Abstract
Red blood cell alloimmunization is a major complication of transfusion therapy. Host immune markers that can predict antibody responders remain poorly described. As regulatory T cells (Tregs) play a role in alloimmunization in mouse models, we analyzed the Treg compartment of a cohort of chronically transfused patients with sickle cell disease (SCD, n = 22) and β-thalassemia major (n = 8) with and without alloantibodies. We found reduced Treg activity in alloantibody responders compared with nonresponders as seen in mice. Higher circulating anti-inflammatory IL-10 levels and lower IFN-γ levels were detected in non-alloimmunized SCD patients. Stimulated sorted CD4+ cells from half of the alloimmunized patients had increased frequency of IL-4 expression compared with nonresponders, indicating a skewed T helper (Th) 2 humoral immune response in a subgroup of antibody responders. All patients had increased Th17 responses, suggesting an underlying inflammatory state. Although small, our study indicates an altered immunoregulatory state in alloantibody responders which may help future identification of potential molecular risk factors for alloimmunization.
Introduction
Red blood cell (RBC) transfusions are often indicated to prevent and treat various complications of sickle cell disease (SCD). The majority of patients have received one or more transfusions by adulthood. Similarly, RBC transfusions remain the main treatment for severe thalassemia. A major complication of transfusion therapy is alloimmunization, which may result in life-threatening delayed hemolytic transfusion reactions in addition to difficulties in obtaining compatible blood for transfusion. Although extended phenotyping for regularly transfused patients has reduced alloimmunization rates in SCD patients [1,2], the cost to benefit ratio remains controversial, partly because not all patients develop alloantibodies [3]. Better characterization of host immunologic factors contributing to RBC alloimmunization [4-8] may help to identify molecular markers in alloantibody responders [9], allowing more cost-effective transfusion strategies [2]. CD4+ regulatory T cells (Tregs) characterized by coexpression of CD25 and FoxP3, are key regulators of immune responses, suppressing the activation and proliferation of multiple cell types including T cells, B cells, and dendritic cells [10]. Our data from mouse models indicate that Tregs are responsible for the magnitude and frequency of alloimmunization [6] and that responders have reduced Treg activity compared with nonresponders [11].
To determine if Tregs are similarly altered in human alloimmunized patients as seen in mice, we have now analyzed the Treg compartment in a cohort of regularly transfused alloimmunized and non-alloimmunized SCD and β-thalassemia major (TM) patients. We have also measured the T helper (Th) responses following nonantigen specific stimulation of sorted CD4+ population as well as some of the circulatory pro- and anti-inflammatory cytokine levels. Although small, our study indicates an altered immunoregulatory state in alloantibody responders, which may help future identification of molecular markers of alloimmunization.
Materials and Methods
Patient population
All the studies were approved by the institutional Review Boards of the New York Blood Center and the Columbia University Medical Center. We studied 22 patients, homozygous for hemoglobin S, receiving either exchange (n = 10) or simple (n = 12) monthly transfusions of leukoreduced units, matched for Kell and Rh antigens for at least 2 years before the study. Ten (seven on simple transfusions and three on exchange transfusions) had a history of alloimmunization (responders) [12]. The antibody responders with SCD consisted of six females and four males with 4/10 teens aged 13, 16, 17, and 19 years old and the rest >20 years of age. A total of 3/10 of the alloimmunized SCD patients were splenectomized. The specificities of alloantibodies in responders included anti-E, -K, -C, -Fya, -Fyb, -S, -VS, and -M. Detectable alloantibodies at the time of the blood collection for the study were only present in 3/10 patients. All patients had a history of having made more than one alloantibody, and in four cases, the patients had also made autoantibodies. The non-alloimmunized patients with SCD consisted of eight males and four females with 4/12 in their teens, two aged 15, one aged 16 and one 17 and the rest >20 years of age. A total of 3/12 of the non-alloimmunized SCD patients were splenectomized. None of the patients with SCD were on hydroxyurea treatment at the time of the study. The estimated total numbers of transfused units for all except two patients was more than 100 units. The exception included two cases (one alloimmunized and one non-alloimmunized) who had received about 50 units. Overall, there were roughly equal numbers of sickle patients in the alloimmunized vs. non-alloimmunized groups who were splenectomized, on exchange vs. simple transfusions, on iron chelation (see below) and similarly exposed to allo-sensitizing events. We also analyzed eight regularly transfused (every 3–4 week), alloantibody-negative TM patients, also receiving leukoreduced blood. The transfused patients with TM consisted of five males and three females, all >20 years old and all alloantibody-negative and splenectomized. All patients on simple transfusions were on iron chelation using deferasirox. Blood drawn just before simple transfusion or from the discard bag following exchange transfusion was used for the studies. Race-matched healthy volunteers were recruited after obtaining consent, consisting of African Americans and Hispanics used as controls for patients with SCD and Caucasians and Asians used as controls for patients with TM.
