Abstract
We report a case of a 73-year-old woman with a brainstem stroke presenting as Wallenberg syndrome. By transoesophageal echocardiography and combined 18F-fluordeoxyglucose positron emission and CT (18F-FDG PET/CT), the diagnosis of large artery vasculitis owing to giant cell arteritis was confirmed. In the absence of classical clinical signs, the examination of the large extracranial vessels by ultrasound and 18F-FDG PET/CT played the key role in detecting a widespread vasculitis.
Background
Strokes resulting from giant cell arteritis (GCA) are rare, but have a high impact on the affected patients. GCA is a severe disease, which can possibly affect all large arteries. With this case presentation we wanted to emphasise that GCA is not only affecting temporal arteries and is a systemic disease with relevant complications as stroke. Furthermore, we wanted to underline the potential of the various imaging methods for the diagnosis of GCA compared to the temporal artery biopsy (TAB).
Case presentation
A 73-year-old woman was transferred to our stroke unit from an external hospital 4 days after onset of a Wallenberg syndrome with right-sided hemiataxia, left-sided dissociated sensory loss, dysphagia and dysarthria. Cranial MRI (cMRI) confirmed an infarction of the right dorsolateral medulla oblongata (figure 1A). The patient had a medical history of hypertension (BP: 140/90 mm Hg at initial presentation), hypothyreosis (TSH 0.7 µU/ml reference value: 0.2–3.1 µU/ml at initial presentation) and nicotine dependence. During 72-h stroke unit monitoring there was no evidence of cardiac arrhythmia. Duplex ultrasound of the extracranial and intracranial vessels showed no relevant stenoses. A transthoracic echocardiography performed in the external hospital showed a slight biventricular diastolic dysfunction and a left ventricular ejection fraction of 95%. Stroke aetiology could not be determined and was labelled as cryptogenic. A secondary prevention was started with acetylsalicylic acid and statin therapy. After 10 days the patient was discharged to a neurological rehabilitation centre. After rehabilitation, residual symptoms included slight hemiataxia and dissociated hypoesthesia.
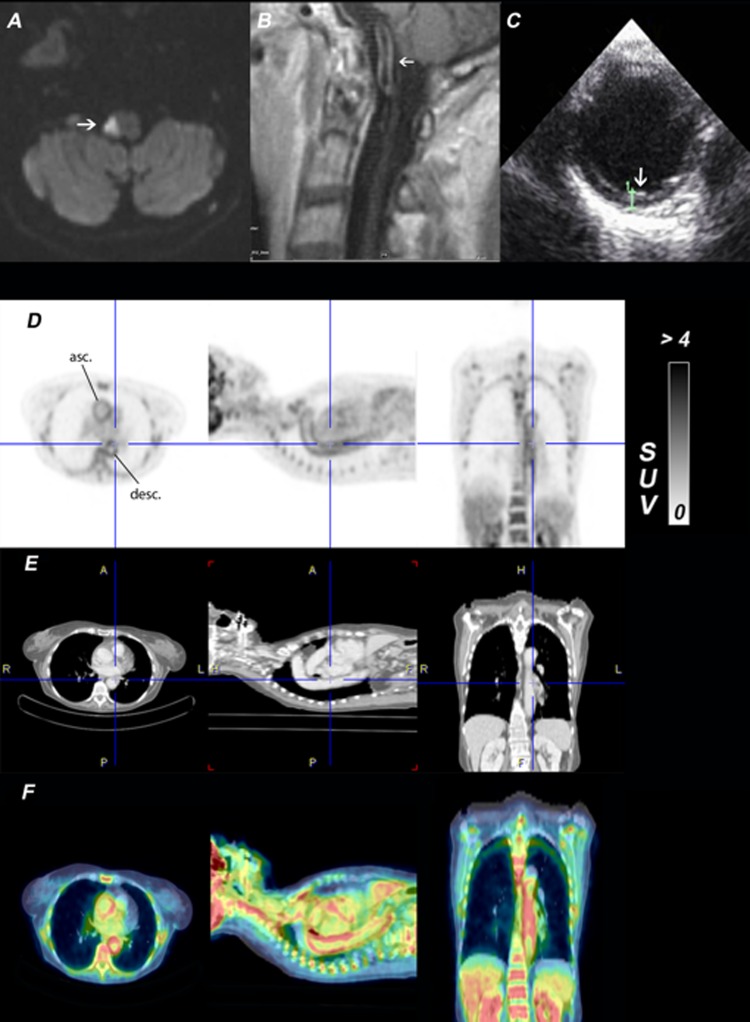
Figure 1.
