Abstract
Teas prepared from the fruits of Ammi visnaga L. (syn. “Khella”) have been traditionally used in Egypt as a remedy to treat kidney stones. It was the aim of our study to evaluate the effect of a Khella extract (KE) as well as the two major constituents khellin and visnagin on renal epithelial injury using LLC-PK1 and Madin-Darby-canine kidney (MDCK) cells. Both cell lines provide suitable model systems to study cellular processes that are possibly involved in the development of a renal stone. LLC-PK1 and MDCK cell lines were exposed to 300 µM oxalate (Ox) or 133 µg/cm2 calcium oxalate monohydrate (COM) in presence or absence of 10, 50, 100 or 200 µg/mL KE. To evaluate cell damage, cell viability was assessed by determining the release of lactate dehydrogenase (LDH). KE (e.g. 100 µg/ml) significantly decreased LDH release from LLC-PK1 (Ox: 8.46± 0.76%; Ox + 100 µg/ml KE: 5.41± 0.94%, p<0.001) as well as MDCK cells (Ox: 30.9± 6.58%; Ox + 100 µg/ml KE: 17.5± 2.50%, p<0.001), which indicated a prevention of cell damage. Similar effects for KE were observed in both cell lines when COM crystals were added. In LLC-PK1 cells khellin and visnagin both decreased the % LDH release significantly in cells that were pretreated with Ox or COM crystals. However, khellin and visnagin exhibited different responses in MDCK cells. Whereas khellin slightly reduced the % LDH release after exposure of the cells to Ox and COM crystals, visnagin significantly decreased % LDH release only after COM crystal exposure. Overall both compounds were more active in LLC-PK1 than in MDCK cells. In summary, exposure of renal epithelial cells to Ox or COM crystals was associated with a significant release of LDH indicating cell injury. Our data demonstrate that KE as well as khellin and visnagin could prevent renal epithelial cell damage caused by Ox and COM and could therefore play a potential role in the prevention of stone formation associated with hyperoxaluria.
Keywords: Ammi visnaga, Nephrolithiasis, Hyperoxaluria, Calcium oxalate, Khellin, Visnagin
Introduction
Kidney stone formation or nephrolithiasis is a complex process that results from a succession of several physico-chemical events including supersaturation, nucleation, growth, aggregation, and retention within the kidneys (Worcester and Coe, 2008). About 5% of American women and 12% of men will develop a kidney stone at some time in their life and prevalence has been rising in both sexes (Coe et al., 2005). In up to 80% of stone formers, the stones are composed mainly of calcium oxalate (Moe, 2006). High oxalate concentrations and calcium oxalate crystals act on renal cells in several ways such as altering the membrane surface to enhance crystal binding, disrupting membrane potential, promoting mitochondria dysfunction, increasing production of reactive oxygen species which finally induces injury to renal epithelial cells (Jonassen et al., 2004). Cellular injury is a predisposing factor for the formation of CaOx stones and products of cell damage can act as heterogeneous nucleators of CaOx crystals both in vitro and in vivo (Khan, 1995; Khan and Kok, 2004; Thamilselvan and Khan, 1998). Once formed, these crystals will grow and aggregate as a result of supersaturation with respect to CaOx (Khan and Kok, 2004).
In spite of substantial progress in the study of the biological and physical manifestations of kidney stones, there is still no satisfactory drug to use in clinical therapy. Also, even though drug treatment has shown some feasibility in many randomized trials, it is not accomplished without side effects (Dellabella et al., 2005). Data from in vitro, in vivo and clinical trials reveal (for review see (Butterweck and Khan, 2009) that phytotherapeutic agents could be useful as either an alternative or an adjunctive therapy in the management of urolithiasis.
The fruits of Ammi visnaga L. (Apiaceae; “Khella”) have traditionally been used in Egypt to relieve pain of kidney stone passage by drinking a tea prepared from the crushed or powdered fruits of khella (Gunaydin and Beyazit, 2004). In a previous study, Khan et al. (Khan et al., 2001) investigated the effects of an Ammi visnaga extract in an animal model of nephrolithiasis and found that the plant extract significantly reduced the incidence of calcium oxalate deposition in the rat kidneys. In addition, the plant extract showed a highly potent diuretic activity. The authors therefore speculated that the diuretic activity might be the possible mechanisms of action of khella extract.
It was the aim of our study to evaluate the effect of an aqueous khella extract (KE) on oxalate and calcium oxalate crystal induced cellular injury using cultured renal epithelial cells which are accepted as powerful tools in experimental studies to explore the mechanisms of nephrolithiasis (Verkoelen et al., 1997). Lethal epithelial cellular injury promotes crystal nucleation, aggregation and retention and nephrolithiasis is a consequence of renal epithelial cell exposure to oxalate and calcium oxalate crystals (Khan, 2006). Further, in order to obtain information about possible active ingredients the two major khella compounds khellin and visnagin were investigated for possible preventive effects on cell damage.
Materials and Methods
Cell lines and Chemicals
MDCK (Madin-Canine Kidney collecting duct tubular epithelium cell line) and LLC-PK1 (Porcine Kidney Proximal Tubular epithelial cell line) cells were purchased from ATCC (Rockville, MD, USA). Khellin (97% purity), visnagin (97% purity), oxalic acid and calcium oxalate monohydrate were purchased from Sigma Aldrich (St. Louis, MO, USA). Dulbecco’s modified eagles’s medium/F-12, calf serum, antibiotics-antimycotic, 0.05% Trypsin, 0.25% ethylenediaminentetraacetic acid, insulin/transferin/selenium mix, hydrocortisone, 3.4 mL triiodo-L-thyronine and prostaglandin E1 were purchased from Fisher Scientific (Fair Lawn, NJ, USA). A CytoTox 96 Non-Radioactive Cytotoxicity assay kit (Cat #: PR-G1780) was purchased from Fisher Scientific (Fair Lawn, NJ, USA). All other reagents were from Sigma (St. Louis, MO, USA) and were of analytical grade. Fruits of Ammi visnaga L. were obtained from a commercial supplier, Caesar and Lorentz, (Caelo) Hilden, Germany. A voucher specimen is deposited at the Department of Pharmaceutics, College of Pharmacy, University of Florida, Gainesville, USA (COP-21-2008).
