Abstract
There is a long-standing debate as to whether the memory process of consolidation is neurochemically similar to or the same as the set of processes involved in retrieval and reconsolidation of that memory. In addition, although we have previously shown that initial memory processing in the hippocampus causes a drainage of hippocampal glucose because of increased local metabolic demand, it is unknown what metabolic changes occur elsewhere in the brain or during subsequent processing of a previously consolidated memory. Male Sprague Dawley rats (3 months old) were implanted with unilateral microdialysis cannulae and in vivo microdialysis of amygdala extracellular fluid (ECF) was performed during both (i) initial learning and (ii) retrieval 24h later of an aversively-motivated avoidance memory task. ECF samples were analyzed for glucose, lactate, pyruvate and glutamate. Results showed close similarity between increases in local glycolysis seen during both consolidation and retrieval, but also suggested that there may perhaps be a difference in amygdalar oxidative phosphorylation stimulated by the two processes. Hence, our data suggest that memory formation places similar metabolic demands across neural systems, and that consolidation may be metabolically different from retrieval.
Keywords: memory systems, glucose, metabolism, hippocampus, amygdala, consolidation
1. Introduction
Despite extensive research on the neuroscience of memory, the similarities and differences between the mechanisms of memory acquisition, consolidation, retrieval, reconsolidation, and extinction remain relatively unclear. Comparatively few studies have attempted to make direct comparisons of the neurobiological mechanisms involved in two or more of these processes. Pharmacological studies have suggested that the impact of specific drug treatments on memory may vary between consolidation and extinction [1, 2] and that the exact location of the processes may vary, for instance between nuclei within the amygdala for conditioned taste aversion processes [3]. However, few or no metabolic and neurochemical measures across processes have been taken. The aim of this study was to confirm that cognitively-induced increases in glucose metabolism take place in regions other than the hippocampus. Further, it was hypothesized that consolidation might differ metabolically from that of retrieval, which would support the suggestion that consolidation and retrieval are distinct neurochemical processes.
Here we report comparison of the metabolic demand and neurochemistry of initial memory consolidation with that of subsequent retrieval. Specifically, we used in vivo microdialysis (mD) to measure local glucose metabolism (via measurement of extracellular glucose, lactate, and pyruvate) and neural activity (extracellular glutamate) in the basolateral amygdala on both day 1 (initial learning) and day 2 (retrieval and reconsolidation) of a passive-avoidance memory task that is well-established to be dependent on basolateral amygdala processing.
The amygdala is well-known for its role in memory, particularly for memories involving emotional arousal or stress [4-6]. At least within the hippocampus, mnemonic processing is metabolically taxing and increases local glucose metabolism, such that extracellular levels of glucose are drained [7-9], suggesting an increase in glucose usage that exceeds local supply; extracellular lactate often rises at such times of increased local activity [10, 11] consistent with an increase in glycolysis. There is a large body of literature showing that administration of exogenous glucose either systemically or directly to any of several relevant brain regions can enhance memory performance, including performance on amygdala-mediated tasks [11-20]. Additionally, increases in glucose supply that enhance memory also reverse the task-associated dip in hippocampal extracellular glucose [8, 10, 11]. However, measurements of amygdala glucose metabolism during memory processing have not previously been reported. We show here that the metabolic processes underlying consolidation and retrieval in the amygdala may be metabolically distinct, with an apparent difference in oxidative metabolism between the two conditions despite similar glycolytic markers.
2. Material and Methods
2.1. Subjects
Male Sprague-Dawley rats (Charles River, Wilmington, MA), 12 weeks old, were housed in pairs on a 12:12h light:dark schedule with food and water available ad libitum. Following surgery, rats were housed in single cages. All procedures were approved by the University at Albany Institutional Animal Care and Use Committee. All rats were allowed to acclimate for at least one week prior to any surgery or testing. Animals were handled routinely from the time of their arrival by the experimenters to minimize any effects of handling stress on experimental measurements.
