Abstract
Within-person changes in estradiol and progesterone predict changes in binge eating tendencies across the menstrual cycle. However, all women have menstrual-cycle fluctuations in hormones, but few experience binge eating. Personality traits may be critical individual difference factors that influence who will engage in emotional eating in the presence of a vulnerable hormonal environment. Women (N=239) provided self-reports of emotional eating and saliva samples for hormone measurement for 45 consecutive days. Negative urgency and negative emotionality were measured once and were examined as moderators of hormone-emotional eating associations. Consistent with prior research, within-person changes in the interaction between estradiol and progesterone predicted emotional eating. Neither negative urgency nor negative emotionality interacted with changes in estradiol and progesterone to predict changes in emotional eating. Additional factors, other than the two personality traits examined, may account for individual differences in within-person associations between hormones and emotional eating.
Keywords: ovarian hormones, personality, negative urgency, eating disorders, emotional eating
1. Introduction
Ovarian hormones are one important set of biological factors involved in the etiology of binge eating and eating disorders (Edler, Lipson, & Keel, 2007; Klump et al., in press-a; Racine et al., 2012). Longitudinal studies across the menstrual cycle find that binge eating and emotional are highest during menstrual cycle phases characterized by high progesterone (i.e., mid-luteal phase) and lowest during phases described by high estradiol (i.e., ovulatory phase) (Edler et al., 2007; Klump, Keel, Culbert, & Edler, 2008; Klump et al., in press-a; Lester, Keel, & Lipson, 2003). Moreover, studies that have directly assayed hormone levels suggest that within-person changes in hormones drive menstrual-cycle fluctuations in binge eating. Pilot work found that binge eating in clinical samples and emotional eating in community samples were predicted by decreases in estradiol and increases in progesterone (Edler et al., 2007; Klump et al., 2008). However, recent research with the largest sample to date suggests that ovarian hormone effects are interactive, such that emotional eating is highest when progesterone and estradiol are high (Klump et al., in press-a). This is consistent with high levels of emotional eating during the mid-luteal phase, a time of high progesterone and relatively high estradiol..
Despite converging evidence demonstrating that ovarian hormones predict binge eating, all normally cycling women have menstrual-cycle changes in estradiol and progesterone, but relatively few engage in dysregulated eating. Individual differences in these relationships clearly exist, and there is a need to examine factors that influence patterns of dysregulated eating across the menstrual cycle in some but not all women in order to develop targeted prevention and interventions for these symptoms.
Personality traits could impact which individuals binge eat in response to a vulnerable hormonal environment, as individual differences in personality predict the development of eating disorder symptoms (Lilenfeld, Wonderlich, Riso, Crosby, & Mitchell, 2006). Impulsivity is perhaps the most important personality trait for binge eating and associated eating disorders. Unlike other traits (e.g., negative emotionality), impulsivity specifically relates to binge eating versus other disordered eating behaviors (e.g., dietary restraint) (Yeomans, Leitch, & Mobini, 2008) and tends to differentiate patients with binge/purge behaviors versus restrictive eating disorders (Claes, Vandereycken, & Vertommen, 2005; Rosval et al., 2006).
Recently, the multidimensional construct of impulsivity has been decomposed into distinct traits that are differentially related to impulsive behaviors (Smith et al., 2007; Whiteside & Lynam, 2001). One type of impulsivity, negative urgency (i.e., the tendency to act rashly in response to negative affect), appears to be most important for binge eating and associated eating disorders. When examined together with other impulsive traits (i.e., lack of planning, lack of perseverance, sensation seeking), negative urgency consistently emerges as the best predictor of binge eating (Anestis, Smith, Fink, & Joiner, 2009; Fischer & Smith, 2008; Fischer, Smith, & Cyders, 2008). Perhaps most importantly, recent longitudinal work suggests that negative urgency is a prospective risk factor for the development of binge eating (Fischer, Peterson, & McCarthy, in press; Pearson, Combs, Zapolski, & Smith, 2012)
To date, no study has investigated whether personality variables critical for binge eating might moderate within-person associations between ovarian hormones and binge eating phenotypes. Individuals high on negative urgency tend to experience strong impulses and have trouble resisting their impulses (Whiteside & Lynam, 2001). The biological drive to binge eat or to eat in response to emotions as a result of menstrual-cycle changes in ovarian hormones may represent a strong urge that is difficult for these individuals to resist. Thus, we hypothesized that ovarian hormones will be more likely to predict within-person changes in emotional eating in individuals with high versus low levels of negative urgency.
