Abstract
Levodopa (L-DOPA) is widely used for symptomatic management in Parkinson’s disease. We recently showed that (−)-epigallocatechin-3-gallate, a tea polyphenol, not only inhibits L-DOPA methylation, but also protects against oxidative hippocampal neurodegeneration. In the present study, we sought to determine several other common dietary phenolics, namely, tea catechins [(+)-catechin and (−)-epicatechin] and a representative flavonoid (quercetin), for their ability to modulate L-DOPA methylation and to protect against oxidative hippocampal injury. A combination of in vitro biochemical assays, cell culture-based mechanistic analyses, and in vivo animal models was used. While both tea catechins and quercetin strongly inhibit human liver catechol-O-methyltransferase (COMT)-mediated O-methylation of L-DOPA in vitro, only (+)-catechin exerts a significant inhibition of L-DOPA methylation in both peripheral compartment and striatum in rats. The stronger in vivo effect of (+)-catechin on L-DOPA methylation compared to the other dietary compounds is due to its better bioavailability in vivo. In addition, (+)-catechin strongly reduces glutamate-induced oxidative cytotoxicity in HT22 mouse hippocampal neurons in vitro through inactivation of the nuclear factor-κB signaling pathway. Administration of (+)-catechin also exerts a strong neuroprotective effect in the kainic acid-induced oxidative hippocampal neurodegeneration model in rats. In conclusion, (+)-catechin is a dietary polyphenolic that may have beneficial effects in L-DOPA-based treatment of Parkinson patients by inhibiting L-DOPA methylation plus reducing oxidative neurodegeneration.
Keywords: Levodopa, Catechol-O-methyltransferase (COMT), (+)-Catechin, Oxidative neuronal damage, COMT inhibition, Neuroprotection
1. INTRODUCTION
The clinical symptoms of Parkinson disease (PD) are largely due to the loss of nigrostriatal dopaminergic neurons and the decrease in striatal dopamine content (Riederer and Wuketich, 1976; Da Prada et al., 1984; Huot et al., 2007). Levodopa (L-DOPA), a natural precursor for dopamine biosynthesis, is commonly used for symptom management in many PD patients. This drug is always administered together with a peripheral dopa decarboxylase inhibitor (e.g., carbidopa) to reduce its rapid conversion to dopamine in peripheral tissues (Bartholini and Pletscher, 1975; Fahn, 2006). When the peripheral dopa decarboxylase is inhibited, the hepatic catechol-O-methyltransferase (COMT)-mediated O-methylation of L-DOPA becomes a major metabolic pathway. Under such conditions, the use of a COMT inhibitor (e.g., tolcapone or entacapone) has been recommended for some patients, which can further improve L-DOPA bioavailability by suppressing enzymatic conversion of L-DOPA to 3-O-methyldopa (3-OMD) (Mannisto and Kaakkola, 1999; Heikkinen et al., 2002; Toulouse and Sullivan, 2008).
Clinical studies have shown that nearly 50% of patients using the L-DOPA + carbidopa treatment develop severe motor fluctuations (also called “wearing-off” phenomenon) and dyskinesia within the first 5 years of treatment (Koller et al., 1999; Ahlskog and Muenter, 2001; Toulouse and Sullivan, 2008). It has been suggested that some of the nervous system complications may result from a combination of the following two changes after chronic L-DOPA administration: One is the relatively large and rapid fluctuations in L-DOPA blood and CNS concentrations, and the other one is the adverse actions exerted by 3-OMD, which is a major L-DOPA metabolite formed in large quantities in both periphery and brain of patients treated with L-DOPA/carbidopa (Feuerstein et al., 1977; Raches and Fahn, 1981; Lee et al., 2008). In partial support of these suggestions, animal studies have shown that 3-OMD can interfere with L-DOPA utilization in the brain, and can also induce neuronal damage via oxidative stress (Raches and Fahn, 1981; Lee et al., 2008). Moreover, 3-OMD was found athigh levels in plasma as well as cerebral spinal fluid of PD patients treated with L-DOPA/carbidopa (Sharpless et al., 1972; Tohgi et al., 1991), and its plasma levels in patients with dyskinesia were significantly higher than patients without dyskinesia (Feuerstein et al., 1977). Theoretically, addition of a COMT inhibitor to the L-DOPA/carbidopa therapy in PD patients will not only reduce L-DOPA concentration fluctuations, but will also greatly reduce 3-OMD levels, both of which would be beneficial to reducing complications. Indeed, many clinical studies have shown that such a three-drug therapy (i.e., L-DOPA + carbidopa + a COMT inhibitor) improves L-DOPA bioavailability and also reduces the occurrence of adverse effects in PD patients (Müller, 2009).
We have previously shown that some of the catechol-containing bioflavonoids and tea catechins are exceptionally good substrates for human COMT (Zhu et al., 2000). In addition, bioflavonoids and tea catechins are also strong inhibitors of human COMT (Lu et al., 2003; Nagai et al., 2004; van Duursen et al., 2004; Chen et al., 2005). In our recent study, (−)-epigallocatechin-3-gallate (EGCG) was found to have beneficial effect on the L-DOPA/carbidopa therapy by inhibiting COMT-mediated L-DOPA methylation (Kang et al., 2010). In the first part of this study, therefore, we sought to further evaluate the effect of two other common tea catechins [(+)-catechin and (−)-epicatechin] and a common flavonoid (quercetin) on L-DOPA methylation to determine their relative effectiveness as naturally-occurring COMT inhibitors (Figure 1). We found that among these dietary compounds, (+)-catechin has the strongest in vivo effect in modulating L-DOPA methylation in a rat model, owing to its favorable pharmacokinetic properties.
Figure 1.
Enzymatic methylation of L-DOPA and its potential modulation by certain dietary phenolics. The upper panel shows that the COMT-catalyzed O-methylation of L-DOPA, which results in the formation of two mono-methylated products. It is hypothesized that certain dietary phenolics such as tea catechins and flavonoids may serve as naturally-occurring inhibitors of human COMT-mediated O-methylation of L-DOPA in vivo. The structures of (+)-catechin, (−)-epicatechin, and quercetin are shown in the lower panel. Partly owing to their strong antioxidant activity, it is also suggested that some of these dietary phenolics may have additional neuroprotective effects. The potential dual beneficial effects of certain dietary phenolics, if found to be true, would be desirable in L-DOPA-based treatment of PD patients.
It is known that PD patients usually also suffer from a variety of non-motor symptoms including depression and dementia (Yamamoto, 2001; Padovani et al., 2006; Eskow et al., 2010). Hippocampus is a brain region that often is associated with chronic damage in PD patients, and as a result, these patients have a six-fold higher risk than age-matched healthy control subjects for developing dementia (Padovani et al., 2006). Deficits in hippocampal-dependent learning have also been observed in parkinsonian animals (Denenberg et al., 2004; Costa et al., 2012). It has been suggested that the use of L-DOPA may help partially restore hippocampal synaptic potentiation via activation of dopamine D1/D5 receptors and thereby ameliorate the cognitive deficit in parkinsonian animals (Costa et al., 2012). Recently, we have reported that EGCG, which can beneficially inhibit L-DOPA methylation in vivo, also has a protective effect against oxidative hippocampal neurodegeneration (Kang et al., 2010). This neuroprotective effect, which is not shared by the currently-approved COMT inhibitors (tolcapone and entacapone) for combination use with L-DOPA in PD patients, is highly desirable. Therefore, in the second part of this study, we also sought to determine whether (+)-catechin has protective effect against hippocampal oxidative neurodegeneration using both in vitro and in vivo models.
2. RESULTS
2.1. PART I – Effect of (+)-catechin, (−)-epicatechin, and quercetin on COMT-mediated L-DOPA methylation
2.1.1. Inhibition of COMT-mediated L-DOPA methylation in vitro
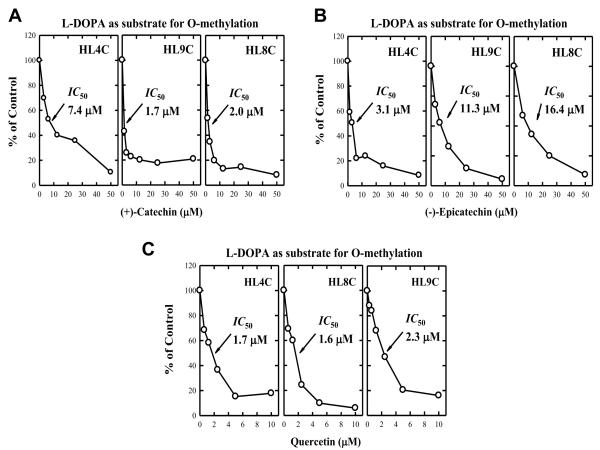
The reaction conditions for the in vitro enzymatic O-methylation of L-DOPA were optimized by determining the effects of different incubation times and enzyme concentrations, and the data were described in our recent study (Kang et al., 2010). Under optimized in vitro metabolism conditions, we then determined the modulating effect of two selected tea catechins and a flavonoid on L-DOPA methylation catalyzed by cytosolic COMT prepared from three representative human liver samples (HL4C, HL8C, and HL9C) (data shown in Figure 2). (+)-Catechin, (−)-epicatechin, and quercetin each inhibited COMT-mediated L-DOPA methylation in a concentration-dependent manner, with IC50 values of 3.7 ± 3.2 μM for (+)-catechin, 10.7 ± 6.7 μM for (−)-epicatechin, and 1.9 ± 0.4 μM for quercetin.
