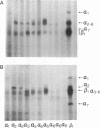
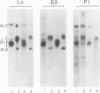
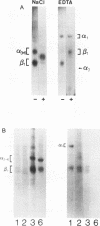
Abstract
A novel integrin, alpha 7 beta 1, that specifically binds with high affinity to laminin has been identified on melanoma cells. This complex was purified from both human and murine melanoma cells by laminin-affinity chromatography, and the alpha 7 subunit was recovered after gel electrophoresis. N-terminal amino acid sequence analysis of the alpha 7 subunit from both human and mouse cells verifies that this integrin is distinct from other alpha chains in the beta 1 family, although strikingly similar to the alpha 6 subunit. By using specific proteolytically derived fragments of laminin, it was determined that the alpha 7 beta 1 complex binds selectively to the E8 region, which represents part of the long arm of laminin. In contrast, the receptor failed to bind to the P1 fragment, which contains the intersection of the short arms of laminin. Although the alpha 7 beta 1 complex was commonly expressed in melanoma cells, this integrin was not detected in normal melanocytes, suggesting that alpha 7 expression may be associated with malignant transformation. These results establish the existence of a novel integrin that binds to the E8 domain of laminin and appears to mediate cell adhesion to this ligand.
Full text
PDF
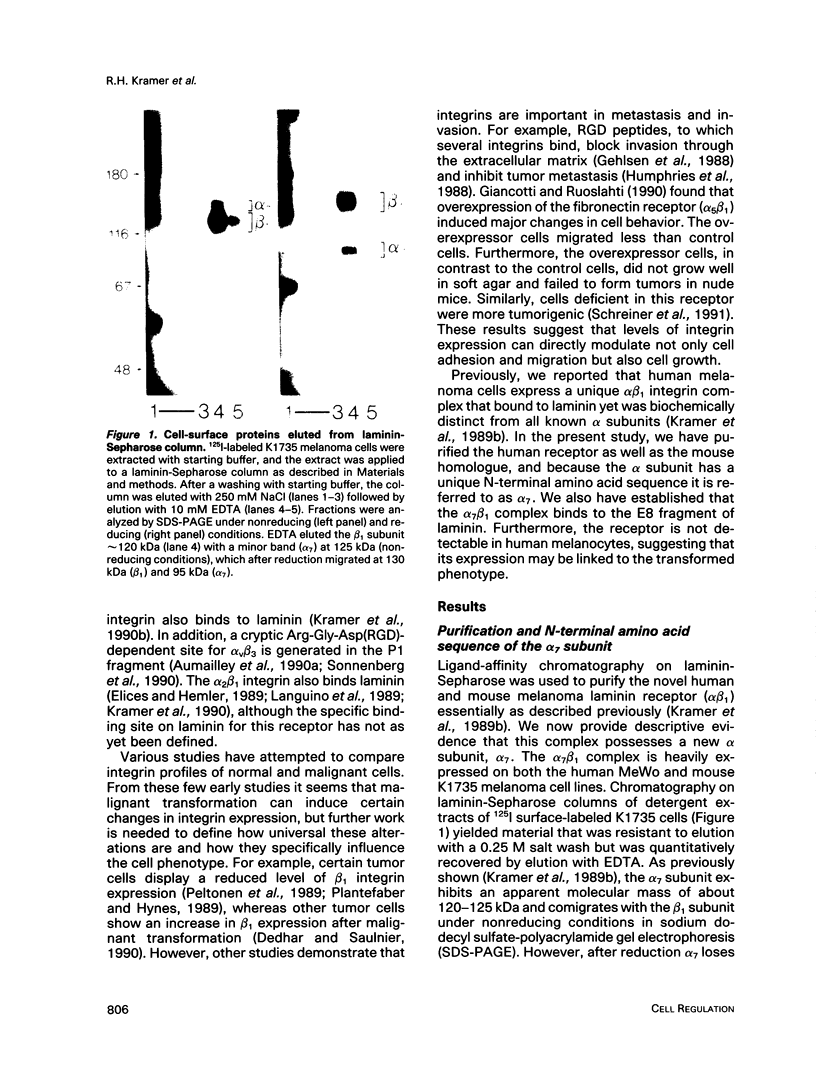


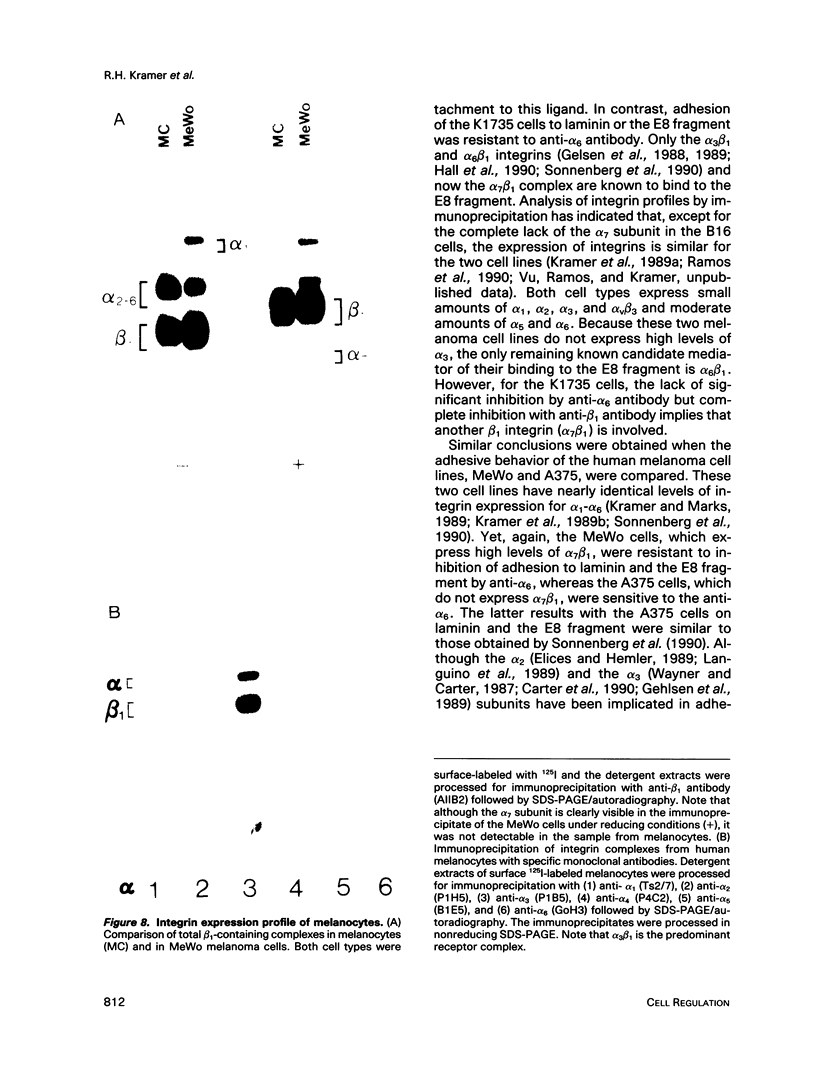




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Argraves W. S., Suzuki S., Arai H., Thompson K., Pierschbacher M. D., Ruoslahti E. Amino acid sequence of the human fibronectin receptor. J Cell Biol. 1987 Sep;105(3):1183–1190. doi: 10.1083/jcb.105.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley M., Timpl R., Sonnenberg A. Antibody to integrin alpha 6 subunit specifically inhibits cell-binding to laminin fragment 8. Exp Cell Res. 1990 May;188(1):55–60. doi: 10.1016/0014-4827(90)90277-h. [DOI] [PubMed] [Google Scholar]
- Barsky S. H., Rao C. N., Williams J. E., Liotta L. A. Laminin molecular domains which alter metastasis in a murine model. J Clin Invest. 1984 Sep;74(3):843–848. doi: 10.1172/JCI111501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Ryan M. C., Gahr P. J. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991 May 17;65(4):599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyman R. I., Turner D. C., Kramer R. H. An alpha 1/beta 1-like integrin receptor on rat aortic smooth muscle cells mediates adhesion to laminin and collagen types I and IV. Arteriosclerosis. 1990 May-Jun;10(3):402–409. doi: 10.1161/01.atv.10.3.402. [DOI] [PubMed] [Google Scholar]
- Dedhar S., Saulnier R. Alterations in integrin receptor expression on chemically transformed human cells: specific enhancement of laminin and collagen receptor complexes. J Cell Biol. 1990 Feb;110(2):481–489. doi: 10.1083/jcb.110.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrig K., Leivo I., Argraves W. S., Ruoslahti E., Engvall E. Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci U S A. 1990 May;87(9):3264–3268. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Hemler M. E. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9906–9910. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg E., Paulsson M., Timpl R., Johansson S. Characterization of a laminin receptor on rat hepatocytes. J Biol Chem. 1990 Apr 15;265(11):6376–6381. [PubMed] [Google Scholar]
