Abstract
Objectives
To evaluate synovitis (clinical vs ultrasound (US)) to predict structural progression in rheumatoid arthritis (RA).
Methods
Patients with RA.
Study design
Prospective, 2-year follow-up.
Data collected
Synovitis (32 joints (2 wrists, 10 metacarpophalangeal, 10 proximal interphalangeal, 10 metatarsophalangeal)) at baseline and after 4 months of therapy by clinical, US grey scale (GS-US) and power doppler (PD-US); x-rays at baseline and at year 2.
Analysis
Measures of association (OR) were tested between structural deterioration and the presence of baseline synovitis, or its persistence, after 4 months of therapy using generalised estimating equation analysis.
Results
Structural deterioration was observed in 9% of the 1888 evaluated joints in 59 patients. Baseline synovitis increased the risk of structural progression: OR=2.01 (1.36–2.98) p<0.001 versus 1.61 (1.06–2.45) p=0.026 versus 1.75 (1.18–2.58) p=0.005 for the clinical versus US-GS versus US-PD evaluation, respectively. In the joints with normal baseline examination (clinical or US), an increased probability for structural progression in the presence of synovitis for the other modality was also observed (OR=2.16 (1.16–4.02) p=0.015 and 3.50 (1.77–6.95) p<0.001 for US-GS and US-PD and 2.79 (1.35–5.76) p=0.002) for clinical examination. Persistent (vs disappearance) synovitis after 4 months of therapy was also predictive of subsequent structural progression.
Conclusions
This study confirms the validity of synovitis for predicting subsequent structural deterioration irrespective of the modality of examination of joints, but also suggests that both clinical and ultrasonographic examinations may be relevant to optimally evaluate the risk of subsequent structural deterioration.
Introduction
In rheumatoid arthritis (RA), structural damage is associated with irreversible pain and functional impairments.1–3 The presence of synovitis (mainly the number of swollen joints at physical examination) has been recognised as one of the most important predisposing factor of subsequent structural damage.4–6
The current objective of RA treatment is to improve patient symptoms such as pain, functional impairment and fatigue, and to prevent subsequent damage. The intensity of patients' symptoms is correlated with the level of inflammation, but at a lower magnitude than objective signs of inflammation, such as the number of swollen joints. Such a switch in the main aim of the current indication of drugs in RA (ie, prevention vs improvement) should result/has resulted in changes when initiating/intensifying disease modifying antirheumatic drug therapy in daily practice.7 8 In particular, specific attention should be given to the presence of synovitis.
Synovitis is usually evaluated via a physical examination.9 During the last decade, ultrasound (US) evaluation of synovitis has demonstrated its superiority versus physical examination in terms of face validity and reliability.10–13 Because the main interest of the clinician in evaluating synovitis is to understand the level of risk of the patient with respect to subsequent structural deterioration, specific attention has to be given to the capacity for US evaluation of synovitis to predict subsequent progression of structural damage.
Several longitudinal studies have demonstrated a link between the presence of synovitis detected by US at a certain point in time and subsequent structural deterioration. However, most of these studies have considered these two variables (eg, synovitis and structural damage) at the patient level, evaluating the correlation between the number of synovitic joints at a certain time-point and the subsequent structural deterioration using a radiological scoring system including multiple and often different joints, such as those included in the modified Sharp Score,14–18 and only a few of them have evaluated this correlation at the level of the joints.19 20
Most of these studies have not compared the magnitude of this link or its predictive validity with regard to the particular modality of evaluation of joints (eg, clinical examination vs US modality). Moreover, most of these studies have not evaluated whether the disappearance of synovitis after treatment in the short term prevents subsequent structural deterioration.
This context prompted us to conduct a 2-year longitudinal study aimed at evaluating the capacity of synovitis to predict subsequent radiological damage with respect to the modality of examination of joints in patients with RA.
Patients-methods
Study design
The study comprised two phases: the first was a prospective, multicentre, international 4-month-duration follow-up of patients with RA requiring a tumour necrosis factor (TNF)-blocker according to the opinion of their treating rheumatologist. The results of this study aimed at evaluating psychometric properties (including reliability, external validity, sensitivity to change and discriminant capacity) of different synovitis scoring systems has been previously reported.21 The second part of the study consisted in a radiological evaluation (hands and feet) 2 years after the inclusion of the patients in the study.
This study was approved by the appropriate ethical committees. All patients gave their written informed consent before enrolling for the study.
Patients
Adult patients fulfilling the 1987 American College of Rheumatology criteria for RA22 were eligible for the study if, in the opinion of the investigators, he/she required a TNF-blocker therapy. The minimum level of disease activity was defined by the number of swollen joints at physical examination ≥6.
Centres
To be eligible, the centres had to fulfil the following criteria: (1) a sonographer (either a radiologist or a rheumatologist) with experience (at least 70 different examinations) in evaluating synovitis; (2) a clinician (either a rheumatologist or a research nurse) with experience in clinical metrology in RA; (3) independent examination of synovitis (eg, for a single patient, the sonographer could not have access to the results of the clinical examination and vice versa); (4) monitoring of the patient during the 4-month period of study by the same investigators (eg, a single sonographer and a single clinician for each individual patient over the 4-month period of the study).