Figure 2.
Inhibition of human liver COMT-mediated O-methylation of L-DOPA by two tea catechins, (+)-catechin and (−)-epicatechin (panels A and B), and a representative flavonoid quercetin (panel C). The incubation mixture consisted of 10 μM L-DOPA, 250 μM [3H-methyl]AdoMet (containing 0.2 μCi), 0.25 mg/mL of human cytosolic protein, 1 mM dithiothreitol, 1.2 mM MgCl2, and a dietary inhibitor (concentration as indicated) in a final volume of 0.25 mL Tris-HCl buffer (10 mM, pH 7.4). Incubations were carried out at 37°C for 10 min. Each point is the mean of duplicate determinations of the 3 human liver (HL) cytosolic samples named HL4C, HL8C and HL9C (with average variations below 5%).
2.1.2. Effect on L-DOPA methylation in vivo
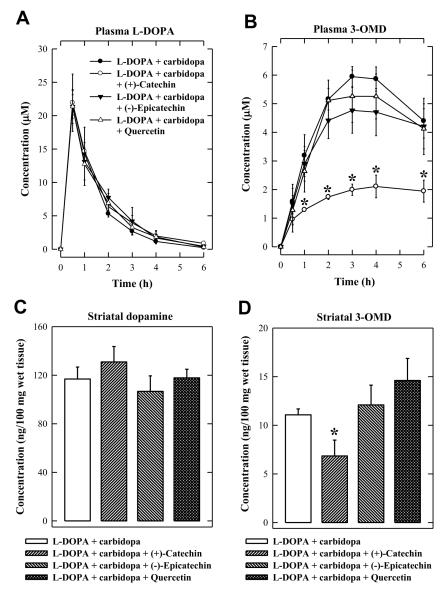
To determine the in vivo modulating effect of these dietary compounds on L-DOPA methylation, rats were injected i.p. with 400 mg/kg (+)-catechin, (−)-epicatechin, or quercetin 2 h before oral administration of L-DOPA/carbidopa (20/5 mg/kg). The doses of L-DOPA and carbidopa were adopted from our recent study (Kang et al., 2010). The plasma L-DOPA levels were not appreciably altered by co-administration of (+)-catechin, (−)-epicatechin, or quercetin (Figure 3A). Administration of (+)-catechin significantly reduced the plasma 3-OMD levels, but (−)-epicatechin or quercetin did not have a similar effect on plasma 3-OMD levels (Figure 3B). The striatal region of the brain was dissected at 6 h after L-DOPA/carbidopa administration and analyzed for dopamine and 3-OMD levels. In the striatum, 3-OMD levels were significantly reduced by (+)-catechin administration (P < 0.05), although dopamine levels were only slightly changed (no statistical significance) (Figure 3C, 3D). In comparison, no significant change in striatal dopamine and 3-OMD levels was observed following administration of (−)-epicatechin or quercetin (Figure 3C, 3D). These data show that only (+)-catechin is an effective inhibitor of L-DOPA methylation in vivo. Notably, very similar observations were made with these dietary compounds in another experiment when slightly different time points were used in collecting blood samples and brain tissues (data not shown).
Figure 3.
Comparison of the effect of (+)-catechin, (−)-epicatecin, and quercetin on the methylation of L-DOPA in rats. (A) Plasma L-DOPA concentrations. (B) Plasma 3-OMD concentrations. (C) Striatal dopamine concentrations. (D) Striatal 3-OMD concentrations. Rats were injected i.p. with 400 mg/kg (+)-catechin, (−)-epicatechin, or quercetin 2 h before oral administration of L-DOPA + carbidopa (20 + 5 mg/kg). Control rats received the vehicle administration only. Each value is the mean ± S.D. * P < 0.05 compared to L-DOPA + carbidopa group.
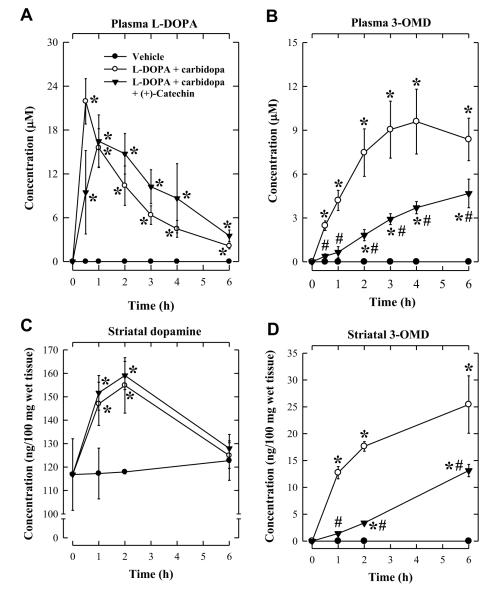
Earlier studies showed that the beneficial effect of COMT inhibition is more pronounced under conditions when a stronger inhibition of dopa decarboxylase is achieved (Kaakkola and Wurtman, 1993; Mannisto and Kaakkola, 1999). Therefore, we further evaluated the in vivo inhibitory efficacy of (+)-catechin on L-DOPA methylation when a 4-fold higher dose of carbidopa (at 20 mg/kg) was used. (+)-Catechin was orally administered 2 h before L-DOPA/carbidopa administration (20/20 mg/kg). Under this condition, a significant increase in L-DOPA AUC0-6 value was observed (from 374.8 ± 81.2 to 570.3 ± 128.9 μg·min/mL) (Table 1). In addition, the time required to reach maximal L-DOPA plasma concentration was modestly shifted to the right, indicating that L-DOPA stayed in circulation longer in rats treated with (+)-catechin than in control animals (Figure 4A). Also, 3-OMD AUC0-6 was slightly increased by approximately 1.6-fold (Table 1). Under this experimental condition, a similar decrease in plasma 3-OMD level was observed in animals treated with (+)-catechin (Figure 4B, Table 1) compared with the experiment when 20 mg/kg L-DOPA and 5 mg/kg carbidopa were used (Figure 3B, Table 1). Although striatal dopamine levels were not significantly affected by (+)-catechin treatment (Figure 4C), striatal 3-OMD levels were markedly reduced by the treatment (Figure 4D).
TABLE 1.
Comparison of the plasma AUC0-6 values of L-DOPA and 3-OMD. Data were obtained from the animal experiments I and II.
| Treatment groups | L-DOPA/carbidopa (at 20 mg/5 mg kg) |
L-DOPA/carbidopa (at 20 mg/20 mg kg) |
||
|---|---|---|---|---|
|
| ||||
| L-DOPA (μg·min/mL) (μg·min/mL) |
3-OMD (μg·min/mL) |
L-DOPA | 3-OMD (μg·min/mL) |
|
| Control | 374.8 ± 81.2 | 377.9 ± 40.4 | 570.3 ± 128.9 | 600.4 ± 130.9 |
| (+)-Catechin | 427.8 ± 88.7 | 141.4 ± 21.8* | 693.1 ± 227.4 | 215.1 ± 38.2* |
| (−)-Epicatechin | 419.1 ± 79.6 | 321.3 ± 63.9 | ND | ND |
| Quercetin | 396.3 ± 104.2 | 343.8 ± 62.6 | ND | ND |
Data are expressed as the mean ± S.D. (N = 4).
P < 0.05 vs. the control group.
ND, not determined.
Figure 4.
Effect of carbidopa on the degree of inhibition of L-DOPA methylation by (+)-catechin in rats. (A) Plasma L-DOPA concentrations. (B) Plasma 3-OMD concentrations. (C) Striatal dopamine levels. (D) Striatal 3-OMD levels. Rats were injected i.p. with 400 mg/kg (+)-catechin 2 h before oral administration of L-DOPA/carbidopa (20/20 mg/kg). The vehicle group received water administration only. Each value is the mean ± S.D. * P < 0.05 compared to vehicle group. # P < 0.05 compared to L-DOPA + carbidopa group.