2.2. Surgeries
Rats were anaesthetized by isoflurane and a single microdialysis guide cannula (CMA12; CMA/Microdialysis) was stereotaxically implanted into the left basolateral amygdala using aseptic surgical technique as detailed previously [7, 10, 21]. Cannula coordinates were −3.0 mm posterior to bregma, +5.0 mm lateral, and 6.0 mm ventral from dura, with nosebar at −4.6 mm above the interaural line. Rats received acetaminophen in their drinking water following surgery and were handled extensively by the researcher for a week long recovery period prior to testing.
2.3 Behavioral testing
To assess consolidation and retrieval, inhibitory avoidance (IA) was used. Animals were placed in a novel, brightly-lit white chamber for 30 seconds, after which a door opened, allowing entry to a dark chamber. Upon entry to the dark chamber on Day 1, animals immediately received a mild foot shock (0.5 mA, 1s); when shocked, animals immediately return to the lit chamber and are then returned to their home cages for 24h. On Day 2, the animals were again placed in the white chamber for 30s before the door was opened; latency to cross into the dark compartment after the door opened (to a maximum of 1500 seconds) was taken as a measure of memory for the aversive experience associated with the dark chamber. The IA used was a 37” trough style chamber with shock floor specifically created and designed with a lid to allow for mD during training and testing (Maze Masters).
2.4 In vivo microdialysis procedures
Microdialysis was performed as previously described [7, 22]. Artificial extracellular fluid (aECF; formulated as previously described [23]) was perfused through the basolateral amygdala on both days before, during, and following testing. On the days of testing, rats were brought into the testing room; a fresh probe with a 2mm membrane length was placed through the guide cannulae into the basolateral amygdala and allowed to equilibrate for one hour while perfused at a rate of 1.5 μl/min; this timing has been shown by us and others to allow for reformation of the blood-brain barrier, stable baseline measurements, and responsiveness to cognitive load [7, 10, 23, 24]. Samples were collected in 20-minute bins at baseline, and then in 10-minute bins during and following placement into the testing apparatus. After the final baseline sample was collected on day two animals were sacrificed and brains were removed for post mortem histology. Glucose, lactate, pyruvate, and glutamate were measured in ECF samples using a CMA 600 Metabolic Analyzer.
2.5 Histology
Immediately following testing, rats were sacrificed by CO2 asphyxiation. Brains were removed, frozen on dry ice, and stored at −80°C. Histological examination examined probe placement. Brains were cut on a Leica cryostat (Nussloch, Germany) at 40 μm sections and mounted to glass slides. Slides were then stained with cresyl violet and were coverslipped. Proper probe placement was verified on a Nikon Eclipse E100 microscope. 18 animals were used in total and 7 were excluded for probes placed outside the BLA; a total of 11 animals were used for metabolic analysis. A total of 6 samples were lost due to analytical error.
2.6 Statistical Analyses
Independent samples t-tests were used to compare metabolic alterations between baseline and maze testing periods, to compare latency to cross between first and second days of behavioral testing, and to compare metabolic fluctuations between the first and second days.