Collected data
Thirty-two joints were systematically evaluated (clinical and ultrasonographic evaluation before (baseline) and after 4 months of TNF-blockers therapy, radiological evaluation at baseline, and after 2 years of follow-up).
The evaluated joints included the metacarpophalangeal (MCP) (×10), the proximal interphalangeal (PIP) (×10), the wrists (×2) and the metatarsophalangeal (MTP) (×10) joints. For each joint, the clinical scoring system was a semiquantitative variable (eg, 0=definitely no synovitis, 1=doubtful, 2=yes moderate, 3=yes obvious and important).
The US evaluation was performed in a darkened room. Systematic multiplanar grey-scale (GS) and power doppler (PD) examinations were carried out with commercially available real-time scanners (eg, ESAOTE Technos MPX, ESAOTE MyLab, TOSHIBA APLIO, PHILIPS HD11, BK Mini Focus) using multifrequency linear transducers (7–12 MHz). Ultrasonography scanning techniques, GS and PD machine settings, and definitions of abnormality were standardised among investigators prior to the study during a 1.5-day meeting.23 It has to be noted that the fingers were evaluated only at the dorsal side. The US scanning scoring method has been described previously.24–29 Synovitis was defined according to the Outcome Measures in Rheumatology Clinical Trials published definitions.26 27 30 31 Both a GS and a PD examination were recorded for each joint.
GS synovitis scoring was evaluated using a 4-grade scale from 0 to 3 with the following subjective definitions for each category:
grade 0 = absence of synovial thickening;
grade 1 = mild synovial thickening;
grade 2 = moderate synovial thickening;
grade 3 = marked synovial thickening.
PD synovitis scoring was evaluated also using a 4-grade scale from 0 to 3 with the following definition for each category:
grade 0 = absence of signal, no intra-articular flow;
grade 1 = mild, one or two vessels signal (including one confluent vessel) for small joints and two to three signals for large joints (including two confluent vessels);
grade 2 = moderate confluent vessels (>grade 1) and less than 50% of normal area;
grade 3 = marked vessels' signals in more than half the synovial area.
The radiological evaluation (plain x-rays of hands, wrists and feet) was performed by a single experienced reader (VD),32–36 aware of the chronologies of the films but unaware of the clinical and US findings.
For each of the 32 joints, and for each-time point (eg, baseline and 24 months), the scoring system for both erosion and joint space narrowing (JSN) was a semiquantitative variable (scored 0=absent, 1=doubtful, 2=obvious but moderate, 3=obvious and important). Moreover, there was also an evaluation concerning the global assessment using a binary variable for each joint and for both erosion and joint space narrowing (scored 0=no change, 1=worsening).
Statistical analysis
The statistical analysis was performed on patients with a complete dataset (clinical and US evaluation at baseline, and after 4 months of follow-up and radiological evaluation at baseline, and after 2 years of follow-up).
The first step of the statistical analysis consisted in the tabulation of the main baseline characteristics of the patients and the evaluated joints using mean and SD for the continuous, and percentages for dichotomous variables.
The probability of structural deterioration with regard to the specific joint region was estimated by calculating the percentage of joints with radiological progression, defined by either the occurrence or worsening in either erosion or joint space narrowing score after the 2-year follow-up period in each of the following four anatomical regions (wrists, MCP, PIP, MTP) without performing any statistical test.
The probability of structural deterioration with regard to the presence of synovitis at baseline was estimated by comparing the percentage of joints with radiological progression defined by either occurrence or worsening in either erosion or JSN in the joints with or without synovitis at baseline for the three different baseline synovitis evaluations (clinical, US-GS and US-PD).
The risk of progression with respect to the presence of synovitis at baseline was estimated by calculating the OR with its 95% CI. These measures of association were tested using generalised estimating equation analysis adjusting for within-patient correlation, and also other factors (age, gender, disease duration, baseline tender joint count, swollen joint count, ESR joint localisation (eg, PIP, MCP, wrists, MTP) and baseline structural damage). Such probability of structural deterioration with regard to the presence of synovitis at baseline either at physical examination, US-GS or US-PD evaluation was initially calculated in the whole population.
The second step of the statistical analysis was focused on the subgroup of joints considered normal by the physicians (one analysis) or the US (another analysis) at baseline, calculating the predictive validity of US evaluation in the group of joints with normal baseline physical examination (one analysis) and also calculating the predictive validity of clinical evaluation in the group of joints with normal US (GS and PD) baseline examination.
The third step tried to check whether a positive short-term treatment effect on synovitis permits to prevent a subsequent structural deterioration. For this purpose, we considered as ‘baseline abnormal joint’, joints with a clinical or US score either >0 or >1 (eg, considering as ‘normal’ the joints with a ‘doubtful’ clinical examination or only with ‘mild synovial thickening’ at US-GS examination, or only ‘mild’ US-PF findings). Thereafter, the joints were split into two categories with regard to the normalisation of the examination after 4 months of anti-TNF therapies, calculating the probability of observing radiological progression after 2 years with respect to this treatment effect.