To determine whether (+)-catechin would have a stronger effect in improving L-DOPA concentration in the striatum of dopamine-deficient rats, the animals were first treated with reserpine to induce CNS dopamine depletion (described in the Methods section). Following oral administration of L-DOPA/carbidopa in these reserpinized animals, plasma L-DOPA and 3-OMD levels were found to be increased to 15.9 ± 2.1 μM and 16.4 ± 1.9 μM, respectively, at 2 h (Figure 5A, 5B). In animals co-treated with (+)-catechin, the plasma levels of L-DOPA were slightly increased to 21.6 ± 3.7 μM (no statistical significance), but plasma 3-OMD levels were significantly reduced in comparison with animals not treated with (+)-catechin (Figure 5A, 5B). Striatal dopamine levels in reserpinized rats were decreased by over 80% compared to normal control animals, but the levels were restored to levels comparable to those in normal rats by co-treatment with (+)-catechin (compare Figure 5C with Figure 4C)). Similarly, striatal 3-OMD levels were also markedly reduced in rats co-treated with (+)-catechin (Figure 5D).
Figure 5.
Effect of (+)-catechin on the methylation of L-DOPA in dopamine-depleted rats. (A) Plasma L-DOPA concentrations. (B) Plasma 3-OMD concentrations. (C) Striatal dopamine levels. (D) Striatal 3-OMD levels. Reserpine (5 mg/kg) was solubilized in water containing lactic acid (1%, v/v) and given to rats 18-20 h before administration of L-DOPA and/or (+)-catechin. Rats were injected i.p. with 400 mg/kg (+)-catechin 2 h before oral administration of L-DOPA/carbidopa (20/20 mg/kg). Each value is the mean ± S.D. * P < 0.05 compared to the reserpinized group. # P < 0.05 compared to L-DOPA + carbidopa group.
2.1.3. Plasma levels of (+)-catechin, (−)-epicatechin and quercetin after i.p. administration
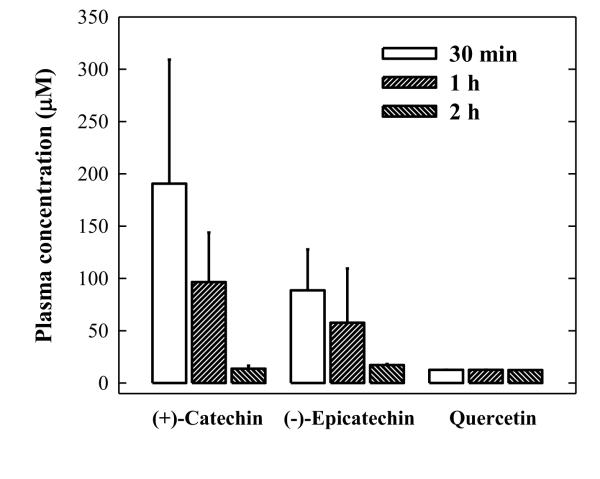
The pharmacokinetics and blood-brain-barrier penetration of some of dietary polyphenolic compounds used in this study have been studied in recent years (Catterall et al., 2003; Loke et al., 2008; Moon et al., 2008; Ferruzzi et al., 2009). The observed stronger effect of (+)-catechin compared to that of (−)-epicatechin and quercetin appears to be consistent with the more favorable bioavailability of (+)-catechin in vivo (Manach et al., 1999). To confirm that the differential bioavailability of these dietary compounds is the key determinant of their in vivo efficacy in modulating L-DOPA metabolism, we conducted an abbreviated experiment to compare the blood levels of these dietary compounds at three selected time points (0.5, 1 and 2 h) in rats injected i.p. with the same dose as used in L-DOPA metabolism experiments. We found that the plasma concentrations of unconjugated (+)-catechin at 30 min after i.p. injection were markedly higher than those of (−)-epicatechin and quercetin (190.6 ± 118.5 μM vs. 88.6 ± 39.2 μM and 12.6 ± 0.1 μM) (Figure 6).
Figure 6.
Plasma concentrations of (+)-catechin, (−)-epicatecin, and quercetin in rats. Rats (N = 4 for each group) were injected i.p. with 400 mg/kg each dietary polyphenolic compound. Blood samples were collected from their tail veins at 0.5, 1, and 2 h after administration of the dietary compound. Each value is the mean ± S.D.
2. 2. PART II – Neuroprotective effect of (+)-catechin
2. 2.1. in vitro protection against glutamate-induced oxidative neuronal cell death
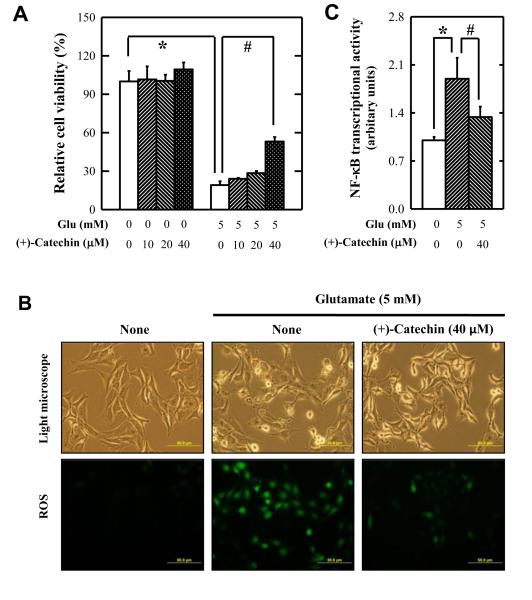
To investigate the effect of (+)-catechin on oxidative stress-induced neuronal cell death, HT22 cells, an immortalized mouse hippocampal neuronal cell line sensitive to glutamate-induced oxidative cell death (Tan et al., 1998; Fukui et al., 2009; Choi et al., 2011), was used as an in vitro model. After 24-h exposure of HT22 cells to 5 mM glutamate, cell viability was reduced by 80%, and co-treatment with (+)-catechin abrogated cell death in a concentration-dependent manner (Figure 7A). In addition, the glutamate-induced accumulation of intracellular ROS was reduced by (+)-catechin treatment (Figure 7B).
Figure 7.
Effect of (+)-catechin against glutamate-induced HT22 cell death. (A) Cell viability. (B) ROS accumulation. (C) Transcriptional activity of NF-κB. HT22 cells were treated with 5 mM glutamate in the presence or absence of (+)-catechin at indicated concentrations. Cell viability, transcriptional activity of NF-κB, and ROS accumulation were determined as described in the Materials and Methods. The experiment was repeated three times, and similar observations were made. DAPI staining was employed as a nuclear counter staining. Each value is the mean ± S.D. * P < 0.05 compared to vehicle-treated group. # P < 0.05 compared to glutamate-treated group.
Our recent study showed that NF-κB activation is involved in glutamate-induced oxidative cytotoxicity in HT22 cells (Kang et al., 2010). To test the mechanistic hypothesis that (+)-catechin protects against neuronal cell death through inactivation of the NF-κB signaling pathway, HT22 cells were selectively transfected with a NF-κB-Luc reporter gene. As expected, NF-κB transcriptional activity was increased in cells treated with 5 mM glutamate (Figure 7C). The presence of (+)-catechin in the cell culture medium significantly attenuated the transcriptional activity of NF-κB in these neuronal cells (Figure 7C).
2.2.2. in vivo study using the kainic acid-induced hippocampal neurodegeneration model
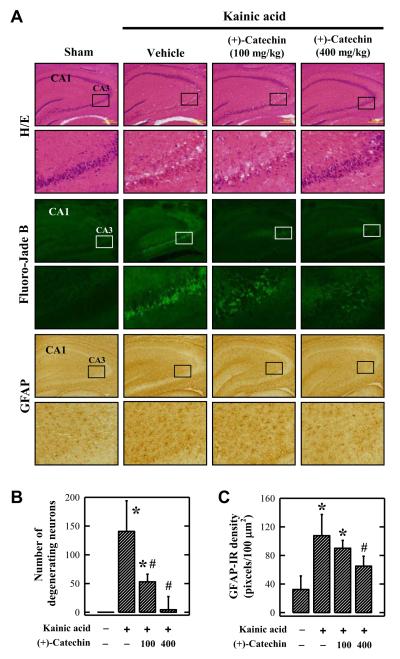
The observed in vitro neuroprotective effect of (+)-catechin was further assessed using the kainic acid-induced hippocampal injury model in vivo. (+)-Catechin was administered to rats i.p. 30 min before intracerebroventricular (i.c.v.) kainic acid injection. Kainic acid (1 μL of 1 mg/mL solution) was injected into the right lateral ventricle of rats, and the animals were sacrificed 24 h later. All rats receiving the i.c.v. injection of kainic acid produced well-characterized seizures (e.g., staring, immobility, and subsequent wet dog shake), and no appreciable behavioral differences were observed in animals that also received (+)-catechin administration. The shrinkage of neuronal cell bodies and formation of peri-neuronal vacuoles were detected in CA3 region at 24 h post kainic acid injection (Figure 8A). Different from the CA3 region, no similar changes were seen in the neighboring CA1 region (Figure 8A). These observations were in agreement with the known selective neurotoxicity of kainic acid in certain hippocampal regions (Nalder et al., 1980). In animals co-treated with (+)-catechin, the extent of neuronal death was markedly reduced (Figure 8A, 8B). In addition, it was observed that there was a modest increase in the expression of GFAP in astrocytes in kainic acid-injected rat brain, and this increase was reduced by (+)-catechin administration (Figure 8A, 8C).