Foxp3 expression analysis and proliferation/Treg suppression assay
For Treg enumeration, freshly collected blood was stained with anti-CD4, −CD25 (BD Biosciences, San Jose, CA), and anti-Foxp3 (clone PCH101 and an isotype control, eBiosciences, San Diego, CA) and analyzed by BD FACSCanto using Diva software (BD, Franklin Lakes, NJ). To assess Treg suppression, CD4+ cells were first enriched by positive selection (Miltenyi Biotech, Auburn, CA) from buffy coats, followed by separation of CD4+CD25hi and CD4+CD25− T cells (94% purity) on a MoFLo cell sorter (Beckman-Coulter, Hialeah, FL). Sorted CD4+CD25− effector cells (5 × 104) were cultured alone or in combination with autologous sorted CD4+CD25hi Tregs in either duplicates or triplicates at various effector to Treg cell ratios and stimulated using Treg Suppression Inspector (Miltenyi Biotec). On Day 4 of culture, 1 μCi of 3H thymidine (Perkin Elmer, Shelton CT) was added, incubated for an additional 16 hr, and the uptake of labeled thymidine was measured by liquid scintillation (PerkinElmer, Waltham, MA). Percent proliferation was determined as (cpm incorporated in the coculture)/cpm of CD4+CD25− cells alone) × 100.
Cytokine analysis
Intracellular cytokine staining was done on CD4+CD25− sorted T cells stimulated for 5 hr with PMA (50 ng/ml) and ionomycin (1 μM) in the presence of brefeldin A (1 μg/ml, BD, San Jose, CA) followed by fixation and permeabilization (Cytofix/Cytoperm, BD Biosciences) and analyzed by flow cytometry. For circulatory cytokine measurements, platelet-poor plasma was prepared [13] from samples on simple transfusions only and analyzed for TGF-β1 (Quantikine ELISA kit, R&D Systems, Minneapolis, MN) and IL-2, IL-6, IL-4, TNF-a, IFN-g, IL-17, and IL-10 (Cytometric Bead Array, BD Biosciences). Plasma cytokine levels of exchange transfusion patients were not measured because of the dilution effect of anticoagulant solutions present in collection bags containing the exchanged units which prevented accurate analysis of cytokine levels.
Statistical analysis
Statistical analyses were performed using Student’s t-test (Treg frequency, cytokine measurements) and 2-way ANOVA for repeated measurements (Treg assay). Differences were considered significant at P < 0.05.
