Abstract
Oral lichen planus (OLP) is a common chronic disease of uncertain aetiology. Treatment of patients with symptomatic OLP represents a therapeutic challenge. Despite numerous existing remedies, there are many treatment failures. The diode laser therapy is used as a possible alternative method in the treatment of lichen planus. The patient with OLP lesions was treated using diode laser (940 nm) for the symptomatic relief of pain and burning sensation. The patient was assessed before, during and after the completion of the treatment weekly. The treatment was performed for 2 months and the patient showed complete remission of burning sensation and pain (visual analog scale 0%). The follow-up was performed for 7 months and no recurrence of burning sensation was found. Diode laser therapy seems to be an effective alternative treatment for relieving the symptoms of OLP.
Background
Recently, lasers have been used in the management of oral lichen planus (OLP) because they improve the efficacy of wound healing and eliminate the potential adverse effects caused by drugs. Diode laser (940 nm) is very effective in providing symptomatic relief of burning sensation in OLR patients.
Case presentation
A 25-year-old man complained of burning sensation (visual analog scale (VAS) 70%) in the right and left buccal mucosa since 1 year while eating hot and spicy food. He had an abusive habit of chewing gutkha. Intraoral examination revealed pigmented areas in both the right and left buccal mucosa. Biopsy was performed to confirm the diagnosis of OLR. After laser therapy sessions for 2 months, the burning sensation completely regressed to VAS 0%. There was no recurrence of burning sensation in the follow-up period of 7 months (figures 1–4).
Figure 1.
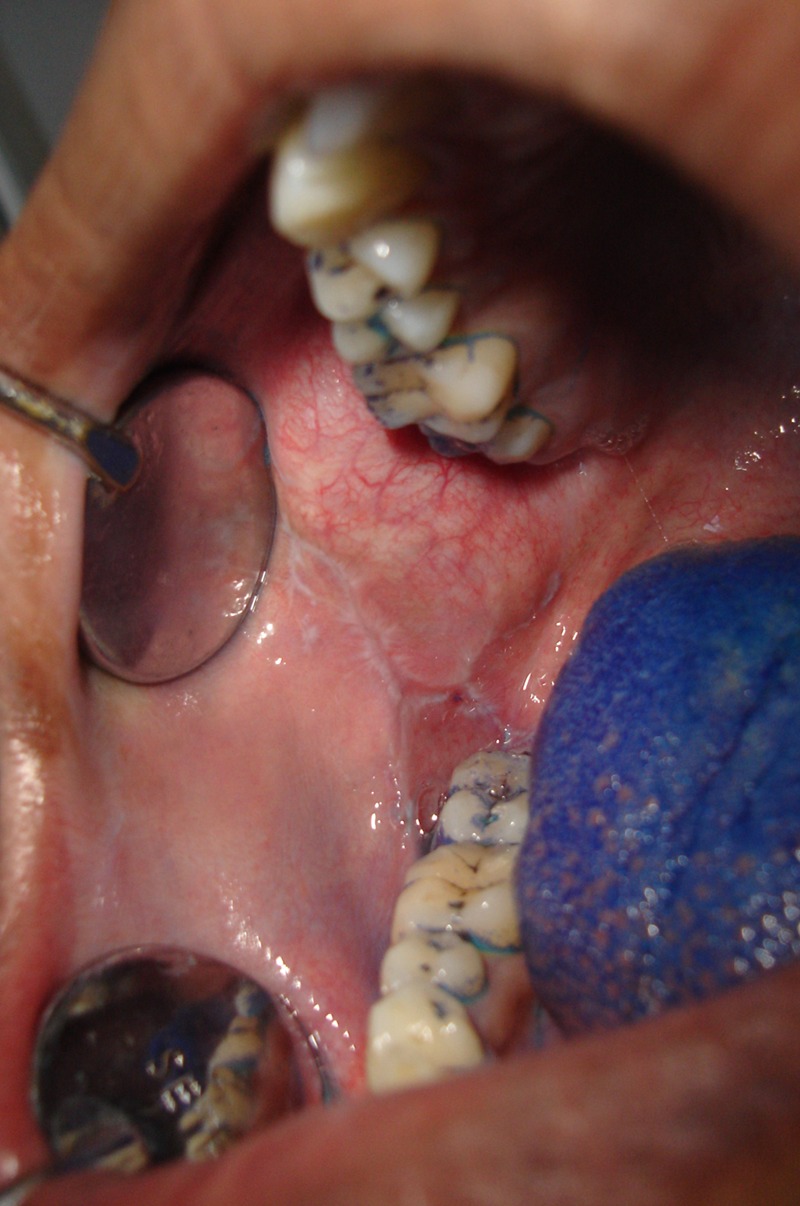
Right buccal mucosa preoperative.
