Abstract
Here we describe a patient with a rare movement disorder, hemichorea–hemiballismus, which is described as a complication of non-ketotic hyperglycaemia. This complication may be seen in individuals with poorly controlled long-standing diabetes mellitus. Proper diagnosis is established with CT and MRI of the brain, which typically show classic findings in the basal ganglia. Treatment focuses on improvement of glycaemic control and usually results in rapid resolution of the movement disorder. Nevertheless, recurrent episodes of hemichorea–hemiballismus, and even more ominous complications such as ischaemic stroke may occur.
Background
Non-ketotic hyperglycaemia hemichorea–hemiballismus (NKHCHB) denotes a rare syndrome characterised by involuntary, non-patterned, continuous and proximal movements on one side of the body, resulting from involvement of the contralateral basal ganglia during episodes of non-ketotic hyperglycaemia. This syndrome has been reported more frequently in elderly Asian women and resolution of symptoms is typically associated with normalisation of serum glucose levels and plasma osmolality. There is only one case in the literature that reports sudden resolution of NKHCHB after an ischaemic stroke 6 months after the beginning of symptoms.1 We describe a case of recurrent NKHCHB associated with ischaemic stroke during the acute phase of this rare metabolically induced movement disorder.
Case presentation
A 52-year-old previously healthy black man was evaluated in the emergency department for sudden-onset hemichoreic-hemiballistic movements of the left upper and lower extremities that started 2 days previously and progressively worsened over time. A detailed clinical examination including a complete neurological evaluation did not reveal other abnormalities. Importantly, there was no postural instability, ataxia, myoclonus, abnormalities on visual field evaluation, facial deviation, dysarthria or muscular weakness. Family history was significant for type 2 diabetes mellitus in two first-degree relatives but there was no family history of neurological or movement disorders.
Serum glucose level was 451 mg/dl (25 mmol/l) with no ketones present either in the blood or urine. Electrolytes: sodium 128 mEq/l, potassium 4.5 mEq/l, chloride 98 mEq/l, bicarbonate 25 mEq/l, calcium 8.9 mEq/l, magnesium 2.1 mg/dl. Measured serum osmolality was 288 mOsm/l. Peripheral blood cell counts, renal and hepatic function were normal. Thyroid-stimulating hormone assay was 2.8 mIU/l and glycated haemoglobin (HbA1c) assay was 10.8%.
Hyperglycaemia was easily corrected with parenteral hydration with isotonic saline solution and subcutaneous therapy with combination of a fast-acting insulin analogue (insulin aspart) and long-acting insulin (glargine). Normalisation of the serum glucose level during the first 24 h of hospitalisation was followed by complete resolution of the movement disorder with no residual neurological deficits noted. The patient was asymptomatic at the time of discharge 48 h after initial presentation and was prescribed metformin, glargine and insulin aspart. Nevertheless, non-compliance with diabetic therapy led to recurrent severe hyperglycaemia (577 mg/dl (27.2 mmol/l)) and left hemichorea–hemiballismus 1 week after the initial episode. This time the movement disorder persisted despite optimal glycaemic control and absence of other metabolic abnormalities. Unenhanced CT of the brain showed hyperatenuation of the right putamen and right peri-sylvian cortex (figure 1) and MRI demonstrated hyperintense signal in the same anatomic areas on T1-weighted images but no evidence of stroke, mass effect or brain oedema (figure 2). On the fifth day of hospitalisation, the movement disorder rapidly resolved but left-sided hemiparesis became progressively evident. Repeat CT scan of the brain demonstrated acute ischaemic stroke involving the right middle cerebral artery territory (figure 3). As the exact timing of the new neurological deficits was difficult to establish, reperfusion therapy was not used and the patient was treated conservatively for ischaemic stroke with good neurological recovery. Diabetes was adequately managed initially with insulin analogues and subsequently with oral medications (metformin and glyburide).
Figure 1.