- Gehlsen K. R., Argraves W. S., Pierschbacher M. D., Ruoslahti E. Inhibition of in vitro tumor cell invasion by Arg-Gly-Asp-containing synthetic peptides. J Cell Biol. 1988 Mar;106(3):925–930. doi: 10.1083/jcb.106.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlsen K. R., Dickerson K., Argraves W. S., Engvall E., Ruoslahti E. Subunit structure of a laminin-binding integrin and localization of its binding site on laminin. J Biol Chem. 1989 Nov 15;264(32):19034–19038. [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Loftus J. C., Plow E. F. Cytoadhesins, integrins, and platelets. Thromb Haemost. 1988 Feb 25;59(1):1–6. [PubMed] [Google Scholar]
- Hall D. E., Reichardt L. F., Crowley E., Holley B., Moezzi H., Sonnenberg A., Damsky C. H. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990 Jun;110(6):2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Clark W. H., Rodeck U., Mancianti M. L., Jambrosic J., Koprowski H. Biology of tumor progression in human melanocytes. Lab Invest. 1987 May;56(5):461–474. [PubMed] [Google Scholar]
- Herlyn M. Human melanoma: development and progression. Cancer Metastasis Rev. 1990 Sep;9(2):101–112. doi: 10.1007/BF00046337. [DOI] [PubMed] [Google Scholar]
- Houghton A. N., Real F. X., Davis L. J., Cordon-Cardo C., Old L. J. Phenotypic heterogeneity of melanoma. Relation to the differentiation program of melanoma cells. J Exp Med. 1987 Mar 1;165(3):812–829. doi: 10.1084/jem.165.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Yamada K. M., Olden K. Investigation of the biological effects of anti-cell adhesive synthetic peptides that inhibit experimental metastasis of B16-F10 murine melanoma cells. J Clin Invest. 1988 Mar;81(3):782–790. doi: 10.1172/JCI113384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Ignatius M. J., Large T. H., Houde M., Tawil J. W., Barton A., Esch F., Carbonetto S., Reichardt L. F. Molecular cloning of the rat integrin alpha 1-subunit: a receptor for laminin and collagen. J Cell Biol. 1990 Aug;111(2):709–720. doi: 10.1083/jcb.111.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. P., Lehmann J. M., Stade B. G., Rothbächer U., Sers C., Riethmüller G. Functional aspects of three molecules associated with metastasis development in human malignant melanoma. Invasion Metastasis. 1989;9(6):338–350. [PubMed] [Google Scholar]
- Kajiji S., Tamura R. N., Quaranta V. A novel integrin (alpha E beta 4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. EMBO J. 1989 Mar;8(3):673–680. doi: 10.1002/j.1460-2075.1989.tb03425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C. E., Steinmayer T., Kaufmann D., Weber L., Bröcker E. B. Identification of a melanoma progression antigen as integrin VLA-2. J Invest Dermatol. 1991 Feb;96(2):281–284. doi: 10.1111/1523-1747.ep12464485. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Cheng Y. F., Clyman R. Human microvascular endothelial cells use beta 1 and beta 3 integrin receptor complexes to attach to laminin. J Cell Biol. 1990 Sep;111(3):1233–1243. doi: 10.1083/jcb.111.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H., Marks N. Identification of integrin collagen receptors on human melanoma cells. J Biol Chem. 1989 Mar 15;264(8):4684–4688. [PubMed] [Google Scholar]