Results
Patients and study course
Of the 77 recruited patients in the first part of the study, one had no baseline US examination and was therefore excluded from this study. Seventeen additional patients did not return for the 2-year follow-up visit resulting in 59 patients with a complete dataset.
The main characteristics of the patients (data provided are either % or mean±SD) (age: 56±12 years old, gender: 81% female, body mass index: 25±5 kg/m2) of their disease (disease duration: 10±8 years, rheumatoid factor positivity: 73%, history of surgery for RA (eg, articular prosthesis, arthrodesis or resection) 24%) and of their treatment (median number of previous disease modifying antirheumatic drugs: 3.0, history of TNF blockers: 32%) were similar to the whole population of 77 screened patients (data not shown). The disease activity evaluated by the disease activity score in 28 joints was quite high at entry (5.1±1.3) and dropped to 3.4±1.3) after 4 months of anti-TNF therapy (etanercept, n=34; adalimumab, n=23, infliximab, n=2). The same improvement was observed in terms of functional disability with a health assessment questionnaire score decreasing from 1.4±0.7 to 1.0±0.7 between baseline and the 4-month follow-up visit, respectively.
Table 1 summarises the main characteristics of the 1888 evaluated joints (eg, 32 joints in 59 patients) confirming a higher sensitivity of US in detecting synovitis, particularly in the feet.
Table 1.
Baseline characteristics of the 1888 evaluated joints of the 59 studied patients with rheumatoid arthritis
| Joints | |||||
|---|---|---|---|---|---|
| Characteristics | Wrists | MCP | PIP | MTP | Total |
| Number | 118 | 590 | 590 | 590 | 1888 |
| Clinical synovitis | |||||
| 0 = absent | 19.7* | 49.0 | 56.8 | 77.1 | 58.4 |
| 1 = doubtful | 23.1 | 13.3 | 11.7 | 12.2 | 13.1 |
| 2 = present, moderate | 38.5 | 23.1 | 21.0 | 8.0 | 18.7 |
| 3 = present, important | 18.8 | 14.6 | 10.5 | 2.7 | 9.9 |
| US grey-scale synovitis† | |||||
| Grade 0 | 17.9 | 49.5 | 62.5 | 44.3 | 50.0 |
| Grade 1 | 35.0 | 15.1 | 11.4 | 26.2 | 18.6 |
| Grade 2 | 27.4 | 13.6 | 10.8 | 15.3 | 14.1 |
| Grade 3 | 19.7 | 21.8 | 15.3 | 14.2 | 17.3 |
| US Power Doppler† | |||||
| Grade 0 | 40.2 | 71.6 | 82.5 | 78.0 | 75.1 |
| Grade 1 | 17.9 | 9.2 | 8.5 | 9.3 | 9.5 |
| Grade 2 | 32.5 | 13.9 | 4.9 | 9.3 | 10.8 |
| Grade 3 | 9.4 | 5.3 | 4.1 | 3.4 | 4.6 |
numbers provided are the percentage of joints in a specific category.
see Methods section for detailed description of the ultrasonographic grading systems.
MCP, metacarpophalangeal; MTP, metatarsophalangeal; PIP, proximal interphalangeal; US, ultrasound.
The probability of observing radiological progression defined by the occurrence of the worsening in either erosion or joint space narrowing was quite low (eg, 9% of the 1888 evaluated joints). Such probability was higher for the wrists (16.2%) and MTP joints (11%) than for the other joints (7.0% and 7.5% for the MCP and PIP, respectively).
Correlations between baseline synovitis evaluations and radiological structural deterioration after 2 years of follow-up.
The online supplementary tables 1, 2 and 3 provide the data observed from the different analyses with respect to the definition of radiological structural deterioration (considering either the changes in radiological erosion, radiological JSN) and also with respect to the individual synovitis scoring systems at baseline.
The data observed when defining radiological progression by the occurrence or the worsening in either erosion or JSN, and when defining the presence of synovitis at baseline by a score of at least 1 on clinical or US scales are summarised in figure 1. Such results were still observed when excluding MTP 1 and 2 of the analyses (data not shown).
Figure 1.