As a control, we investigated whether the personality trait of negative emotionality (i.e., a stable disposition towards experiencing negative affect, negative interpersonal interactions, and withdrawal behaviors; Patrick, Curtin, & Tellegen, 2002) may similarly moderate hormone-emotional eating associations. Negative emotionality has been identified as a risk factor for the development of disordered eating symptoms (Leon, Fulkerson, Perry, & Cudeck, 1993; Leon, Fulkerson, Perry, Keel, & Klump, 1999; Martin et al., 2000) as well as eating disorder diagnoses (Cervera et al., 2003; Killen et al., 1996). Moreover, negative emotionality is correlated with both binge eating and emotional eating in community samples (Heaven, Mulligan, Merrilees, Woods, & Fairooz, 2001; Klump, McGue, & Iacono, 2002). Although both negative emotionality and negative urgency involve negative affect, negative urgency includes the additional component of rash action that is hypothesized to be particularly important for dysregulated eating in response to a risky hormonal milieu. Therefore, examining negative emotionality may help determine whether any moderating effects are specific to negative urgency or are more general and present for other personality traits associated with eating symptomatology.
Finally, consistent with previous research (Klump et al., in press-a), we included daily negative affect as a covariate in all models. State levels of negative affect are strong proximal predictors of binge eating/emotional eating (Haedt-Matt et al., submitted; Smyth et al., 2007), and levels of negative affect are thought to vary across the menstrual cycle (Dennerstein & Burrows, 1979; Ivey & Bardwick, 1968). Moreover, high negative affect appears to be associated with stronger relationships between estradiol levels and emotional eating across the menstrual cycle (Hu, Boker, Neale, & Klump, submitted). Thus, we wanted to ensure that trait-level, personality characteristics moderated the direct effects of ovarian hormones on emotional eating, independent of state negative affect.
2. Methods
This study was reviewed and approved by the Michigan State University Institutional Review Board and was carried out in accordance with the APA Code of Ethics.
2.1 Participants
Participants included 239 (132 monozygotic; 107 dizygotic) same-sex female twins between the ages of 16 and 22 years drawn from the Twin Study of Hormones and Behavior Across the Menstrual Cycle (Klump et al., in press-a) within the Michigan State University Twin Registry (MSUTR). MSUTR twins were recruited using birth record methods previously described (Klump & Burt, 2006). Because of the focus on ovarian hormones, a number of exclusion criteria were necessary to capture natural hormonal variation (see Klump et al., in press-a for details). Despite these criteria, participants were demographically representative of the recruitment region (83.3% Caucasian; 15.1% African American; 0.8% Asian/Pacific Islander; 0.8% Native American; http://www.michigan.gov/mdch). Moreover, they did not meaningfully differ on levels of impulsivity, negative emotionality, or binge eating when compared to participants from previous MSUTR studies without similar exclusion criteria (average d = .11; d’s = .01-.20).
2.2 Procedures
Participants provided saliva samples and behavioral data for 45 consecutive days. Saliva samples were collected every morning within 30 minutes of waking (see Klump et al., 2008 for details). Behavioral questionnaires were completed each evening (after 5:00 pm) and included items assessing daily emotional eating and negative affect. The timing of daily data collection was such that ovarian hormone measurements clearly preceded behavioral ratings each day. In addition, three in-person visits occurred at the start of the study, mid-way through data collection (~day 23), and at the end of data collection (~day 45). Each visit included a re-assessment of study eligibility, completion of questionnaires, height/weight measurements, and collection of materials. Questionnaires assessing the personality traits of negative urgency and negative emotionality were administered one time during the first study visit (see below). Between visits, staff called/emailed participants once/week to answer questions and confirm protocol adherence. This was effective at minimizing missing data (≤ 6%) and drop-outs (< 3%) as well as identifying participants who were no longer eligible (e.g., due to pregnancy, < 3%).
2. 3 Measures
Sample characteristics for all study measures are presented in Table 1. Although daily, longitudinal data was examined for some variables, we present averages across data collection in order to characterize the sample for comparisons in future studies.
Table 1.