Extract preparation
The preparation of the extract was performed according to the traditional use of khella as a tea. An infusion of khella fruits was prepared using the following method: 20 g fruits were grinded (medium powder size) using a regular coffee grinder (Smart Grind CBG5, Black and Decker, Miramar, FL, USA). 200 ml of boiling water (the water was heated to 90 – 100°C) was poured over the grinded fruits and then steeped for 5 min at room temperature. The aqueous extract was filtered through No.4 filter paper (Whatman International Ltd, Maidstone, England) and was freeze dried (FreeZone 6, Labconco, USA). It was kept in amber colored bottles under argon atmosphere and stored at −20°C until use. Since many extracts contain oxalate, citrate and calcium the amount of these compounds need to be determined in order to exclude false negative effects. Oxalate (≤ 0.01%), citrate (≤ 0.01%) and calcium (≤ 11.6%) concentrations in the extract were determined by commercial kits (Oxalate: Trinity Biotech, New Jersey, USA; citrate: R-Biopharm, Michigan, USA; calcium: Quantichrom, CA, USA) according to manufacturer’s instructions.
Quantitative determination of khellin and visnagin in KE by HPLC
50 mg of KE were dissolved in 5 ml methanol and filtered through 0.45 µm. The samples were analyzed using reverse-phase HPLC with diode array detector Shimadzu VP series HPLC system (Kyoto, Japan) with diode array detection. LiChrocart RP-select (250 mm×4 mm i.d., 5µm particle diameter) reversed-phase column, with a pre-column (4.6 mm. i.d. × 2.5 cm) was used for the investigation. The mobile phase consisted of the following solvents: (A) = water, (B) = THF and (C) = methanol. The gradient elution program was applied as followed: 5–13% B from 0–20 min with constant 5% C balanced with A, 13–22% B and 5–7% C from 20–52 min balanced with A and then 22-5% B and 7-5% C balanced with A from 52–60 min. The injection volume for samples was 50 µl. HPLC was carried out at a constant temperature 30°C and a mobile phase flow rate of 1 ml/min. The detection wavelength was set at 330 nm. Khellin and visnagin were identified by their spectrum at 330 nm using reference compounds. Quantification of khellin and visnagin was performed using six-point regression curve, each point in triplicate. Peak area was correlated with the concentrations according to the calibration curves. Calibration was carried out by an external standardization method. The method was validated over the range of concentration of the target compounds present in the KEs. The validation parameters of linearity, sensitivity, specificity, precision, accuracy and stability were determined.
Calibration curves (n = 9) operating in the range of 0.05 - 1.0 mg/ml for both Khella components were linear (r2 > 0.99). The LLOQ of khellin and visnagin were 0.01 mg/ml. The precision intra-and inter-day for khellin and visnagin were satisfactory with CV values between 0.93 and 12.98%. Similarly, the accuracy of the assay was between 93.45 and 105.78% for all compounds tested at three different concentrations.
Cell culture experiment
MDCK and LLC-PK1 (Porcine Kidney Proximal Tubular epithelial cell line, were exposed to oxalate (Ox) at a concentration of 300 µmol and calcium oxalate monohydrate (COM) crystals at a concentration of 133 µg/cm2. These concentrations were based on the previously published data (Thamilselvan et al., 1999; Thamilselvan and Khan, 1998). LLC-PK1 and MDCK were maintained in growth medium (500 ml Dulbecco’s modified eagles’s medium/F-12, 50 ml newborn calf serum and 10 ml antibiotics-antimycotic) in 75 cm2 T-Flask (Corning, Corning, New York). The cells were subcultured by disassociation with 0.05% Trypsin and 0.25% ethylenediamine tetraacetic acid and seeded to 24 well-plate tissue culture dishes.
After achieving confluence (5 to 6 days after seeding) the cells were weaned from growth medium to acclimation medium (500 ml Dulbecco’s modified eagles’s medium/F-12, 10 ml antibiotics-antimycotic solution, 5 ml insulin/transferin/selenium mix, 1 ml hydrocortisone, 3.4 mL triiodo-L-thyronine and 1 ml prostaglandin E1).
Cells were divided into the following groups (n = 6 per group): Control (acclimation medium), Control + 300 µM oxalate (Ox), 300 µM Ox + 10, 50, 100 and 200 µg/ml khella extract (KE); Control + 133 µg/cm2 calcium oxalate monohydrate (COM), 133 µg/cm2 COM + 10, 50, 100 and 200 µg/ml KE. The cells were exposed for 1, 3, 6 and 12 hours with or without khella extract then the used media from individual wells were recovered and centrifuged to remove crystal and cellular debris. Best results were obtained at 6 h of incubation. The media were analyzed for lactate dehydrogynase (LDH) to determine cell viability through membrane damage. Exposure of khellin and visnagin to LLC-PK1 and MDCK were conducted using the same Ox and COM concentrations as mentioned above. The single compounds were dosed based on their amount in 100 mg extract and were tested in concentrations of 2.8 and 1.7 µg/ml, respectively.
Lactate Dehydrogynase (LDH) Assay
Medium was aliquoted to designated wells of 96-well plate. A CytoTox 96 Non-Radioactive Cytotoxicity assay kit (Cat #: PR-G1780, Fisher Scientific, Fair Lawn, NJ, USA) was used to determine the % LDH release. Substrates supplied with the kit were added to all samples (duplicate), positive control (MDCK or LLC-PK1 cell lysed with lysis solution supplied with the kit) and blank (acclimation medium). The plate was incubated at room temperature for 30 minutes in the dark. Stop solution supplied with the kit was added to all samples, positive control and blanks. Optical density absorbency was read at 490 nm using a microplate reader (Synergy HT, Biotek instrument INC., VT).