Results
Treg frequency and activity in transfused patients
To determine whether Treg frequency and activity vary between alloimmunized and non-alloimmunized patients, we compared the peripheral Treg compartment of a cohort of regularly transfused subjects. Consistent with previous studies [14,15], the CD4+ frequency was significantly reduced in all the transfused populations regardless of alloimmunization status (Fig. 1A). We found no differences in Foxp3 levels (Fig. 1B,C) or Treg frequencies (Fig. 1D,E) between the transfused patient groups and healthy controls (all P > 0.1). We next compared the functional activity of CD4+CD25hi Tregs by purifying and measuring their ability to suppress proliferation of autologous purified CD4+CD25− cells. The proliferation capacity of CD4+CD25− T cells from alloimmunized and non-alloimmunized SCD patients was not statistically different (30,568 ± 7,682 cpm vs. 22,958 ± 4,058 cpm, P= 0.4) and comparable with race-matched healthy controls (30,191 ± 5,568 cpm). Similarly, CD4+CD25− T cells from non-alloimmunized TM patients and race-matched controls were equally proliferative (10,830± 1,780 cpm vs. 11,890± 2,099 cpm). Purified CD4+CD25hi Tregs from patients and controls, when cultured alone, were equally characteristically anergic [10] (data not shown). However, in cocultures of CD4+CD25hi Tregs and CD4+CD25− T effector cells, proliferation rates were lower in non-alloimmunized SCD or TM patients compared with those of alloimmunized sickle patients (Fig. 2, P < 0.05). These data indicate that CD4+CD25hi Tregs from non-alloimmunized SCD or TM patients can suppress proliferation of autologous CD4+CD25− cells more effectively than Tregs from alloimmunized SCD patients, suggesting that Treg activity is reduced in responders compared with nonresponders, as we have previously described in our mouse model [11].
Figure 1.
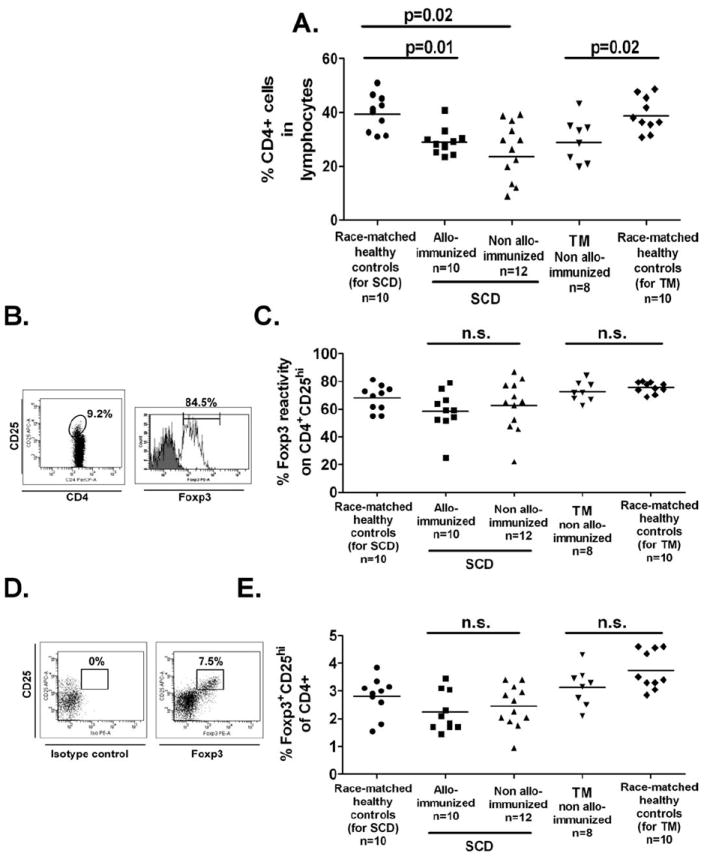
Phenotypic analysis of Treg compartment in chronically transfused SCD and TM patients. (A) For Treg enumeration, CD4+ lymphocyte population in peripheral blood was analyzed by flow cytometry and are shown for transfused SCD patients with (squares, n = 12) or without (triangles, n = 10) alloantibodies, and transfused non-alloimmunized TM patients (upside-down triangles, n = 8). Race-matched African American and Hispanic (circles, n = 10 for SCD), and Caucasian and Asian (diamonds, n = 10 for TM) volunteers constituted the healthy control groups. Statistically significant differences between groups are indicated by P values. (B) Foxp3 expression levels with respect to the antibody isotype control in the CD4+CD25hi cell population as defined by the gating strategy were measured and (C) are shown for each of the patient and control groups. Similarly, (D) frequencies of Foxp3+CD25hi of the CD4+ T-cell population were analyzed based on the Foxp3 isotype control and (E) are shown for patient and control groups. Differences between groups were not statistically significant (“n.s.”).
