Abstract
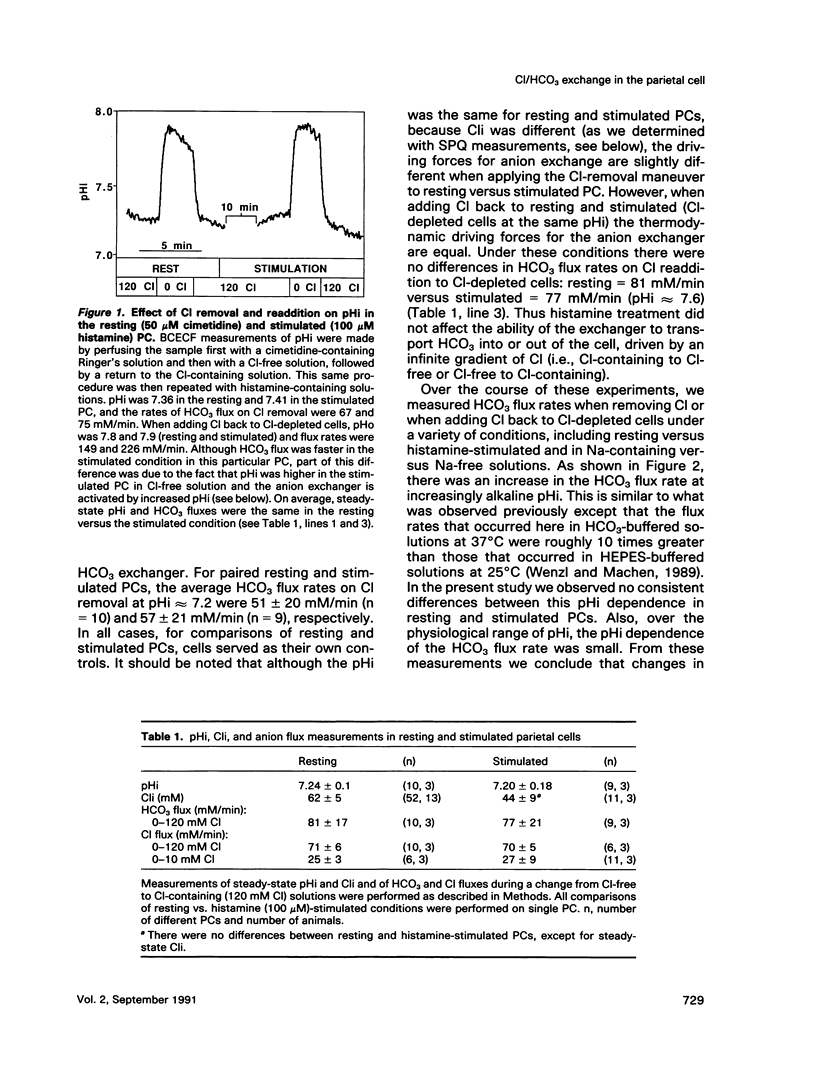
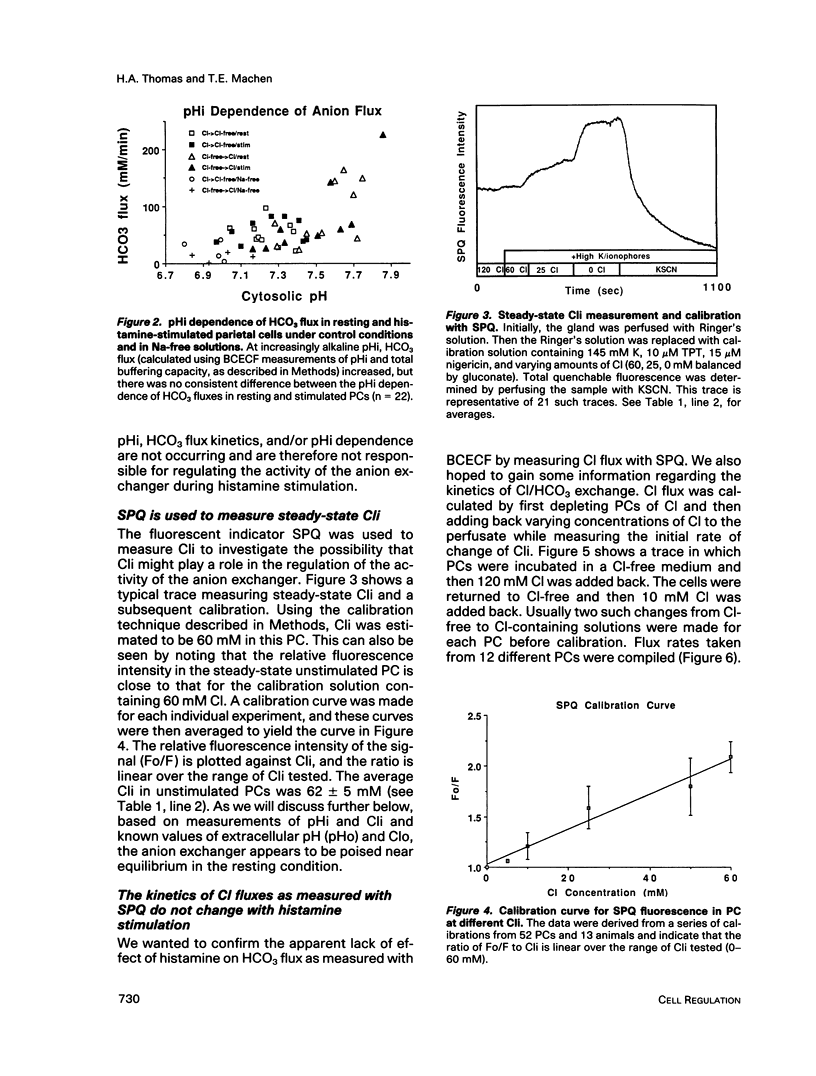
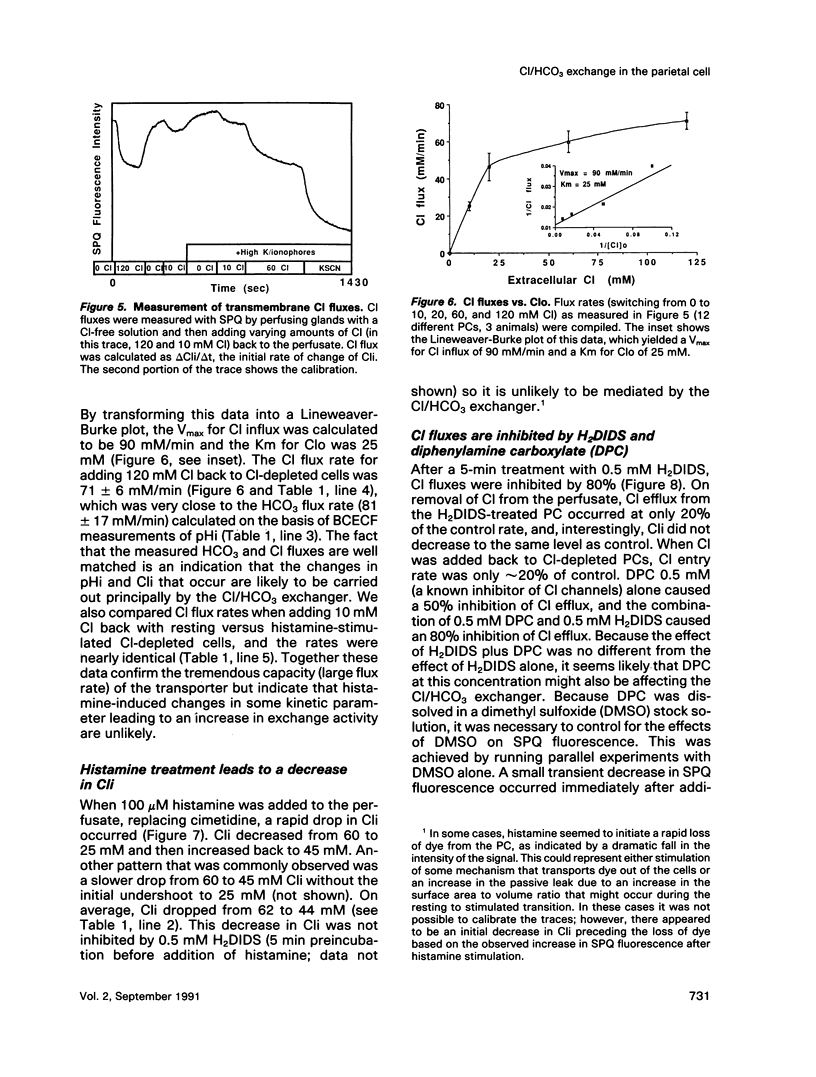
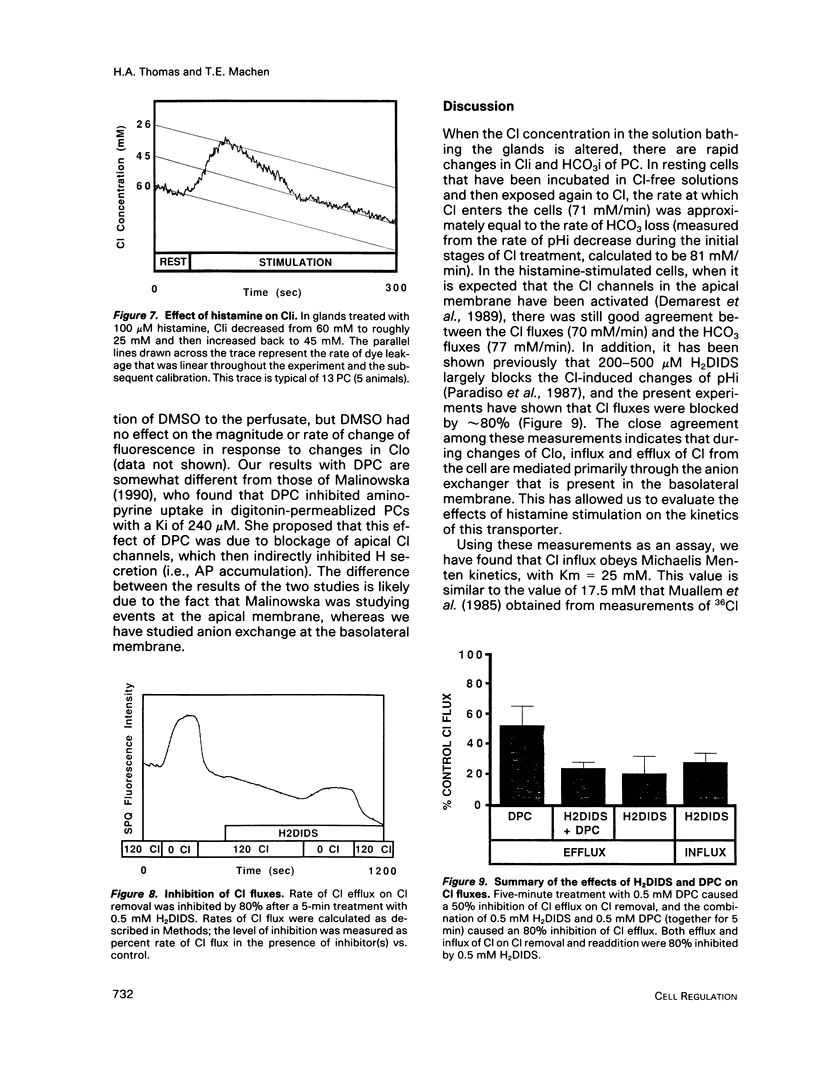
Microspectrofluorimetry of the fluorescent indicators 2',7'-bis-(2-carboxyethyl)-5(and-6)carboxyfluorescein and 6-methoxy-N-(3-sulfopropyl)-quinolinium was used to measure intracellular pH (pHi), intracellular Cl (Cli), and transmembrane fluxes of HCO3 and Cl in single parietal cells (PC) in isolated rabbit gastric glands incubated in HCO3/CO2-buffered solutions. Steady-state pHi was 7.2 in both resting (50 microM cimetidine) and stimulated (100 microM histamine) PCs. Transmembrane anion (HCO3 or Cl) flux rates during Cl removal from or readdition to the perfusate were the same in resting and stimulated PCs. These rates increased at alkaline pHi, though this pHi dependence was small in the physiological range. Maximum velocity (Vmax) for Cl influx or HCO3 efflux was 80-110 mM/min at pHi 7.6-7.8, and the Km for extracellular concentrations of Cl (Clo) was 25 mM; in the physiological range (pHi 7.1-7.3), Vmax for anion fluxes was approximately 50 mM/min. Steady-state Cli in the unstimulated PC was 62 +/- 5 mM, but on histamine stimulation, Cli decreased rapidly to 25 mM and then increased back to a steady-state level of 44 mM. HCO3 fluxes due to Cl removal or readdition were completely blocked by 0.5 mM 4,4'-diisothiocyanatodihydrostilbene-2,2'-disulfonic acid (H2DIDS), but Cl fluxes were only inhibited by 80%. H2DIDS did not inhibit the decrease in Cli that occurred with histamine treatment. Diphenylamine carboxylate (0.5 mM) inhibited Cl flux by only 50% and caused no additional inhibition of Cl flux when used in