Abstract
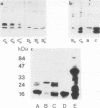
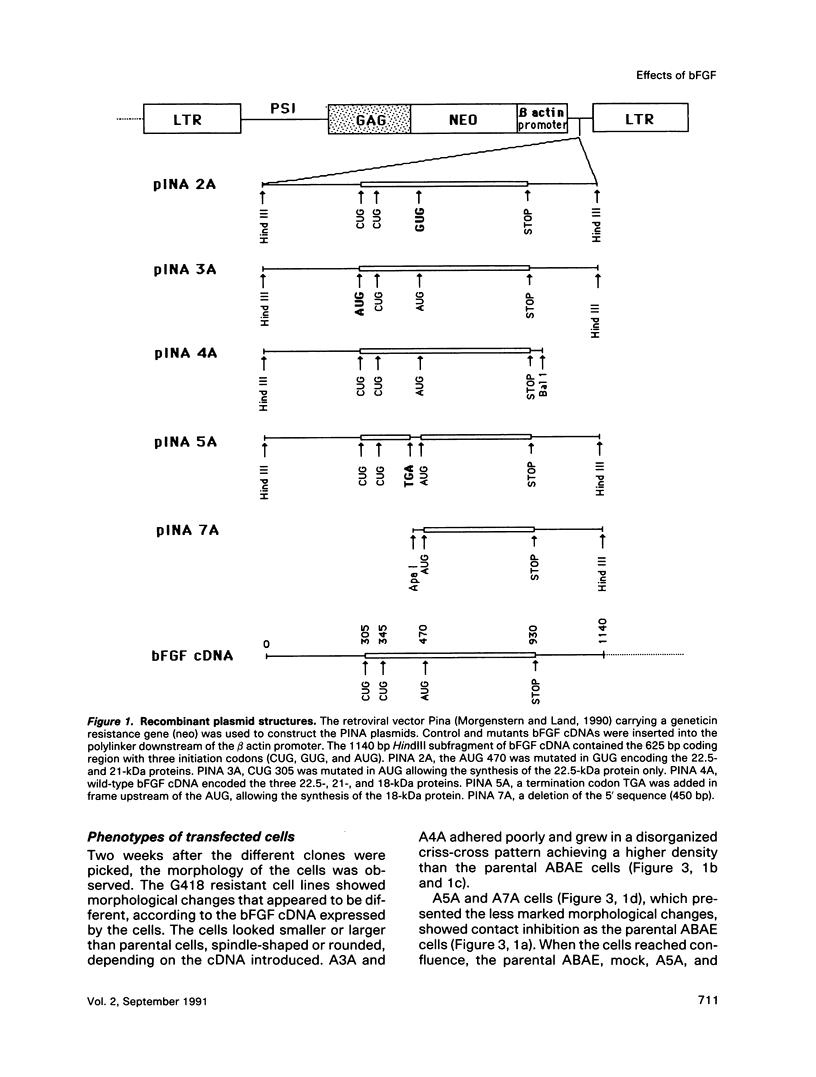
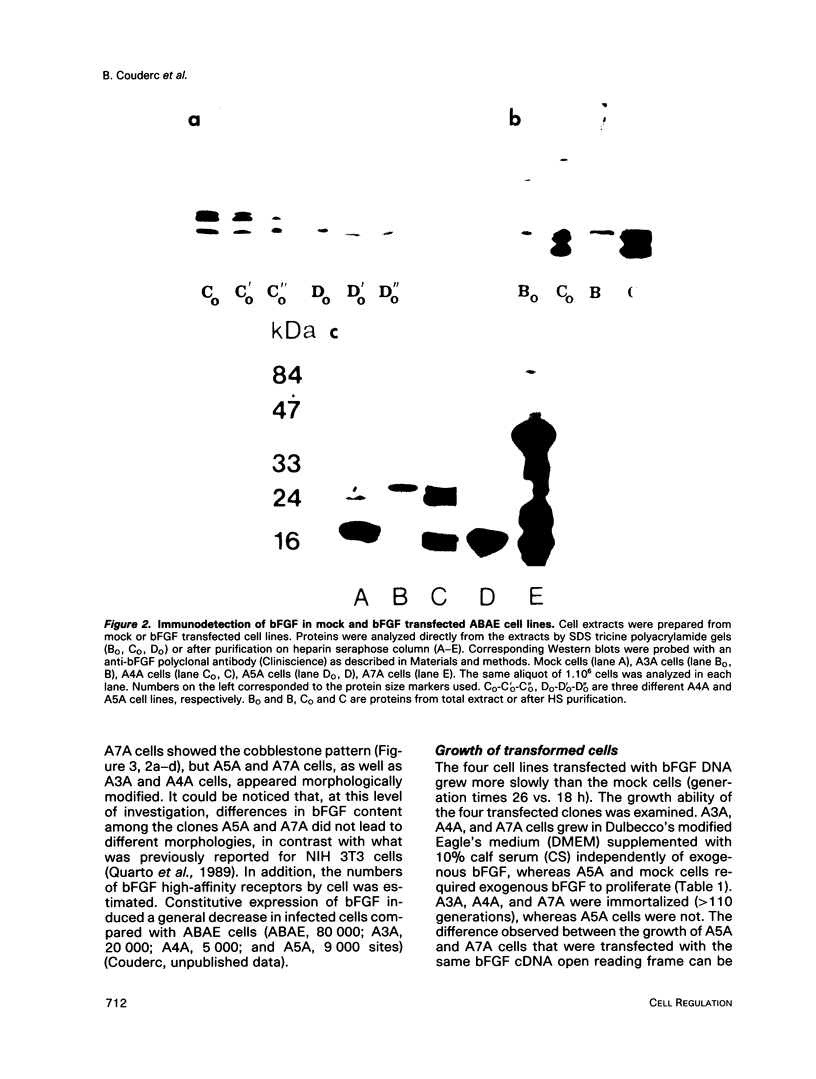
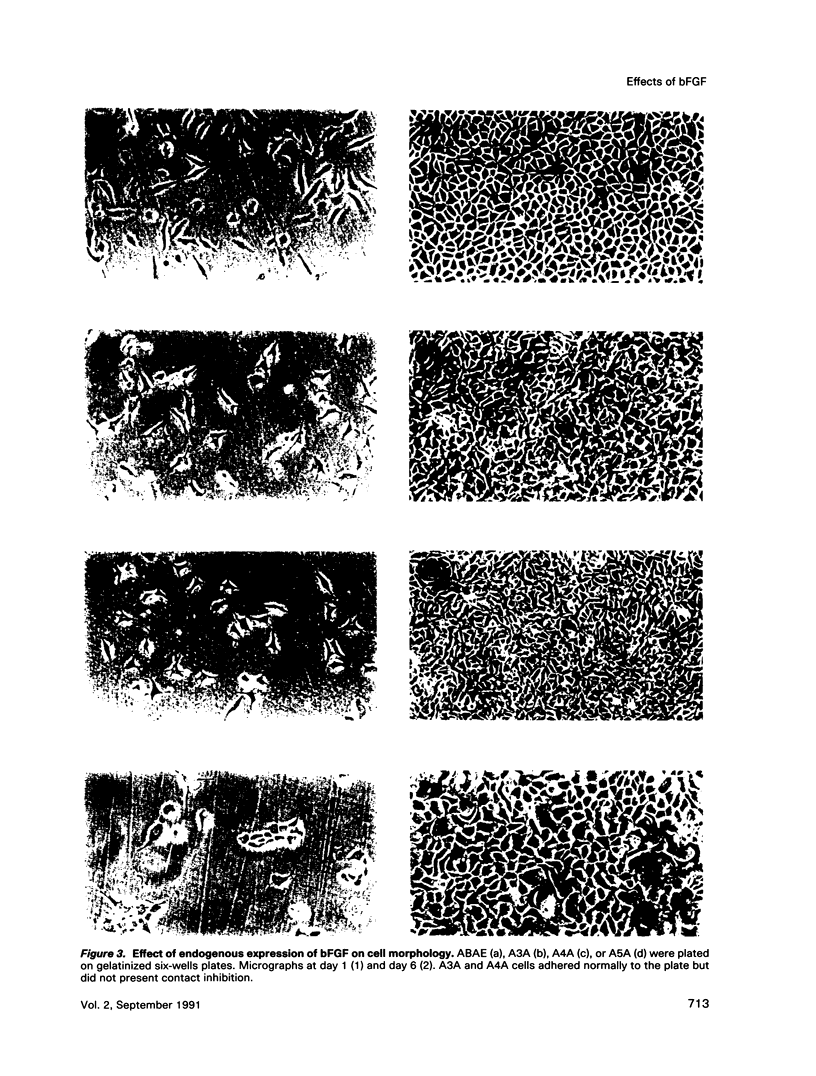
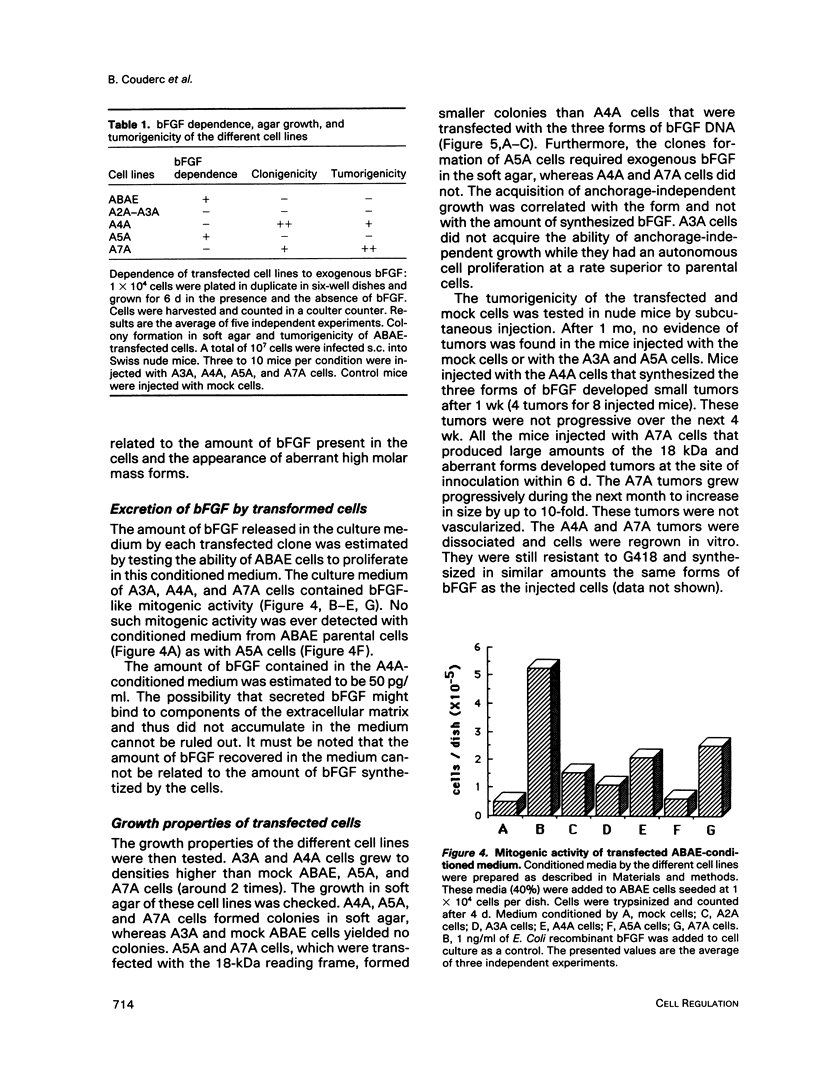
Normal adult bovine aortic endothelial cells were infected with various recombinant retroviruses expressing one, two, or three human basic fibroblast growth factor (bFGF) proteins normally synthesized by an alternative use of translation initiation codons. We show here that the constitutive expression of the AUG-initiated from (18 kDa) leads the transfected cells to form colonies in soft agar. The expression of the high molar weight (HMW) forms (22.5 and 21 kDa) initiated at one of the two CUG initiation codons allows cell immortalization, whereas the tumorigenic potential is reached when the three forms are constitutively expressed. Furthermore, we provide evidence that constitutive expression of (HMW) bFGF forms has a down-regulation effect on bFGF synthesis from the gene naturally active in parental endothelial cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Aubin R. J., Weinfeld M., Paterson M. C. Factors influencing efficiency and reproducibility of polybrene-assisted gene transfer. Somat Cell Mol Genet. 1988 Mar;14(2):155–167. doi: 10.1007/BF01534401. [DOI] [PubMed] [Google Scholar]