Figure 2.
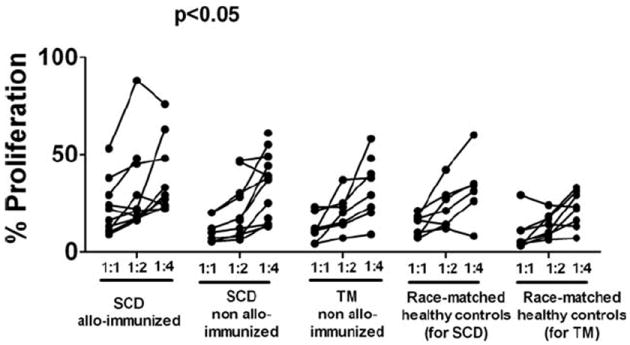
Functional activity of circulating Tregs in chronically transfused SCD and TM patients. Proliferation of sorted CD4+CD25− T effector cells cocultured with autologous sorted CD4+CD25hi Tregs at 1:1, 1:2, and 1:4 (Treg: CD4+CD25−) ratios following stimulation using microbeads precoupled with anti-CD2, anti-CD3, and anti-CD28 (Treg Suppression Inspector) is shown as percentages of CD4+CD25− T cell proliferation when cultured alone (see Materials and Methods section). For some samples, we were unable to obtain sufficient numbers of sorted Tregs, and in those cases, the cocultures were performed at only one ratio of Treg: CD4+CD25−. The average percent proliferation values for alloimmunized transfused SCD patients (n = 12) were significantly higher, when compared with that of non-alloimmunized (SCD patients, n = 10 and TM patients, n = 8, both P < 0.05), indicating that the alloimmunized Tregs are less suppressive. The relative proliferation values of race-matched healthy controls (n = 10 for each of the groups) are also shown.
Th immune responses in transfused cohort
Classically, Th1 cells, characterized by IFN-γ production, regulate cellular immunity, whereas Th2 cells with signature IL-4 secretion regulate humoral immunity [16]. The recently identified Th17 cells, defined by expression of IL-17, promote inflammation and mediate extracellular bacteria clearance [17]. To determine whether the responder state correlated with altered Th subsets, we analyzed the functional phenotype of sorted CD4+ T cells following nonantigen specific stimulation with PMA and ionomycin. Higher proportion of IL-4+CD4+ cells were detected in 5/10 alloimmunized SCD patients compared with non-alloimmunized patients with SCD (Fig. 3A, P = 0.04) or TM (Fig. 3A, P = 0.01), indicating a skewed Th2 response in a subgroup of the antibody responders. Interestingly, the five alloimmunized patients with increased Th2 responses had lower proliferation rate and therefore better Treg activity at 1:1 ratio of Tregs:T effector cells compared with the five alloimmunized patients with lower Th2 responses (Fig. 3B). Although differences in Th1 and Th17 between alloimmunized and non-alloimmunized patients were not statistically significant, IL-17+CD4+, but not IFN-γ+CD4+, cell frequencies were higher in our transfused SCD and TM cohorts compared with healthy race-matched controls (Fig. 3C,D).
Figure 3.