Figure 8.
Effect of orally-administered (+)-catechin on kainic acid-induced rat hippocampal injury. (A) Histopathological and histochemical analyses of brain tissue slides. (B) Number of degenerating neurons based on Fluoro-Jade B staining. (C) Relative density of GFAP-immunoreactive (IR) astrocytes in CA3 region. Rats were orally administered 400 mg/kg of (+)-catechin 30 min before kainic acid injection. Kainic acid (1 μL of 1 mg/mL solution) was injected into the right lateral ventricle (anterior/posterior, −1.0; rostral, 1.6; dorsal/ventral, 4.5) using a microliter syringe. Each value is the mean ± S.D. * P < 0.05 compared to vehicle-treated group. # P < 0.05 compared to kainic acid-treated group.
3. DISCUSSION
In recent years, a number of therapeutically-useful drugs have been rationally designed and synthesized according to the new knowledge gained from studying the actions of various biologically-active chemicals derived from plants and medicinal herbs (Matthews et al., 1999; Schmidt et al., 2008). One of the advantages of these naturally-occurring substances is their potential to interact with multiple targets in the body to elicit desirable beneficial outcomes (Schmidt et al., 2008). With this paradigm in mind, we recently have reported the dual beneficial effects of EGCG on L-DOPA methylation and hippocampal neurodegeneration (Kang et al., 2010). In the present study, we focused on evaluating whether these beneficial effects are associated with some of other well-known natural phenolics, which share certain degrees of structural similarity to EGCG. We found that although the three compounds tested in this study are highly effective in vitro for inhibiting COMT-mediated O-methylation of L-DOPA, only (+)-catechin was effective in vivo, due to its favorable bioavailability. In addition, (+)-catechin has a strong in vivo protective effect against oxidative hippocampal neurodegeneration, as it was also recently reported for EGCG (Kang et al., 2010). Together, these results suggest that (+)-catechin may be another potential dietary compound for clinical use in PD patients. In addition, the results of this study may shed some light on the structural features in the design of novel COMT-inhibitors that also retain beneficial neuroprotective functions.
3.1. Effect of natural phenolics on L-DOPA methylation in vitro and in vivo
We found that (+)-catechin, (−)-epicatechin, and quercetin each can effectively inhibit, in a concentration-dependent manner, the in vitro methylation of L-DOPA catalyzed by human liver cytosolic COMT. Using a rat model, we also showed that the plasma and striatal 3-OMD levels (an in vivo marker of L-DOPA methylation) were effectively reduced by co-treatment with (+)-catechin, whereas (−)-epicatechin and quercetin did not show an appreciable effect. The stronger inhibitory effect of (+)-catechin than the other two dietary compounds on L-DOPA methylation in vivo agrees with the more favorable bioavailability of (+)-catechin.
Treatment of animals with reserpine sometimes has been used as a means to deplete catecholamine content in various brain regions, including a marked reduction in striatal dopamine content. This animal model has previously been used in evaluating some of the antiparkinsonian drugs (Dawson et al., 2000; Segovia et al., 2003). When the modulating effect of (+)-catechin on L-DOPA methylation and dopamine content was examined in reserpinized rats, we found that the increases in plasma and striatal 3-OMD levels following administration of L-DOPA/carbidopa were strongly blunted by (+)-catechin. Notably, the reduced striatal dopamine levels in reserpinized rats, which were approximately 20% of the levels found in non-reserpinized control rats, were markedly increased by co-treatment with (+)-catechin. This observation, along with earlier clinical studies in PD patients (Tedroff et al., 1996), supports the notion that the ability of the administered L-DOPA to increase synaptic dopamine content depends on the degree of striatal dopamine deficiency. It is expected that in the striatum of PD patients where there is a severe dopamine deficiency, a greater increase in dopamine content likely will be seen when these patients are jointly treated with L-DOPA/carbidopa plus a dietary COMT inhibitor such as (+)-catechin or EGCG.
Compared to tolcapone and entacapone, the in vivo potency and efficacy of (+)-catechin in inhibiting L-DOPA methylation (based on inhibition of 3-OMD formation) are weaker, which likely is due to the following two factors: (i) (+)-catechin has a lower potency in directly inhibiting human liver COMT-mediated L-DOPA methylaton, and (ii) (+)-catechin likely has a lower bioavailability in vivo (Chen et al., 1997; Lu et al., 2003) compared to tolcapone and entacapone.
Lastly, it is of note that while we observed a strong reduction in 3-OMD plasma level, there was no appreciable increase in L-DOPA plasma level in rats co-treated with (+)-catechin. Similar observations were also made in earlier clinical studies with tolcapone and entacapone in human subjects (Sêdek et al., 1997; Cedarbaum et al., 1991; Keränen et al., 1993; Heikkinen et al., 2001; Blandini et al., 2003). Therefore, it is reasonable to suggest that while the use of (+)-catechin as an adjunct will help prolong the therapeutic effects of L-DOPA in PD patients, it will not increase the peak concentrations of L-DOPA nor aggravate its concentration fluctuations. These effects would be highly beneficial to reducing the occurrence of neural toxicity such as dyskinesia and hallucinations.
3.2. Neuroprotective effect of (+)-catechin in vitro and in vivo
To characterize the mechanism of the neuroprotective effect of (+)-catechin, HT22 cells, an immortalized mouse hippocampal neuronal cell line, was used. The mechanism of glutamate-induced oxidative stress and neurotoxicity in these neuronal cells has been well characterized in recent years (Tan et al., 1998; Fukui et al., 2009; Fukui et al., 2010; Choi et al., 2011). In addition, it is known that ROS-mediated cellular oxidative stress often results in NF-κB activation (Post et al., 1998). NF-κB is usually sequestered in cytoplasmic compartment in complex with an inhibitory protein, IκB. Various extracellular signals can induce phosphorylation and rapid degradation of IκB to release NF-κB. After IκB is released, NF-κB then migrates into nucleus where it binds to specific DNA sequences and turns on gene transcription (Gloire et al., 2006). Our results show that (+)-catechin can reduce glutamate-induced HT22 cell death by protecting these cells from ROS insult through reducing NF-κB activation, and its effect was slightly weaker than EGCG under the same experimental conditions (Kang et al., 2010).
In addition, we have investigated the effect of (+)-catechin on neurodegeneration using the kainic acid-induced rat hippocampal injury model. Induction of oxidative stress has been identified as a major mechanism for hippocampal neurodegeneration (Nalder et al., 1980, Floreani et al., 1997; Shin et al., 2009). Our results show that i.c.v. injection of kainic acid induces neuronal loss as well as activation of glial cells in hippocampal CA3 region as described before (Nalder et al., 1980; Matsuoka et al., 1999), and treatment with (+)-catechin not only protects hippocampal neuronal cells, but also reduces GFAP-positive astrocytes. These data suggest that (+)-catechin has a strong neuroprotective effect against kainic acid-induced hippocampal neurodegeneration in rats. This protective effect is of considerable interest and clinical relevance because hippocampus is a brain region that often suffers chronic neurodegeneration in PD patients. It is estimated that PD patients have a six-fold higher risk than age-matched healthy control subjects for developing dementia (Padovani et al., 2006). Hence, the observed neuroprotective effect of (+)-catechin may help alleviate hippocampal neurodegeneration in PD patients.
Here it is of note that in this study, we have also examined the effect of (+)-catechin in the 6-hydroxydopamine-induced neuronal damage model, and it did not show any appreciable protective effect (data not shown). Similarly, an earlier study by others also reported that EGCG, a major tea catechin, was ineffective in the 6-hydroxydopamine-induced neurotoxicity model (Leaver et al., 2009). It is apparent that the neuroprotective effect of catechin is only seen under certain experimental conditions.