Statistical analysis
Statistical calculations were carried out with one-way ANOVA with Newman-Keuls post-hoc test for multiple comparisons (GraphPad Prism 4, San Diego, USA). The results were considered significant if the probability of error was < 0.05. Data are expressed as mean ± SD of 6 individual experiments.
Results
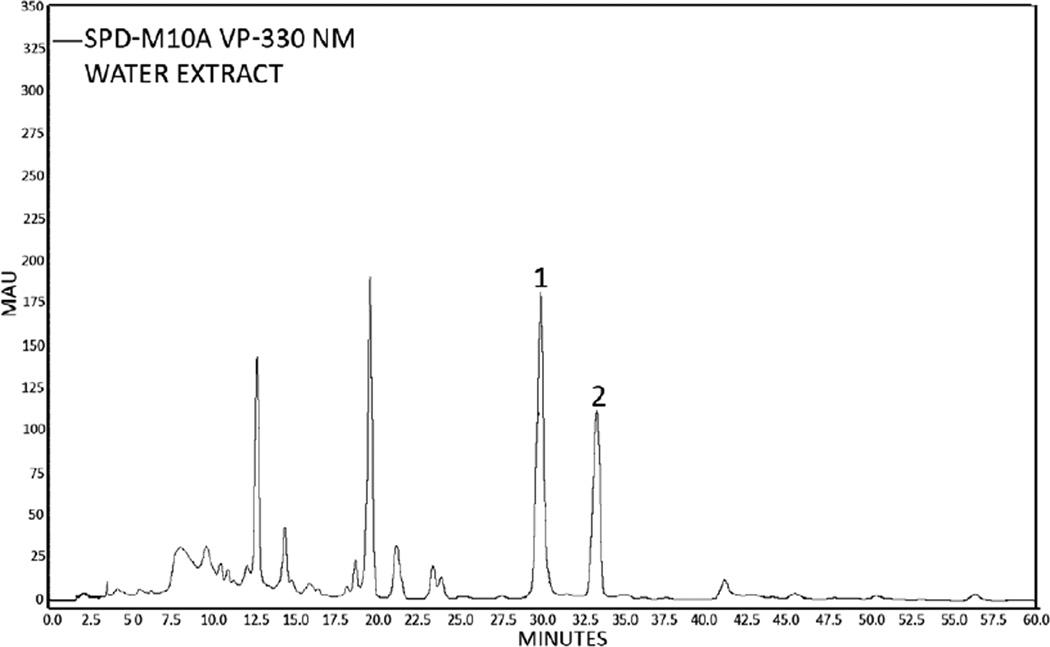
The marker compounds khellin and visnagin were quantitatively determined in an aqueous extract prepared from the crushed fruits of Ammi visnaga L. using HPLC. A representative chromatogram of KE is shown in Fig. 1. The quantitative amount of the marker compounds khellin and visnagin were 2.88 and 1.72 mg per 100 mg of the extract, respectively.
Fig. 1.
HPLC-Chromatogram of an aqueous khella extract (1 = khellin, 2 = visnagin).
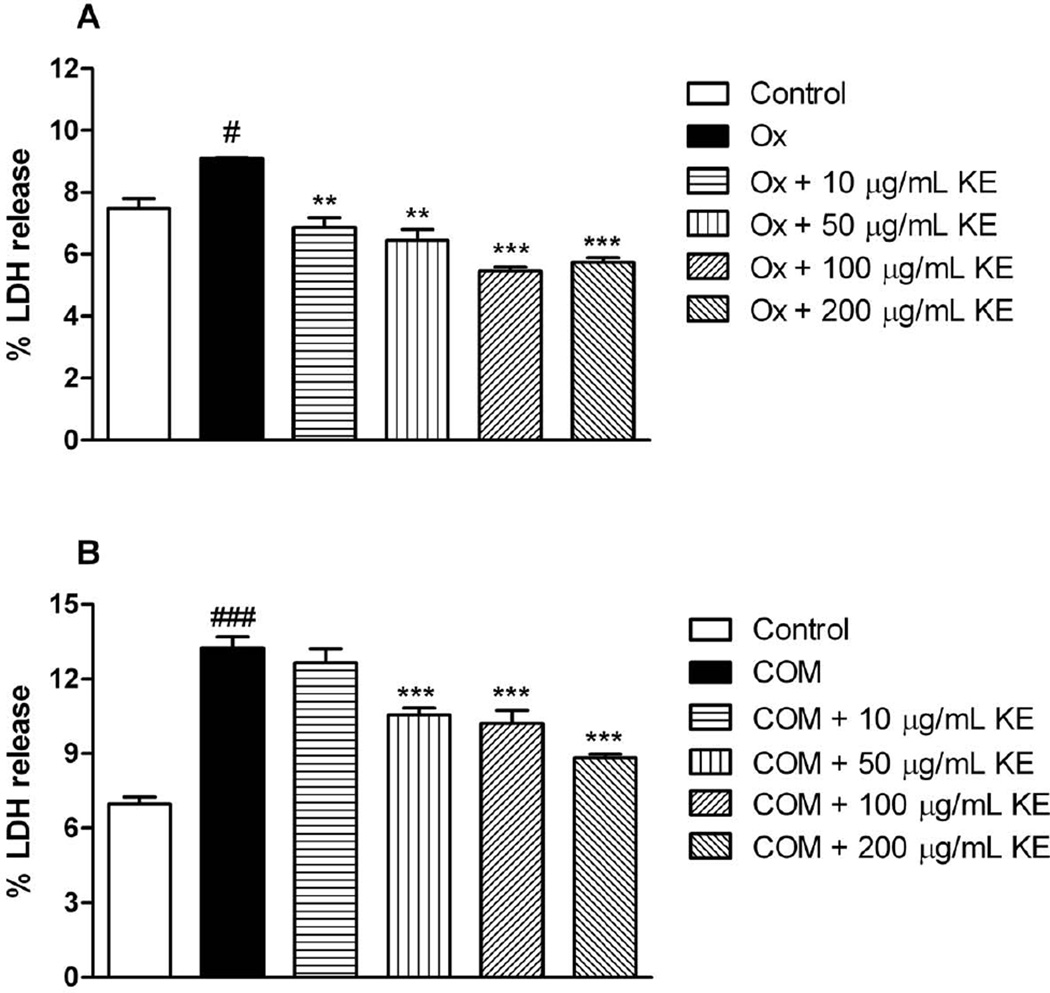
Fig. 2A illustrates the % LDH release from oxalate exposure to LLC-PK1 in presence or absence of KE. Exposure to oxalate significantly increased % LDH release. At every concentration, the % LDH release after treatment with KE was significantly decreased when compared to the oxalate group. Fig. 2B displays the % LDH release after exposure of COM crystals to LLC-PK1 in presence or absence of KE. After treatment with 50, 100 and 200 µg/ml KE the % LDH release significantly decreased if compared to the COM crystal group.