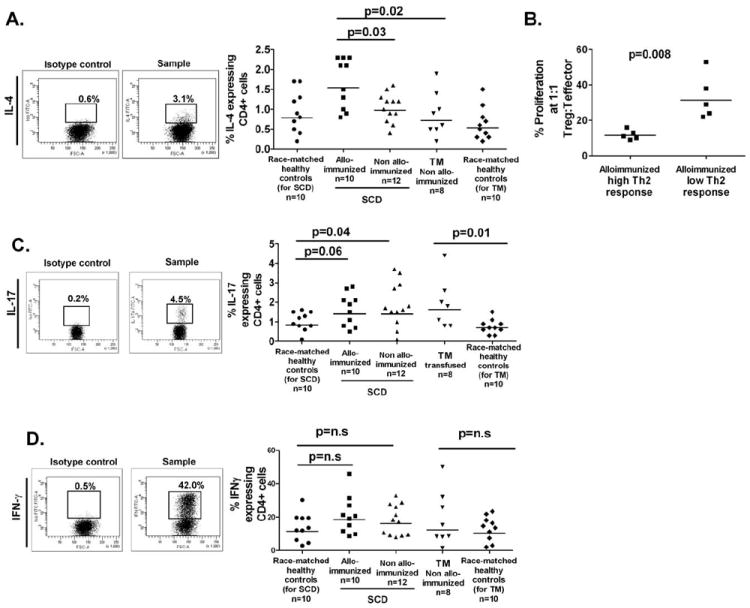
Th1/2/17 subset frequency following stimulation of sorted CD4+ cells. (A) Sorted CD4+CD25− T cell populations from patients and race-matched controls were stimulated with PMA/ionomycin (5 hr) in presence of the Golgi inhibitor and the frequencies of CD4+ cells expressing intracellular IL-4, were measured by flow cytometry according to the gating strategy shown. In alloimmunized sickle patients, a subgroup of patients (5/10) with higher frequency of IL-4 expressing CD4 cells was detected. (B) The Treg activity as measured by proliferation in cocultures at 1:1 ratio of Tregs:T effectors cells relative to effector cells alone are shown for the subgroup of alloimmunized sickle patients with either higher frequency of IL-4 expressing CD4+ cells (“alloimmunized high Th2 response”), when compared with the rest of the alloimmunized sickle patients (“alloimmunized low Th2 response”). The subgroup with higher Th2 responses had lower proliferation frequency and therefore better Treg activity. (C) The gating strategy and frequencies of stimulated CD4+ sorted cells expressing IL-17 and (D) IFN-γ are shown for each group. Statistical significant differences are indicated by P values (“n.s.,” not significant).
Circulatory cytokine levels in transfused population
We next measured various pro- and anti-inflammatory plasma cytokine levels. For these studies, we restricted our analysis to those patients who had been on simple transfusions because the plasma from patients on exchange transfusions was diluted with the anticoagulant solution present in the collection bags containing the exchanged units which prevented accurate measurements of plasma cytokine levels. Elevated circulating TGF-β and IL-6 levels were found in both alloimmunized and non-alloimmunized transfused patients, being significantly different from controls (Fig. 4A,B) as has been previously reported [18,19]. We found increased levels of IL-10 in non-alloimmunized patients with SCD, when compared with alloimmunized patients (Fig. 4C, P = 0.02), whereas increased levels of IFN-γ were found in alloimmunized, when compared with non-alloimmunized group (Fig. 4D, P = 0.03). As we did not detect higher frequency of CD4+IFN-γ+ cells in alloimmunized patients (Fig. 3D), the increased plasma IFN-γ levels does not reflect pure Th function but is likely to be due to increased secretions by CD8 and monocytes. There were no significant differences among groups for the other cytokines examined (IL-2, IL-4, IL-17, and TNF-α, data not shown).
Figure 4.