Sample Characteristics
| Variable | Mean (SD) | Range |
|---|---|---|
| Age | 18.09 (1.74) | 16-22 |
| Negative Urgency | 2.03 (0.54) | 1-3.67 |
| Negative Emotionality | 35.53 (15.32) | 13-89 |
| Emotional Eating | 0.33 (0.42) | 0-3 |
| Estradiol (pg/ml) | 3.04 (1.32) | 0.95-10.63 |
| Progesterone (pg/ml) | 123.85 (62.98) | 18.73-368.00 |
| Negative Affect | 14.83 (3.50) | 10-28 |
| Body mass index (BMI) | 24.20 (5.83) | 16.71-45.83 |
Values for emotional eating, estradiol, progesterone, and negative affect are average values across the 45-day data collection period. Values for BMI are average values across the three study visits.
2.3.1 Emotional Eating
The Dutch Eating Behavior Questionnaire (DEBQ) Emotional Eating scale (van Strien, Frijters, Bergers, & Defares, 1986) was completed daily and includes thirteen items assessing eating in response to negative affective cues (e.g., “Did you have a desire to eat when you were discouraged?”). Similar to previous research (Klump et al., 2008), instructions for this scale were modified with permission to ask about emotional eating over the current day. Eating in response to negative emotions is thought to be a core feature of binge eating. Importantly, this scale correlates with established measures of binge eating (r’s = .55-.69) (Racine, Culbert, Larson, & Klump, 2009; van Strien, 2000) and has demonstrated validity in distinguishing between individuals with bulimia nervosa/binge eating, overweight individuals, and college students. Internal consistency of DEBQ Emotional Eating was excellent in the current study (average α = .90).
2.3.2 Negative Urgency
The UPPS-P Impulsive Behavior Scale (Lynam, Smith, Whiteside, & Cyders, 2006) was administered one time, and the 12-item Negative Urgency Scale was examined. Internal consistency for this scale was high in the current study (α = .85) and previous work (Fischer & Smith, 2008). In addition, good convergent and discriminant validity have been demonstrated as reflected by the pattern of correlations between negative urgency and the other UPPS-P impulsive traits across self-report and interview assessments (Smith et al., 2007).
2.3.3 Negative Emotionality
The Multidimensional Personality Questionnaire-Brief Form (MPQ-BF; Patrick et al., 2002) was administered one time and was used to assess negative emotionality. The higher order Negative Emotionality factor is a weighted sum of the 11 primary trait scales, with the traits of Stress Reaction, Alienation, and Aggression having the highest loadings. Negative Emotionality exhibits expected convergent and discriminant relationships with scales from other personality inventories (e.g., r with Big Five Neuroticism = .70) (Church, 1994), and has been examined extensively as a personality correlate of eating disorder symptoms (e.g., Klump et al., 2002).
2.3.4. Ovarian Hormones
Estradiol and progesterone were measured via saliva. Saliva sampling is less invasive and is associated with higher compliance and more robust hormone-behavior associations than blood spots (Edler et al., 2007). Saliva samples were analyzed by Salimetrics, LLC (State College, PA, USA) using high-sensitivity enzyme immunoassay techniques that show excellent intra- and inter-assay coefficients of variation (see Klump et al., in press-a for details). In order to conserve resources and capture key periods of hormonal variation (i.e., mid-follicular through pre-menstrual phase), we only assayed every second saliva sample during menstrual bleeding and the early follicular phase when hormone levels are expected to be low and stable.
2.3.5 Covariates
2.3.5.1 Negative affect
The Positive and Negative Affect Schedule (Watson, Clark, & Tellegen, 1988) was used to measure daily levels of negative affect (e.g., irritability, nervousness). This scale exhibits excellent internal consistency (average α = .85 in this study) as well as good convergent and discriminant validity (Watson et al., 1988),
2.3.5.2 Body mass index (BMI)
BMI (weight (in kilograms)/height(in meters) squared) was calculated using weight and height measured during each of the three study visits using a digital scale and wall-mounted ruler, respectively. BMI was included as a covariate given its association with binge eating (Stice, Presnell, & Spangler, 2002) and ovarian hormone levels (Ukkola et al., 2001).
2.4 Statistical Analyses
2.4.1 Data preparation
Similar to each previous study of hormone-binge eating associations (Klump et al., 2008; Klump et al., in press-a), five-day rolling averages were calculated for hormones, negative affect, emotional eating, and BMI. Rolling averages minimize random variation in behavioral data due to environmental circumstances (Gladis & Walsh, 1987) and smooth the pattern of hormone variability (Kassam et al., 1996; Waller et al., 1998). Given that BMI was assessed at three time points rather than daily, we calculated rolling averages using visit 1 BMI for days in-between visits 1 and 2, visit 2 BMIs for days in-between visits 2 and 3, and visit 3 BMI for the last assessment day (day 45) All rolling averages were converted to within-person standardized scores based on each participant’s overall mean and standard deviation across data collection. These scores index the degree to which changes in a woman’s ovarian hormones, relative to her equilibrium, predict changes away from her equilibrium for emotional eating. Negative urgency and negative emotionality were standardized according to the sample mean and standard deviation.