(A) 1.5 tesla MRI, diffusion weighted imaging shows diffusion restriction in the right dorsolateral medulla oblongata (white arrow). (B) 1.5 tesla MRI, T1 postcontrast agent shows vessel-wall enhancement and thickening of the basilar artery (white arrow). (C) Transoesophageal ultrasound shows the thoracic aorta with a wall thickening of 2.9 mm (white arrow). (D) Three orthogonal slices of the 18F-FDG whole-body PET. The colour table is scaled to the range 0–4 of the standardised uptake value. There is a significantly increased uptake of 18F-FDG in the wall of the thoracic aorta (asc., ascending; desc., descending aorta). (E) Coregistered body CT imaging. (F) PET/CT fusion images.
Three months later the patient was readmitted to our emergency department because of a sudden onset of diplopia and left facial palsy and the residual symptoms of the first stroke with a slight right-sided hemiataxia and a left-sided dissociated sensory loss. The diplopia and the left facial palsy resolved within the first 24 h. Upon cMRI there was no evidence for a new infarction, and a transitory ischaemic attack (TIA) of the brainstem was assumed. Transoesophageal echocardiography revealed a thickening of the thoracic ascending part of the aorta (figure 1C), suggestive of a vasculitis of the large arteries, eg, GCA.
The patient never had any history of headaches, impaired vision or jaw claudication. C reactive protein levels were elevated to 0.65 mg/dl (reference value <0.5 mg/dl), erythrocyte sedimentation rate (ESR) was elevated to 40 mm during the first hour (reference value: <10 mm during the first hour). Thus, the inflammatory markers C reactive protein and ESR were elevated but not highly suggestive for a GCA, since ESR exceeds 50 mm in the first hour in typical presentations of GCA. TAB was refused by the patient. To detect inflammatory alterations in the aortic wall, combined 18F-fluorodeoxyglucose positron emission and CT (18F-FDG PET/CT) were performed. CT showed not only the wall thickening of the ascending aorta, as described in transoesophageal echocardiography, but also a thickened wall of the descending thoracic aorta indicating a widespread affection of the large arteries (figure 1E). According to the CT results, 18F-FDG PET showed a corresponding hypermetabolism in the walls of all large arteries, especially in the ascending and descending thoracic aorta (figure 1DF). A critical re-evaluation of the first cMRI, performed 3 months back, also revealed a mural wall thickening of the basilar artery (figure 1B).
Facing the diagnosis of GCA, we initiated a steroid therapy with 150 mg methylprednisolone per day. Clinical deficits improved within the first day after the steroid therapy was started. The imaging findings and the good response to steroid therapy confirmed the diagnosis of a GCA. A cyclophosphamide treatment was refused by the patient, thus we started an immunosuppressive therapy with methotrexate 15 mg once a week. Corticosteroids were tapered without relapse to 5 mg methylprednisolone per day. Since there were no signs of recurrent stroke episodes or GCA activity (eg, headaches, fever or visual disorders) at the clinical follow-up examination, 18F-FDG PET/CT and transoesophageal echocardiography were not repeated. The C reactive protein value in the follow-up after 1 year was 0.72 mg/dl (reference value <0.5 mg/dl), ESR was not repeated in the follow-up after 1 year.
Discussion
GCA is mainly a T-cell-mediated granulomatous vasculitis of the large- and medium-sized vessels commonly affecting the temporal arteries in patients over the age of 50 years.1 According to the American College of Rheumatology (ACR) criteria (table 1) main symptoms of GCA are new onset of stinging headaches or vision disorders.2
Table 1.