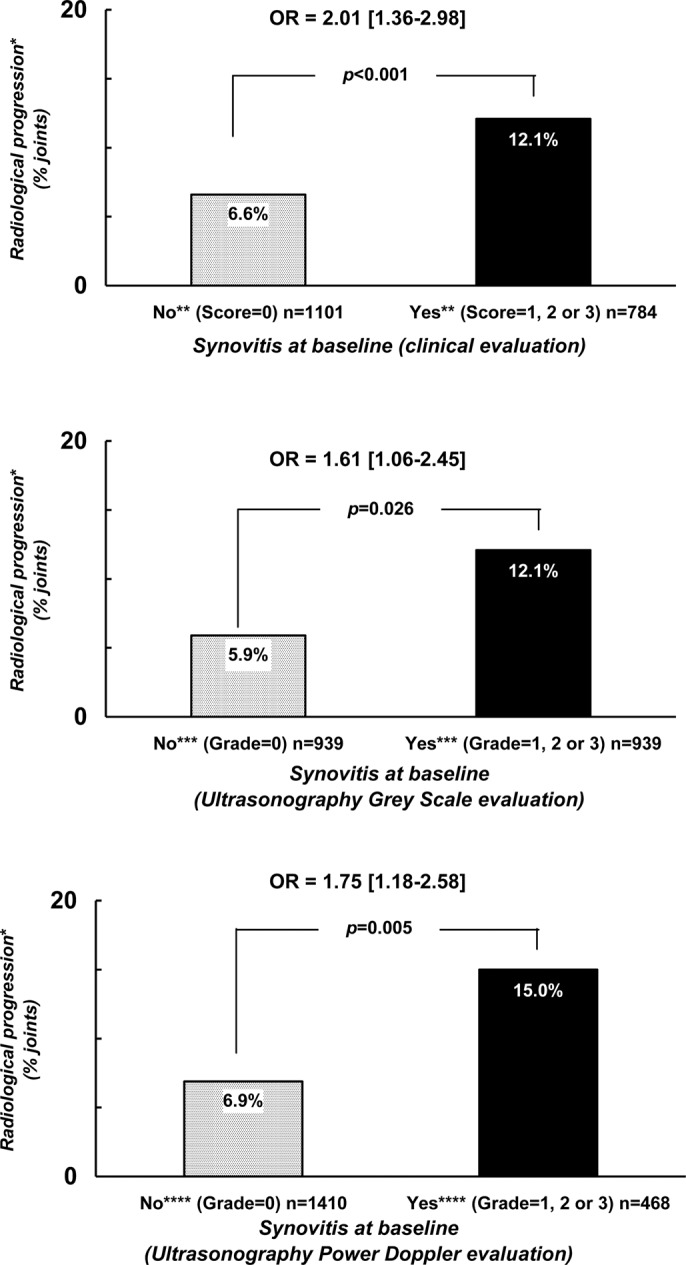
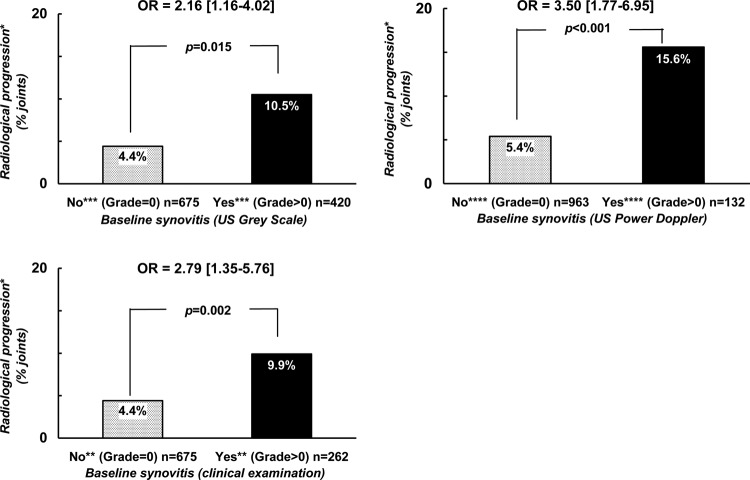
Probability of radiological progression after a 2-year follow-up period with regard to the presence of baseline synovitis defined by either clinical or ultrasonographic examination. *Radiological progression = occurrence or worsening of erosion or joint space narrowing. **Score of ‘clinical’ synovitis (0 = no synovitis; 1 = doubtful synovitis; 2 = obvious and moderate synovitis; 3 = obvious and important synovitis. ***Score of ultrasonography grey-scale evaluation: 0 = absence of synovial thickening; 1 = mild synovial thickening; 2 = moderate synovial thickening; 3 = marked synovial thickening. ****Score of ultrasonographic Power Doppler evaluation: 0 = absence of signal, no intra-articular flow; 1 = mild, one or two vessels' signals (including one confluent vessel) for small joints and two to three signals for large joints (including two confluent vessels); 2 = moderate confluent vessels (>grade 1) and less than 50% of normal area; 3 = marked vessels' signals in more than half the synovial area.
Regardless of what technique was used to assess synovitis, the presence of synovitis was predictive of subsequent structural deterioration. However, the observed OR with the 95% CI did not demonstrate a statistically significant difference in the magnitude of this link between the different methods of synovitis evaluation.
With respect to the subgroup analyses: the first subgroup of interest was defined by the 1101 joints the investigators considered as normal at physical examination (23 wrists, 288 MCP, 335PIP and 455 MTP). Structural deterioration defined by either the occurrence or the worsening in either erosion or joint space narrowing was observed in 6.6% of the joints, but more frequently in the presence of any synovitis (grade >0) detected by US, either by GS (10.5%) or by PD (15.6%) (figure 2A) with OR of 2.16 (1.16–4.02), p=0.015 and of 3.50 (1.77–6.95), p<0.001, respectively.
Figure 2.