Fig. 2.
% LDH release from exposure oxalate (Ox) or calcium monohydrate oxalate (COM) crystals to LLC-PK1 cells in presence or absence of KE (#p < 0.05 vs. control, ###p < 0.05 vs. control; ** p< 0.01, *** p< 0.001 vs. Ox or COM, respectively). Data are expressed as mean ± SD (n = 6).
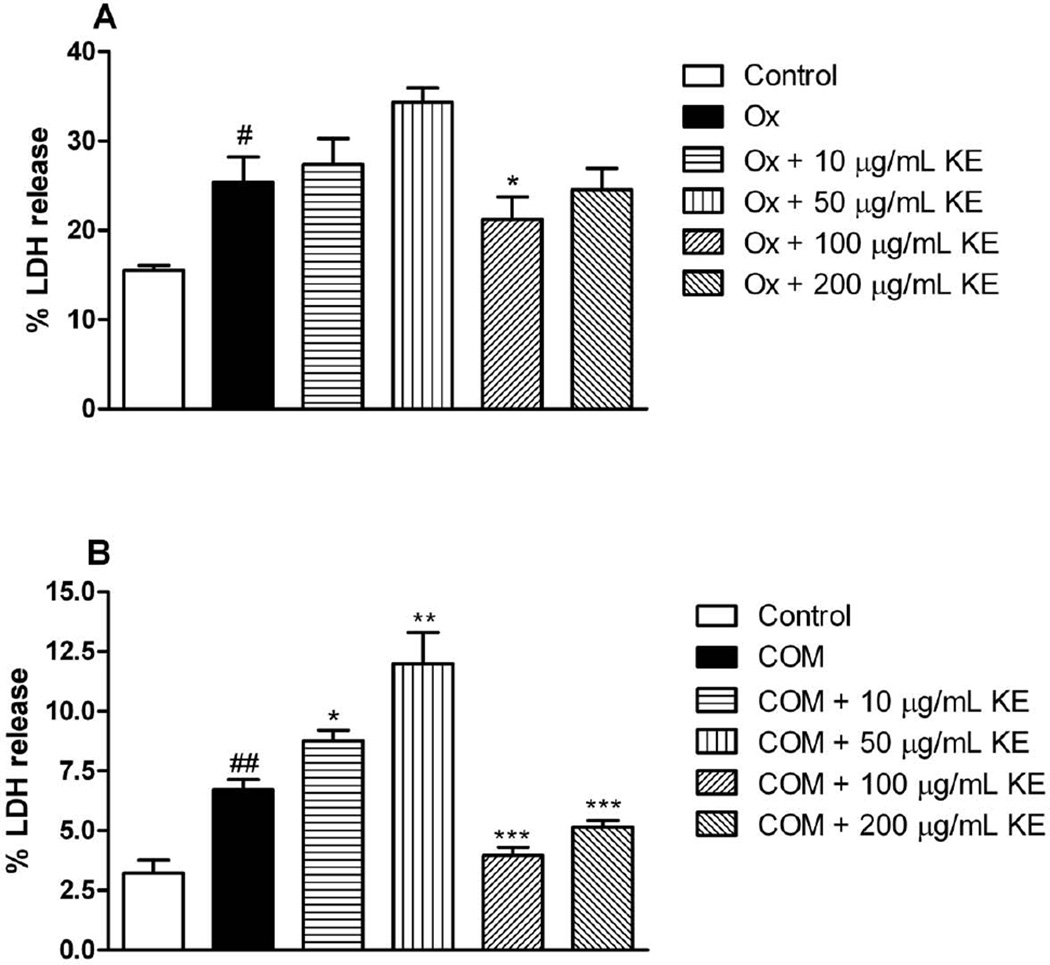
As illustrated in Fig. 3A 100 and 200 µg/ml of KE significantly reduced the % LDH release from MDCK cells when they were exposed to oxalate. Interestingly, 10 and 50 further increased %LDH release after exposure to COM (Fig. 3B) indicating inefficiency of KE at these concenrtrations to stop crystal induced injury. However, 100 and 200 µg/ml of KE significantly reduced the leakage of LDH (Fig. 3B).
Fig. 3.
LDH release from exposure oxalate (Ox) or calcium monohydrate oxalate (COM) crystals to MDCK cells in presence or absence of KE (#p < 0.05 vs. control, ##p < 0.01 vs. control; * p<0.05,** p< 0.01, *** p< 0.001 vs. Ox or COM, respectively). Data are expressed as mean ± SD (n = 6).
Since 100 of KE on the release of LDH in both cell lines revealed more consistent effects this concentration was chosen to calculate the corresponding amount of khellin and visnagin. Based on the result from the quantitative HPLC analysis, the amount of khellin and visnagin were calculated as 2.88 and 1.72 µg/ml, respectively and used for further in vitro studies.