2.4.2 Statistical models
Hierarchical linear models (HLMs) were used as they are able to examine interactive effects of predictors (i.e., hormones and personality traits) while controlling for covariates as well as for the non-independence of the repeated measures and twin data. We allowed residual errors for emotional eating to correlate between members of a twin pair, and we estimated a time-specific dyadic correlation that allowed twin’s residual errors to correlate from day-to-day. Each time-varying predictor was included as a random effect in order to model random slopes and the relationship between these slopes within twin pairs. However, there was no evidence that random slopes were correlated across twins, so an identity covariance matrix, which estimates a single variance for both twins in a pair, was used.
Given the substantial correlation between negative urgency and negative emotionality in our data set (r = .60), we ran two separate HLMs to examine each personality trait as an independent moderator of hormone-emotional eating associations. Models focused on testing the main effects of estradiol and progesterone, as well as all two-way interactions (estradiol × personality trait, progesterone × personality trait, estradiol × progesterone) and the three-way interaction (estradiol × progesterone × personality trait). Significant two-way and/or three-way interactions between ovarian hormones and personality traits would indicate that the strength of hormone-emotional eating associations differs according to level of negative urgency/negative emotionality.
3. Results
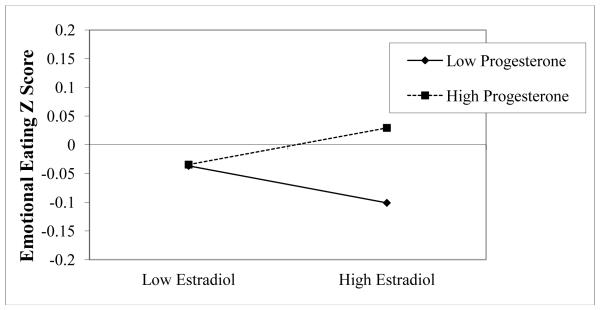
Results from HLMs examining negative urgency and negative emotionality as moderators of ovarian hormone-emotional eating relationships are presented in Table 2. Within-person changes in estradiol and progesterone did not exhibit main effects on emotional eating, but there were significant estradiol × progesterone interactions, such that the presence of high estradiol and high progesterone was associated with greater levels of emotional eating. The nature and magnitude of this interaction in both models (see Figure 1 for an example in the negative urgency model) was very similar to that observed in a previous report by our group (Klump et al., in press-a) that examined a smaller subset (82%) of the current sample.
Table 2.
Personality as a Moderator of Within-person Associations between Ovarian Hormones and Emotional Eating
| b (SE) | t | df | p | |
|---|---|---|---|---|
| Negative Urgency as a Moderator | ||||
| Intercept | −.04 (.01) | −3.57 | 223 | <.001 |
| Estradiol | 0 (.02) | −0.01 | 226 | .99 |
| Progesterone | .03 (.02) | 1.44 | 224 | .15 |
| Estradiol*Progesterone | .03 (.01) | 2.18 | 215 | .030 |
| Negative Urgency | −.006 (.01) | −0.38 | 218 | .70 |
| Estradiol*Negative Urgency | .01 (.02) | 0.49 | 228 | .62 |
| Progesterone*Negative Urgency | −.006 (.02) | −0.26 | 228 | .79 |
| Estradiol*Progesterone*Negative Urgency | −.006 (.01) | −0.38 | 218 | .70 |
| Covariates | ||||
| Negative Affect | .18 (.02) | 7.31 | 224 | <.001 |
| BMI | .06 (.03) | 1.91 | 220 | .06 |
| Negative Emotionality as a Moderator | ||||
| Intercept | −.03 (.01) | −3.52 | 230 | .001 |
| Estradiol | −.001 (.02) | −0.47 | 229 | .96 |
| Progesterone | .03 (.02) | 1.54 | 228 | .12 |
| Estradiol*Progesterone | .03 (.01) | 2.25 | 223 | .026 |
| Negative Emotionality | −.004 (.01) | −0.42 | 198 | .68 |
| Estradiol*Negative Emotionality | −.004 (.02) | −0.21 | 223 | .84 |
| Progesterone*Negative Emotionality | −.007 (.02) | −0.33 | 224 | .74 |
| Estradiol*Progesterone*Negative Emotionality | .01 (.01) | 0.86 | 221 | .39 |
| Covariates | ||||
| Negative Affect | .18 (.02) | 7.35 | 227 | <.001 |
| BMI | .05 (.03) | 2.24 | 224 | .09 |
Figure 1.