conjunction with H2DIDS. Transmembrane anion fluxes during solution Cl removal or readdition occurred 80% through the anion exchanger at the basal membrane and 20% through other pathway(s), presumably the Cl channel in the apical membrane. We conclude that the increase in transport activity via the Cl/HCO3 exchanger that occurs during histamine-induced increases in HCl secretion is due mostly to the decrease in Cli. In the resting cell with Cli = 62 mM, Clo = 120 mM, pHi = 7.2, and extracellular pH = 7.4, the anion exchanger is poised near its thermodynamic equilibrium. During histamine stimulation Cli drops from 62 mM to 44 mM, the thermodynamic equilibrium of the anion exchanger at the basolateral membrane is disturbed, and the anion exchanger then exchanges cellular HCO3 for extracellular Cl. Cli serves a crucial regulatory role in stimulus-secretion coupling in the PC.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Cox J. V., Moon R. T., Lazarides E. Anion transporter: highly cell-type-specific expression of distinct polypeptides and transcripts in erythroid and nonerythroid cells. J Cell Biol. 1985 May;100(5):1548–1557. doi: 10.1083/jcb.100.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest J. R., Loo D. D., Sachs G. Activation of apical chloride channels in the gastric oxyntic cell. Science. 1989 Jul 28;245(4916):402–404. doi: 10.1126/science.2474200. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Oelmann M., Schaaf P., Wagner M., Wagner S. Band 3 is the basolateral anion exchanger of dark epithelial cells of turtle urinary bladder. Am J Physiol. 1987 May;252(5 Pt 1):C570–C574. doi: 10.1152/ajpcell.1987.252.5.C570. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Zinke K., Schauer U., Appell K. C., Low P. S. Identification of immunoreactive forms of human erythrocyte band 3 in nonerythroid cells. Eur J Cell Biol. 1984 May;34(1):144–150. [PubMed] [Google Scholar]
