinetics, the in vitro drug release data was fitted to First order plots, Higuchi diffusion plots and Peppas log-log plots [Figure 3]. The regression coefficient values for this entire graph showed good linearity by both the methods. Slope values of the Peppas log-log plot were also calculated. In almost all the cases slope values of the Peppas plots were in between 0.45 and 0.89 suggesting that the drug was released by non-fickian (anomalous) release mechanism i.e., the drug was released by the combination of both diffusion and erosion controlled drug release. Among all the formulations, formulations prepared by thin film hydration method TNS1, TNS2 and TNS4 showed higher drug release. TNS4 gave highest entrapment efficiency as compared to TNS1 and TNS2. It also showed that particle size for TNS4 is less compared to TNS1. So after considering all these parameters, formulation TNS4 was found to be the best having vesicle size of 5.94 ± 2.14 μm, entrapment efficiency of 69.39 ± 0.94% and drug release of 66.06 ± 1.72% at the end of 24 h [Figure 4]. Finally, the promising formulation TNS4 was centrifuged and pellets were obtained and incorporated into a 1% Carbopol gel.
Figure 3.
In vitro drug release profiles of formulations using Span 40, Span 60 and cholesterol
Figure 4.
In vitro release plot of Ketoconazole plain gel, Marketed ointment and Ketoconazole niosomal gel (TNS4)
Niosomal gel
Carbopol was chosen to prepare the niosomal gel in this study, which is due to its hydrophilic nature and bioadhesive properties, which may result in an increased residence time of a drug at the site of absorption by interacting with the mucosa.
The improved stability of niosomes after incorporation into the gel base may be due to prevention of fusion of niosomes. The higher drug skin retention in case of niosomal gel may be due to, creation of reservoir effect of drug in the skin and thereby increasing the drug retention capacity into the skin.
This niosomal gel formulation (TNS4) was evaluated for appearance, pH, viscosity, drug content, in vitro drug release and antifungal activity. The niosomal gel formulation is off-white in color. The pH of the gel was 5.56 ± 0.05 and viscosity of the gel was 8370 cps. The drug content of the niosomal gel was found to be 96.42 ± 0.60%. The prepared niosomal gel formulation is also subjected to in vitro release study and the release data was compared to Ketoconazole plain gel and marketed ointment (Phytoral). The niosomal gel formulation, Ketoconazole plain gel and marketed ointment released 36.18 ± 1.50, 73.21 ± 1.41 and 82.06 ± 1.62% of drug respectively at the end of 12 h. For the characterization of the release kinetics the in vitro drug release data was fitted to First order plots, Higuchi diffusion plots and Peppas log-log plots. The regression coefficient values for this entire graph showed good linearity. Slope values of the Peppas log-log plot were also calculated. The release exponent for niosomal gel was 0.7761, Ketoconazole plain gel was 0.7160 and marketed ointment was 0.753, which indicated a coupling of the diffusion and erosion mechanism-so-called anomalous diffusion and may indicate that the drug release is controlled by more than one process.
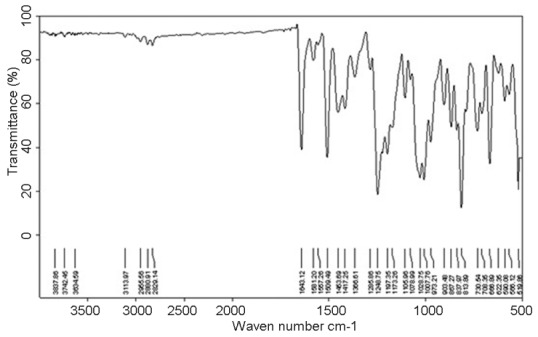
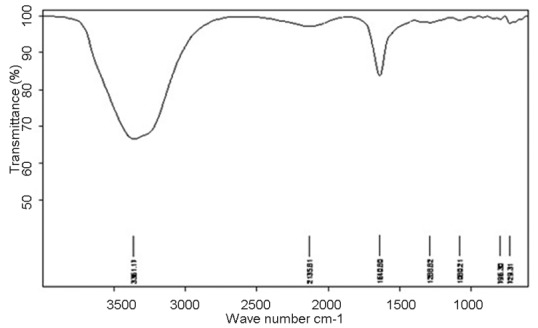
IR study
The IR spectrum of the pure drug shows characteristic peaks at 1,643, 813 and 1,285/cm due to C = O, –Cl and aromatic groups respectively. Formulation TNS4 also exhibited similar peaks at 1,640, 796 and 1,288/cm respectively for the above groups. This confirms undisturbed structure of the drug in the formulations [Figures 5 and 6].
Figure 5.
Infra red spectrum of Ketoconazole
Figure 6.
Infra red spectrum of formulation TNS4
In vitro antifungal activity
Based on in vitro characterization studies, Ketoconazole niosomal gel formulation was further evaluated for antifungal activity by cup-plate method. A result of zone of inhibition of Ketoconazole niosomal gel formulation (F) was compared to Ketoconazole plain gel (P) and marketed ointment (M). Results of F, P and M were found to be 25.3 ± 0.57, 19.6 ± 1.52 and 23.3 ± 3.78 mm respectively. Results indicate that F exhibits maximum antifungal activity after 24 h. These niosomes acts as carriers for drug delivery to the particular site of action, the antifungal activity is created by the drug incorporated in the niosomes.