Interaction between Estradiol and Progesterone in the Prediction of Emotional Eating in the Negative Urgency Model. “Emotional Eating Z Score” = 5-day rolling average calculated within subjects, then averaged across participants.
As shown in Table 2, the personality traits of negative urgency and negative emotionality did not significantly interact with changes in estradiol, progesterone, or the estradiol-progesterone interaction to predict changes in emotional eating across the menstrual cycle. Therefore, contrary to hypotheses, these personality traits were not significant moderators of within-person associations between ovarian hormones and emotional eating in our sample.
4. Discussion
The current study was the first to examine whether personality traits might moderate within-person associations between ovarian hormones (i.e., estradiol and progesterone) and emotional eating symptoms across the menstrual cycle in a community sample of women. We hypothesized that negative urgency might be a particularly important personality trait, given robust associations between negative urgency and binge eating previously reported (Fischer et al., 2008). In addition, individuals high on negative urgency have trouble resisting strong impulses including, possibly, a biological drive towards dysregulated eating. However, our results did not support the hypothesis that negative urgency influences the strength of associations between within-person changes in ovarian hormones and emotional eating. The personality trait of negative emotionality was also not a significant moderator of hormone-emotional eating relationships. Therefore, individual differences on emotion-based rash action or chronic negative affectivity do not appear to explain why some individuals engage in emotional eating across the menstrual cycle while others do not.
In order to inform future research of this kind, it is important to explore potential reasons for our lack of hypothesized hormone-negative urgency interactions. First, controlling for daily levels of negative affect may have impacted our ability to detect moderating effects. Because the rash action of individuals high on negative urgency is thought to be dependent on the presence of momentary increases in negative affect, we may have partialled out key parts of the negative urgency construct by including negative affect in the models. However, negative urgency-hormone interactions remained non-significant in post-hoc HLMs that did not control for negative affect (data not shown), suggesting that including negative affect as a covariate is unlikely to have meaningfully impacted our results.
Second, changes in one’s propensity for impulsive action across time may be more relevant than stable individual differences for influencing the strength of hormone-emotional eating associations. According to an interactional perspective on personality (Endler & Parker, 1992), the occurrence of rash action is dependent on a person’s personality predisposition towards impulsive behavior as well as situational variables, including environmental, social, and even biological circumstances. Indeed, research demonstrates that impulsive behavior varies in response to alcohol intake (Dougherty, Marsh-Richard, Hatzis, Nouvion, & Mathias, 2008), manipulations of the serotonin system (Walderhaug et al., 2002), and phase of the menstrual cycle (Howard, Gifford, & Lumsden, 1988; Pine & Fletcher, 2011). Although most of this research has used laboratory-based behavioral tasks (e.g., Go/NoGo task), a self-report measure of state impulsivity has recently been developed (Iribarren, Jiménez-Giménez, García-de Cecilia, & Rubio-Valladolid, 2011) and could be incorporated into future daily diary and ecological momentary assessment studies of dysregulated eating. These research designs could explicitly test whether one’s immediate inclination to engage in emotion-based rash action significantly influences emotional eating in the presence of a vulnerable hormonal milieu.
Future studies must also consider additional risk variables that could moderate hormone-emotional eating associations. In the only other study of this kind, Klump et al. (in press-b) investigated whether body weight regulation variables (i.e., BMI, dietary restraint) might influence menstrual-cycle relationships between ovarian hormones and emotional eating, but null findings were also reported. The one exception was the presence of a trend-level interaction between dietary restraint and estradiol that appeared to explain pre-menstrual increases in binge eating/emotional eating in women with more severe eating pathology (e.g., women with bulimia nervosa) (Edler et al., 2007; Lester et al., 2003).
