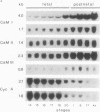
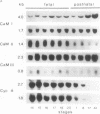
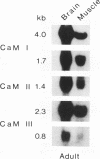
Abstract
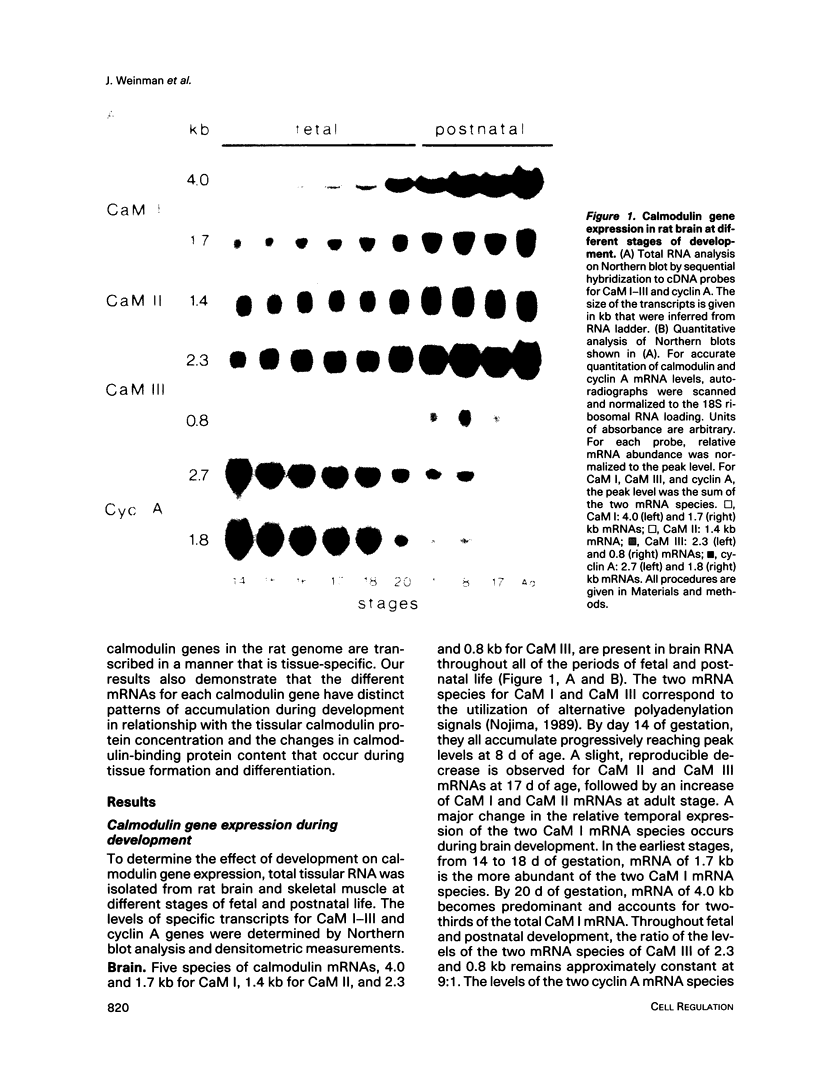
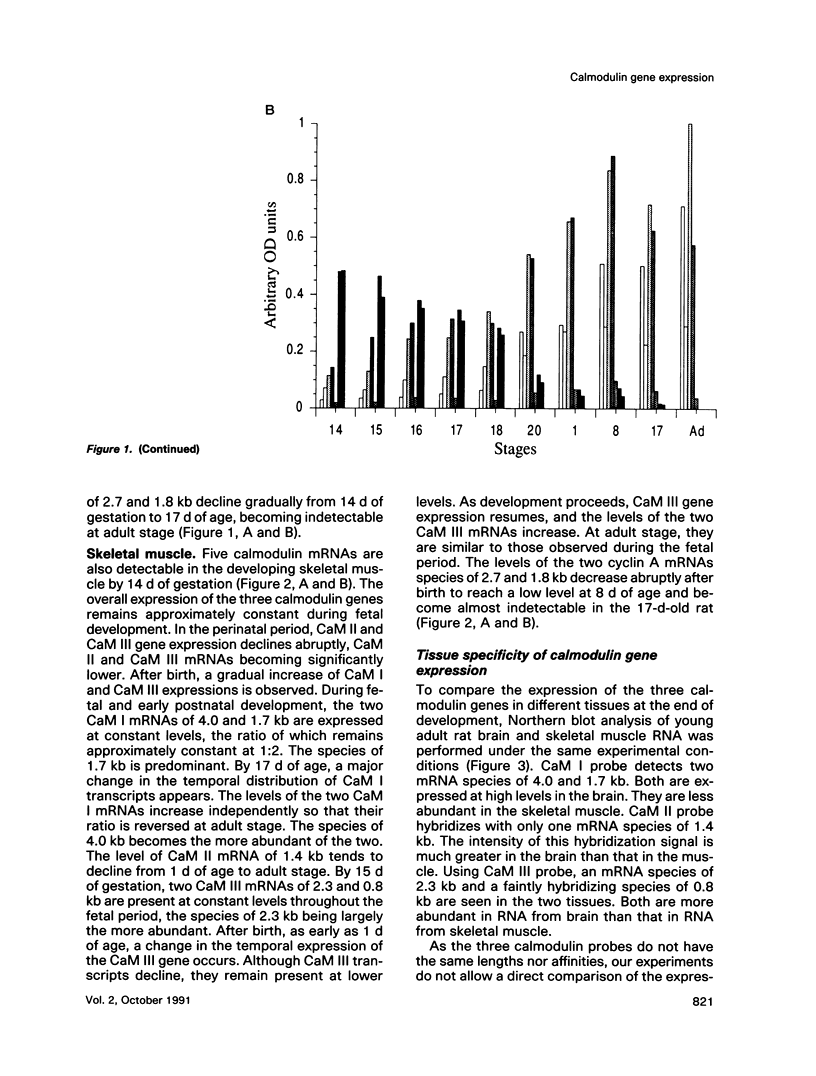
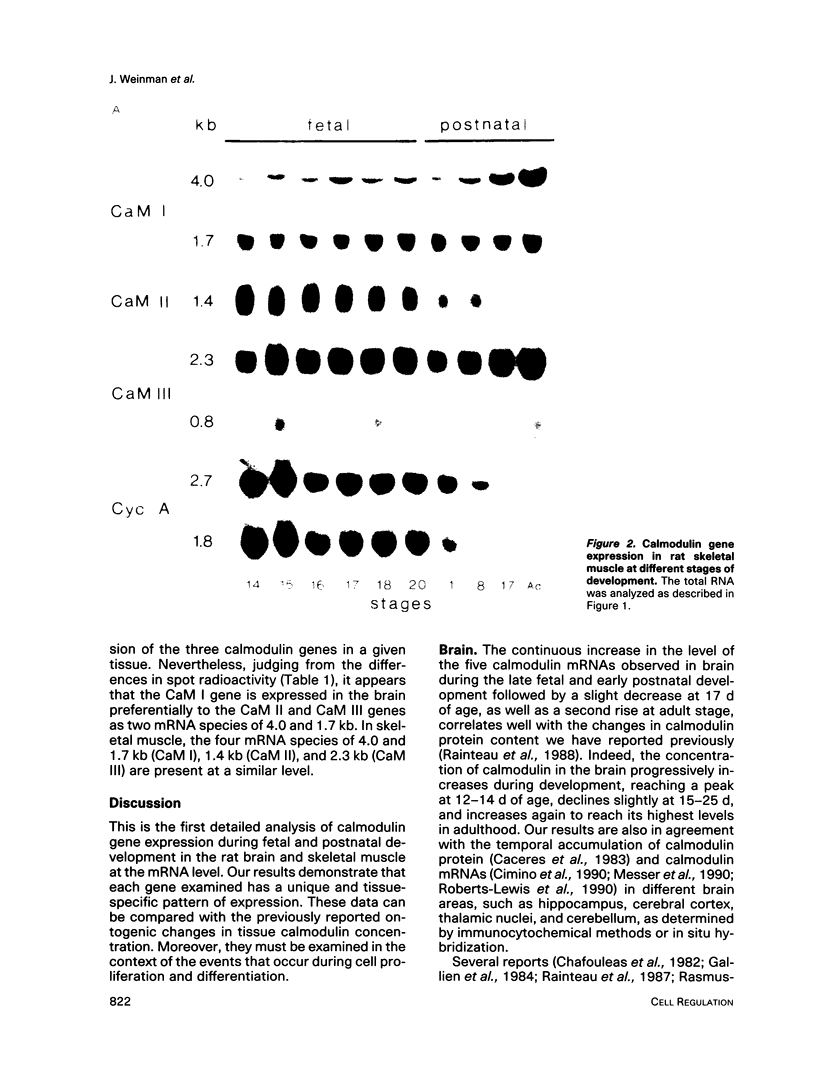
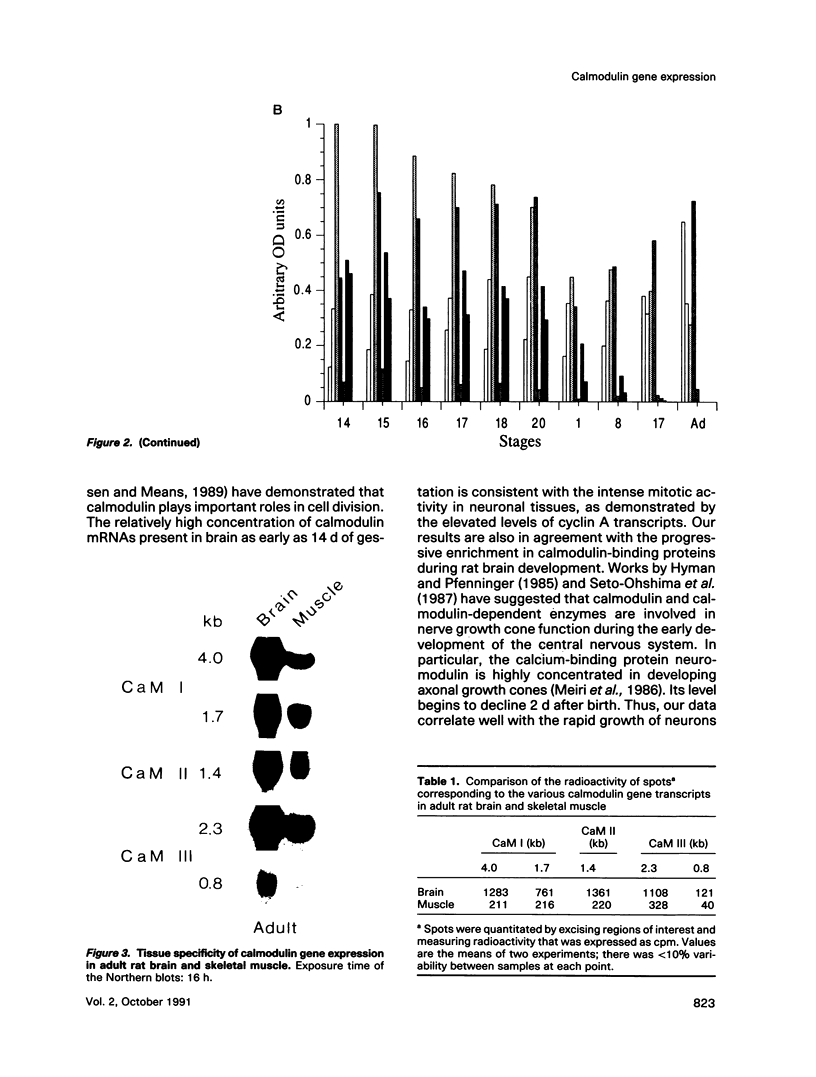
Three different calmodulin genes that encode the identical protein have been identified in the rat (Nojima, 1989); however, calmodulin gene expression at the various stages of tissue differentiation and maturation has not been previously determined. We have quantitated the content of mRNAs encoding calmodulin in the developing brain and skeletal muscle using RNA blot analysis with three specific cDNA probes. Our results show that five species of calmodulin mRNAs: 4.0 and 1.7 kb for CaM I, 1.4 kb for CaM II, and 2.3 and 0.8 kb for CaM III are detectable at all ages in the brain as well as in skeletal muscle but exhibit a tissue-specific developmental pattern of expression. The comparison of the temporal pattern of calmodulin gene expression with both mitotic activity, as demonstrated by cyclin A mRNA levels, and differentiation and maturation of specific brain or muscle regions is consistent with calmodulin involvement in development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu Y. S., Sack J. S., Greenhough T. J., Bugg C. E., Means A. R., Cook W. J. Three-dimensional structure of calmodulin. Nature. 1985 May 2;315(6014):37–40. doi: 10.1038/315037a0. [DOI] [PubMed] [Google Scholar]