Figure 2.

Biolase diode laser.
Figure 3.
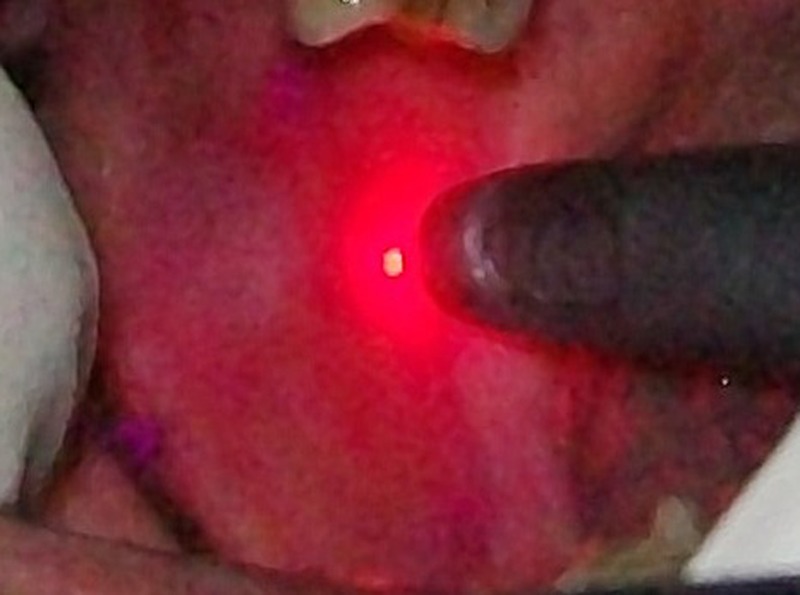
Intraoperative.
Figure 4.
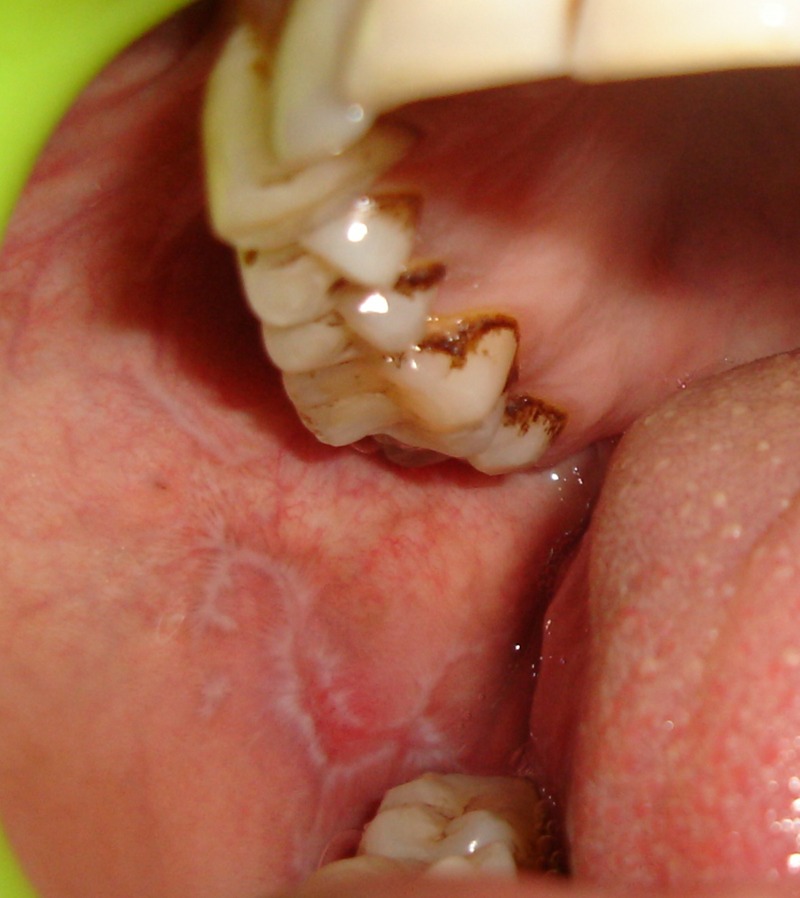
Right buccal mucosa after 2 months.