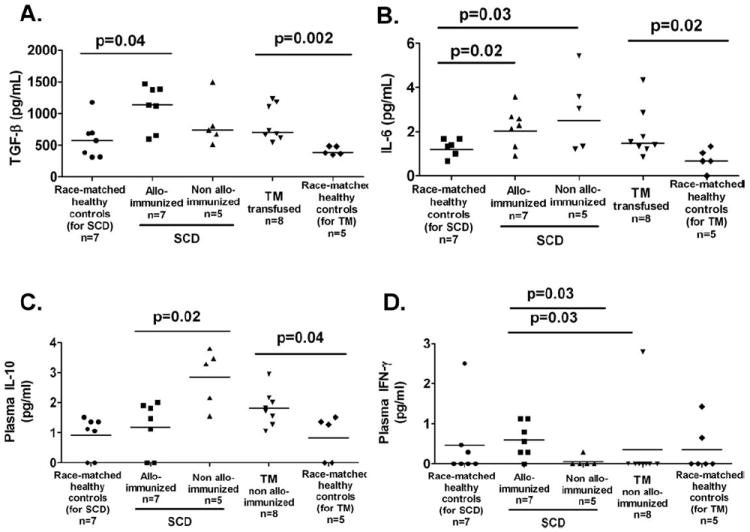
Plasma cytokine expression patterns in transfused patients with SCD and TM. The analysis of plasma cytokine measurements in patients on exchange transfusions were excluded because the anticoagulant solution present in the collection bags containing the exchanged units diluted the plasma. The cytokine measurements were therefore restricted to those patients who had been on simple transfusions only. (A) Circulating levels of TGF-β1 were measured by ELISA using platelet poor plasma from patients on simple transfusions. Platelet poor plasma was also prepared from some of the healthy controls analyzed. Elevated levels were detected in the transfused patient groups, when compared with race-matched controls as indicated by the P values. Platelet poor plasma from the same samples as in A were used to simultaneously measure levels of circulating IL-6 (B) and IL-10 (C) and (D) IFN-γ using a cytometric bead assay (CBA). IL-6 levels were significantly higher in transfused populations relative to race-matched healthy controls. IL-10 and IFN-γ levels were higher in alloimmunized, when compared with non-alloimmunized patients with SCD (P = 0.02, and P = 0.03, respectively). We did not find significant differences among the different groups for the other cytokines that were tested using the CBA (IL-2, IL-4, TNF-α, and IL-17, data not shown).
Discussion
In this small study of chronically transfused patients with SCD and thalassemia, we have found reduced peripheral Treg function in transfused antibody responders compared with nonresponders similar to what we previously reported in mice [11]. Furthermore, increased frequency of IL-4 expressing CD4+ cells was detected in a subset of alloimmunized patients, consistent with skewed Th2 humoral responses in this subgroup. At this time, it is unclear what distinguishes the two subgroups of alloimmunized patients. The elevated Th2 response is not likely to be due to altered Treg activity as we found greater Treg activity in this subgroup compared with the alloimmunized group with lower Th2 responses (Fig. 3B). The study had very small numbers to determine if this could be related to either iron overload or its treatment. Three of the five patients with increased Th2 responses received exchange transfusions and were not on iron chelation (deferasirox), while none of the five patients on simple transfusions (all of whom were on the iron chelator, deferasirox) had such increased Th2 responses. However, it should be noted that in non-alloimmunized patients, the frequencies of IL-4 expressing CD4+ cells in nonresponder sickle subgroup on simple (n = 5) vs. exchange transfusions (n = 5) were not different (0.94 ± 0.18%, vs. 1.15 ± 0.12%, P = 0.3). Future studies using larger cohorts are needed to determine whether iron overload or the use of deferasirox have an effect on altering Th2 responses in alloimmunized patients.
We found reduced levels of CD4+ T cell frequency in our cohort regardless of alloimmunization status. The exact cause for lower CD4+ in our cohort remains unknown, but it may be part of the sickle/thalassemia immune pathology or as a result of immune response to repeated transfusion. Indeed, reduced frequency helper T cells have been previously reported in patients with SCD [20-22] and with thalassemia major [23], although blood transfusions have also been associated with reduced CD4+/CD8+ ratios [14,15].
TGF-β in the presence of inflammatory cytokines including IL-6 promotes human Th17 differentiation [24]. Statistically significant increases in IL-17 expressing CD4+ cells as well as circulating TGF-β and IL-6 levels were found in our transfused patients regardless of alloimmunization status, although we did not find elevated levels of circulating IL-17 (data not shown) which may be partially due to detection limits of our assay. TGF-β and IL-6 have been reported to be increased not only in transfused patients [18,19] but also in nontransfused patients with SCD [25] and splenectomized TM patients [26], indicating that the underlying disease pathology is probably responsible for the increased levels of these cytokines. It remains to be determined if the pro-inflammatory Th17 frequencies are also elevated in nontransfused patients, which would be consistent with the described pro-inflammatory states in these diseases [27,28].