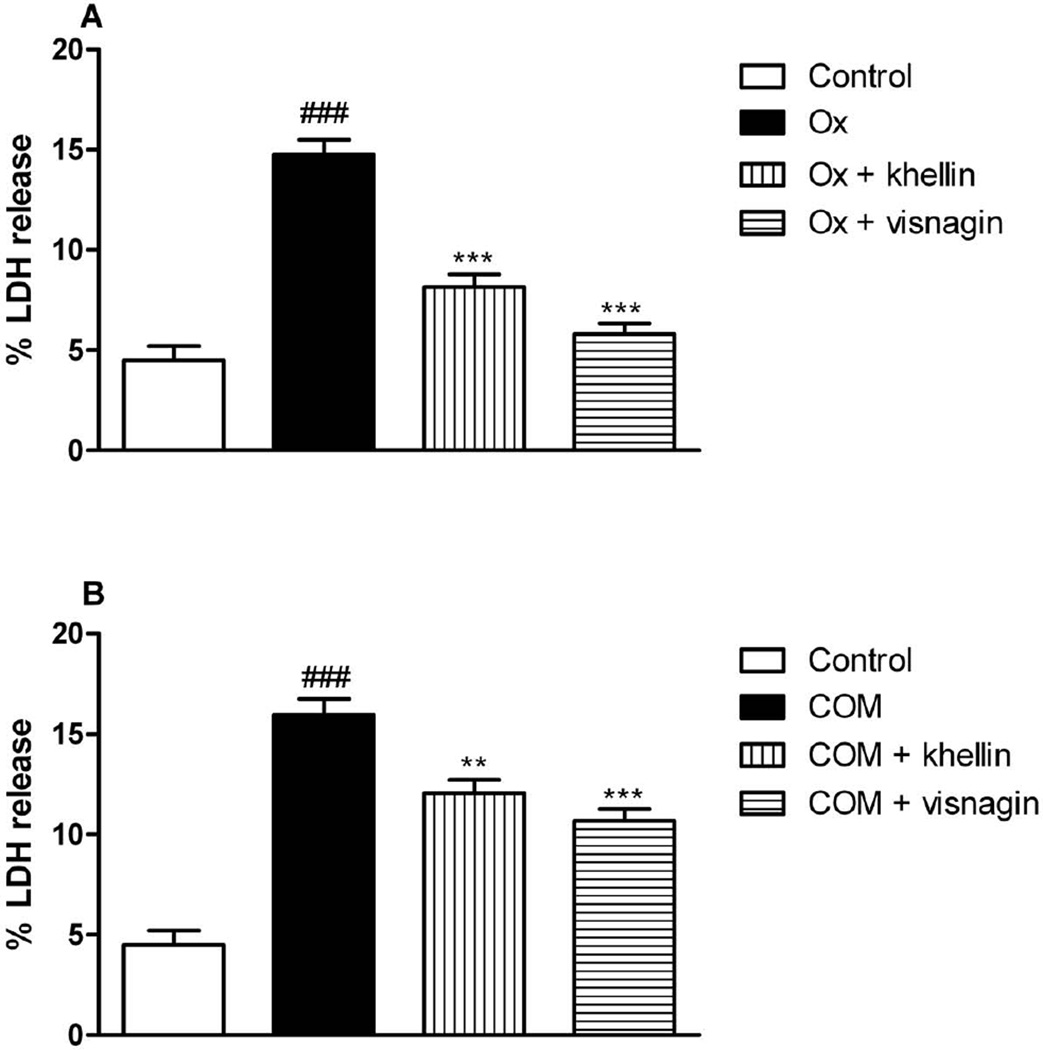
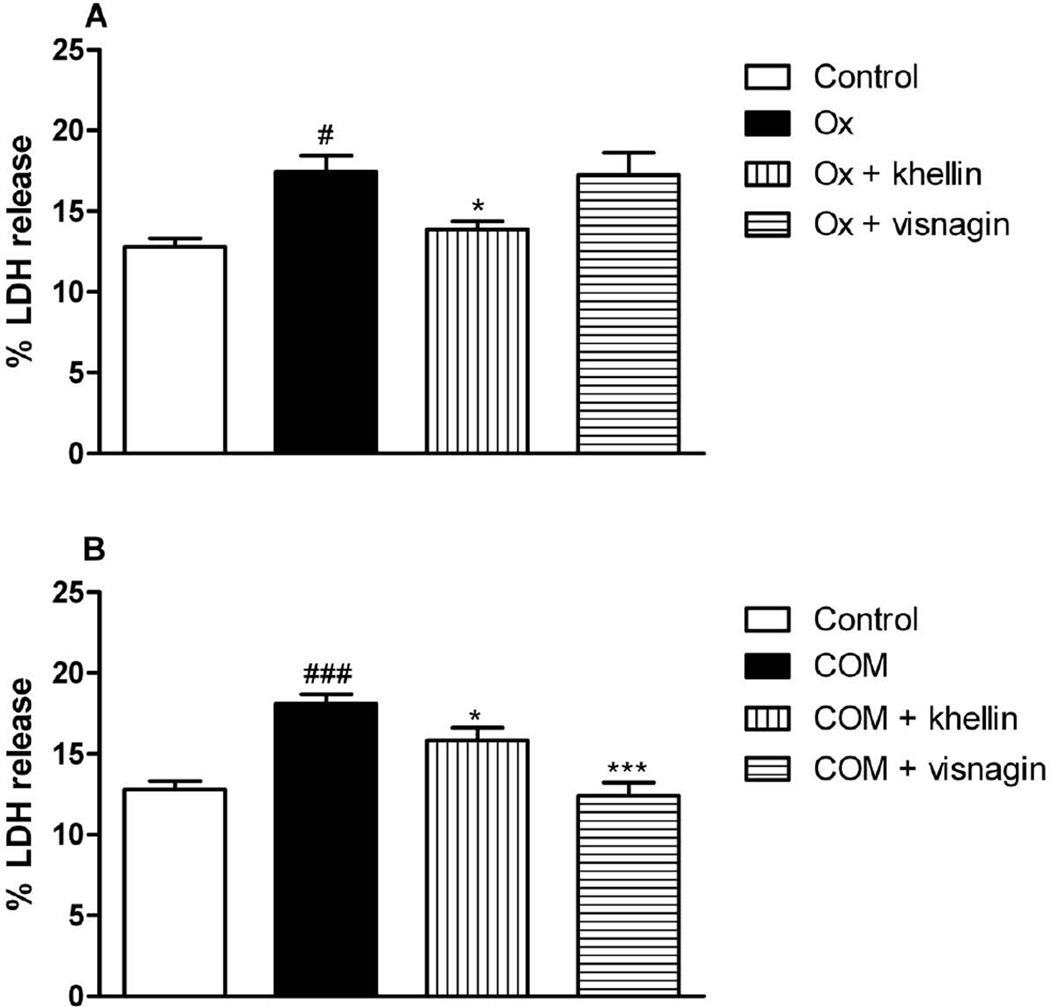
Fig. 4 demonstrates the exposure of Ox (Fig. 4A) or COM (Fig. 4B) crystals to LLC-PK1 in presence or absence of khellin or visnagin. Both compounds significantly decreased the % LDH release compared to the Ox or COM control groups. After exposure of MDCK cells to Ox LDH leakage was slightly decreased by khellin, but not by visnagin (Fig. 5A). When COM was added to MDCK cells both compounds decreased the % LDH release, but visnagin seemed to be slightly more active than khellin in the tested concentration (Fig. 5B).