Investigations
- Blood examinations showed a normal profile
- Haemoglobin% 11.6 g%
- Total leucocyte count 6200 cells/mm3.
- Neutrophils 71%
- Lymphocytes 25%
- Eosinophils 03%
- Monocytes 01%
- Basophils 00%
- Erythrocyte sedimentation rate (Wintrob's method) 18 mm/h
- Bleeding time (Ivy's method) 2 min 48 s
- Clotting time (Lee and White method) 6 min 52 s
Histopathological examination revealed epithelium and underlying connective tissue stroma. The epithelium is stratified squamous parakeratinised in nature. The epithelium shows saw tooth rete ridges with basal cell degeneration. Few areas showing basal cell hyperplasia are also visualised. Connective tissue juxtaepithelially shows a dense band of inflammatory infiltrate predominantly lymphocytes. In the deeper submucosa, muscle fibres and nerve bundles cut in longitudinal and cross sections, vascular spaces and adipose cells are also visualised.
Treatment
Diode laser (940 nm) therapy was given twice weekly for 2 months.
Outcome and follow-up
Burning sensation gradually reduced from VAS 70% to VAS 0% in 2 months and no recurrence was found in 7 months follow-up time.
| Site | Before therapy | 1st week | 2 weeks | 3 weeks | 4 weeks | 5 weeks | 6 weeks | 7 weeks | 8 weeks | 7 months |
|---|---|---|---|---|---|---|---|---|---|---|
| B.M | VAS 75% | VAS 60% | VAS 50% | VAS 40% | VAS 35% | VAS 20% | VAS 15% | VAS 10% | VAS 0% | VAS 0 |
B.M, buccal mucosa; VAS, visual analog scale.
Discussion
OLP is a chronic inflammatory1 disease characterised by relapses and remissions. It is a cell-mediated immune condition of unknown aetiology, in which T lymphocytes accumulate beneath the epithelium of the oral mucosa and increase the rate of differentiation of the stratified squamous epithelium, resulting in hyperkeratosis and erythema with or without ulceration.
The T cells kill the target cell either by synthesis and extracellular release of cytotoxic proteins as perforin and granzymes, producing pores in the target cell membrane and so kill cell by osmotic lysis, or by stimulating the target cell, through mechanisms that are not well understood to undergo apoptosis.2
Despite numerous existing remedies, there are many treatment failures. One of the current approaches to the management of OLP includes diode laser therapy, a promising modality with minimum side-effects.
Clinical applications of the low-level laser therapy (LLLT) started to appear in the 1980s and became the most popular lasers which are relatively inexpensive diode units.3–6
The GaAs (gallium arsenide; 904 nm) diode laser and the GaAlAs (gallium aluminium arsenide; 780–890 nm).7
They have significant neuropharmacological effects on the synthesis, release and metabolism of a range of neurochemicals, including serotonin and acetylcholine at the central level and histamine and prostaglandin at the peripheral level.8
The pain influence has also been explained by the LLLT effect on enhanced synthesis of endorphin, decreased C-fibre activity, bradykinin and altered pain threshold.
It causes vasodilation and increases local blood flow which brings in oxygen and makes a greater movement of immune cells into the tissue.9
Diode is a solid active medium laser, manufactured from semiconductor crystals using some combination of aluminium or indium, gallium and arsenic. The available wavelengths for dental use range from about 800 nm for the active medium containing aluminium to 980 nm for the active medium composed of indium. All of the diode wavelengths are highly absorbed by pigmented tissue and are deeply penetrating.10
The principle of using diode is to supply direct biostimulative light energy to the body's cells. Cellular photoreceptors (eg, cytochromophores and antenna pigments) can absorb diode laser light and pass it on to mitochondria, which promptly produce the cell's fuel, ATP. It may have significant neuropharmacological effects on the synthesis, release and metabolism of a range of neurochemicals, including serotonin and acetylcholine at the central level and histamine and prostaglandin at the peripheral level. It has effect on enhanced synthesis of endorphin, decreased C-fibre activity, bradykinin and altered pain threshold.11
Diode laser is used in various dental procedures like non-surgical and surgical periodontal treatments, and in several clinical conditions like temporomandibular disorders, hypersensitive dentine, postextraction and bone-healing therapy, traumatic ulcerations, herpes simplex virus infection of the lips, aphthous ulcers, mucositis, paresthesiae and trigeminal neuralgia.