- Baldin V., Roman A. M., Bosc-Bierne I., Amalric F., Bouche G. Translocation of bFGF to the nucleus is G1 phase cell cycle specific in bovine aortic endothelial cells. EMBO J. 1990 May;9(5):1511–1517. doi: 10.1002/j.1460-2075.1990.tb08269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blam S. B., Mitchell R., Tischer E., Rubin J. S., Silva M., Silver S., Fiddes J. C., Abraham J. A., Aaronson S. A. Addition of growth hormone secretion signal to basic fibroblast growth factor results in cell transformation and secretion of aberrant forms of the protein. Oncogene. 1988 Aug;3(2):129–136. [PubMed] [Google Scholar]
- Bugler B., Amalric F., Prats H. Alternative initiation of translation determines cytoplasmic or nuclear localization of basic fibroblast growth factor. Mol Cell Biol. 1991 Jan;11(1):573–577. doi: 10.1128/mcb.11.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Dickson C., Peters G. Potential oncogene product related to growth factors. 1987 Apr 30-May 6Nature. 326(6116):833–833. doi: 10.1038/326833a0. [DOI] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Sommer A. Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Fibroblast growth factor. Mol Cell Endocrinol. 1986 Aug;46(3):187–204. doi: 10.1016/0303-7207(86)90001-8. [DOI] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Lapeyre B., Amalric F., Ghaffari S. H., Rao S. V., Dumbar T. S., Olson M. O. Protein and cDNA sequence of a glycine-rich, dimethylarginine-containing region located near the carboxyl-terminal end of nucleolin (C23 and 100 kDa). J Biol Chem. 1986 Jul 15;261(20):9167–9173. [PubMed] [Google Scholar]