Fig. 4.
% LDH release from exposure oxalate (Ox) or calcium monohydrate oxalate (COM) crystals to LLC-PK1 cells in presence or absence of khellin or visnagin (###p < 0.05 vs. control; ** p< 0.01, *** p< 0.001 vs. Ox or COM, respectively). Data are expressed as mean ± SD (n = 6).
Fig. 5.
% LDH release from exposure oxalate (Ox) or calcium monohydrate oxalate (COM) crystals to MDCK cells in presence or absence of khellin or visnagin (#p < 0.05 vs. control, ###p < 0.05 vs. control; ** p< 0.01, *** p< 0.001 vs. Ox or COM, respectively). Data are expressed as mean ± SD (n = 6).
Discussion
Hyperoxaluria is a major risk factor for calcium oxalate nephrolithiasis, and calcium oxalate urinary stones are the most common type of urinary stone. High levels of oxalate cause a variety of changes in the renal epithelial cells, such as an increase in free radical production and a decrease in antioxidant status (Huang et al., 2002; Selvam, 2002; Thamilselvan et al., 2003), followed by cell injury (Selvam, 2002; Thamilselvan et al., 2003) and cell death (Schepers et al., 2005a, b). These changes are considered significant predisposing factors for the facilitation of crystal adherence and retention (Hackett et al., 1990; Thamilselvan et al., 2000) within the kidneys and promotion of kidney stone formation. Thus, cultured renal epithelial cells may serve as a tool to investigate the mechanisms involved in human kidney stone formation and also to screen new agents with antiurolithiatic activity (Verkoelen et al., 1997). A number of cell lines with characteristics of distinct nephronal segments are available (Verkoelen et al., 1997). Over the past few decades two continuous renal epithelial cell lines have been most used for studying nephrolithiasis, the Mardin-Darby canine kidney collecting duct tubular epithelial cells (MDCK) and porcine kidney proximal tubular epithelial cells (LLC-PK1). MDCK cells have been widely used as a model system for the distal/collecting duct and LLC-PK1 cells have retained many characteristics of the proximal tubule (Gstraunthaler et al., 1985; Wiessner et al., 2001). As mentioned earlier, the interaction between renal epithelial cells and oxalate or calcium oxalate crystals plays a significant role in the formation, retention and development of calcium oxalate stone disease (Thamilselvan et al., 2003). Oxalate, a major constituent of CaOx stones, has been shown to exert cytotoxic effects on renal tubular epithelial cells, attributable to increased oxidative stress within the cells (Hackett et al., 1990). Oxalate seems to induce cell death mediated by both apoptosis and cellular necrosis, since oxalate exposure leads to the formation of apoptotic bodies as well as induces changes in membrane integrity, the release of cellular enzymes, and membrane-lipid peroxidation (Khan et al., 1990).
In the present study, oxalate or calcium oxalate crystals exposure resulted in a significant increase of LDH release. The cellular injury potentiates calcium oxalate crystal formation and retention (Thamilselvan et al., 2003). Our data demonstrated that LLC-PK1 and MDCK cells were injured when exposed to Ox or CaOx crystals, which correlates with results from previous studies (Koul et al., 1996; Scheid et al., 2000; Scheid et al., 1996; Thamilselvan and Khan, 1998). 100 and 200 µg/ml of KE extract reduced LDH release from LLC-PK1 and MDCK cells after exposure to Ox or COM crystals most effectively if compared to 10 or 50 µg/ml. The magnitude of protection provided by KE to exposure of OX and COM in LLC-PK1 cells was comparable whereas in MDCK cells the extract seemed to be more effective against COM crystal induced injury, although an initial increase in % LDH release was observed for 10 and 50 µg/ml, respectively. The increase may be a result of the insufficiency of 10 or 50 µg/ml to provide the protection. It is suggested that KE protects against Ox or COM crystal induced cell damage to renal epithelial cells but that the response is more definite in LLC-PK1 than in MDCK cells. Our results further demonstrated that khellin and visnagin decreased the % LDH release from both LLC-PK1 and MDCK when exposed to Ox or COM crystals. Like for the extract, the protective effect was more pronounced in LLC-PK1 than in MDCK cells, indicating that both compounds respond to the different cell lines in a distinctive manner. As mentioned previously, LLC-PK1 cells are considered to be of proximal tubular origin where as MDCK cells are related to the distal tubules and collecting ducts (Gstraunthaler et al., 1990). In the proximal tubules, urine is generally undersaturated with respect to CaOx, and crystals do not form except in cases of acute or primary hyperoxaluria. In normal conditions, urine becomes metastably supersaturated when it reaches the collecting ducts (Thamilselvan et al., 1999). Another interesting aspect is that the single compounds were not more protective than the khella extract. In fact, especially in MDCK cells, when they were exposed to COM, KE was more protective than khellin or visnagin suggesting that the extract may contain additional unknown compounds which are responsible for the effect. The exact mechanism of action of KE for the prevention of cell damage has not been elucidated. In renal tubular epithelial cells, oxalate-induced cell injury and apoptotic changes are associated with an increased generation of free radicals, including O2·, hydroxyl radical (OH·), hydrogen peroxide (H2O2), peroxide radical (RO·) and singlet molecular oxygen (Sarica et al., 2001). A positive correlation between urinary oxalate levels and renal tubular epithelial cell injury as well as between oxalate levels and lipid peroxides has been discovered in experimental urolithiasis rats (Selvam, 2002) and patients with kidney stones (Huang et al., 2002). It can be speculated that KE as well as khellin and visnagin suppress the production of radical oxygen species, which might be followed by suppression of stone formation due to the prevention of renal tubular epithelial cell injury.