Lastly, it is noteworthy that recent epidemiological studies suggest that regular tea or coffee drinking is associated with a reduced risk of PD in humans (Hu et al., 2007; Kandinov et al., 2009). The potential beneficial effects of tea polyphenols (and possibly some of the coffee polyphenolic compounds) on brain catecholamine metabolism and neuronal survival as observed in this study may partially contribute to the epidemiological observations. Although the daily intake of tea and coffee polyphenols through regular tea and coffee drinking may not reach therapeutically-effective concentrations, it should be noted that tea and coffee also contain many other phenolic antioxidants (Scalbert and Williamson, 2000; Ferruzzi et al., 2009). The collective neuroprotective effect exerted by these polyphenolic compounds could be significant. Moreover, many of these dietary phenolics (e.g., catechins, quercetin, and caffeic acid) also contain the same catecholic structures as does L-DOPA, which make them good substrates and also effective inhibitors of COMT (Zhu et al., 2000; Zhu, 2002 & 2004; Lu et al., 2003; Nagai et al., 2004; Bai et al., 2007; Zhu et al., 2008). Theoretically, a collective inhibition of COMT-mediated O-methylation of endogenous catecholamines (including dopamine and L-DOPA) by these dietary phenolics could be quite significant in the human body. In partial support of this suggestion, it is known that drinking tea and coffee is associated with a number of physiological effects in humans, such as increased heart rate and contractility, increased urination, and strong central nervous system stimulation. These effects are all known to be associated with elevated levels of endogenous catecholamines. While some of these effects associated with tea and coffee often are conveniently attributed to the small amounts of theophylline and caffeine contained in these beverages, the contribution of COMT inhibition has also been suggested in recent years (Zhu, 2002 & 2004).
CONCLUSIONS
In the present study, we showed that while both tea catechins and flavonoids can strongly inhibit L-DOPA methylation in vitro, only (+)-catechin was found to exert a significant inhibition of L-DOPA methylation in vivo. Notably, administration of (+)-catechin produces a greater increase in striatum dopamine content in dopamine-depleted rats than in normal rats, suggesting that this dietary polyphenol may have a greater beneficial effect in PD patients who have a reduced striatal dopamine content. The stronger in vivo effect of (+)-catechin compared to other dietary phenolics tested in this study is attributable to its more favorable bioavailability. In addition, we showed that (+)-catechin has a strong protective effect against oxidative hippocampal neurodegeneration in vivo. Based on these observations, it is suggested that (+)-catechin is a dietary compound that may have beneficial effects in L-DOPA-based treatment of PD patients by jointly inhibiting L-DOPA methylation and reducing oxidative neuronal damage.
4. MATERIALS AND METHODS
4.1. Chemicals
(+)-Catechin, (−)-epicatechin, quercetin, L-DOPA, carbidopa, 3-OMD, dopamine, 3,4-dihydroxybenzylamine hydrobromide (DHBA), glutamate, kainic acid and 2′,7′-dichlorofluorescein diacetate (H2-DCF-DA) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). [3H-Methyl]AdoMet (specific activity = 11.2-13.5 Ci mmol−1, purity > 97%) was purchased from New England Nuclear Research Products (Boston, MA, USA). The plasmid pNF-κB-Luc carrying a firefly luciferase cDNA driven by5× NF-κB-binding sites was purchased from Stratagene (La Jolla, CA, USA). All other reagents used in this study were obtained from standard suppliers and were of analytical grade or better.
4.2. in vitro modulation of L-DOPA methylation by tea catechins and flavonoids
The procedures for collecting human liver samples were approved by the Institutional Review Boards (IRBs). The methods for preparing cytosols from these collected tissue specimens were described in detail earlier (Nagai et al., 2004). For assaying the in vitro O-methylation of L-DOPA catalyzed by human liver cytosolic COMT, the incubation mixture consisted of 10 μM L-DOPA as a substrate, 250 μM [3H-methyl]AdoMet (containing 0.2 μCi) as a methyl donor, 0.25 mg/mL of human cytosolic protein as the enzyme source, and a dietary inhibitor [(+)-catechin, (−)-epicatechin, or quercetin] in a final volume of 0.25 mL Tris-HCl buffer (10 mM, pH 7.4) supplemented with 1.2 mM MgCl2 and 1 mM dithiothreitol. Incubations were carried out at 37°C for 10 min. Each measurement is the mean of duplicate determinations (with an average variation below 5%).
4.3. in vitro protective effect of (+)-catechin against oxidative stress-induced neuronal cell death
Glutamate-sensitive HT22 murine hippocampal neuronal cells (a gift from Dr. David Schubert at the Salk Institute, La Jolla, CA) were maintained in DMEM supplemented with 10% (v/v) FBS and antibiotics (penicillin-streptomycin), and incubated at 37°C under 5% CO2. Cells were subcultured once every 2 days. Cells were seeded in 96-well plates at a density of 5000 cells per well. Stock solutions of glutamate (1 M in DMEM without serum) and (+)-catechin (100 mM in 200 proof ethanol) were diluted in the culture medium immediately before addition into each culture well at desired final concentrations, and the treatment lasted up to 24 h.
The MTT assay was used for assessing cell viability as described in our recent study (Fukui et al., 2009). The production of ROS in HT22 cells with or without glutamate treatment was detected using the H2-DCF-DA method. H2-DCF-DA (1 μM) was added to each well, and incubated for 20 min at 37°C. Then the supernatant was removed and PBS was added. Intracellular ROS accumulation was observed and photographed under a fluorescence microscope (AXIO, Carl Zeiss Corporation, Germany). For detection of transcriptional activity of NF-κB, HT22 cells in 24-well culture plates were transfected with 0.05 μg pNF-κB-Luc plasmid using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Twenty-four h after transfection, cells were treated with 5 mM glutamate with or without 40 μM (+)-catechin for 8 h. Then cells were harvested and the luciferase activity was determined by a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). Firefly luciferase activity was normalized to cellular protein concentrations.
4.4. Animal experiments
4.4.1. Animals
All procedures involving the use of live animals as described in this study were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center, and strictly followed the guidelines for humane care of animals set forth by the U.S. National Institutes of Health. Male Sprague-Dawley rats, weighing 250-270 g, were purchased from Harlan (Indianapolis, IN) and kept in a plastic bottomed cage with a 12-h light/12-h dark cycle, with controlled room temperature (at 25°C) and humidity (60%). They were allowed free access to laboratory pellet chow and water. After arrival, the animals were allowed to acclimatize to the new environment for one week before they were used in the experimentation.
4.4.2. Animal experiment I – Compare the effect of (+)-catechin, (−)-epicatechin, and quercetin on L-DOPA methylation
Rats were randomly divided into various experimental groups with no significant difference in average body weights. Rats were injected i.p. 400 mg/kg (+)-catechin, (−)-epicatechin, or quercetin 2 h before oral administration of L-DOPA/carbidopa (20/5 mg/kg). The in vivo experimental conditions (dose, time and administration routes) were adopted from our recent study (Kang et al., 2010). Control rats received the same i.p. and/or oral administrations of vehicle only. Blood samples were collected from the tail vein under light anesthesia (isoflurane inhalation) at 0.5, 1, 2, 3, 4 and 6 h after L-DOPA/carbidopa administration. About 100 μL blood was drawn, collected in a heparin-coated tube, and plasma was obtained following rapid centrifugation and immediately frozen at −80°C until analysis. After the final blood collection, rats were euthanized with CO2 followed by decapitation. The striatal region of the brain was dissected, weighed, and kept at −80°C until analysis.
Plasma L-DOPA and its metabolites were measured using the method of Karimi et al., (2006) with minor modifications. Plasma samples (50 μL) from each rat were spiked with 5 μL internal standard (DHBA, 100 μM). To precipitate proteins, 20 μL of 1.2 M perchloric acid was added to each tube. The tubes were briefly vortexed, placed on ice for 10 min, and centrifuged for 4 min at 1250 g at 4°C. Thirty microliters of the supernatant were added to 60 μL potassium citrate buffer (0.2 M, pH 3.8) to precipitate perchlorate. After that, each tube was again vortexed for 1 min, left on ice for 10 min, centrifuged for 4 min at 1250 g at 4°C, and an aliquot of the supernatant was injected into HPLC for analysis of composition. Similarly, to determine the striatal 3-OMD and dopamine levels, the thawed brain tissues were homogenized using a PowerGen-700 homogenizer (Fisher Scientific, Pitsburgh, PA, USA) in 0.4 M perchloric acid, and the homogenates were centrifuged and filtered for HPLC analysis.
The HPLC system consisted of a Shimadzu pump (LC-10AT model), an electrochemical detector (Coulochem III, ESA Bioscience, Chelmsford, MA, USA), and a HR-80 C-18 reverse-phase column (3 μm, 80 × 4.6 mm, ESA Bioscience). The mobile phase consisted of 50 mM sodium phosphate, 1 mM sodium dodecyl sulfate, 0.67 μL triethylamine, 13.3 μM EDTA, and 8% acetonitrile in water, adjusted to pH 3.0 with phosphoric acid. Before use, the mobile phase was filtered through the 0.45-μm (pore size) filter (Millipore, Bedford, MA, USA), and degassed under vacuum. An isocratic elution at a flow rate of 1 mL/min was used. L-DOPA and its metabolites were identified by comparing their retention times with those of standard compounds. Concentrations were calculated with the aid of an internal standard (DHBA). Linearity of the detector responses were tested for all the compounds of interest, and the coefficients of correlation were >0.999. The relative standard deviation for the intra-day repeatability was well below 5%, representing good precision of the analytical method used.