- Marics I., Adelaide J., Raybaud F., Mattei M. G., Coulier F., Planche J., de Lapeyriere O., Birnbaum D. Characterization of the HST-related FGF.6 gene, a new member of the fibroblast growth factor gene family. Oncogene. 1989 Mar;4(3):335–340. [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990 Jun 25;18(12):3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats A. C., De Billy G., Wang P., Darlix J. L. CUG initiation codon used for the synthesis of a cell surface antigen coded by the murine leukemia virus. J Mol Biol. 1989 Jan 20;205(2):363–372. doi: 10.1016/0022-2836(89)90347-1. [DOI] [PubMed] [Google Scholar]
- Prats H., Kaghad M., Prats A. C., Klagsbrun M., Lélias J. M., Liauzun P., Chalon P., Tauber J. P., Amalric F., Smith J. A. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1836–1840. doi: 10.1073/pnas.86.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarto N., Talarico D., Sommer A., Florkiewicz R., Basilico C., Rifkin D. B. Transformation by basic fibroblast growth factor requires high levels of expression: comparison with transformation by hst/K-fgf. Oncogene Res. 1989;5(2):101–110. [PubMed] [Google Scholar]
- Renko M., Quarto N., Morimoto T., Rifkin D. B. Nuclear and cytoplasmic localization of different basic fibroblast growth factor species. J Cell Physiol. 1990 Jul;144(1):108–114. doi: 10.1002/jcp.1041440114. [DOI] [PubMed] [Google Scholar]