- Forte J. G., Black J. A., Forte T. M., Machen T. E., Wolosin J. M. Ultrastructural changes related to functional activity in gastric oxyntic cells. Am J Physiol. 1981 Nov;241(5):G349–G358. doi: 10.1152/ajpgi.1981.241.5.G349. [DOI] [PubMed] [Google Scholar]
- Green J., Yamaguchi D. T., Kleeman C. R., Muallem S. Cytosolic pH regulation in osteoblasts. Regulation of anion exchange by intracellular pH and Ca2+ ions. J Gen Physiol. 1990 Jan;95(1):121–145. doi: 10.1085/jgp.95.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illsley N. P., Verkman A. S. Membrane chloride transport measured using a chloride-sensitive fluorescent probe. Biochemistry. 1987 Mar 10;26(5):1215–1219. doi: 10.1021/bi00379a002. [DOI] [PubMed] [Google Scholar]
- Ito S., Schofield G. C. Studies on the depletion and accumulation of microvilli and changes in the tubulovesicular compartment of mouse parietal cells in relation to gastric acid secretion. J Cell Biol. 1974 Nov;63(2 Pt 1):364–382. doi: 10.1083/jcb.63.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M., Tracey C. M., Goodman J. R., Cone J. C., Bassel P. S. Polypeptides immunologically related to band 3 are present in nucleated somatic cells. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6882–6886. doi: 10.1073/pnas.80.22.