- Kramer R. H., McDonald K. A., Crowley E., Ramos D. M., Damsky C. H. Melanoma cell adhesion to basement membrane mediated by integrin-related complexes. Cancer Res. 1989 Jan 15;49(2):393–402. [PubMed] [Google Scholar]
- Kramer R. H., Vu M., Cheng Y. F., Ramos D. M. Integrin expression in malignant melanoma. Cancer Metastasis Rev. 1991 May;10(1):49–59. doi: 10.1007/BF00046843. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984 Mar 16;67(2):379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- Languino L. R., Gehlsen K. R., Wayner E., Carter W. G., Engvall E., Ruoslahti E. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. J Cell Biol. 1989 Nov;109(5):2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. Migration and differentiation of neural crest cells. Curr Top Dev Biol. 1980;16:31–85. doi: 10.1016/s0070-2153(08)60153-2. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Lotan R., Raz A. Lectins in cancer cells. Ann N Y Acad Sci. 1988;551:385–398. doi: 10.1111/j.1749-6632.1988.tb22372.x. [DOI] [PubMed] [Google Scholar]
- McGregor B. C., McGregor J. L., Weiss L. M., Wood G. S., Hu C. H., Boukerche H., Warnke R. A. Presence of cytoadhesins (IIb-IIIa-like glycoproteins) on human metastatic melanomas but not on benign melanocytes. Am J Clin Pathol. 1989 Oct;92(4):495–499. doi: 10.1093/ajcp/92.4.495. [DOI] [PubMed] [Google Scholar]
- Peltonen J., Larjava H., Jaakkola S., Gralnick H., Akiyama S. K., Yamada S. S., Yamada K. M., Uitto J. Localization of integrin receptors for fibronectin, collagen, and laminin in human skin. Variable expression in basal and squamous cell carcinomas. J Clin Invest. 1989 Dec;84(6):1916–1923. doi: 10.1172/JCI114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantefaber L. C., Hynes R. O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989 Jan 27;56(2):281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- Ramos D. M., Berston E. D., Kramer R. H. Analysis of integrin receptors for laminin and type IV collagen on metastatic B16 melanoma cells. Cancer Res. 1990 Feb 1;50(3):728–734. [PubMed] [Google Scholar]
- Rossino P., Gavazzi I., Timpl R., Aumailley M., Abbadini M., Giancotti F., Silengo L., Marchisio P. C., Tarone G. Nerve growth factor induces increased expression of a laminin-binding integrin in rat pheochromocytoma PC12 cells. Exp Cell Res. 1990 Jul;189(1):100–108. doi: 10.1016/0014-4827(90)90262-9. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Giancotti F. G. Integrins and tumor cell dissemination. Cancer Cells. 1989 Dec;1(4):119–126. [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Pierschbacher M., Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82(Pt A):803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Schreiner C., Fisher M., Hussein S., Juliano R. L. Increased tumorigenicity of fibronectin receptor deficient Chinese hamster ovary cell variants. Cancer Res. 1991 Mar 15;51(6):1738–1740. [PubMed] [Google Scholar]