The cellular subset producing IL-10, which include T and B cells and monocytes/macrophages [29], in our patient cohort remains to be identified, although monocytes/macrophages, known to be activated in patients with SCD [30], are possible candidates. IL-10 is a Th2 cytokine with stimulatory effects on B cell differentiation and antibody production but also possesses anti-inflammatory activity, inhibiting IFN-γ production of T cells and antigen presentation by macrophages [29]. As we found higher levels of circulating IL-10 but reduced plasma levels of IFN-γ in non-alloimmunized compared with alloimunized sickle patients, we speculate that IL-10 may be playing a role in disabling antigen presentation/T cell activation [29] in non-alloimmunized SCD patients. Future studies are needed to directly address this hypothesis.
Despite our small patient cohort and lack of data on the untransfused patient counterparts, our findings indicate altered immunoregulation as evidenced by reduced Treg activity or increased Th2 responses in antibody responders. If confirmed in larger cohorts, this information may help in identification of T cell associated molecular markers that may predict responders in advance, thereby enabling design of preventative strategies to reduce alloimmunization-associated morbidity and mortality.
Acknowledgments
Contract grant sponsor: National Heart, Lung, and Blood Institute; Contract grant number: R21HL097350.
The authors are grateful to Genia Billote (CUMC) with help with obtaining IRB approval for the studies at the Columbia University Medical Center and performing some of the clinical analysis; to Maureen Licursi (CUMC) for obtaining consent, and coordinating patient sample collection and clinical data analysis and to Hannah Stephens (NYPH/ CUMC) for coordinating the exchange transfusion samples. They thank Dr. Susanne Heck (NYBC), Dr. Wu He (NYBC), Peter Lopez (NYU), and Michael Gregory (NYU) for performing the cell sorting.
Footnotes
Author Contributions
W.B., H.Z., and X.L. performed research, analyzed and interpreted data, and edited the article; M.T.L. recruited the sickle cell patients, performed clinical data analysis, and edited the article; J.S. coordinated the exchange transfusion samples from sickle patients and edited the article; S.S. recruited the TM patients, performed clinical data analysis, and edited the article and was the PI for the IRB study at the Columbia University Medical Center; K.Y. designed, directed, performed data analysis, and wrote the article.
Conflict of interest: Nothing to report
References
- 1.Vichinsky EP, Luban NL, Wright E, et al. Prospective RBC phenotype matching in a stroke-prevention trial in sickle cell anemia: A multicenter transfusion trial. Transfusion. 2001;41:1086–1092. doi: 10.1046/j.1537-2995.2001.41091086.x. [DOI] [PubMed] [Google Scholar]
- 2.Josephson CD, Su LL, Hillyer KL, Hillyer CD. Transfusion in the patient with sickle cell disease: A critical review of the literature and transfusion guidelines. Transfus Med Rev. 2007;21:118–133. doi: 10.1016/j.tmrv.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 3.King KE, Shirey RS. Transfusion management of patients with sickle cell disease: The continuing dilemma. Transfusion. 2010;50:2–4. doi: 10.1111/j.1537-2995.2009.02527.x. [DOI] [PubMed] [Google Scholar]
- 4.Bauer MP, Wiersum-Osselton J, Schipperus M, et al. Clinical predictors of alloimmunization after red blood cell transfusion. Transfusion. 2007;47:2066–2071. doi: 10.1111/j.1537-2995.2007.01433.x. [DOI] [PubMed] [Google Scholar]
- 5.Hendrickson JE, Desmarets M, Deshpande SS, et al. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46:1526–1536. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Heck S, Yazdanbakhsh K. Prevention of red cell alloimmunization by CD25 regulatory T cells in mouse models. Am J Hematol. 2007;82:691–696. doi: 10.1002/ajh.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noizat-Pirenne F, Tournamille C, Bierling P, et al. Relative immunogenicity of Fya and K antigens in a Caucasian population, based on HLA class II restriction analysis. Transfusion. 2006;46:1328–1333. doi: 10.1111/j.1537-2995.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoppe C, Klitz W, Vichinsky E, Styles L. HLA type and risk of alloimmunization in sickle cell disease. Am J Hematol. 2009;84:462–464. doi: 10.1002/ajh.21442. [DOI] [PubMed] [Google Scholar]