Probability of radiological progression after a 2-year follow-up period with regard to the presence of synovitis at baseline and the modality of joint examination in two different groups of joints. *Radiological progression = occurrence or worsening of erosion or joint space narrowing. **Score of ‘clinical’ synovitis (0 = no synovitis; 1 = doubtful synovitis; 2 = obvious and moderate synovitis; 3 = obvious and important synovitis. ***Score of ultrasonography grey-scale evaluation: 0 = absence of synovial thickening; 1 = mild synovial thickening; 2 = moderate synovial thickening; 3 = marked synovial thickening. ****Score of ultrasonographic Power Doppler evaluation: 0 = absence of signal, no intra-articular flow; 1 = mild, one or two vessels' signals (including one confluent vessel) for small joints and two to three signals for large joints (including two confluent vessels); 2 = moderate confluent vessels (>grade 1) and less than 50% of normal area; 3 = marked vessels' signals in more than half the synovial area.
The second subgroup of interest was defined by the 938 joints that US considered as normal according to both GS and PD techniques (21 wrists, 290 MCP, 369 PIP, 258 MTP). Structural deterioration defined by either the occurrence or the worsening in either erosion or JSN was observed in 5.9% of the joints, but more frequently in the presence of any synovitis (grade >0) detected by physical examination (9.9%) with an OR of 2.79 (1.35–5.76), p=0.005 (figure 2B).
Finally, we also evaluated the probability of observing radiological progression after 2 years of follow-up with respect to the persistence of synovitis after 4 months of therapy. The two analyses conducted (the one conducted in the joints defined as abnormal by a score above 0 and the one by a score above 1), showed a trend or statistical significance suggesting more frequent structural progression in case of persistent synovitis. In the analysis considering an abnormal baseline joint as a joint with a score of at least 1 (784, 939 and 468 joints at clinical, US-GS and US-PD evaluations, respectively), radiological progression was observed more frequently in the case of persistent synovitis (16.6% vs 8.9% for clinical evaluation (OR=1.26 (0.79–2.02), p=0.336; 17.1% vs 5.9% for US-GS evaluation (OR=2.41 (1.24–4.67), p=0.010) and 20.7% versus 11.9% for US-PD evaluation (OR=1.63 (0.75–3.57), p=0.218 in joints with, versus without persistent synovitis, respectively. These findings were even more pronounced when considering abnormal baseline joints by a score of at least two (figure 3) (538, 589 and 289 joints at clinical, US-GS and US-PD evaluations, respectively). Radiological progression was observed more frequently in case of persistent synovitis after 4 months (15.7% vs 7.5% for clinical evaluation (OR=1.70 (0.93–3.12), p=0.086); 19.3% versus 6.5% for US-GS evaluation (OR=3.14 (1.50–6.55), p=0.002) and 21.1% vs 8.0% for US-PD evaluation (OR=2.79 (1.19–6.56), p=0.019) in joints with, versus without persistent synovitis, respectively.
Figure 3.
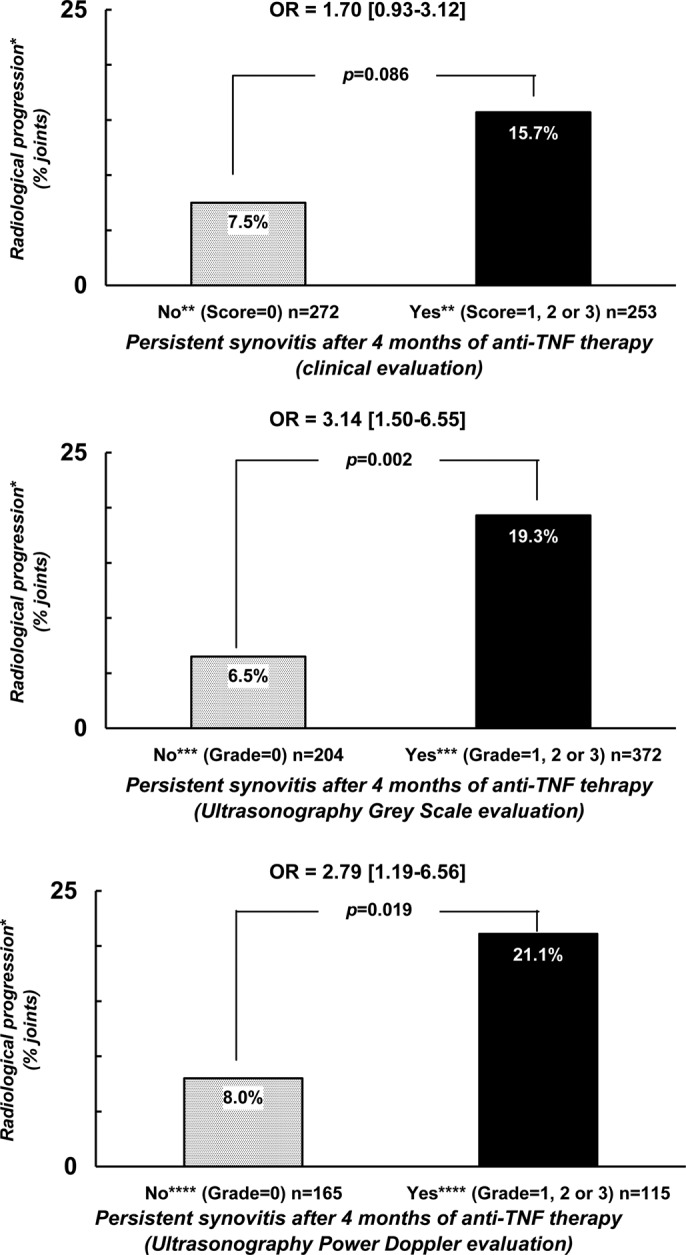
Probability of radiological progression after a 2-year follow-up period with regard to the capacity of a treatment to normalise the joint examination in terms of synovitis. *Radiological progression = occurrence or worsening of erosion or joint space narrowing. **Score of ‘clinical’ synovitis (0 = no synovitis; 1 = doubtful synovitis; 2 = obvious and moderate synovitis; 3 = obvious and important synovitis. ***Score of ultrasonography grey-scale evaluation: 0 = absence of synovial thickening; 1 = mild synovial thickening; 2 = moderate synovial thickening; 3 = marked synovial thickening. ****Score of ultrasonographic Power Doppler evaluation: 0 = absence of signal, no intra-articular flow; 1 = mild, one or two vessels' signals (including one confluent vessel) for small joints and two to three signals for large joints (including two confluent vessels); 2 = moderate confluent vessels (>grade 1) and less than 50% of normal area; 3 = marked vessels' signals in more than half the synovial area.