For measuring the plasma levels of unconjugated (+)-catechin, (−)-epicatechin, and quercetin, a gas chromatography/mass spectrometry (GS/MS) method (Donovan et al., 1999; Loke et al., 2008) was adopted. Briefly, taxifolin (10 μg) was added to the collected plasma samples as an internal standard, and the plasma was extracted twice with ethyl acetate (1 mL). One microliter of transmethylated sample was injected into an Agilent gas chromatography (model-6890N) coupled with an Agilent mass spectrometer (model-5975B). A capillary column (HP5-MS, 30 m × 0.25 mm, 0.25 μm film thickness) was used, with helium as carrier gas. For elution, the column oven temperature was set at 180°C, ramped to 260°C at 4°C/min, then further ramped to 300°C at 5°C/min and held at 300°C for 5 min.
4.4.3. Animal experiment II – Determine the influence of carbidopa dose on the inhibitory efficacy of (+)-catechin on L-DOPA methylation
Rats were randomly divided into various experimental groups with no significant difference in average body weights. Rats were i.p. injected 400 mg/kg (+)-catechin 2 h before oral administration of L-DOPA/carbidopa (20/20 mg/kg). Control rats received the same i.p. and/or oral administrations of the corresponding vehicles only. Blood samples were collected from the tail vein with light anesthesia (isoflurane inhalation) at 0.5, 1, 2, 3, 4 and 6 h after L-DOPA + carbidopa administration. The plasma and striatal tissues were prepared and analyzed as described above in animal experiment I.
4.4.4. Animal experiment III – Determine the effect of (+)-catechin on L-DOPA methylation in dopamine-depleted rats
Rats were randomly divided into various experimental groups. To induce dopamine-depletion, reserpine (5 mg/kg) was solubilized in water containing lactic acid (1%, v/v) and given to rats (i.p.) 18-20 h before drug administration (Rivas et al., 1999). The reserpinized rats were then injected i.p. 400 mg/kg (+)-catechin 2 h before oral administration of L-DOPA/carbidopa (20/20 mg/kg). Control rats received the same i.p. and/or oral administrations of vehicles only. Animals were euthanized at2h after L-DOPA/carbidopa administration, and the plasma and striatal tissues were prepared and analyzed as described above in animal experiment I.
4.4.5. Animal experiment IV – Determine the protective effect of (+)-catechin on kainic acid-induced rat hippocampal injury
In these experiments, male Sprague-Dawley rats were randomly divided into multiple experimental groups, with very similar average body weight for each group. Animals were administered orally 100 or 400 mg/kg body weight of (+)-catechin 30 min before kainic acid injection. Kainic acid (1 μL of 1 mg/mL solution) was injected into the right lateral ventricle (anterior/posterior, −1.0; rostral, 1.6; dorsal/ventral, 4.5) using a microliter syringe under anesthesia with ketamine/xylazine (50/5 mg/kg, s.c.). The needle was withdrawn 5 min later and scalp was sutured. Control rats were injected with 1 μL of saline instead of kainic acid (He et al., 2006). Neuronal loss in the hippocampus (based on morphological evaluations) was analyzed at 24 h after kainic acid treatment. At the end of the experiment right before the brain tissue was collected for analysis, the animals received ketamine and xylazine (50 and 5 mg/kg, s.c.) for anesthesia, and then perfused with 0.1 M neutral phosphate-buffered formalin (4% final concentration of formaldehyde) via the ascending aorta, with the descending aorta clamped off.
The collected brain tissues were post-fixed overnight in 0.1 M neutral phosphate-buffered formalin (containing 4% formaldehyde). After cryoprotection in 30% sucrose/phosphate buffer, tissues were frozen in liquid nitrogen and sectioned serially (30 μm) through the entire brain. The sections were collected in 0.1 M neutral phosphate buffer, mounted on slides, then air-dried on a slide warmer at 50°C for at least 30 min, and stained with hematoxylin and eosin (H/E) for histopathological analysis.
Fluoro-Jade B staining was performed by following the protocols described by Schmued and Hopkins (2000) with minor modifications. Briefly, the slides were transferred to a solution of 0.06% potassium permanganate for 10 min. The staining solution was prepared from a 0.01% stock solution of Fluoro-Jade B that was made by adding 10 mg of the dye powder to 100 mL distilled water. After 20 min in the staining solution, slides were rinsed and placed on a slide warmer until they were fully dry. The cell bodies of Fluoro-Jade B-positive neurons were clearly visible. The number of stained neurons was counted in the obtained images using the Axiovision image analysis software (Carl Zeiss, Inc., Thornwood, NY). For analysis of the hippocampus, stained neurons of three sections from each rat were counted in the dorsal hippocampal region (between −1.5 and −2.5 mm from bregma).
Since glial cell activation, as evidenced by anti-glial fibrillary acidic protein (GFAP) upregulation, often was present in neuronal damage, tissue sections were also incubated successively with a rabbitmonoclonal anti-GFAP antibody (1:800, Sigma-aldrich), biotin-conjugated goat polyclonal anti-rabbit second antibody (1:250, Vector Laboratories, CA, USA), and horseradish peroxidase-conjugated avidin-biotin complex (Vector Laboratories). Sections were then exposed to the 3,3′-diaminobenzidine (DAB) substrate kit (Vector Laboratories) for detection. To perform quantitative analysis of GFAP immunostaining, 3-4 sections per animal were selected and images were captured and analyzed using the Axiovision image analysis software. One field (100 μm × 100 μm) in each slide within the midpoint of hippocampal CA3 regions were selected for quantification, and the intensity of GFAP immunoreactivity was evaluated by means of a relative optical density value (Georgievska et al., 2006).
4.5. Data analysis
Wherever possible, data were expressed as mean ± S.D. (standard deviation). Statistical significance was determined through analysis of variance (ANOVA) followed by a multiple comparison test with a Bonferroni adjustment. P values of less than 0.05 were considered statistically significant.
HIGHLIGHTS.
(+)-Catechin strongly inhibits L-DOPA methylation metabolism in vitro and in vivo.
(+)-Catechin also exerts a strong neuroprotective effect against oxidative damage in vitro and in vivo.
(+)-Catechin may be used as an adjuvant in the treatment of human Parkinson disease.
ACKNOWLEDGEMENTS
This study was supported in part by a grant from a grant from the National Institutes of Health (grant#ES 015242).
This study was supported, in part, by a grant from the National Institutes of Health (ES015242).