3. Results
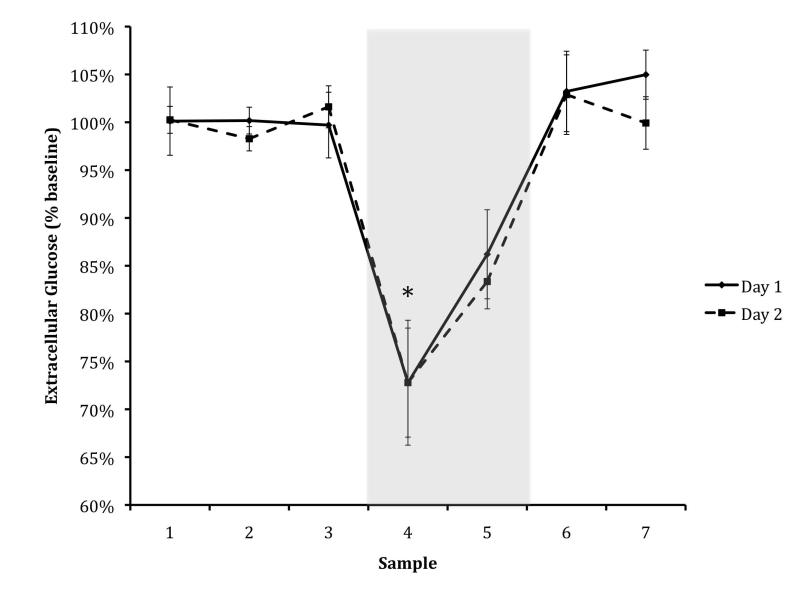
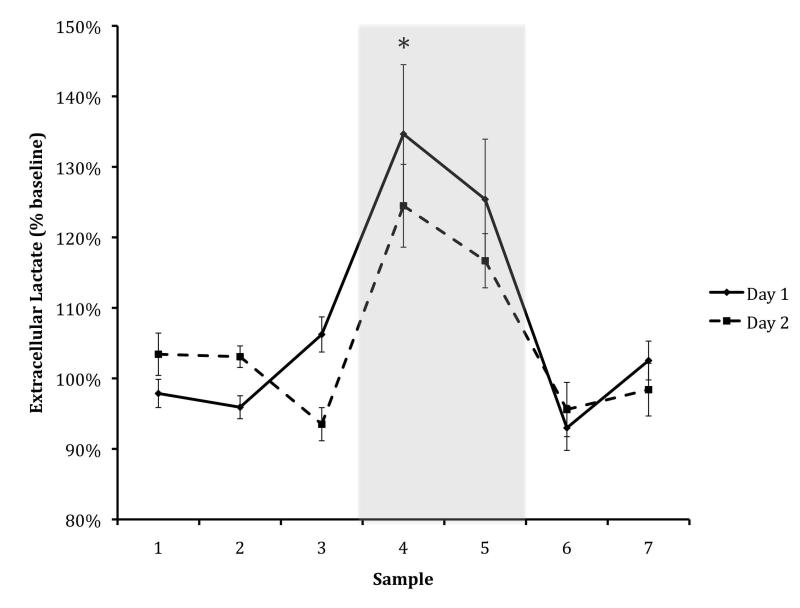
Animals entered the dark chamber rapidly on Day 1 (average of 10 sec, S.E.M. = 1.14) and displayed excellent retention of inhibitory avoidance conditioning, with no animal entering the dark chamber on Day 2 in less than 600s (average of 880 sec, S.E.M. = 57.00); t(10) = −15.311, p < 0.001. On both Day 1 and Day 2, there was a marked dip in ECF glucose (to 70% of baseline, S.E.M. Day 1 = 0.07, S.E.M. Day 2 = 0.06; Day 1: t(42) = 5.813, p < 0.001; Day 2: t(41) = 7.463, p < 0.001) whose start coincided with the start of the task (Figure 1). Concurrent with this decrease in ECF glucose, there was a marked rise in ECF lactate during the task period on both days (to 130% of baseline, S.E.M. = 0.10; Day 1, to 120% of baseline, S.E.M. = 0.10; Day 2); Day 1: t(42) = −4.978, p < 0.001; Day 2: t(42) = −4.033, p < 0.001).
Figure: 1. Glucose Day One & Day Two.
Amygdala ECF glucose levels in 12-week-old animals, shown as percent of baseline level. The black line denotes Day 1 (training) and the dashed line denotes Day 2 (testing); glucose levels did not differ significantly across days for any sample. Asterisks indicate significant difference from baseline for both days (p < .05). The shaded rectangle indicates the maze-testing period. Data are presented as mean +/− S.E.M.