Discussion
This study confirms the ability of synovitis to predict subsequent structural deterioration in patients with RA. The data observed in the whole set of joints suggest that such predictive validity is of similar magnitude whatever the modality of the joint examination (ie, whether clinical or US evaluation). The data observed in the subgroups of joints (with normal clinical or US baseline examination) suggest that both clinical and US evaluations are relevant to optimally evaluate the risk of subsequent structural deterioration. Finally, these data emphasise the importance of persistent synovitis since the probability of a 2-year structural progression was higher in cases of persistent synovitis after 4 months of anti-TNF therapy.
All the investigators involved in the collection of synovitis outcome measures were experienced in their particular areas. Moreover, all the investigators participated at a 1-day training session on assessment of clinical and US synovitis outcome measures.37 The information given above can be seen as the strength of this study but may also reduce the generalisation to other sets of investigators.
The findings observed in our study are in accordance with previous studies showing a link between the presence of synovitis at a single point in time, and a subsequent structural deterioration. Most of the studies evaluating the predictive validity of synovitis detected by US have considered the two variables (synovitis and radiological changes) at the patient level by considering, for example, the total number of joints with synovitis and a summed radiological scoring system.14–16 18 We have focused our study by evaluating the risk of structural progression at the level of the joints. Both approaches are important. For example, in a prospective longitudinal evaluation of patients with RA in remission or in low disease activity state for at least 2 months, subclinical activity observed by PD signals on US were able to predict subsequent disease relapse.16
Our study was focused on the evaluation of the relationship between US and physical examination of joints in their detection of synovitis. It is well known that synovitis detected by US is probably more valid in terms of face validity, and in terms of sensitivity rather than physical examination.10–12 In this study, we confirmed the higher sensitivity for US examination in detecting synovitis, in particular in the front region of the feet (table 1). However, the predictive validity of an outcome measure is probably one of the most clinically relevant issues when considering potential surrogate markers. Our study confirms the predictive validity of synovitis in terms of subsequent structural deterioration, but does not demonstrate the superiority of US in comparison with physical examination. However, our study suggests that both the clinical and the US examinations are relevant to optimally evaluate the risk of subsequent structural deterioration. Such findings centre around the analyses of the subgroups of joints with either subclinical synovitis (synovitis detected only at US examination) or clinically detected non-US synovitis (synovitis detected only at clinical examination). In this study, we have evaluated the clinical and therapeutic parameters during the first 4 months of the study, but we were not in a position to collect such information during the remaining 20 months of the study. Such lack of information might limit the interpretation of the observed data. Another potential limitation of this study is its relatively small sample size. The findings concerning non-US synovitis might be explained by the particular design of our study with experienced clinicians or research nurses to identify patients with synovitis who have participated at the training session prior to the meeting. If this explanation is the correct one, such findings reinforce the interest of a better training to any health professionals (including the rheumatologists) in this area.38–40 Moreover, clinical examination might have considered periarticular phenomena such as tenosynovitis in their joint count which have not been evaluated by US examination, and which might help predict long-term structural progression.
However, such findings (the non-superiority of US vs clinical examination) might also be explained by the difficulty of US examination justifying the current efforts to improve the training of ultrasonographers in this area.41–43 It might also reflect the particular US protocol employed in this study. In particular, the use of different US machines with relevant differences in terms of GS and PD performances, and also the limitation of the evaluation of the fingers at the dorsal side44 might explain the non-superiority of US examination. Moreover, the wrist (which includes 17 different joints) has been evaluated in this study as a single joint.
In this study, we have also confirmed that a positive treatment effect (the disappearance of synovitis in the short term) is of benefit for the joint in terms of reducing subsequent structural deterioration. Our study confirms the one reported by Fukae et al,17 in which the researchers have split the finger joints into two categories regarding the presence or absence of improvement in PD-US vascularity of at least 70% after 8 weeks of either adalimumab or tocilizumab therapy. They showed that the risk of subsequent radiological progression was lower in the ‘responder’ joints. The confirmation of such findings in our study re-emphasises the importance of synovitis detection in daily practice, and also the definition of absence of synovitis as one of the major targets of our therapy.7 8
Further studies are required in other sets of patients, and by other clinicians and ultrasonographers, in order to confirm our findings, in particular with respect to both the clinical and US definitions of synovitis.