1990 American College of Rheumatology criteria for the classification of giant cell (temporal) arteritis (traditional format)2
| Criterion | Definition |
|---|---|
| Age at disease onset >50 years | Development of symptoms or findings beginning at age 50 or later. |
| New headache | New onset of or new type of localised pain in the head. |
| Temporal artery abnormality | Temporal artery tenderness to palpation or decreased pulsation, unrelated to arteriosclerosis of cervical arteries. |
| Elevated ESR | >50 mm in the first hour by the Westergren method. |
| Abnormal artery biopsy | Biopsy specimen with artery showing vasculitis characterised by a predominance of mononuclear cell infiltration or granulomatous inflammation, usually with multinucleated giant cells. |
With respect to the ACR criteria about 40% of the GCA patients may have an atypical presentation.3
If patients meet three of the five items of the ACR diagnostic criteria, the sensitivity and specificity to distinguish GCA from other types of vasculitis amount to 94 and 91%, respectively.4 However, the positive predictive value for the ACR criteria to detect GCA in an unselected population is below 50%.4 The main purpose of the ACR criteria is the distinction of the different vasculitis types but not a definition of diagnostic criteria for detection of each entity. Our patient presenting with brainstem stroke did not meet any of the GCA criteria and thus would have been missed.
In the literature, the prevalence of strokes associated with GCA ranges from 3 to 7%.5 In 0.15% of the patients with the first-ever stroke, GCA has been identified as the stroke cause.6 Strokes in GCA occur predominantly in the vertebrobasilar territory owing to inflammation mainly of the extracranial parts of the vertebral arteries.5 Therefore, GCA as a rare disease may be considered as an important differential diagnosis in case of vertebrobasilar strokes. This patient with a brainstem stroke showed no sign of vertebral artery involvement but a wall thickening of the basilar artery on cMRI. Till date, GCA-associated strokes have not yet been considered in the ACR criteria. However, this might be a step forward towards improved diagnosis of GCA in unselected populations.
TAB is still frequently considered as a diagnostic gold-standard to confirm the diagnosis of GCA and to justify a long-term corticosteroid therapy. However, TAB is currently discussed controversially, as it is invasive, has few consequences for further therapeutic strategies7 and has a high false-negative rate of 15–40%.8
In the recent years, multimodal imaging methods have become more important in the diagnosis of GCA. The ‘halo sign’ as a circumferential hypoechogenic wall thickening owing to vessel wall inflammation as detected by colour-coded ultrasound has a sensitivity and specificity of 68 and 91%, respectively, to diagnose GCA.9 Of late, MRI has emerged as an alternative for the diagnosis of GCA. High-resolution MRI with field strengths of 1.5 and 3 T reveals wall thickening of the superficial temporal artery with a high sensitivity and specificity of around 81 and 97%, respectively.10
The diagnostic benefit of 18F-FDG PET in addition to MRI or CT is the characterisation of the full spatial extent of the inflammation by detection of increased 18F-FDG uptake owing to the activation of inflammatory cells in large vessels.11 A recent meta-analysis for the diagnostic performance of 18F-FDG PET in GCA reported a sensitivity of 0.80, a specificity of 0.89, a positive predictive value of 0.85 and a negative predictive value of 0.88.12 Whereas it is not helpful in the intracranial and temporal vessels, it gives a comprehensive overview of the inflammation of all extracranial arteries.
The advantage of hybrid imaging (PET/CT or PET/MRI) is the combination of morphological and functional information. In the case of GCA, the finding of wall thickening alone does not necessarily prove an inflammatory process. The additional information of increased 18F-FDG tracer uptake suggests inflammation as the cause of wall thickening. Thus, hybrid imaging in GCA provides an additional diagnostic approach and could also be a sensitive longitudinal marker of disease activity under the therapy.13 However, PET imaging has a relevant radiation exposure for patients, and should only be used for follow-up examination in specific cases as a missing response to treatment or an unclear aetiology.
The use of C reactive protein and ESR in the diagnosis and follow-up of a GCA might represent a fast and barely invasive method. For a positive TAB, an elevated C reactive protein and elevated ESR provide a sensitivity of 86.9 and 84.1%, respectively.14 In the literature, the OR of a concordantly elevated ESR and C reactive protein for positive TAB is reported as 3.06 (95% CI 2.03 to 4.62), whereas the OR for concordantly normal ESR and C reactive protein is reported as 0.49 (95% CI 0.29 to 0.83).14 Since neither C reactive protein nor ESR was suggestive of a GCA in this patient, they could not be used as reliable parameters in the follow-up. C reactive protein after 1 year was only elevated to 0.72 mg/dl (reference value <0.5 mg/dl).