- Billingsley M. L., Polli J. W., Balaban C. D., Kincaid R. L. Developmental expression of calmodulin-dependent cyclic nucleotide phosphodiesterase in rat brain. Brain Res Dev Brain Res. 1990 May 1;53(2):253–263. doi: 10.1016/0165-3806(90)90015-q. [DOI] [PubMed] [Google Scholar]
- Caceres A., Bender P., Snavely L., Rebhun L. I., Steward O. Distribution and subcellular localization of calmodulin in adult and developing brain tissue. Neuroscience. 1983 Oct;10(2):449–461. doi: 10.1016/0306-4522(83)90145-8. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Bolton W. E., Hidaka H., Boyd A. E., 3rd, Means A. R. Calmodulin and the cell cycle: involvement in regulation of cell-cycle progression. Cell. 1982 Jan;28(1):41–50. doi: 10.1016/0092-8674(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. S., VanTuinen P., Reeves A. A., Philip B. A., Caskey C. T. Isolation of cDNA clones for the catalytic gamma subunit of mouse muscle phosphorylase kinase: expression of mRNA in normal and mutant Phk mice. Proc Natl Acad Sci U S A. 1987 May;84(9):2886–2890. doi: 10.1073/pnas.84.9.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cimino M., Chen J. F., Weiss B. Ontogenetic development of calmodulin mRNA in rat brain using in situ hybridization histochemistry. Brain Res Dev Brain Res. 1990 Jun 1;54(1):43–49. doi: 10.1016/0165-3806(90)90063-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gallien C. L., Weinman J., Rainteau D., Weinman S. Changes in calmodulin level after fertilization and during first cleavage in the egg of the urodelan amphibian Pleurodeles waltlii. Exp Cell Res. 1984 Dec;155(2):397–405. doi: 10.1016/0014-4827(84)90200-3. [DOI] [PubMed] [Google Scholar]