Supplementary Material
Acknowledgments
The authors would like to thank the different investigators who contributed to the recruitment and/or the monitoring of the patients (Jean-David Albert, Pierre Bourgeois, Maxime Breban, Françoise Carbonnelle, Tiffen Couchouron, Laurent Grange, Pascal Guggenbuhl, Cécile Hacquard-Bouder, Christophe Hudry, Rachida Inaoui, Catherine Le Bourlout, Damien Loeuille, Jean-Marcel Meadeb, Anne Miquel).
Footnotes
Funding: This study was supported by an unrestricted grant from ABBOTT, France.
Competing interests:
Patient Consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Drossaers-Bakker KW, de Buck M, van Zeben D, et al. Long-term course and outcome of functional capacity in rheumatoid arthritis: the effect of disease activity and radiologic damage over time. Arthritis Rheum 1999;42:1854–60 [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D. Patients with rheumatoid arthritis in clinical care. Ann Rheum Dis 2004;63:221–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aletaha D, Smolen J, Ward MM. Measuring function in rheumatoid arthritis: Identifying reversible and irreversible components. Arthritis Rheum 2006;54:2784–92 [DOI] [PubMed] [Google Scholar]
- 4.van Leeuwen MA, van der Heijde DM, van Rijswijk MH, et al. Interrelationship of outcome measures and process variables in early rheumatoid arthritis. A comparison of radiologic damage, physical disability, joint counts, and acute phase reactants. J Rheumatol 1994;21:425–9 [PubMed] [Google Scholar]
- 5.Aletaha D, Nell VP, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolen JS, Van Der Heijde DM, St Clair EW, et al. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum 2006;54:702–10 [DOI] [PubMed] [Google Scholar]
- 7.Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung PP, Ruyssen-Witrand A, Gossec L, et al. Reliability of patient self-evaluation of swollen and tender joints in rheumatoid arthritis: A comparison study with ultrasonography, physician, and nurse assessments. Arthritis Care Res (Hoboken) 2010;62:1112–9 [DOI] [PubMed] [Google Scholar]
- 10.Backhaus M, Kamradt T, Sandrock D, et al. Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum 1999;42:1232–45 [DOI] [PubMed] [Google Scholar]
- 11.Kane D, Balint PV, Sturrock RD. Ultrasonography is superior to clinical examination in the detection and localization of knee joint effusion in rheumatoid arthritis. J Rheumatol 2003;30:966–71 [PubMed] [Google Scholar]
- 12.Wakefield RJ, Green MJ, Marzo-Ortega H, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis 2004;63:382–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szkudlarek M, Narvestad E, Klarlund M, et al. Ultrasonography of the metatarsophalangeal joints in rheumatoid arthritis: comparison with magnetic resonance imaging, conventional radiography, and clinical examination. Arthritis Rheum 2004;50:2103–12 [DOI] [PubMed] [Google Scholar]
- 14.Bøyesen P, Haavardsholm EA, van der Heijde D, et al. Prediction of MRI erosive progression: a comparison of modern imaging modalities in early rheumatoid arthritis patients. Ann Rheum Dis 2011;70:176–9 [DOI] [PubMed] [Google Scholar]
- 15.Lillegraven S, Bøyesen P, Hammer HB, et al. Tenosynovitis of the extensor carpi ulnaris tendon predicts erosive progression in early rheumatoid arthritis. Ann Rheum Dis 2011;70:2049–50 [DOI] [PubMed] [Google Scholar]
- 16.Foltz V, Gandjbakhch F, Etchepare F, et al. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low levels of disease activity. Arthritis Rheum 2012;64:67–76 [DOI] [PubMed] [Google Scholar]
- 17.Fukae J, Isobe M, Kitano A, et al. Radiographic prognosis of finger joint damage predicted by early alteration in synovial vascularity in patients with rheumatoid arthritis: Potential utility of power doppler sonography in clinical practice. Arthritis Care Res (Hoboken) 2011;63:1247–53 [DOI] [PubMed] [Google Scholar]
- 18.Naredo E, Collado P, Cruz A, et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum 2007;57:116–24 [DOI] [PubMed] [Google Scholar]
- 19.Molenaar ET, Voskuyl AE, Dinant HJ, et al. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum 2004;50:36–42 [DOI] [PubMed] [Google Scholar]
- 20.Rau R. Is remission in rheumatoid arthritis associated with radiographic healing? Clin Esp Rheum 2006;24:S41–4 [PubMed] [Google Scholar]
- 21.Dougados M, Jousse-Joulin S, Mistretta F, et al. Evaluation of several ultrasonography scoring systems for synovitis and comparison to clinical examination: results from a prospective multicentre study of rheumatoid arthritis. Ann Rheum Dis 2010;69:828–33 [DOI] [PubMed] [Google Scholar]