Nonetheless, several intriguing moderator possibilities remain untested. For example, individuals with a genetic predisposition for binge eating may be particularly vulnerable to dysregulated eating during certain menstrual-cycle phases (Klump et al., in press-a). Ovarian hormones are potent regulators of genes in various neurotransmitter systems involved in food intake/binge eating (e.g., serotonin) (Östlund, Keller, & Hurd, 2003), and menstrual-cycle fluctuations in emotional eating may result from hormone-induced changes in gene transcription. However, these effects may only occur in women possessing risk alleles of candidate genes for binge eating phenotypes, possibly accounting for individual differences in the strength of hormone-binge eating associations (Klump et al., in press-a).
The possibility that genomic effects of ovarian hormones account for menstrual cycle changes in dysregulated eating is further supported by the fact that hormone-emotional eating associations are independent of several important risk variables. Studies consistently show that these relationships are independent of BMI and daily changes in negative affect (Edler et al., 2007; Klump et al., 2008; Klump et al., in press-a). Our study also suggests that hormone effects cannot be accounted for by negative urgency or negative emotionality. That is, estradiol-progesterone interactions continued to significantly predict within-person changes in emotional eating after controlling for these personality traits. Taken together, ovarian hormone effects on binge eating phenotypes appear to be direct, and our findings are informative for continuing to build mechanistic models regarding the precise role of ovarian hormones in binge eating development/maintenance.
Strengths of this study included daily hormone and behavioral data collection across 45 days in a large sample that allowed us to investigate potential moderating effects of personality traits. However, we examined a non-clinical sample using emotional eating as our dependent variable, and it is unclear whether our results would generalize to clinical samples of women with binge eating/eating disorders. Identifying whether personality traits moderate hormone-binge eating associations in clinical samples is an interesting question for future research. In addition, most participants (ages 16-22 years) were not through the peak period of risk for eating disorders, which can extend up until at least age 25 (Lewinsohn, Striegel-Moore, & Seeley, 2000). Nonetheless, emotional eating is present as early as childhood (Blissett, Haycraft, & Farrow, 2010) and predicts later binge eating (Stice et al., 2002), suggesting that our sample likely includes a number of “at risk” individuals.
Highlights.
Individual Differences in the Relationship between Ovarian Hormones and Emotional Eating Across the Menstrual Cycle: A Role for Personality?
- Individual differences exist for ovarian hormone-emotional eating relationships
- Negative urgency and negative emotionality did not moderate these relationships
- Changes in propensity for impulsive action may instead moderate associations
- Ovarian hormone interactions predict emotional eating independent of personality
Acknowledgments
Role of funding source This research was supported by grants from the National Institute of Mental Health (NIMH) (1 R01 MH 0820-54) (KLK, PKK, SAB, CLS, MN, SB) and the Canadian Institutes of Health Research (CIHR) (MDR-96630) (SER). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or CIHR. These funding agencies had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions Drs. Klump, Keel, Burt, Sisk, Neale, and Boker were involved in conceptualizing and designing the protocol for the data collection, obtaining funding for the data collection, and editing the manuscript. Ms. Racine conceptualized the current study, conducted literature searches, completed the statistical analyses, and wrote the first draft of the manuscript. Dr. Klump was involved in all stages of the research project and provided extensive feedback and guidance throughout the project and on manuscript drafts. All authors approve the final manuscript.
Conflicts of Interest All authors declare that they have no conflicts of interest to disclose.