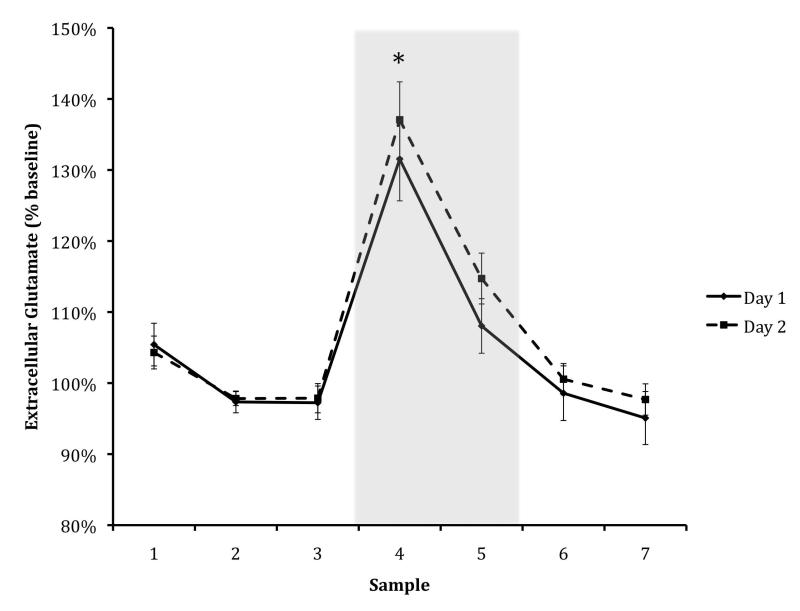
There were no significant differences in the magnitude and duration of either change across days suggesting a similar elevation in local glycolysis on each day. Further, on both Day 1 and Day 2 there was a similar, marked rise in glutamate during the task period (to 130% of baseline, S.E.M. Day 1 = 0.06, S.E.M. Day 2 = 0.05); Day 1: t(41) = −7.578, p < 0.001; Day 2: t(42) = −10.1735, p < 0.001) during the IA task, consistent with the expected increase in neural processing (Figure 3).
Figure: 3. Glutamate Day One & Day Two.
Amygdala ECF glutamate levels in 12-week-old animals, shown as percent of baseline level. The black line denotes Day 1 (training) and the dashed line denotes Day 2 (testing); glutamate levels did not differ significantly across days for any sample. Asterisks indicate significant difference from baseline for both days (p < .05). The shaded rectangle represents the maze-testing period. Data are means +/− S.E.M.
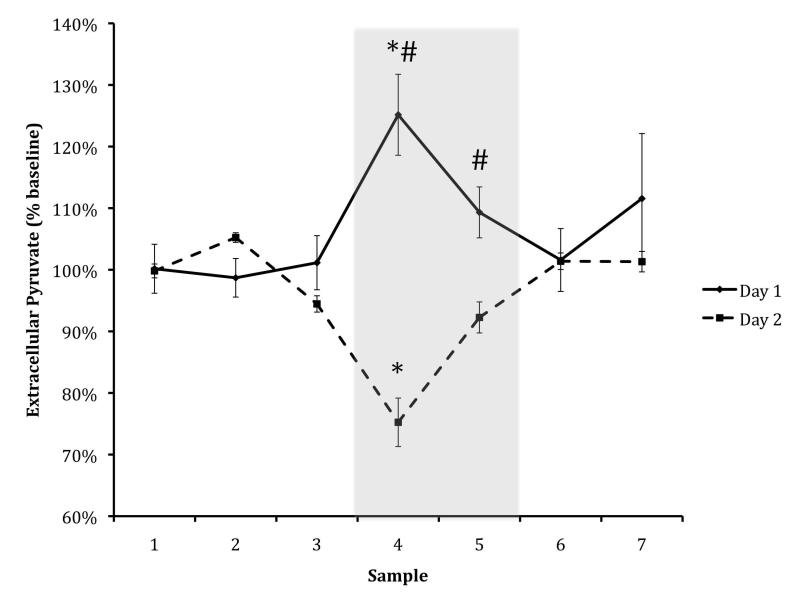
Interestingly, on Day 1 there was a marked rise in pyruvate during the training period, while on Day 2 there was a marked dip during the testing period (to 120% of baseline, S.E.M. Day 1 = 0.0.07; t(42) = −4.749, p < 0.001, and 80% of baseline, S.E.M. Day 2 = 0.04; t(41) = 3.849, p < 0.001, respectively). There was also a significant difference in both maze collection samples (4 and 5) across days (t(19) = 5.00, p < 0.001; t(20) = 2.51, p = 0.02, respectively; Figure 4) . This difference across days suggests that although both initial learning and retrieval are metabolically demanding, there may perhaps be a difference in amygdalar oxidative phosphorylation between the two processes.