Here F exhibits best antifungal activity because F form is in niosomal formulation while the others are plain gel forms.
CONCLUSION
The results of this study showed that CHO content and the type of surfactant altered the entrapment efficiency and drug release rate from niosomes. Formulation having surfactant and CHO ratio 1:0.2 exhibited higher drug release. From all these studies, it can be concluded that a gel formulation containing niosomes loaded with Ketoconazole showed prolonged action than formulations containing Ketoconazole in non-niosomal form and it can be developed successfully to improve the antifungal activity.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Hardman JG, Limbird LE. Goodman and Gilman's, The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001. Anti microbial Agents: Anti fungal Agents; pp. 1301–2. [Google Scholar]
- 2.Manosroi A, Khanrin P, Lohcharoenkal W, Werner RG, Götz F, Manosroi W, et al. Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. Int J Pharm. 2010;392:304–10. doi: 10.1016/j.ijpharm.2010.03.064. [DOI] [PubMed] [Google Scholar]
- 3.Choi MJ, Maibach HI. Liposomes and niosomes as topical drug delivery systems. Skin Pharmacol Physiol. 2005;18:209–19. doi: 10.1159/000086666. [DOI] [PubMed] [Google Scholar]
- 4.Sankar V, Ruckmani K, Jailani S, Ganesan K, Sharavanan SP. Recent trends and developments: Niosome drug delivery system. Indiana Pharm. 2010;9:16–8. [Google Scholar]
- 5.Malhotra M, Jain NK. Niosomes as drug carriers. Indian Drugs. 1994;31:81–6. [Google Scholar]
- 6.Hao YM, Li K. Entrapment and release difference resulting from hydrogen bonding interactions in niosome. Int J Pharm. 2011;403:245–53. doi: 10.1016/j.ijpharm.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Di Marzio L, Marianecci C, Petrone M, Rinaldi F, Carafa M. Novel pH-sensitive non-ionic surfactant vesicles: Comparison between Tween 21 and Tween 20. Colloids Surf B Biointerfaces. 2011;82:18–24. doi: 10.1016/j.colsurfb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Vyas SP. Theory and Practice in Novel Drug Delivery System. 1st ed. New Delhi: CBS Publishers and Distributors; 2009. pp. 284–98. [Google Scholar]
- 9.Barakat HS, Darwish IA, El-Khordagui LK, Khalafallah NM. Development of naftifine hydrochloride alcohol-free niosome gel. Drug Dev Ind Pharm. 2009;35:631–7. doi: 10.1080/03639040802498864. [DOI] [PubMed] [Google Scholar]
- 10.Essa EA. Effect of formulation and processing variables on the particle size of sorbitan monopalmitate niosome. Asian J Pharm. 2010;4:227–33. [Google Scholar]
- 11.Gupta KS, Nappinnai M, Gupta VR. Formulation and evaluation of topical meloxicam niosomal gel. Int J Biopharm. 2010;1:7–13. [Google Scholar]
- 12.Tavano L, Muzzalupo R, Cassano R, Trombino S, Ferrarelli T, Picci N. New sucrose cocoate based vesicles: Preparation, characterization and skin permeation studies. Colloids Surf B Biointerfaces. 2010;75:319–22. doi: 10.1016/j.colsurfb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Srinivas S, Anand Kumar Y, Hemanth A, Anitha M. Preparation and evaluation of niosomes containing aceclofenac. Dig J Nanomater Bios. 2010;5:249–54. [Google Scholar]
- 14.Hayes, Chetwynd A. Antifungal Ketoconazole composition for topical use. EP1309352B1 (Patent) 2002 [Google Scholar]
- 15.Ning M, Guo Y, Pan H, Chen X, Gu Z. Preparation, in vitro and in vivo evaluation of liposomal/niosomal gel delivery systems for clotrimazole. Drug Dev Ind Pharm. 2005;31:375–83. doi: 10.1081/ddc-54315. [DOI] [PubMed] [Google Scholar]
- 16.Patel RP, Patel H, Baria AH. Formulation and evaluation of carbopol gel containing liposome of Ketoconazole. Int J Drug Del Technol. 2009;1:42–5. [Google Scholar]
- 17.Gupta GD, Goud RS. Release rate of tenoxicam from acrypol gels. Indiana Pharm. 2005;4:69–75. [Google Scholar]
- 18.Staub I, Schapoval EE, Bergold AM. Microbiological assay of ketoconazole in shampoo. Int J Pharm. 2005;292:195–9. doi: 10.1016/j.ijpharm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Nesseem DI. Formulation and evaluation of itraconazole via liquid crystal for topical delivery system. J Pharm Biomed Anal. 2001;26:387–99. doi: 10.1016/s0731-7085(01)00414-9. [DOI] [PubMed] [Google Scholar]