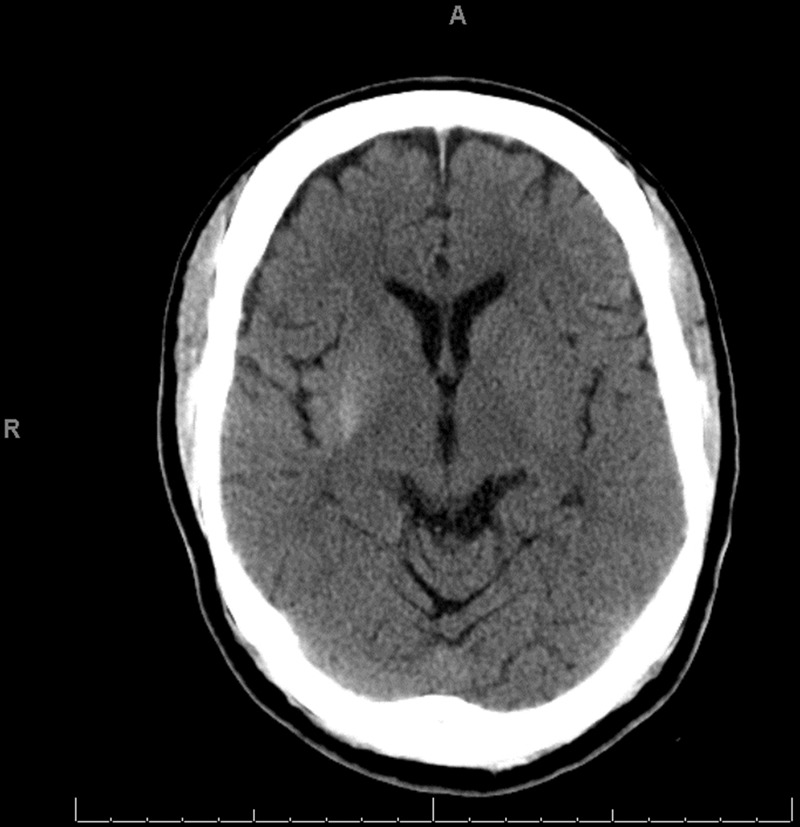
CT demonstrating hyperatenuation of the right putamen and right perisylvian cortex.
Figure 2.
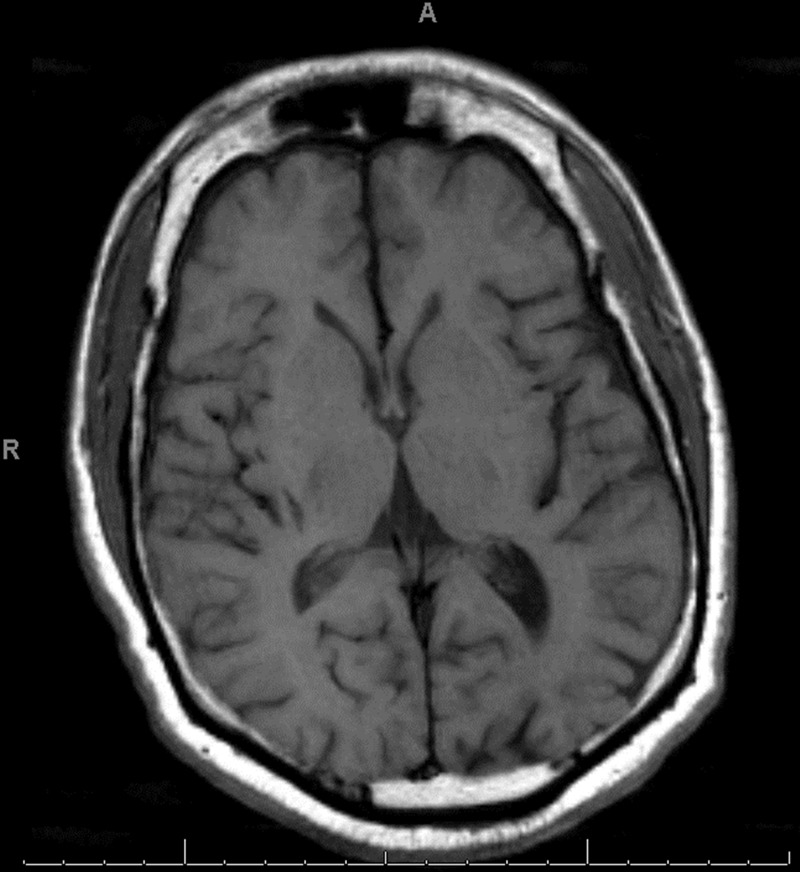
T1-weighted MRI showing a subtle hyperintense signal in the right putamen.
Figure 3.