ABBREVIATIONS USED
- L-DOPA
Levodopa
- PD
Parkinson disease
- COMT
catechol-O-methyltransferase
- 3-OMD
3-O-methyldopa
- CNS
central nervous system
- H2-DCF-DA
2′,7′-dichlorofluorescein diacetate
- AdoMet
S-adenosyl-L-methionine
- ROS
reactive oxygen species
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- EGCG
epigallocatechin gallate
- GFAP
glial fibrillary acidic protein
Footnotes
CONFLICT OF INTEREST STATEMENT All the authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesia and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Bai H, Shim JY, Zhu BT. Biochemical and molecular modeling studies of the O-methylation of various endogenous and exogenous catechol substrates catalyzed by recombinant human soluble and membrane-bound catechol-O-methyltransferases. Chem. Res. Toxicol. 2007;20:1409–1425. doi: 10.1021/tx700174w. [DOI] [PubMed] [Google Scholar]
- Bartholini G, Pletscher A. Decarboxylase inhibitors. Pharmacol. Ther. 1975;21:407–421. doi: 10.1016/0306-039x(75)90047-1. [DOI] [PubMed] [Google Scholar]
- Blandini F, Nappi G, Fancellu R, Mangiagalli A, Samuele A, Riboldazzi G, Calandrella D, Pacchetti C, Bono G, Martignoni E. Modifications of plasma and platelet levels of L-DOPA and its direct metabolites during treatment with tolcapone or entacapone in patients with Parkinson’s disease. J. Neural. Transm. 2003;110:911–922. doi: 10.1007/s00702-003-0004-z. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson’s disease is associated with hippocampal atrophy. Mov. Disord. 2003;18:784–790. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- Catterall F, King LJ, Clifford MN, Ioannides C. Bioavailability of dietary doses of 3H-labelled tea antioxidants (+)-catechin and (−)-epicatechin in rat. Xenobiotica. 2003;33:743–753. doi: 10.1080/0049825031000108315. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Leger G, Guttman M. Reduction of circulating 3-O-methyldopa by inhibition of catechol-O-methyltransferase with OR-611 and OR-462 in Cynomolgus monkeys: implications for the treatment of Parkinson’s disease. Clin. Neuropharmacol. 1991;14:330–342. doi: 10.1097/00002826-199108000-00005. [DOI] [PubMed] [Google Scholar]
- Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997;25:1045–1050. [PubMed] [Google Scholar]
- Chen D, Wang CY, Lambert JD, Ai N, Welsh WJ, Yang CS. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochem. Pharmacol. 2005;69:1523–1531. doi: 10.1016/j.bcp.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Kang KS, Fukui M, Zhu BT. Critical role of the JNK-p53-GADD45α apoptotic cascade in mediating oxidative cytotoxicity in hippocampal neurons. Br. J. Pharmacol. 2011;162:175–192. doi: 10.1111/j.1476-5381.2010.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert FC. Pharmacological characteristics of tremor, rigidity and hypokinesia induced by reserpine in rat. Neuropharmacology. 1987;26:1431–1440. doi: 10.1016/0028-3908(87)90110-9. [DOI] [PubMed] [Google Scholar]
- Costa C, Sgobio C, Siliquini S, Tozzi A, Tantucci M, Ghiglieri V, Di Filippo M, Pendolino V, de Iure A, Marti M, Morari M, Spillantini MG, Latagliata EC, Pascucci T, Puglisi-Allegra S, Gardoni F, Di Luca M, Picconi B, Calabresi P. Mechanisms underlying the impairment of hippocampal long-term potentiation and memory in experimental Parkinson’s disease. Brain. 2012;135:1884–1899. doi: 10.1093/brain/aws101. [DOI] [PubMed] [Google Scholar]
- Da Prada M, Keller HH, Pieri L, Kettler R, Haefely WE. The pharmacology of Parkinson’s disease: basic aspects and recent advances. Experientia. 1984;40:1165–1304. doi: 10.1007/BF01946641. [DOI] [PubMed] [Google Scholar]
- Dawson L, Chadha A, Megalou M, Duty S. The group II metabotropic glutamate receptor agonist, DCG-IV, alleviates akinesia following intranigral or intraventricular administration in the reserpine-treated rat. Br. J. Pharmacol. 2000;129:541–546. doi: 10.1038/sj.bjp.0703105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denenberg VH, Kim DS, Palmiter RD. The role of dopamine in learning, memory, and performance of a water escape task. Behav. Brain Res. 2004;148:73–78. doi: 10.1016/s0166-4328(03)00183-9. [DOI] [PubMed] [Google Scholar]
- Donovan JL, Bell JR, Kasim-Karakas S, German JB, Walzem RL, Hansen RJ, Waterhouse AL. Catechin is present as metabolites in human plasma after consumption of red wine. J. Nutr. 1999;129:1662–1668. doi: 10.1093/jn/129.9.1662. [DOI] [PubMed] [Google Scholar]
- Eskow Jaunarajs KL, Dupre KB, Ostock CY, Button T, Deak T, Bishop C. Behavioral and neurochemical effects of chronic L-DOPA treatment on nonmotor sequelae in the hemiparkinsonian rat. Behav. Pharmacol. 2010;21:627–637. doi: 10.1097/FBP.0b013e32833e7e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. Levodopa in the treatment of Parkinson’s disease. J. Neural. Transm. 2006;71:1–15. doi: 10.1007/978-3-211-33328-0_1. [DOI] [PubMed] [Google Scholar]
- Floreani M, Skaper SD, Facci L, Lipartiti M, Giusti P. Melatonin maintains glutathione homeostasis in kainic acid-exposed rat brain tissues. FASEB J. 1997;11:1309–1315. doi: 10.1096/fasebj.11.14.9409550. [DOI] [PubMed] [Google Scholar]
- Ferruzzi MG, Lobo JK, Janle EM, Cooper B, Simon JE, Wu QL, Welch C, Ho L, Weaver C, Pasinetti GM. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: implications for treatment in Alzheimer’s disease. J. Alzheimers Dis. 2009;18:113–124. doi: 10.3233/JAD-2009-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein C, Serre F, Gavend M, Pellat J, Perret J, Tanche M. Plasma O-methyldopa in levodopa-induced dyskinesias. A bioclinical investigation. Acta Neurol. Scand. 1977;56:508–524. doi: 10.1111/j.1600-0404.1977.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Fukui M, Song JH, Choi JY, Choi HJ, Zhu BT. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur. J. Pharmacol. 2009;617:1–11. doi: 10.1016/j.ejphar.2009.06.059. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Carlsson T, Lacar B, Winkler C, Kirik D. Dissociation between short-term increased graft survival and long-term functional improvements in Parkinsonian rats overexpressing glial cell line-derived neurotrophic factor. Eur. J. Neurosci. 2004;20:3121–3130. doi: 10.1111/j.1460-9568.2004.03770.x. [DOI] [PubMed] [Google Scholar]
- Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Goetz CG. Influence of COMT inhibition on levodopa pharmacology and therapy. Neurology. 1998;50:S26–S30. doi: 10.1212/wnl.50.5_suppl_5.s26. [DOI] [PubMed] [Google Scholar]
- He X, Jenner AM, Ong WY, Farooqui AA, Patel SC. Lovastatin modulates increased cholesterol and oxysterol levels and has a neuroprotective effect on rat hippocampal neurons after kainate injury. J. Neuropathol. Exp. Neurol. 2006;65:652–663. doi: 10.1097/01.jnen.0000225906.82428.69. [DOI] [PubMed] [Google Scholar]
- Heikkinen H, Nutt JG, LeWitt PA, Koller WC, Gordin A. The effects of different repeated doses of entacapone on the pharmacokinetics of L-Dopa and on the clinical response to L-Dopa in Parkinson’s disease. Clin. Neuropharmacol. 2001;24:150–157. doi: 10.1097/00002826-200105000-00006. [DOI] [PubMed] [Google Scholar]
- Heikkinen H, Varhe A, Laine T, Puttonen J, Kela M, Kaakkola S, Reinikainen K. Entacapone improves the availability of L-dopa in plasma by decreasing its peripheral metabolism independent of L-dopa/carbidopa dose. Br. J. Clin. Pharmacol. 2002;54:363–371. doi: 10.1046/j.1365-2125.2002.01654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Bidel S, Jousilahti P, Antikainen R, Tuomilehto J. Coffee and tea consumption and the risk of Parkinson’s disease. Mov. Disord. 2007;22:2242–2248. doi: 10.1002/mds.21706. [DOI] [PubMed] [Google Scholar]
- Huot P, Lévesque M, Parent A. The fate of striatal dopaminergic neurons in Parkinson’s disease and Huntington’s chorea. Brain. 2007;130:222–232. doi: 10.1093/brain/awl332. [DOI] [PubMed] [Google Scholar]
- Kaakkola S, Wurtman RJ. Effects of catechol-O-methyltransferase inhibitors and L-3,4-dihydroxyphenylalanine with or without carbidopa on extracellular dopamine in rat striatum. J. Neurochem. 1993;60:137–144. doi: 10.1111/j.1471-4159.1993.tb05831.x. [DOI] [PubMed] [Google Scholar]