REFERENCES
- Anestis MD, Smith AR, Fink EL, Joiner TE. Dysregulated eating and distress: Examining the specific role of negative urgency in a clinical sample. Cognitive Therapy and Research. 2009;33:390–397. [Google Scholar]
- Blissett J, Haycraft E, Farrow C. Inducing preschool children’s emotional eating: Relations with parental feeding practices. The American Journal of Clinical Nutrition. 2010;92:359–365. doi: 10.3945/ajcn.2010.29375. [DOI] [PubMed] [Google Scholar]
- Cervera S, Lahortiga F, Angel Martínez-González M, Gual P, Irala-Estévez J, Alonso Y. Neuroticism and low self-esteem as risk factors for incident eating disorders in a prospective cohort study. International Journal of Eating Disorders. 2003;33:271–280. doi: 10.1002/eat.10147. [DOI] [PubMed] [Google Scholar]
- Church AT. Relating the Tellegen and five-factor models of personality structure. Journal of Personality and Social Psychology. 1994;67:898–909. doi: 10.1037//0022-3514.67.5.898. [DOI] [PubMed] [Google Scholar]
- Claes L, Vandereycken W, Vertommen H. Impulsivity-related traits in eating disorder patients. Personality and Individual Differences. 2005;39:739–749. [Google Scholar]
- Dennerstein L, Burrows G. Affect and the menstrual cycle. Journal of Affective Disorders. 1979;1:77–92. doi: 10.1016/0165-0327(79)90027-2. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug and Alcohol Dependence. 2008;96:111–120. doi: 10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychological Medicine. 2007;37:131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Endler NS, Parker JDA. Interactionism revisited: Reflections on the continuing crisis in the personality area. European Journal of Personality. 1992;6:177–198. [Google Scholar]
- Fischer S, Peterson CM, McCarthy D. A prospective test of the influence of negative urgency and expectancies on binge eating and purging. Psychology and Addictive Behaviors. doi: 10.1037/a0029323. (in press) [DOI] [PubMed] [Google Scholar]
- Fischer S, Smith GT. Binge eating, problem drinking, and pathological gambling: Linking behavior to shared traits and social learning. Personality and Individual Differences. 2008;44:789–800. [Google Scholar]
- Fischer S, Smith GT, Cyders MA. Another look at impulsivity: A meta-analytic review comparing specific dispositions to rash action in their relationship to bulimic symptoms. Clinical Psychology Review. 2008;28:1413–1425. doi: 10.1016/j.cpr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. American Journal of Psychiatry. 1987;144:1592–1595. doi: 10.1176/ajp.144.12.1592. [DOI] [PubMed] [Google Scholar]
- Haedt-Matt AA, Keel PK, Racine SE, Burt SA, Hu JY, Boker S, et al. Does emotional eating regulate affect? Concurrent and prospective associations and implications for risk models of binge eating. doi: 10.1002/eat.22247. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaven PCL, Mulligan K, Merrilees R, Woods T, Fairooz Y. Neuroticism and conscientiousness as predictors of emotional, external, and restrained eating behaviors. International Journal of Eating Disorders. 2001;30:161–166. doi: 10.1002/eat.1068. [DOI] [PubMed] [Google Scholar]
- Howard R, Gifford M, Lumsden J. Changes in an electrocortical measure of impulsivity during the menstrual cycle. Personality and individual differences. 1988;9:917–918. [Google Scholar]
- Hu JY, Boker S, Neale M, Klump KL. Latent differential equation with moderators: Simulation and application. Psychological Methods. doi: 10.1037/a0032476. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iribarren M, Jiménez-Giménez M, García-de Cecilia J, Rubio-Valladolid G. Validation and psychometric properties of the State Impulsivity Scale (SIS) Actas españolas de psiquiatría. 2011;39:49–60. [PubMed] [Google Scholar]
- Ivey ME, Bardwick JM. Patterns of affective fluctuation in the menstrual cycle. Psychosomatic Medicine. 1968;30:336–345. doi: 10.1097/00006842-196805000-00008. [DOI] [PubMed] [Google Scholar]
- Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environmental Health Perspectives. 1996;104:408–413. doi: 10.1289/ehp.96104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Taylor CB, Hayward C, Haydel KF, Wilson DM, Hammer L, et al. Weight concerns influence the development of eating disorders: A 4-year prospective study. Journal of Consulting and Clinical Psychology. 1996;64:936–940. doi: 10.1037//0022-006x.64.5.936. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): Genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9:971–977. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Burt SA, Racine SE, Neale M, Sisk C, et al. Ovarian hormones and emotional associations across the menstrual cycle: An examination of the potential moderating effects of body mass index and dietary restraint. (in press-b) [DOI] [PMC free article] [PubMed]
- Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: Exploring associations in community samples. Psychological Medicine. 2008;38:1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk C, et al. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of Abnormal Psychology. doi: 10.1037/a0029524. (in press-a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Genetic relationships between personality and eating attitudes and behaviors. Journal of Abnormal Psychology. 2002;111:380–389. doi: 10.1037//0021-843x.111.2.380. [DOI] [PubMed] [Google Scholar]