- Sonnenberg A., Linders C. J., Modderman P. W., Damsky C. H., Aumailley M., Timpl R. Integrin recognition of different cell-binding fragments of laminin (P1, E3, E8) and evidence that alpha 6 beta 1 but not alpha 6 beta 4 functions as a major receptor for fragment E8. J Cell Biol. 1990 Jun;110(6):2145–2155. doi: 10.1083/jcb.110.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Argraves W. S., Arai H., Languino L. R., Pierschbacher M. D., Ruoslahti E. Amino acid sequence of the vitronectin receptor alpha subunit and comparative expression of adhesion receptor mRNAs. J Biol Chem. 1987 Oct 15;262(29):14080–14085. [PubMed] [Google Scholar]
- Taichman D. B., Cybulsky M. I., Djaffar I., Longenecker B. M., Teixidó J., Rice G. E., Aruffo A., Bevilacqua M. P. Tumor cell surface alpha 4 beta 1 integrin mediates adhesion to vascular endothelium: demonstration of an interaction with the N-terminal domains of INCAM-110/VCAM-1. Cell Regul. 1991 May;2(5):347–355. doi: 10.1091/mbc.2.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Elices M. J., Crouse C., Hemler M. E. The primary structure of the alpha 4 subunit of VLA-4: homology to other integrins and a possible cell-cell adhesion function. EMBO J. 1989 May;8(5):1361–1368. doi: 10.1002/j.1460-2075.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Hemler M. E. The primary structure of the VLA-2/collagen receptor alpha 2 subunit (platelet GPIa): homology to other integrins and the presence of a possible collagen-binding domain. J Cell Biol. 1989 Jul;109(1):397–407. doi: 10.1083/jcb.109.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Strominger J. L., Hemler M. E. The very late antigen family of heterodimers is part of a superfamily of molecules involved in adhesion and embryogenesis. Proc Natl Acad Sci U S A. 1987 May;84(10):3239–3243. doi: 10.1073/pnas.84.10.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura R. N., Rozzo C., Starr L., Chambers J., Reichardt L. F., Cooper H. M., Quaranta V. Epithelial integrin alpha 6 beta 4: complete primary structure of alpha 6 and variant forms of beta 4. J Cell Biol. 1990 Oct;111(4):1593–1604. doi: 10.1083/jcb.111.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Aumailley M., Gerl M., Mann K., Nurcombe V., Edgar D., Deutzmann R. Structure and function of the laminin-nidogen complex. Ann N Y Acad Sci. 1990;580:311–323. doi: 10.1111/j.1749-6632.1990.tb17940.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Paulsson M., Dziadek M., Fujiwara S. Basement membranes. Methods Enzymol. 1987;145:363–391. doi: 10.1016/0076-6879(87)45021-0. [DOI] [PubMed] [Google Scholar]
- Tsuji T., Yamamoto F., Miura Y., Takio K., Titani K., Pawar S., Osawa T., Hakomori S. Characterization through cDNA cloning of galactoprotein b3 (Gap b3), a cell surface membrane glycoprotein showing enhanced expression on oncogenic transformation. Identification of Gap b3 as a member of the integrin superfamily. J Biol Chem. 1990 Apr 25;265(12):7016–7021. [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]