- Kandinov B, Giladi N, Korczyn AD. Smoking and tea consumption delay onset of Parkinson’s disease. Parkinsonism Relat. Disord. 2009;15:41–46. doi: 10.1016/j.parkreldis.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Kang KS, Wen Y, Yamabe N, Fukui M, Bishop SC, Zhu BT. Dual beneficial effects of (−)-epigallocatechin gallate on levodopa methylation and hippocampal neurodegeneration: in vitro and in vivo studies. PLoS One. 2010;5:e11951. doi: 10.1371/journal.pone.0011951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Carl JL, Loftin S, Perlmutter JS. Modified high-performance liquid chromatography with electrochemical detection method for plasma measurement of levedopa, 3-O-methyldopa, dopamine, carbidopa and 3,4-dihydroxyphenyl acetic acid. J. Chromatogr. B. 2006;836:120–123. doi: 10.1016/j.jchromb.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Keränen T, Gordin A, Harjola VP, Karlsson M, Korpela K, Pentikäinen PJ, Rita H, Seppälä L, Wikberg T. The effect of catechol-O-methyl transferase inhibition by entacapone on the pharmacokinetics and metabolism of levodopa in healthy volunteers. Clin. Neuropharmacol. 1993;16:145–56. doi: 10.1097/00002826-199304000-00007. [DOI] [PubMed] [Google Scholar]
- Koller WC, Hutton JT, Tolosa E, Capilldeo R. Immediate-release and controlled-release carbidopa/levodopa in PD: a 5 year randomized multicenter study. Neurology. 1999;53:1012–1019. doi: 10.1212/wnl.53.5.1012. [DOI] [PubMed] [Google Scholar]
- Leaver KR, Allbutt HN, Creber NJ, Kassiou M, Henderson JM. Oral pre-treatment with epigallocatechin gallate in 6-OHDA lesioned rats produces subtle symptomatic relief but not neuroprotection. Brain Res. Bull. 2009;80:397–402. doi: 10.1016/j.brainresbull.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Lee EY, Chen H, King J, Charlton C. The role of 3-O-methyldopa in the side effects of L-DOPA. Neurochem. Res. 2008;33:401–411. doi: 10.1007/s11064-007-9442-6. [DOI] [PubMed] [Google Scholar]
- Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, Croft KD. Pure dietary flavonoids quercetin and (−)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008;88:1018–1025. doi: 10.1093/ajcn/88.4.1018. [DOI] [PubMed] [Google Scholar]
- Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab. Dispos. 2003;31:572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- Manach C, Texier O, Morand C, Crespy V, Régérat F, Demigné C, Rémésy C. Comparison of the bioavailability of quercetin and catechin in rats. Free Radic. Biol. Med. 1999;27:1259–1266. doi: 10.1016/s0891-5849(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology and clinical efficacy of the new selective COMT inhibitors. Pharmacol. Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- Matsuoka Y, Kitamura Y, Okazaki M, Terai K, Taniguchi T. Kainic acid-induced activation of nuclear factor-kB in rat hippocampus. Exp. Brain Res. 1999;124:215–222. doi: 10.1007/s002210050616. [DOI] [PubMed] [Google Scholar]
- Matthews HB, Lucier GW, Fisher KD. Medicinal herbs in the United States: research needs. Environ. Health Perspect. 1999;107:773–778. doi: 10.1289/ehp.99107773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon YJ, Wang L, DiCenzo R, Morris ME. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008;29:205–217. doi: 10.1002/bdd.605. [DOI] [PubMed] [Google Scholar]
- Müller T. Levodopa/carbidopa and entacapone in the treatment of Parkinson’s disease: efficacy, safety and patient preference. Patient Prefer. Adherence. 2009;3:51–59. doi: 10.2147/ppa.s4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Conney AH, Zhu BT. Strong inhibitory effects of common tea catechins and bioflavonoids on the O-methylation of catechol estrogens catalyzed by human liver cytosolic catechol-O-methyltransferase. Drug Metab. Dispos. 2004;32:497–504. doi: 10.1124/dmd.32.5.497. [DOI] [PubMed] [Google Scholar]
- Nalder JV, Perry BW, Gentry C, Cotman CW. Degeneration of hippocampal CA3 pyramidal cells induced by intraventricular kainic acid. J. Comp. Neurol. 1980;192:333–359. doi: 10.1002/cne.901920209. [DOI] [PubMed] [Google Scholar]
- Padovani A, Costanzi C, Gilberti N, Borroni B. Parkinson’s disease and dementia. Neurol. Sci. 2006;27:S40–S43. doi: 10.1007/s10072-006-0546-6. [DOI] [PubMed] [Google Scholar]
- Post A, Holsboer F, Behl C. Induction of NF-κB activity during haloperidol-induced oxidative toxicity in clonal hippocampal cells: suppression of NF-κB and neuroprotection by antioxidants. J. Neurosci. 1998;18:8236–8246. doi: 10.1523/JNEUROSCI.18-20-08236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raches A, Fahn S. O-methyldopa interferes with striatal utilization of levodopa. Ann. Neurol. 1981;10:94–95. [Google Scholar]
- Riederer P, Wuketich S. Time course of nigrostriatal degeneration in parkinson’s disease. A detailed study of influential factors in human brain amine analysis. J. Neural. Transm. 1976;38:277–301. doi: 10.1007/BF01249445. [DOI] [PubMed] [Google Scholar]
- Rivas E, de Ceballos ML, Nieto O, Fontenla JA. In vivo effects of new inhibitors of catechol-O-methyl transferase. Br. J. Pharmacol. 1999;126:1667–1673. doi: 10.1038/sj.bjp.0702474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Ribnicky DM, Poulev A, Logendra S, Cefalu WT, Raskin I. A natural history of botanical therapeutics. Metabolism. 2008;57:S3–S9. doi: 10.1016/j.metabol.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Sêdek G, Jorga K, Schmitt M, Burns RS, Leese P. Effect of tolcapone on plasma levodopa concentrations after coadministration with levodopa/carbidopa to healthy volunteers. Clin. Neuropharmacol. 1997;20:531–541. doi: 10.1097/00002826-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Segovia G, Mora F, Crossman AR, Brotchie JM. Effects of CB1 cannabinoid receptor modulating compounds on the hyperkinesia induced by high-dose levodopa in the reserpine-treated rat model of Parkinson’s disease. Mov. Disord. 2003;18:138–149. doi: 10.1002/mds.10312. [DOI] [PubMed] [Google Scholar]
- Sharpless NS, Muenter MD, Tyce GM, Owen CA., Jr. 3-Methoxy-4-hydroxyphenylalanine (3-O-methyldopa) in plasma during oral L-dopa therapy of patients with Parkinson’s disease. Clin. Chim. Acta. 1972;37:359–369. doi: 10.1016/0009-8981(72)90456-1. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Jeong JH, Kim AY, Koh YH, Nah SY, Kim WK, Ko KH, Kim HJ, Wie MB, Kwon YS, Yoneda Y, Kim HC. Protection against kainate neurotoxicity by ginsenosides: attenuation of convulsive behavior, mitochondrial dysfunction, and oxidative stress. J. Neurosci. Res. 2009;15:710–722. doi: 10.1002/jnr.21880. [DOI] [PubMed] [Google Scholar]
- Tan S, Wood M, Maher P. Oxidative stress induces a form of programmed cell death with characteristics of both necrosis and apoptosis in neuronal cells. J. Neurochem. 1998;71:95–105. doi: 10.1046/j.1471-4159.1998.71010095.x. [DOI] [PubMed] [Google Scholar]
- Tedroff J, Pedersen M, Aquilonius SM, Hartvig P, Jacobsson G, Långström B. Levodopa-induced changes in synaptic dopamine in patients with Parkinson’s disease as measured by [11C]raclopride displacement and PET. Neurology. 1996;46:1430–1436. doi: 10.1212/wnl.46.5.1430. [DOI] [PubMed] [Google Scholar]
- Toulouse A, Sullivan AM. Progress in Parkinson’s disease-Where do we stand? Prog. Neurobiol. 2008;85:376–392. doi: 10.1016/j.pneurobio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Abe T, Kikuchi T, Takahashi S, Nozaki Y. The significance of3-O-methyldopa concentrations in the cerebrospinal fluid in the pathogenesis of wearing-off phenomenon in Parkinson’s disease. Neurosci. Lett. 1991;132:19–22. doi: 10.1016/0304-3940(91)90422-p. [DOI] [PubMed] [Google Scholar]
- van Duursen MB, Sanderson JT, de Jong PC, Kraaij M, van den Berg M. Phytochemicals inhibit catechol-O-methyltransferase activity in cytosolic fractions from healthy human mammary tissues: implications for catechol estrogen-induced DNA damage. Toxicol. Sci. 2004;81:316–324. doi: 10.1093/toxsci/kfh216. [DOI] [PubMed] [Google Scholar]
- Yamamoto M. Depression in Parkinson’s disease: its prevalence, diagnosis, and neurochemical background. J. Neurol. 2001;248:III5–III 11. doi: 10.1007/pl00022917. [DOI] [PubMed] [Google Scholar]
- Zhu BT, Patel UK, Cai MX, Conney AH. O-Methylation of tea polyphenols catalyzed by human placental cytosolic catechol-O-methyltransferase. Drug Metab. Dispos. 2000;28:1024–1030. [PubMed] [Google Scholar]
- Zhu BT. Catechol-O-methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: Importance in pathophysiology and pathogenesis. Curr. Drug Metab. 2002;3:321–349. doi: 10.2174/1389200023337586. [DOI] [PubMed] [Google Scholar]
- Zhu BT. CNS dopamine oxidation and catechol-O-methyltransferase (COMT): Importance in the etiology, pharmacotherapy, and dietary prevention of Parkinson’s disease. Int. J. Mol. Med. 2004;13:343–354. [PubMed] [Google Scholar]
- Zhu BT, Shim JY, Nagai M, Bai HW. Molecular mechanism for the high-potency inhibition of human catechol-O-methyltransferase by (−)-epigallocatechin-3-O-gallate. Xenobiotica. 2008;38:130–146. doi: 10.1080/00498250701744641. [DOI] [PubMed] [Google Scholar]