- Leon GR, Fulkerson JA, Perry CL, Cudeck R. Personality and behavioral vulnerabilities associated with risk status for eating disorders in adolescent girls. Journal of Abnormal Psychology. 1993;102:438. doi: 10.1037//0021-843x.102.3.438. [DOI] [PubMed] [Google Scholar]
- Leon GR, Fulkerson JA, Perry CL, Keel PK, Klump KL. Three to four year prospective evaluation of personality and behavioral risk factors for later disordered eating in adolescent girls and boys. Journal of Youth and Adolescence. 1999;28:181–196. [Google Scholar]
- Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: Relation to menstrual-cycle phase and cortisol levels. Psychological Medicine. 2003;33:51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Striegel-Moore RH, Seeley JR. Epidemiology and natural course of eating disorders in young women from adolescence to young adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1284–1292. doi: 10.1097/00004583-200010000-00016. [DOI] [PubMed] [Google Scholar]
- Lilenfeld LRR, Wonderlich S, Riso LP, Crosby R, Mitchell J. Eating disorders and personality: A methodological and empirical review. Clinical Psychology Review. 2006;26:299–320. doi: 10.1016/j.cpr.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Smith GT, Whiteside SP, Cyders MA. The UPPS-P: Assessing five personality pathways to impulsive behavior. Purdue University; West Lafayette, IN: 2006. [Google Scholar]
- Martin GC, Wertheim EH, Prior M, Smart D, Sanson A, Oberklaid F. A longitudinal study of the role of childhood temperament in the later development of eating concerns. International Journal of Eating Disorders. 2000;27:150–162. doi: 10.1002/(sici)1098-108x(200003)27:2<150::aid-eat3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Östlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Pearson CM, Combs JL, Zapolski TCB, Smith GT. A longitudinal transactional risk model for early eating disorder onset. Journal of Abnormal Psychology. 2012;121:707–718. doi: 10.1037/a0027567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine KJ, Fletcher BC. Women’s spending behaviour is menstrual-cycle sensitive. Personality and individual differences. 2011;50:74–78. [Google Scholar]
- Racine SE, Culbert KM, Keel PK, Sisk CL, Burt SA, Klump KL. Differential associations between ovarian hormones and disordered eating symptoms across the menstrual cycle in women. International Journal of Eating Disorders. 2012;45:333–344. doi: 10.1002/eat.20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Culbert KM, Larson CL, Klump KL. The possible influence of impulsivity and dietary restraint on associations between serotonin genes and binge eating. Journal of Psychiatric Research. 2009;43:1278–1286. doi: 10.1016/j.jpsychires.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosval L, Steiger H, Bruce K, Israël M, Richardson J, Aubut M. Impulsivity in women with eating disorders: Problem of response inhibition, planning, or attention? International Journal of Eating Disorders. 2006;39:590–593. doi: 10.1002/eat.20296. [DOI] [PubMed] [Google Scholar]
- Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS, McCarthy DM. On the validity and utility of discriminating among impulsivity-like traits. Assessment. 2007;14:155–170. doi: 10.1177/1073191106295527. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Wonderlich SA, Heron KE, Sliwinski MJ, Crosby RD, Mitchell JE, et al. Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. Journal of Consulting and Clinical Psychology. 2007;75:629–638. doi: 10.1037/0022-006X.75.4.629. [DOI] [PubMed] [Google Scholar]
- Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls: A 2-year prospective investigation. Health Psychology. 2002;21:131–138. [PubMed] [Google Scholar]
- Ukkola O, Gagnon J, Rankinen T, Thompson P, Hong Y, Leon A, et al. Age, body mass index, race and other determinants of steroid hormone variability: The HERITAGE Family Study. European journal of endocrinology. 2001;145:1–9. doi: 10.1530/eje.0.1450001. [DOI] [PubMed] [Google Scholar]
- van Strien T. Ice-cream consumption, tendency toward overeating, and personality. International Journal of Eating Disorders. 2000;28:460–464. doi: 10.1002/1098-108x(200012)28:4<460::aid-eat16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- van Strien T, Frijters JER, Bergers G, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5:295–315. [Google Scholar]
- Walderhaug E, Lunde H, Nordvik JE, Landro NI, Refsum H, Magnusson A. Lowering of serotonin by rapid tryptophan depletion increases impulsiveness in normal individuals. Psychopharmacology. 2002;164:385–391. doi: 10.1007/s00213-002-1238-4. [DOI] [PubMed] [Google Scholar]
- Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. American Journal of Epidemiology. 1998;147:1071–1080. doi: 10.1093/oxfordjournals.aje.a009401. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- Yeomans MR, Leitch M, Mobini S. Impulsivity is associated with the disinhibition but not restraint factor from the Three Factor Eating Questionnaire. Appetite. 2008;50:469–476. doi: 10.1016/j.appet.2007.10.002. [DOI] [PubMed] [Google Scholar]