In conclusion, our present data indicate that an aqueous extract of Ammi visnaga L., as well as the main compounds khellin and visnagin could prevent cell damage caused by Ox or COM crystals in renal epithelial cells. Our preliminary in vitro data are promising since they have shown for the first time a suppression of cell injury induced by oxalate exposure for KE and its major compounds khellin and visnagin. The extract as well as the single compounds could therefore play a potential role in the prevention of stone formation associated with hyperoxaluria. However, one safety concern regarding the use of khellin or visnagin as alternative treatments in nephrolithiasis could be that based on their furochromone structure both compounds could intercalate with DNA molecules. Indeed, interstrand DNA crosslinks induced by khellin and visnagin have been observed in vitro at high UVA doses (Abeysekera et al., 1983; Cassuto et al., 1977; Vedaldi et al., 1988), but not in mammalian cells (Morliere et al., 1988). In addition, it has been shown that khellin is also a poor type I (radicals) and type II (singlet oxygen) photodynamic sensitizer and is less genotoxic in yeast than bifunctional 5-methoxypsoralen (5-MOP) (Morliere et al., 1988). Furthermore khellin and visnagin behave as monofunctional agents and it is proven that furanocoumarine bioadducts (e.g. 5-MOP) are more genotoxic than monoadducts and therefore more effective for inducting recombination (Cassuto et al., 1977). These properties of visnagin and khellin are of particular interest since this confers a low toxicity to these compounds. Yet, further preclinical and clinical studies which support the use of Ammi visnaga as alternative or an adjunctive therapy in the management of nephrolithiasis are needed.
List of Abbreviations
- KE
Khella extract
- Ox
Oxalate
- COM
Calcium oxalate monohydrate
- CaOx
Calcium oxalate
- MDCK
Madin-Canine Kidney collecting duct tubular epithelium cell line
- LLC-PK1
Porcine Kidney Proximal Tubular epithelial cell line
- LDH
Lactate dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeysekera BF, Abramowski Z, Towers GH. Genotoxicity of the natural furochromones, khellin and visnagin and the identification of a khellin-thymine photoadduct. Photochem Photobiol. 1983;38:311–315. doi: 10.1111/j.1751-1097.1983.tb02677.x. [DOI] [PubMed] [Google Scholar]
- Butterweck V, Khan SR. Herbal Medicines in the Management of Urolithiasis: Alternative or Complementary? Planta Med. 2009;75:1095–1103. doi: 10.1055/s-0029-1185719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassuto E, Gross N, Bardwell E, Howard-Flanders P. Genetic effects of photoadducts and photocross-links in the DNA of phage lambda exposed to 360 nm light and trimethylpsoralen or khellin. Biochim Biophys Acta. 1977;475:589–600. doi: 10.1016/0005-2787(77)90319-7. [DOI] [PubMed] [Google Scholar]
- Coe FL, Evan A, Worcester E. Kidney stone disease. Journal of Clinical Investigation. 2005;115:2598–2608. doi: 10.1172/JCI26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellabella M, Milanese G, Muzzonigro G. Medical-expulsive therapy for distal ureterolithiasis: randomized prospective study on role of corticosteroids used in combination with tamsulosin-simplified treatment regimen and health-related quality of life. Urology. 2005;66:712–715. doi: 10.1016/j.urology.2005.04.055. [DOI] [PubMed] [Google Scholar]
- Gstraunthaler G, Pfaller W, Kotanko P. Biochemical characterization of renal epithelial cell cultures (LLC-PK1 and MDCK) Am J Physiol. 1985;248:F536–F544. doi: 10.1152/ajprenal.1985.248.4.F536. [DOI] [PubMed] [Google Scholar]
- Gstraunthaler G, Steinmassl D, Pfaller W. Renal cell cultures: a tool for studying tubular function and nephrotoxicity. Toxicol Lett. 1990;53:1–7. doi: 10.1016/0378-4274(90)90085-z. [DOI] [PubMed] [Google Scholar]
- Gunaydin K, Beyazit N. The chemical investigations on the ripe fruits of Ammi visnaga (LAM.) Lamarck growing in Turkey. Natural Product Research. 2004;18:169–175. doi: 10.1080/14786410310001608091. [DOI] [PubMed] [Google Scholar]
- Hackett RL, Shevock PN, Khan SR. Cell injury associated calcium oxalate crystalluria. J Urol. 1990;144:1535–1538. doi: 10.1016/s0022-5347(17)39793-8. [DOI] [PubMed] [Google Scholar]
- Huang HS, Ma MC, Chen J, Chen CF. Changes in the oxidant-antioxidant balance in the kidney of rats with nephrolithiasis induced by ethylene glycol. J Urol. 2002;167:2584–2593. [PubMed] [Google Scholar]
- Jonassen JA, Cao LC, Honeyman T, Scheid CR. Intracellular events in the initiation of calcium oxalate stones. Nephron Experimental Nephrology. 2004;98:E61–E64. doi: 10.1159/000080258. [DOI] [PubMed] [Google Scholar]