In this case in the absence of typical ACR criteria, the transoesophageal echocardiography and the 18F-FDG PET/CT were suggestive of a GCA. Owing to the clear response to the steroid therapy and the complementary information from the different imaging methods, a confirmation of the GCA diagnosis was possible without TAB. 18F-FDG PET/CT finally unmasked the ‘hidden giant’.
According to our opinion, the advisable diagnostic imaging algorithm should be tailored according to the risk and cost of the specific procedures. Ultrasound represents a fast and non-invasive method as the first screening in patients with clinically suspected GCA. MRI or CT angiography can confirm vessel wall thickening, MRI may also depict silent infarcts. 18F-FDG PET/CT has its major advantages in atypical cases to visualise widespread vessel wall inflammation.
Learning points.
This case emphasises that giant cell arteritis (GCA) is a systemic disease, which can involve potentially all large arteries.
Given the fact that 40% of GCA patients have an atypical presentation, there is a need for advanced diagnostic pathways.
In the future, the complementary use of different imaging methods may become a potential alternative for TAB in the detection of the ‘hidden giant’.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Borchers AT, Gershwin ME. Giant cell arteritis: a review of classification, pathophysiology, geoepidemiology and treatment. Autoimmun Rev 2012;11:A544–54 [DOI] [PubMed] [Google Scholar]
- 2.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122–8 [DOI] [PubMed] [Google Scholar]
- 3.Zwicker J, Atkins EJ, Lum C, et al. An atypical presentation of giant cell arteritis. CMAJ 2011;183:E301–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao JK, Allen NB, Pincus T. Limitations of the 1990 American College of Rheumatology classification criteria in the diagnosis of vasculitis. Ann Intern Med 1998;129:345–52 [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Gomez-Acebo I, et al. Strokes at time of disease diagnosis in a series of 287 patients with biopsy-proven giant cell arteritis. Medicine (Baltimore) 2009;88:227–35 [DOI] [PubMed] [Google Scholar]
- 6.Wiszniewska M, Devuyst G, Bogousslavsky J. Giant cell arteritis as a cause of first-ever stroke. Cerebrovasc Dis 2007;24:226–30 [DOI] [PubMed] [Google Scholar]
- 7.Quinn EM, Kearney DE, Kelly J, et al. Temporal artery biopsy is not required in all cases of suspected giant cell arteritis. Ann Vasc Surg 2012;26:649–54 [DOI] [PubMed] [Google Scholar]
- 8.Chong EWT, Robertson AJ. Is temporal artery biopsy a worthwhile procedure? ANZ J Surg 2005;75:388–91 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt WA, Kraft HE, Borkowski A, et al. Color duplex ultrasonography in large-vessel giant cell arteritis. Scand J Rheumatol 1999;28:374–6 [DOI] [PubMed] [Google Scholar]
- 10.Markl M, Uhl M, Wieben O, et al. High resolution 3T MRI for the assessment of cervical and superficial cranial arteries in giant cell arteritis. J Magn Reson Imaging 2006;24:423–7 [DOI] [PubMed] [Google Scholar]
- 11.Belhocine T, Blockmans D, Hustinx R, et al. Imaging of large vessel vasculitis with (18)FDG PET: illusion or reality? A critical review of the literature data. Eur J Nucl Med Mol Imaging 2003;30:1305–13 [DOI] [PubMed] [Google Scholar]
- 12.Besson FL, Parienti JJ, Bienvenu B, et al. Diagnostic performance of 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2011;38:1764–72 [DOI] [PubMed] [Google Scholar]
- 13.Treglia G, Mattoli MV, Leccisotti L, et al. Usefulness of whole-body fluorine-18-fluorodeoxyglucose positron emission tomography in patients with large-vessel vasculitis: a systematic review. Clin Rheumatol 2011; 30:1265–75 [DOI] [PubMed] [Google Scholar]
- 14.Kermani TA, Schmidt J, Crowson CS, et al. Utility of erythrocyte sedimentation rate and C-reactive protein for the diagnosis of giant cell arteritis. Semin Arthritis Rheum 2012;41:866–71 [DOI] [PMC free article] [PubMed] [Google Scholar]