Figure: 4. Pyruvate Day One & Day Two.
Amygdala ECF pyruvate levels in 12-week-old animals, shown as percent of baseline level. The black line denotes Day 1 (training) and the dashed line denotes Day 2 (testing); pyruvate levels differed significantly across days during maze testing. Asterisks indicate significant difference from baseline for both days (p < .05); hash tags indicate significant differences in sample across days (p < .05). The shaded rectangle represents the maze-testing period. Data are means +/− S.E.M.
4. Discussion
The task-associated dip in ECF glucose and concurrent rise in ECF lactate seen on both days closely resemble those previously observed in the hippocampus during spatial memory processing [7, 11, 23], suggesting that the metabolic demands of mnemonic processing may be similar across brain regions, potentially with optimal memory performance being limited by available glucose supply, as we have previously seen in the hippocampus [7, 9, 25, 26]. Second, the processes underlying consolidation and retrieval in the amygdala are at least superficially similar neurochemically, with almost identical fluctuations in glucose, lactate and glutamate. Third, however, the markedly different pyruvate changes observed between the two days suggests that consolidation and retrieval differ either in the demands placed on oxidative metabolism, or in coupling of activity to blood flow and hence oxygen supply, or both; the exact reason for this difference remains to be determined.
Comparison of the first 10-minutes of training with the second 10-minutes of training revealed significant differences for all measures. This change is likely indicative of a decrease in metabolic load and/or a decline in the neural activity associated with post-learning consolidation.
The present study did not include a manipulation that would induce a reconsolidation impairment at the time of the retention test, so we are unable to confirm whether or not the retention test elicited activation of the mechanisms involved in memory reconsolidation. However, most studies of retrieval and reconsolidation involve re-exposing the animal to some aspect of the training (e.g., CS in fear conditioning) or providing a nonreinforced training trial (e.g., water maze with escape platform removed) [27-30]. Such protocols, by nature, elicit new learning through either extinction or a conditioning trial. Under such conditions, animals presumably retrieve the old consolidated memory of training and then update that memory. Because of the new learning, this approach makes it very difficult to determine the role of memory retrieval in reconsolidation. By adjusting reactivation protocols, some researchers have attempted to eliminate the role of new learning at reactivation. These studies have implied that the act of retrieving an old consolidated memory from storage is sufficient to elicit mechanisms of reconsolidation [30-34]. Based on this literature, we suggest that the first 10 minutes of the retention test, during which no animal had crossed into the dark chamber, may reflect memory retrieval and reconsolidation processes in the basolateral nucleus of the amygdala.
Due to the nature of the microdialysis testing, the animal is probed on Day 1 and for a second time on Day 2. This may produce glial scar tissue buildup, although the literature suggests that repeat measurements can reliably be taken for up to three days after initial probing [24, 35]. The fact that very similar changes in glucose and lactate were seen across days suggests that any impairment due to measurement was slight at most. It is potentially possible that the differences in pyruvate seen across days might be related to this repeated probing. The rise and dip in pyruvate are only seen during and immediately following testing, and if changes in oxidation were due to the probing and subsequent testing alterations in oxidative phosphorylation would be likely to be during baseline measures and would presumably persist through testing; therefore, it is unlikely that changes observed in oxidative metabolism across days are due to the microdialysis protocol. One possible confound that must be borne in mind is that any effect of re-probing (for instance, on oxidative phosphorylation) might only manifest during high metabolic demand, such as during maze testing.
A more likely explanation for the observed differences in ECF pyruvate across days, if not purely due to a difference in metabolic processing, is a differential alteration of local cerebral blood flow, presumably with greater flow and hence greater oxygen supply being seen on Day 2. The fact that processing on Day 2 might be more effective at coupling neural activity to vascular supply than on day one is a potentially interesting finding, and would if correct offer new insight into the mechanisms underpinning consolidation versus reconsolidation. Measurement of local cerebral blood flow under conditions similar to those used here could be used to address this question.