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapf R., Berry C. A., Verkman A. S. Estimation of intracellular chloride activity in isolated perfused rabbit proximal convoluted tubules using a fluorescent indicator. Biophys J. 1988 Jun;53(6):955–962. doi: 10.1016/S0006-3495(88)83176-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudrycki K. E., Newman P. R., Shull G. E. cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl-/HCO3- exchanger. J Biol Chem. 1990 Jan 5;265(1):462–471. [PubMed] [Google Scholar]
- Lee H. C., Breitbart H., Berman M., Forte J. G. Potassium-stimulated ATPase activity and hydrogen transport in gastric microsomal vesicles. Biochim Biophys Acta. 1979 May 3;553(1):107–131. doi: 10.1016/0005-2736(79)90034-8. [DOI] [PubMed] [Google Scholar]
- Machen T. E., Townsley M. C., Paradiso A. M., Wenzl E., Negulescu P. A. H and HCO3 transport across the basolateral membrane of the parietal cell. Ann N Y Acad Sci. 1989;574:447–462. doi: 10.1111/j.1749-6632.1989.tb25183.x. [DOI] [PubMed] [Google Scholar]
- Malinowska D. H. Cl- channel blockers inhibit acid secretion in rabbit parietal cells. Am J Physiol. 1990 Oct;259(4 Pt 1):G536–G543. doi: 10.1152/ajpgi.1990.259.4.G536. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H. Effects of growth factors on intracellular pH regulation. Annu Rev Physiol. 1986;48:363–376. doi: 10.1146/annurev.ph.48.030186.002051. [DOI] [PubMed] [Google Scholar]
- Morell G., Steplock D., Shenolikar S., Weinman E. J. Identification of a putative Na(+)-H+ exchanger regulatory cofactor in rabbit renal BBM. Am J Physiol. 1990 Dec;259(6 Pt 2):F867–F871. doi: 10.1152/ajprenal.1990.259.6.F867. [DOI] [PubMed] [Google Scholar]
- Muallem S., Blissard D., Cragoe E. J., Jr, Sachs G. Activation of the Na+/H+ and Cl-/HCO3- exchange by stimulation of acid secretion in the parietal cell. J Biol Chem. 1988 Oct 15;263(29):14703–14711. [PubMed] [Google Scholar]