- Rogelj S., Weinberg R. A., Fanning P., Klagsbrun M. Basic fibroblast growth factor fused to a signal peptide transforms cells. Nature. 1988 Jan 14;331(6152):173–175. doi: 10.1038/331173a0. [DOI] [PubMed] [Google Scholar]
- Sasada R., Kurokawa T., Iwane M., Igarashi K. Transformation of mouse BALB/c 3T3 cells with human basic fibroblast growth factor cDNA. Mol Cell Biol. 1988 Feb;8(2):588–594. doi: 10.1128/mcb.8.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Mergia A., Abraham J. A., Fiddes J. C., Gospodarowicz D. Basic fibroblast growth factor in human rhabdomyosarcoma cells: implications for the proliferation and neovascularization of myoblast-derived tumors. Proc Natl Acad Sci U S A. 1987 Feb;84(3):842–846. doi: 10.1073/pnas.84.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A., Moscatelli D., Rifkin D. B. An amino-terminally extended and post-translationally modified form of a 25kD basic fibroblast growth factor. Biochem Biophys Res Commun. 1989 May 15;160(3):1267–1274. doi: 10.1016/s0006-291x(89)80140-8. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Zhan X., Bates B., Hu X. G., Goldfarb M. The human FGF-5 oncogene encodes a novel protein related to fibroblast growth factors. Mol Cell Biol. 1988 Aug;8(8):3487–3495. doi: 10.1128/mcb.8.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]