This study sampled exclusively from the left BLA using a 2mm probe. There has been mixed evidence as to the lateralization of amygdalar involvement in mnemonic processing [36-39], so that studies across both hemispheres might potentially uncover differences; however, previous studies from our lab and others [40, 41] to which the present data were designed to be compared have focused primarily on the left hemisphere, hence the unilateral design used here. The 2mm probe used here has been used successfully in BLA microdialysis studies [42-44], but it is possible that the data reported here reflect changes not only within the BLA but also in surrounding brain tissue.
5. Conclusion
Consistent with previous hippocampal data, avoidance training and testing caused an acute elevation of local metabolism within the basolateral amygdala, causing a decrease in ECF glucose and increased ECF lactate and glutamate. To our knowledge, these are the first microdialysis measurements of local metabolism in the amygdala during affective processing, and also the first attempt to compare neurochemical processes in the amygdala during consolidation and retrieval.
Highlights.
Mnemonic processing within the amygdala was assessed using in vivo microdialysis
Similarities in local glycolysis were seen during both consolidation and retrieval
Differences in oxidative phosphorlyation were also seen during mnemonic processing
These are the first microdialysis measurements of local metabolism in the amygdala
We are the first to compare neurochemical processes during mnemonic processing
Figure: 2. Lactate Day One & Day Two.
Amygdala ECF lactate levels in 12-week-old animals, shown as percent of baseline level. The black line denotes Day 1 (training) and the dashed line denotes Day 2 (testing). Asterisks indicate significant difference from baseline for both days (p < .05). The shaded rectangle represents the maze-testing period. Data are means +/− S.E.M.
Acknowledgement
Support provided, in part, by a Professional Development Grant to RWF from The College of Saint Rose, and by NIDDK R01 077106 to ECM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Myskiw JC, Fiorenza NG, Izquierdo LA, Izquierdo I. Molecular mechanisms in hippocampus and basolateral amygdala but not in parietal or cingulate cortex are involved in extinction of one-trial avoidance learning. Neurobiology of learning and memory. 2010;94:285–91. doi: 10.1016/j.nlm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- [2].Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:4787–95. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bahar A, Dorfman N, Dudai Y. Amygdalar circuits required for either consolidation or extinction of taste aversion memory are not required for reconsolidation. The European journal of neuroscience. 2004;19:1115–8. doi: 10.1111/j.0953-816x.2004.03215.x. [DOI] [PubMed] [Google Scholar]
- [4].Bush DE, Caparosa EM, Gekker A, Ledoux J. Beta-adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Frontiers in behavioral neuroscience. 2010;4:154. doi: 10.3389/fnbeh.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiology of learning and memory. 2002;78:539–52. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- [6].Werka T, Skar J, Ursin H. Exploration and avoidance in rats with lesions in amygdala and piriform cortex. Journal of comparative and physiological psychology. 1978;92:672–81. doi: 10.1037/h0077505. [DOI] [PubMed] [Google Scholar]
- [7].McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2881–5. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McNay EC, Gold PE. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56:B66–71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- [9].McNay EC, Canal CE, Sherwin RS, Gold PE. Modulation of memory with septal injections of morphine and glucose: effects on extracellular glucose levels in the hippocampus. Physiology & behavior. 2006;87:298–303. doi: 10.1016/j.physbeh.2005.10.016. [DOI] [PubMed] [Google Scholar]