- Goldenring J. R., McGuire J. S., Jr, DeLorenzo R. J. Identification of the major postsynaptic density protein as homologous with the major calmodulin-binding subunit of a calmodulin-dependent protein kinase. J Neurochem. 1984 Apr;42(4):1077–1084. doi: 10.1111/j.1471-4159.1984.tb12713.x. [DOI] [PubMed] [Google Scholar]
- Greeb J., Shull G. E. Molecular cloning of a third isoform of the calmodulin-sensitive plasma membrane Ca2+-transporting ATPase that is expressed predominantly in brain and skeletal muscle. J Biol Chem. 1989 Nov 5;264(31):18569–18576. [PubMed] [Google Scholar]
- Harris A. J., Fitzsimons R. B., McEwan J. C. Neural control of the sequence of expression of myosin heavy chain isoforms in foetal mammalian muscles. Development. 1989 Dec;107(4):751–769. doi: 10.1242/dev.107.4.751. [DOI] [PubMed] [Google Scholar]
- Hyman C., Pfenninger K. H. Intracellular regulators of neuronal sprouting: calmodulin-binding proteins of nerve growth cones. J Cell Biol. 1985 Sep;101(3):1153–1160. doi: 10.1083/jcb.101.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. T., Shields S., Conway K., Yip R., Burgin K. Developmental changes in calmodulin-kinase II activity at brain synaptic junctions: alterations in holoenzyme composition. J Neurochem. 1987 Dec;49(6):1927–1940. doi: 10.1111/j.1471-4159.1987.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Larson R. E., Espindola F. S., Espreafico E. M. Calmodulin-binding proteins and calcium/calmodulin-regulated enzyme activities associated with brain actomyosin. J Neurochem. 1990 Apr;54(4):1288–1294. doi: 10.1111/j.1471-4159.1990.tb01961.x. [DOI] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Meiri K. F., Pfenninger K. H., Willard M. B. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci U S A. 1986 May;83(10):3537–3541. doi: 10.1073/pnas.83.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer A., Plummer-Siegard J., Eisenberg B. Staggerer mutant mouse Purkinje cells do not contain detectable calmodulin mRNA. J Neurochem. 1990 Jul;55(1):293–302. doi: 10.1111/j.1471-4159.1990.tb08851.x. [DOI] [PubMed] [Google Scholar]
- Nojima H. Structural organization of multiple rat calmodulin genes. J Mol Biol. 1989 Jul 20;208(2):269–282. doi: 10.1016/0022-2836(89)90388-4. [DOI] [PubMed] [Google Scholar]
- Rainteau D. P., Weinman S. J., Kabaktchis C. A., Smith V. L., Kaetzel M. A., Dedman J. R., Weinman J. S. The expression of the 35- and 67-kDa calcimedins is dependent on thyroid hormone. J Biol Chem. 1988 Sep 15;263(26):12844–12848. [PubMed] [Google Scholar]
- Rainteau D., Sharif A., Bourrillon R., Weinman S. Calmodulin in lymphocyte mitogenic stimulation and in lymphoid cell line growth. Exp Cell Res. 1987 Feb;168(2):546–554. doi: 10.1016/0014-4827(87)90027-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen C. D., Means A. R. Calmodulin is required for cell-cycle progression during G1 and mitosis. EMBO J. 1989 Jan;8(1):73–82. doi: 10.1002/j.1460-2075.1989.tb03350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Lewis J. M., Cimino M., Krause R. G., 2nd, Tyrrell D. F., Jr, Davis L. G., Weiss B., Lewis M. E. Anatomical localization of calmodulin mRNA in the rat brain with cloned cDNA and synthetic oligonucleotide probes. Synapse. 1990;5(3):247–254. doi: 10.1002/syn.890050311. [DOI] [PubMed] [Google Scholar]