- Khan SR. Calcium-Oxalate Crystal Interaction with Renal Tubular Epithelium, Mechanism of Crystal Adhesion and Its Impact on Stone Development. Urological Research. 1995;23:71–79. doi: 10.1007/BF00307936. [DOI] [PubMed] [Google Scholar]
- Khan SR. Renal tubular damage/dysfunction: key to the formation of kidney stones. Urol Res. 2006;34:86–91. doi: 10.1007/s00240-005-0016-2. [DOI] [PubMed] [Google Scholar]
- Khan SR, Kok DJ. Modulators of urinary stone formation. Front Biosci. 2004;9:1450–1482. doi: 10.2741/1347. [DOI] [PubMed] [Google Scholar]
- Khan SR, Shevock PN, Hackett RL. Membrane-associated crystallization of calcium oxalate in vitro. Calcif Tissue Int. 1990;46:116–120. doi: 10.1007/BF02556095. [DOI] [PubMed] [Google Scholar]
- Khan ZA, Assiri AM, Al-Afghani HM, Maghrabi TM. Inhibition of oxalate nephrolithiasis with Ammi visnaga (AI-Khillah) Int.Urol.Nephrol. 2001;33:605–608. doi: 10.1023/a:1020526517097. [DOI] [PubMed] [Google Scholar]
- Koul H, Kennington L, Honeyman T, Jonassen J, Menon M, Scheid C. Activation of c-myc gene mediates the mitogenic effects of oxalate in LLC-PK1 cells, a line of renal epithelial cells. Kidney Int. 1996;50:1525–1530. doi: 10.1038/ki.1996.467. [DOI] [PubMed] [Google Scholar]
- Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006;367:333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- Morliere P, Honigsmann H, Averbeck D, Dardalhon M, Huppe G, Ortel B, Santus R, Dubertret L. Phototherapeutic, photobiologic, and photosensitizing properties of khellin. J Invest Dermatol. 1988;90:720–724. doi: 10.1111/1523-1747.ep13083852. [DOI] [PubMed] [Google Scholar]
- Sarica K, Yagci F, Bakir K, Erbagci A, Erturhan S, Ucak R. Renal tubular injury induced by hyperoxaluria: evaluation of apoptotic changes. Urol Res. 2001;29:34–37. doi: 10.1007/s002400000150. [DOI] [PubMed] [Google Scholar]
- Scheid C, Honeyman T, Kohjimoto Y, Cao LC, Jonassen J. Oxalate-induced changes in renal epithelial cell function: role in stone disease. Mol Urol. 2000;4:371–382. [PubMed] [Google Scholar]
- Scheid C, Koul H, Hill WA, Luber-Narod J, Jonassen J, Honeyman T, Kennington L, Kohli R, Hodapp J, Ayvazian P, Menon M. Oxalate toxicity in LLC-PK1 cells, a line of renal epithelial cells. J Urol. 1996;155:1112–1116. [PubMed] [Google Scholar]
- Schepers MS, van Ballegooijen ES, Bangma CH, Verkoelen CF. Crystals cause acute necrotic cell death in renal proximal tubule cells, but not in collecting tubule cells. Kidney Int. 2005a;68:1543–1553. doi: 10.1111/j.1523-1755.2005.00566.x. [DOI] [PubMed] [Google Scholar]
- Schepers MS, van Ballegooijen ES, Bangma CH, Verkoelen CF. Oxalate is toxic to renal tubular cells only at supraphysiologic concentrations. Kidney Int. 2005b;68:1660–1669. doi: 10.1111/j.1523-1755.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- Selvam R. Calcium oxalate stone disease: role of lipid peroxidation and antioxidants. Urol Res. 2002;30:35–47. doi: 10.1007/s00240-001-0228-z. [DOI] [PubMed] [Google Scholar]
- Thamilselvan S, Byer KJ, Hackett RL, Khan SR. Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol. 2000;164:224–229. [PubMed] [Google Scholar]
- Thamilselvan S, Hackett RL, Khan SR. Cells of proximal and distal tubular origin respond differently to challenges of oxalate and calcium oxalate crystals. J Am Soc Nephrol. 1999;10(Suppl 14):S452–S456. [PubMed] [Google Scholar]
- Thamilselvan S, Khan SR. Oxalate and calcium oxalate crystals are injurious to renal epithelial cells: results of in vivo and in vitro studies. J Nephrol. 1998;11(Suppl 1):66–69. [PubMed] [Google Scholar]
- Thamilselvan S, Khan SR, Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res. 2003;31:3–9. doi: 10.1007/s00240-002-0286-x. [DOI] [PubMed] [Google Scholar]
- Vedaldi D, Caffieri S, Dall'Acqua F, Andreassi L, Bovalini L, Martelli P. Khellin, a naturally occurring furochromone, used for the photochemotherapy of skin diseases: mechanism of action. Farmaco Sci. 1988;43:333–346. [PubMed] [Google Scholar]
- Verkoelen CF, van der Boom BG, Schroder FH, Romijn JC. Cell cultures and nephrolithiasis. World J Urol. 1997;15:229–235. doi: 10.1007/BF01367660. [DOI] [PubMed] [Google Scholar]
- Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS. Mechanisms of calcium oxalate crystal attachment to injured renal collecting duct cells. Kidney Int. 2001;59:637–644. doi: 10.1046/j.1523-1755.2001.059002637.x. [DOI] [PubMed] [Google Scholar]
- Worcester EM, Coe FL. Nephrolithiasis. Prim Care. 2008;35:369–391. doi: 10.1016/j.pop.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]