- [10].McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiology of learning and memory. 2010;93:546–53. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McNay EC, Williamson A, McCrimmon RJ, Sherwin RS. Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes. 2006;55:1088–95. doi: 10.2337/diabetes.55.04.06.db05-1314. [DOI] [PubMed] [Google Scholar]
- [12].Canal CE, Stutz SJ, Gold PE. Glucose injections into the dorsal hippocampus or dorsolateral striatum of rats prior to T-maze training: modulation of learning rates and strategy selection. Learn Mem. 2005;12:367–74. doi: 10.1101/lm.88205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gold PE. Role of glucose in regulating the brain and cognition. The American journal of clinical nutrition. 1995;61:987S–95S. doi: 10.1093/ajcn/61.4.987S. [DOI] [PubMed] [Google Scholar]
- [14].Krebs-Kraft DL, Parent MB. Hippocampal infusions of glucose reverse memory deficits produced by co-infusions of a GABA receptor agonist. Neurobiology of learning and memory. 2008;89:142–52. doi: 10.1016/j.nlm.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee MK, Graham SN, Gold PE. Memory enhancement with posttraining intraventricular glucose injections in rats. Behavioral neuroscience. 1988;102:591–5. doi: 10.1037//0735-7044.102.4.591. [DOI] [PubMed] [Google Scholar]
- [16].McNay EC, Gold PE. Memory modulation across neural systems: intra-amygdala glucose reverses deficits caused by intraseptal morphine on a spatial task but not on an aversive task. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:3853–8. doi: 10.1523/JNEUROSCI.18-10-03853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pych JC, Kim M, Gold PE. Effects of injections of glucose into the dorsal striatum on learning of place and response mazes. Behavioural brain research. 2006;167:373–8. doi: 10.1016/j.bbr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [18].Korol DL, Gold PE. Glucose, memory, and aging. The American journal of clinical nutrition. 1998;67:764S–71S. doi: 10.1093/ajcn/67.4.764S. [DOI] [PubMed] [Google Scholar]
- [19].Manning CA, Parsons MW, Gold PE. Anterograde and retrograde enhancement of 24-h memory by glucose in elderly humans. Behavioral and neural biology. 1992;58:125–30. doi: 10.1016/0163-1047(92)90351-4. [DOI] [PubMed] [Google Scholar]
- [20].McNay EC, Gold PE. Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Behavioral and cognitive neuroscience reviews. 2002;1:264–80. doi: 10.1177/1534582302238337. [DOI] [PubMed] [Google Scholar]
- [21].McNay EC, Gold PE. Extracellular glucose concentrations in the rat hippocampus measured by zero-net-flux: effects of microdialysis flow rate, strain, and age. Journal of neurochemistry. 1999;72:785–90. doi: 10.1046/j.1471-4159.1999.720785.x. [DOI] [PubMed] [Google Scholar]
- [22].McNay EC, Sandusky LA, Pearson-Leary J. Hippocampal Insulin Microinjection and In vivo Microdialysis During Spatial Memory Testing. Journal of visualized experiments : JoVE. 2013 doi: 10.3791/4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McNay EC, Sherwin RS. From artificial cerebro-spinal fluid (aCSF) to artificial extracellular fluid (aECF): microdialysis perfusate composition effects on in vivo brain ECF glucose measurements. Journal of neuroscience methods. 2004;132:35–43. doi: 10.1016/j.jneumeth.2003.08.014. [DOI] [PubMed] [Google Scholar]
- [24].Benveniste H, Diemer NH. Cellular reactions to implantation of a microdialysis tube in the rat hippocampus. Acta neuropathologica. 1987;74:234–8. doi: 10.1007/BF00688186. [DOI] [PubMed] [Google Scholar]
- [25].McNay EC, Cotero VE. Mini-review: impact of recurrent hypoglycemia on cognitive and brain function. Physiology & behavior. 2010;100:234–8. doi: 10.1016/j.physbeh.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McNay EC, McCarty RC, Gold PE. Fluctuations in brain glucose concentration during behavioral testing: dissociations between brain areas and between brain and blood. Neurobiology of learning and memory. 2001;75:325–37. doi: 10.1006/nlme.2000.3976. [DOI] [PubMed] [Google Scholar]
- [27].Land C, Bunsey M, Riccio DC. Anomalous properties of hippocampal lesion-induced retrograde amnesia Psychobiology. 2000;28:476–85. [Google Scholar]