- 22.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24 [DOI] [PubMed] [Google Scholar]
- 23.Jousse-Joulin S, d'Agostino MA, Marhadour T, et al. Reproducibility of joint swelling assessment by sonography in patients with long-lasting rheumatoid arthritis (SEA-Repro study part II). J Rheumatol 2010;37:938–45 [DOI] [PubMed] [Google Scholar]
- 24.Karim Z, Wakefield RJ, Quinn M, et al. Validation and reproducibility of ultrasonography in the detection of synovitis in the knee: a comparison with arthroscopy and clinical examination. Arthritis Rheum 2004;50:387–94 [DOI] [PubMed] [Google Scholar]
- 25.Walther M, Harms H, Krenn V, et al. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum 2001;44:331–8 [DOI] [PubMed] [Google Scholar]
- 26.Naredo E, Bonilla G, Gamero F, et al. Assessment of inflammatory activity in rheumatoid arthritis: a comparative study of clinical evaluation with grey scale and power Doppler ultrasonography. Ann Rheum Dis 2005;64:375–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naredo E, Collado P, Cruz A, et al. Longitudinal power Doppler ultra-sonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum 2007;57:16–24 [DOI] [PubMed] [Google Scholar]
- 28.Alasaarela EM, Alasaarela EL. Ultrasound evaluation of painful rheumatoid shoulders. J Rheumatol 1994;21:1642–8 [PubMed] [Google Scholar]
- 29.Scheel AK, Hermann KG, Kahler E, et al. A novel ultrasonographic synovitis scoring system suitable for analyzing finger joint inflammation in rheumatoid arthritis. Arthritis Rheum 2005;52:733–43 [DOI] [PubMed] [Google Scholar]
- 30.Schmidt WA, Schmidt H, Schicke B, et al. Standard reference values for musculoskeletal ultrasonography. Ann Rheum Dis 2004;63:988–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 2005;32:2485–7 [PubMed] [Google Scholar]
- 32.Devauchelle-Pensec V, Berthelot JM, Jousse S, et al. Performance of hand radiographs in predicting the diagnosis in patients with early arthritis. J Rheumatol 2006;33:1511–5 [PubMed] [Google Scholar]
- 33.Devauchelle Pensec V, Saraux A, Berthelot JM, et al. Ability of foot radiographs to predict rheumatoid arthritis in patients with early arthritis. J Rheumatol 2004;31:66–70 [PubMed] [Google Scholar]
- 34.Devauchelle Pensec V, Saraux A, Berthelot JM, et al. Ability of hand radiographs to predict a further diagnosis of rheumatoid arthritis in patients with early arthritis. J Rheumatol 2001;28:2603–7 [PubMed] [Google Scholar]
- 35.Devauchelle-Pensec V, Josseaume T, Samjee I, et al. Ability of oblique foot radiographs to detect erosions in early arthritis: results in the ESPOIR cohort. Arthritis Rheum 2008;59:1729–34 [DOI] [PubMed] [Google Scholar]
- 36.D'Agostino MA, Saraux A, Chary-Valckenaere I, et al. Can we improve the diagnosis of spondyloarthritis in patients with uncertain diagnosis? The EchoSpA prospective multicenter French cohort. Joint Bone Spine. 2012 doi: 10.1016/j.jbspin.2012.02.007. [Epub ahead of print 27 March 2012] [DOI] [PubMed] [Google Scholar]
- 37.Marhadour, Jousse-Joulin S, Chalès G, et al. Reproducibility of joint swelling assessment in long-lasting rheumatoid arthritis: influence on disease activity score-28 values (SEA-Repro study part I). J Rheumatol 2010;37:932–7 [DOI] [PubMed] [Google Scholar]
- 38.Scott DL, Antoni C, Choy EH, et al. Joint counts in routine practice. Rheumatology (Oxford) 2003;42:919–23 [DOI] [PubMed] [Google Scholar]
- 39.Klinkhoff AV, Bellamy N, Bombardier C, et al. An experiment in reducing interobserver variability of the examination for joint tenderness. J Rheumatol 1988;15:492–4 [PubMed] [Google Scholar]
- 40.Scott DL, Choy EH, Greeves A, et al. Standardising joint assessment in rheumatoid arthritis. Clin Rheumatol 1996;15:579–82 [DOI] [PubMed] [Google Scholar]
- 41.Scheel AK, Schmidt WA, Hermann KG, et al. Interobserver reliability of rheumatologists performing musculoskeletal ultrasonography: results from a EULAR “Train the trainers” course. Ann Rheum Dis 2005;64:1043–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naredo E, Möller I, Moragues C, et al. Interobserver reliability in musculoskeletal ultrasonography: results from a “Teach the Teachers” rheumatologist course. Ann Rheum Dis 2006;65:14–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wakefield RJ, D'Agostino MA, Iagnocco A, et al. The OMERACT Ultrasound Group: status of current activities and research directions. J Rheumatol 2007;34: 848–51 [PubMed] [Google Scholar]
- 44.Vlad V, Berghea F, Libianu S, et al. Ultrasound in rheumatoid arthritis: volar versus dorsal synovitis evaluation and scoring. BMC Musculoskelet Disord 2011;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.