- Muallem S., Burnham C., Blissard D., Berglindh T., Sachs G. Electrolyte transport across the basolateral membrane of the parietal cells. J Biol Chem. 1985 Jun 10;260(11):6641–6653. [PubMed] [Google Scholar]
- Negulescu P. A., Harootunian A., Tsien R. Y., Machen T. E. Fluorescence measurements of cytosolic free Na concentration, influx and efflux in gastric cells. Cell Regul. 1990 Feb;1(3):259–268. doi: 10.1091/mbc.1.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negulescu P. A., Reenstra W. W., Machen T. E. Intracellular Ca requirements for stimulus-secretion coupling in parietal cell. Am J Physiol. 1989 Feb;256(2 Pt 1):C241–C251. doi: 10.1152/ajpcell.1989.256.2.C241. [DOI] [PubMed] [Google Scholar]
- Paradiso A. M., Negulescu P. A., Machen T. E. Na+-H+ and Cl(-)-OH-(HCO3-) exchange in gastric glands. Am J Physiol. 1986 Apr;250(4 Pt 1):G524–G534. doi: 10.1152/ajpgi.1986.250.4.G524. [DOI] [PubMed] [Google Scholar]
- Paradiso A. M., Townsley M. C., Wenzl E., Machen T. E. Regulation of intracellular pH in resting and in stimulated parietal cells. Am J Physiol. 1989 Sep;257(3 Pt 1):C554–C561. doi: 10.1152/ajpcell.1989.257.3.C554. [DOI] [PubMed] [Google Scholar]
- Paradiso A. M., Tsien R. Y., Demarest J. R., Machen T. E. Na-H and Cl-HCO3 exchange in rabbit oxyntic cells using fluorescence microscopy. Am J Physiol. 1987 Jul;253(1 Pt 1):C30–C36. doi: 10.1152/ajpcell.1987.253.1.C30. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardet C., Counillon L., Franchi A., Pouysségur J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science. 1990 Feb 9;247(4943):723–726. doi: 10.1126/science.2154036. [DOI] [PubMed] [Google Scholar]