Treatment with diode laser is non-invasive and non-pharmaceutical.
Earlier studies conducted by Soliman et al in 2005 on 25 patients of OLP using diode laser (980 nm) observed marked clinical improvement in 64% patients with complete remission of symptoms, with recurrence observed in 12% of patients after 3 months. Cafaro et al in 2010 conducted a study on 13 patients of OLP using 904 nm pulsed infrared laser and observed that all patients reported a complete resolution of symptoms at the end of the laser sessions. Jajarm et al in 2011 conducted a comparative pilot study of low-intensity laser (LIL) versus topical corticosteroids in the treatment of erosive atrophic OLP and observed that LIL therapy (LILT) was as effective as topical corticosteroids but LILT did not exhibit unwanted side effects. The results of earlier studies that used excimer lasers were not satisfactory. Passeron et al used excimer lasers (308 nm) on four patients with OLP. Kollner et al also studied the effect of excimer lasers on eight patients with OLP, and only one patient responded completely after 12 sessions. In a study by Trehan et al, an excimer laser was used in eight patients with OLP who had previously failed to respond to traditional treatment and five patients improved more than 75%.
The patient of OLP was treated with diode laser at our institute and 100% resolution of symptoms was observed within 2 months and there were no side effects. No recurrence of symptoms was observed in the follow-up period of 7 months.
Learning points.
Early clinical detection of oral lichen planus (OLR) in the patient.
Early histopathological confirmation of OLR.
Preoperative and postoperative evaluation of burning sensation in the OLR patient.
Early institution of diode laser (940 nm) therapy.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bhattacharyya I, Cohen DM, Silverman S. Red and white lesions of the oral mucosa. In: Greenberg MS, Glick M, eds. Burket's oral medicine: diagnosis and treatment. 10th edn Ontario: B.C Decker, 2008;85–125 [Google Scholar]
- 2.Soliman M, Kharbotly AEL, Saafan A. Management of oral lichen planus using diode laser (980 nm). A clinical study. Egypt Dermatol Online Jl 2005;1:1–12 [Google Scholar]
- 3.Calderhead G, Ohshiro T, Itoh E, et al. The Nd:YAG and GaAlAs lasers: a comparative analysis in pain therapy. Laser Acupunc 1982;21:1–4 [Google Scholar]
- 4.Kleinkort JA, Foley RA. Laser acupuncture: its use in physical therapy. Am J Acupunc 1984;12:51 [Google Scholar]
- 5.Goujon C, Divol J, Moulin GL. Preliminary results of mid laser treatment of chronic ulcerations of legs. Lasers Surg Med 1985;5:78 [Google Scholar]
- 6.Kamikawa K, Kyoto J. Double blind experiences with mid-lasers in Japan. Proceedings of the International Congress on Lasers in Medicine and Surgery; Bologna, 1985:165–9 [Google Scholar]
- 7.Sun G, Tuner J. Low-level laser therapy in dentistry. Dent Clin N Am 2004;48:1061–76 [DOI] [PubMed] [Google Scholar]
- 8.Montesinos M. Experimental effects of low power laser in encephalon and endorphin synthesis. J Eur Med Laser Assoc 1988;1:2–7 [Google Scholar]
- 9.Luciana A L, Josepa R, Renato AZ, et al. Comparison of the low intensity laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluency. Laser Surg. Med 2001;29:179–84 [DOI] [PubMed] [Google Scholar]
- 10.Coluzzi DJ. Fundamentals of dental lasers: science and instruments. Dent Clin N Am 2004;48:751–70 [DOI] [PubMed] [Google Scholar]
- 11.Sun G, Tuner J. Low-level laser therapy in dentistry. Dent Clin N Am 2004;48:1061–76 [DOI] [PubMed] [Google Scholar]