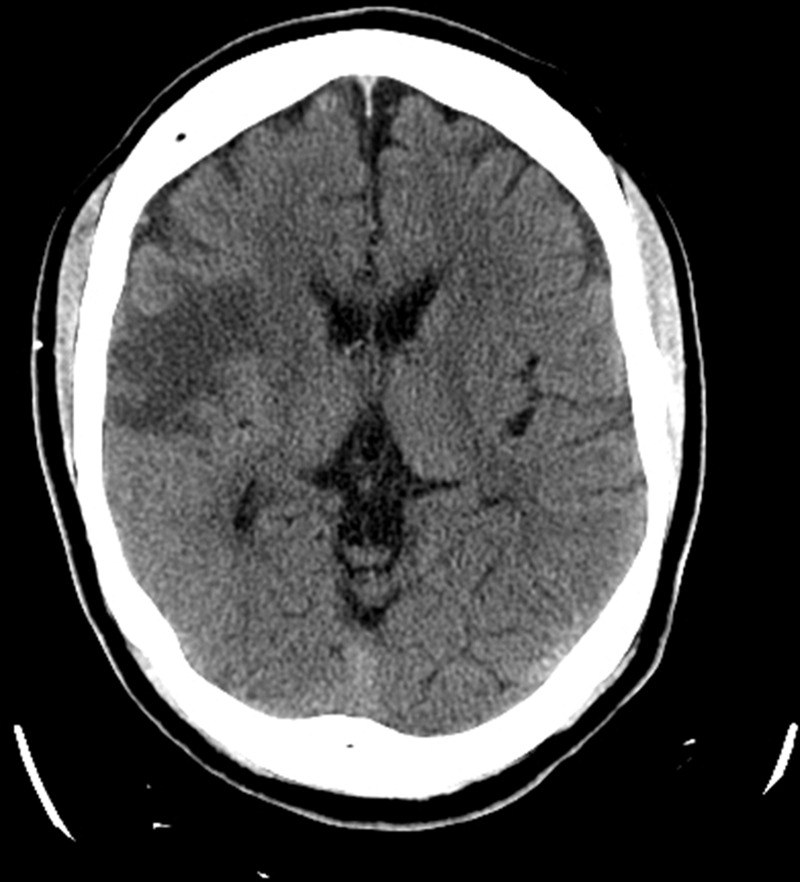
CT demonstrating a large ischaemic stroke in the right middle cerebral artery territory.
Differential diagnosis
Cerebrovascular disease: stroke involving the caudate, striatum, lenticular nucleus and thalamus; transient ischaemic attack; arteriovenous malformations, venous angiomas
Seizure disorders
Dystonia
Myoclonus
Infectious: bacterial, viral, fungal, mycobacterial, parasitic, prions
Metabolic derangements: hyperthyroidism, liver failure, renal failure, hyponatremia, hypocalcaemia, hypomagnesaemia, hypo/hyperglycaemia
Drugs: phenytoin intoxication
Neurodegenerative disorders: multiple sclerosis, systemic lupus erythematosus with central nervous system involvement
Tumours: primary brain tumours, metastatic brain tumours
Psychiatric disorders
Treatment
Adequate glycaemic control with metformin and glyburide (insulin therapy discontinued 1 week after discharge). Conservative medical treatment for ischaemic stroke including antiplatelet therapy and statin.
Outcome and follow-up
No significant neurological deficits from the stroke were noted after 4 weeks of physical rehabilitation. HbA1c was rechecked 3 months after discharge and was 7.2%. No recurrent abnormal movements of the extremities occurred.
Discussion
The first association of hemichorea and non-ketotic hyperglycaemia was reported in 1960.2 Poorly controlled diabetic patients with acute hyperglycaemic crises may present with this rare movement disorder known as NKHCHB; characterised by a typical triad: hemichorea–hemiballismus, contralateral radiological abnormalities in the striatum and rapid resolution of the symptoms after glycaemic normalisation with insulin and hydration.3–5
Cross-sectional brain imaging with CT scans typically demonstrate hyperatenuation in the striatum contralateral to the affected site, without mass effect, oedema or volume loss.3 6 The putamen is involved in all cases, the head of the caudate nucleus in most cases, and the globus pallidus in a minority of patients.3 MRI typically shows hyperintensity in the contralateral putamen in T1-weighted sequences. Findings in T2-weighted images are more variable, ranging from hyperintensity and isointensity to hypointensity.3 4 These changes in the basal ganglia have been also noted in other conditions such as hepatic encephalopathy, long-term parenteral nutrition, hyperglycaemia, postcardiac arrest encephalopathy, hypoglycaemic-induced coma and mild focal ischaemia.1 These imaging findings usually resolve completely in few months; nonetheless, persistent abnormalities have been reported for up to 6 years.7 Single-photon emission CT and positron emission tomography may show a decrease in the ratio of blood flow and reduced rates of brain glucose metabolism in the basal ganglia contralateral to the chorea.6
The exact pathophysiology of NKHCHB remains largely unknown; nevertheless, focal metabolic derangements secondary to hyperglycaemia-induced hyperviscosity, microvascular ischaemic injury and resulting hypoxia, alterations in dopaminergical activity in predisposed patients, and reactive gliosis have been postulated as hypotheses of the acute putaminal dysfunction seen in this syndrome.3–9 The striatum and subthalamic nucleus are the usual sites of injury resulting in reduced pallidal activity and thalamic disinhibition, which manifest as the classic movement disorder observed in this syndrome.