- Sardet C., Franchi A., Pouysségur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989 Jan 27;56(2):271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- Schuster V. L., Bonsib S. M., Jennings M. L. Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol. 1986 Sep;251(3 Pt 1):C347–C355. doi: 10.1152/ajpcell.1986.251.3.C347. [DOI] [PubMed] [Google Scholar]
- Thomas H. A., Machen T. E., Smolka A., Baron R., Kopito R. R. Identification of a 185-kDa band 3-related polypeptide in oxyntic cells. Am J Physiol. 1989 Sep;257(3 Pt 1):C537–C544. doi: 10.1152/ajpcell.1989.257.3.C537. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Townsley M. C., Machen T. E. Na-HCO3 cotransport in rabbit parietal cells. Am J Physiol. 1989 Sep;257(3 Pt 1):G350–G356. doi: 10.1152/ajpgi.1989.257.3.G350. [DOI] [PubMed] [Google Scholar]
- Ueda S., Loo D. D., Sachs G. Regulation of K+ channels in the basolateral membrane of Necturus oxyntic cells. J Membr Biol. 1987;97(1):31–41. doi: 10.1007/BF01869612. [DOI] [PubMed] [Google Scholar]
- Wagner S., Vogel R., Lietzke R., Koob R., Drenckhahn D. Immunochemical characterization of a band 3-like anion exchanger in collecting duct of human kidney. Am J Physiol. 1987 Aug;253(2 Pt 2):F213–F221. doi: 10.1152/ajprenal.1987.253.2.F213. [DOI] [PubMed] [Google Scholar]
- Wenzl E., Machen T. E. Intracellular pH dependence of buffer capacity and anion exchange in the parietal cell. Am J Physiol. 1989 Nov;257(5 Pt 1):G741–G747. doi: 10.1152/ajpgi.1989.257.5.G741. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M., Forte J. G. Stimulation of oxyntic cell triggers K+ and Cl- conductances in apical H+-K+-ATPase membrane. Am J Physiol. 1984 May;246(5 Pt 1):C537–C545. doi: 10.1152/ajpcell.1984.246.5.C537. [DOI] [PubMed] [Google Scholar]