- [28].Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, Dudai Y, et al. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–89. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- [29].Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–6. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- [30].Rossato JI, Bevilaqua LR, Myskiw JC, Medina JH, Izquierdo I, Cammarota M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem. 2007;14:36–46. doi: 10.1101/lm.422607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Artinian J, De Jaeger X, Fellini L, de Saint Blanquat P, Roullet P. Reactivation with a simple exposure to the experimental environment is sufficient to induce reconsolidation requiring protein synthesis in the hippocampal CA3 region in mice. Hippocampus. 2007;17:181–91. doi: 10.1002/hipo.20256. [DOI] [PubMed] [Google Scholar]
- [32].Flint RW, Jr., Marino CL. Cycloheximide impairs reconsolidation of a contextually reactivated memory in a conditioned taste aversion paradigm. Behavioral neuroscience. 2007;121:433–8. doi: 10.1037/0735-7044.121.2.433. [DOI] [PubMed] [Google Scholar]
- [33].Flint RW, Jr., Valentine S, Papandrea D., Jr. Reconsolidation of a long-term spatial memory is impaired by cycloheximide when reactivated with a contextual latent learning trial in male and female rats. Neuroscience. 2007;148:833–44. doi: 10.1016/j.neuroscience.2007.07.022. [DOI] [PubMed] [Google Scholar]
- [34].Rodriguez-Ortiz CJ, Garcia-DeLaTorre P, Benavidez E, Ballesteros MA, Bermudez-Rattoni F. Intrahippocampal anisomycin infusions disrupt previously consolidated spatial memory only when memory is updated. Neurobiology of learning and memory. 2008;89:352–9. doi: 10.1016/j.nlm.2007.10.004. [DOI] [PubMed] [Google Scholar]
- [35].Benveniste H, Drejer J, Schousboe A, Diemer NH. Regional cerebral glucose phosphorylation and blood flow after insertion of a microdialysis fiber through the dorsal hippocampus in the rat. Journal of neurochemistry. 1987;49:729–34. doi: 10.1111/j.1471-4159.1987.tb00954.x. [DOI] [PubMed] [Google Scholar]
- [36].LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- [37].Lalumiere RT, McGaugh JL. Memory enhancement induced by post-training intrabasolateral amygdala infusions of beta-adrenergic or muscarinic agonists requires activation of dopamine receptors: Involvement of right, but not left, basolateral amygdala. Learn Mem. 2005;12:527–32. doi: 10.1101/lm.97405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Funayama ES, Grillon C, Davis M, Phelps EA. A double dissociation in the affective modulation of startle in humans: effects of unilateral temporal lobectomy. Journal of cognitive neuroscience. 2001;13:721–9. doi: 10.1162/08989290152541395. [DOI] [PubMed] [Google Scholar]
- [39].Stevenson CW. Role of amygdala-prefrontal cortex circuitry in regulating the expression of contextual fear memory. Neurobiology of learning and memory. 2011;96:315–23. doi: 10.1016/j.nlm.2011.06.005. [DOI] [PubMed] [Google Scholar]
- [40].McReynolds JR, Donowho K, Abdi A, McGaugh JL, Roozendaal B, McIntyre CK. Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiology of learning and memory. 2010;93:312–21. doi: 10.1016/j.nlm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. The European journal of neuroscience. 2002;16:1223–6. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- [42].Howland JG, Taepavarapruk P, Phillips AG. Glutamate receptor-dependent modulation of dopamine efflux in the nucleus accumbens by basolateral, but not central, nucleus of the amygdala in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1137–45. doi: 10.1523/JNEUROSCI.22-03-01137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ahn S, Phillips AG. Modulatin by central and basolateral amygdalar nuclei of dopaminergic correlates of feeding to satiety in the rat nucleus accumbens and medial prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:10958–65. doi: 10.1523/JNEUROSCI.22-24-10958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Buffalari DM, Grace AA. Anxiogenic modulation of spontaneous and evoked neuronal activity in the basolateral amygdala. Neuroscience. 2009;163:1069–77. doi: 10.1016/j.neuroscience.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]