There is only a single previous report of NKHBC that ended in basal ganglion infarction 6 months after onset.1 We describe the second case of this disorder ceasing with ischaemic stroke 2 weeks after the onset of symptoms for the first time, and 5 days after recurrence of NKHBC. Although NKHBC and ischaemic brain injury may share a similar pathophysiology within a spectrum of ischaemic vascular disease, the development of macrovascular ischaemia resulting in gross infarction of the brain appears to be extremely unusual.
In conclusion, hemichorea–hemiballismus may be observed during hyperglycaemic crises and clinicians should be able to recognise this rare syndrome and its characteristic neuroimaging findings. Poorly controlled diabetes increases the risk of cerebrovascular ischaemia and reduces cerebral blood flow, which is the most common cause of hemiballismus and hemichorea originating from acute dysfunction in the basal ganglia. Rapid glucose-lowering therapy and hydration resolves symptoms quickly in most patients. Sudden cease of movements and appearance of new focal neurological sings require clinical reassessment and possibly neuroimaging studies to look for ischaemic or haemorrhagical insults.
Learning points.
Hyperglycaemia should be considered as a differential diagnosis in patients presenting with hemichorea–hemiballismus, and every patient presenting with this movement disorder should have a serum glucose level measured.
Rapid resolution of hyperglycaemic and/or hyperosmolar state typically results in rapid resolution of the movement disorder.
A high-level of suspicion for superimposed ischaemic brain injury should exist in patients with Non-ketotic hyperglycaemia hemichorea–hemiballismus, particularly when new focal neurological findings appear.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kim YD, Cho HJ, Song IU, et al. Complete disappearance of hemichorea-hemiballism due to hyperglycemia following acute ischemic stroke. Eur Neurol 2011;66:339–42 [DOI] [PubMed] [Google Scholar]
- 2.Bedwell SF. Some observations on hemiballismus. Neurology 1960;10:619–22 [DOI] [PubMed] [Google Scholar]
- 3.Battisti C, Forte F, Rubenni E, et al. Two cases of hemichorea-hemiballism with nonketotic hyperglycemia: a new point of view. Neurol Sci 2009;30:179–83 [DOI] [PubMed] [Google Scholar]
- 4.Wintermark M, Fischbein NJ, Mukherjee P, et al. Unilateral putaminal CT, MR, and diffusion abnormalities secondary to nonketotic hyperglycemia in the setting of acute neurologic symptoms mimicking stroke. AJNR Am J Neuroradiol 2004;25:975–6 [PMC free article] [PubMed] [Google Scholar]
- 5.Dewey RB, Jankovic J. Hemiballism-Hemichorea. Clinical and pharmacologic findings in 21 patients. Arch Neurol 1989;46:862–7 [DOI] [PubMed] [Google Scholar]
- 6.Qi X, Yan YY, Gao Y, et al. Hemichorea associated with non-ketotic hyperglycaemia: a case report. Diabetes Res Clin Pract 2012;95:e1–3 [DOI] [PubMed] [Google Scholar]
- 7.Oh SH, Lee KY, Im JH, et al. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci 2002;200:57–62 [DOI] [PubMed] [Google Scholar]
- 8.Chang KH, Tsou JC, Cheng ST, et al. Temporal features of magnetic resonance imaging and spectroscopy in non-ketotic hyperglycemic chorea-ballism patients. Eur J Neurol 2010;17:589–93 [DOI] [PubMed] [Google Scholar]
- 9.Narayanan S. Hyperglycemia-induced hemiballismus hemichorea: a case report and brief review of the literature. J Emerg Med 2012;43:442–4 [DOI] [PubMed] [Google Scholar]