- 9.Zimring JC, Welniak L, Semple JW, et al. Current problems and future directions of transfusion-induced alloimmunization: Summary of an NHLBI working group. Transfusion. 2011;51:435–441. doi: 10.1111/j.1537-2995.2010.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 11.Bao W, Yu J, Heck S, Yazdanbakhsh K. Regulatory T-cell status in red cell alloimmunized responder and nonresponder mice. Blood. 2009;113:5624–5627. doi: 10.1182/blood-2008-12-193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: Evidence for a distinct population of immunologic responders. Blood. 2008;112:2546–2553. doi: 10.1182/blood-2008-03-146415. [DOI] [PubMed] [Google Scholar]
- 13.Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116:4639–4645. doi: 10.1182/blood-2010-04-281717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan J, Sarnaik S, Gitlin J, Lusher J. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984;64:308–310. [PubMed] [Google Scholar]
- 15.Kessler CM, Schulof RS, Goldstein AL, et al. Abnormal T-lymphocyte subpopulations associated with transfusion of blood-derived products. Lancet. 1983;1:991–992. doi: 10.1016/s0140-6736(83)92116-5. [DOI] [PubMed] [Google Scholar]
- 16.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 17.Dong C. TH17 cells in development: An updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 18.Walter PB, Fung EB, Killilea DW, et al. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254–263. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter PB, Macklin EA, Porter J, et al. Inflammation and oxidant-stress in beta-thalassemia patients treated with iron chelators deferasirox ICL670. or deferoxamine: An ancillary study of the Novartis CICL670A0107 trial. Haematologica. 2008;93:817–825. doi: 10.3324/haematol.11755. [DOI] [PubMed] [Google Scholar]
- 20.Adedeji MO. Lymphocyte subpopulations in homozygous sickle cell anaemia. Acta Haematol. 1985;74:10–13. doi: 10.1159/000206155. [DOI] [PubMed] [Google Scholar]
- 21.Kaaba SA, al Harbi SA. Reduced levels of CD2+ cells and T-cell subsets in patients with sickle cell anaemia. Immunol Lett. 1993;37:77–81. doi: 10.1016/0165-2478(93)90135-o. [DOI] [PubMed] [Google Scholar]
- 22.Koffi KG, Sawadogo D, Meite M, et al. Reduced levels of T-cell subsets CD4+ and CD8+ in homozygous sickle cell anaemia patients with splenic defects. Hematol J. 2003;4:363–365. doi: 10.1038/sj.thj.6200310. [DOI] [PubMed] [Google Scholar]
- 23.Neri A, Brugiatelli M, Iacopino P, Callea V, Ronco F. Natural killer cell activity and T subpopulations in thalassemia major. Acta Haematol. 1984;71:263–269. doi: 10.1159/000206598. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human TH.17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Croizat H, Nagel RL. Circulating cytokines response and the level of erythropoiesis in sickle cell anemia. Am J Hematol. 1999;60:105–115. doi: 10.1002/(sici)1096-8652(199902)60:2<105::aid-ajh4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Moshtaghi-Kashanian GR, Gholamhoseinian A, Hoseinimoghadam A, Rajabalian S. Splenectomy changes the pattern of cytokine production in beta-thalassemic patients. Cytokine. 2006;35:253–257. doi: 10.1016/j.cyto.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Rachmilewitz EA, Shohet SB, Lubin BH. Lipid membrane peroxidation in beta-thalassemia major. Blood. 1976;47:495–505. [PubMed] [Google Scholar]
- 28.Platt OS. Sickle cell anemia as an inflammatory disease. J Clin Invest. 2000;106:337–338. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore KW, de Waal MR, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Ann Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 30.Belcher JD, Marker PH, Weber JP, et al. Activated monocytes in sickle cell disease: Potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96:2451–2459. [PubMed] [Google Scholar]