- Seto-Ohshima A., Yamazaki Y., Kawamura N., Kitajima S., Sano M., Mizutani A. The early expression of immunoreactivity for calmodulin in the nervous system of mouse embryos. Histochemistry. 1987;86(4):337–343. doi: 10.1007/BF00494990. [DOI] [PubMed] [Google Scholar]
- Slaughter G. R., Means A. R. Analysis of expression of multiple genes encoding calmodulin during spermatogenesis. Mol Endocrinol. 1989 Oct;3(10):1569–1578. doi: 10.1210/mend-3-10-1569. [DOI] [PubMed] [Google Scholar]
- Slaughter G. R., Meistrich M. L., Means A. R. Expression of RNAs for calmodulin, actins, and tubulins in rat testis cells. Biol Reprod. 1989 Feb;40(2):395–405. doi: 10.1095/biolreprod40.2.395. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Cohen P. Protein phosphatase-2B from rabbit skeletal muscle: a Ca2+-dependent, calmodulin-stimulated enzyme. Methods Enzymol. 1988;159:409–416. doi: 10.1016/0076-6879(88)59040-7. [DOI] [PubMed] [Google Scholar]
- Tallant E. A., Cheung W. Y. Calmodulin-dependent protein phosphatase: a developmental study. Biochemistry. 1983 Jul 19;22(15):3630–3635. doi: 10.1021/bi00284a014. [DOI] [PubMed] [Google Scholar]
- Tuana B. S., MacLennan D. H. Isolation of the calmodulin-dependent protein kinase system from rabbit skeletal muscle sarcoplasmic reticulum. FEBS Lett. 1988 Aug 1;235(1-2):219–223. doi: 10.1016/0014-5793(88)81266-3. [DOI] [PubMed] [Google Scholar]
- Wang J., Chenivesse X., Henglein B., Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990 Feb 8;343(6258):555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]