Abstract
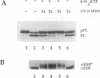
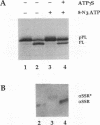
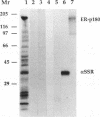
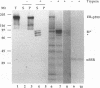
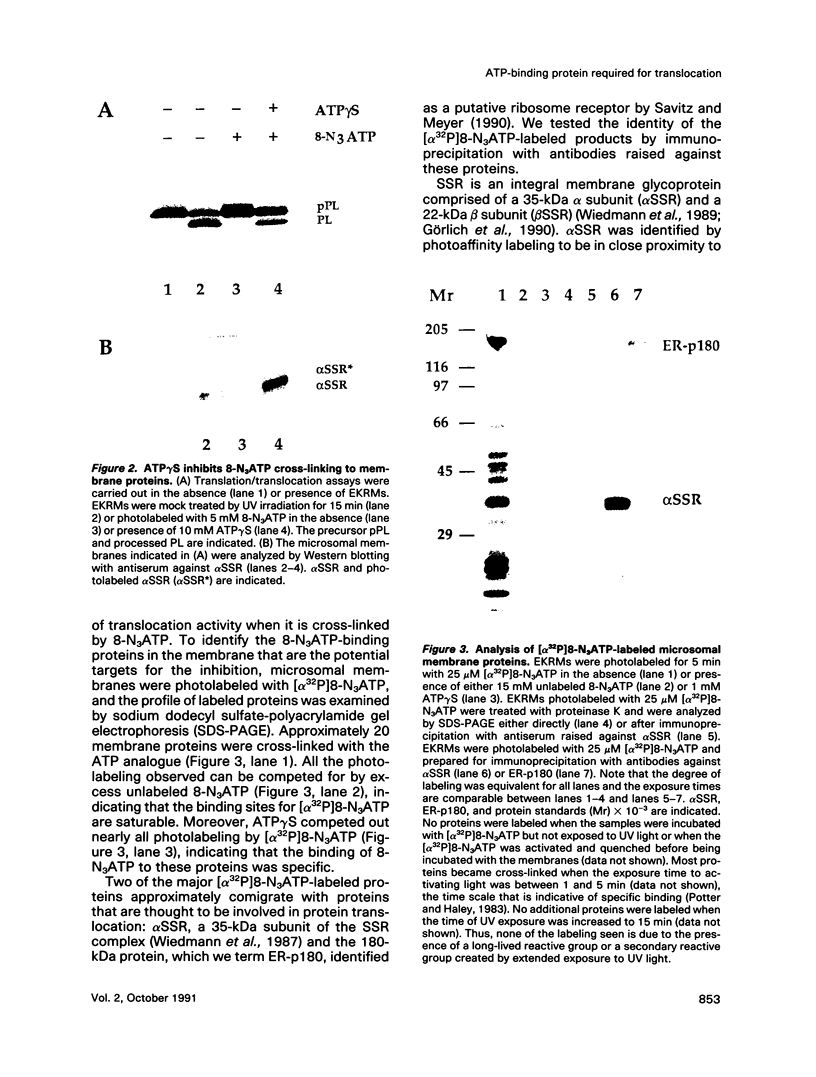
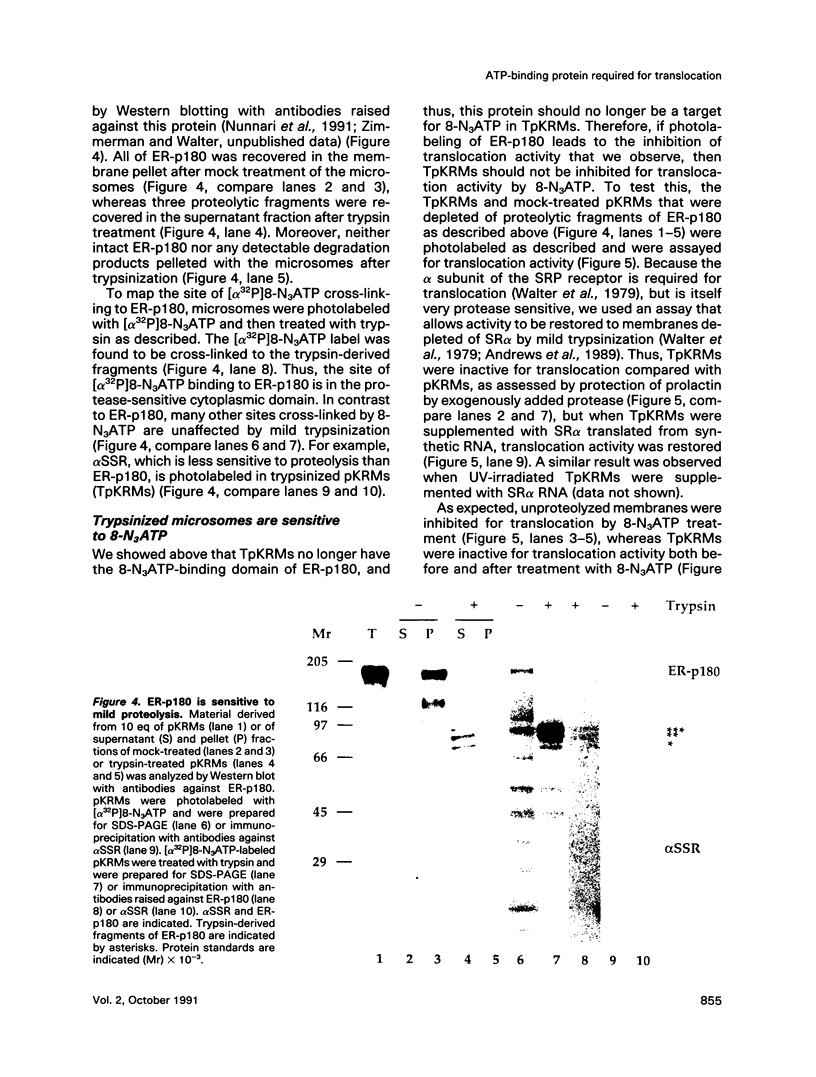
The role of nucleotides in providing energy for polypeptide transfer across the endoplasmic reticulum (ER) membrane is still unknown. To address this question, we treated ER-derived mammalian microsomal vesicles with a photoactivatable analogue of ATP, 8-N3ATP. This treatment resulted in a progressive inhibition of translocation activity. Approximately 20 microsomal membrane proteins were labeled by [alpha 32P]8-N3ATP. Two of these were identified as proteins with putative roles in translocation, alpha signal sequence receptor (SSR), the 35-kDa subunit of the signal sequence receptor complex, and ER-p180, a putative ribosome receptor. We found that there was a positive correlation between inactivation of translocation activity and photolabeling of alpha SSR. In contrast, our data demonstrate that the ATP-binding domain of ER-p180 is dispensable for translocation activity and does not contribute to the observed 8-N3ATP sensitivity of the microsomal vesicles.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Sabatini D. D., Blobel G. Ribosome-membrane interaction. Nondestructive disassembly of rat liver rough microsomes into ribosomal and membranous components. J Cell Biol. 1973 Jan;56(1):206–229. doi: 10.1083/jcb.56.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. W., Lauffer L., Walter P., Lingappa V. R. Evidence for a two-step mechanism involved in assembly of functional signal recognition particle receptor. J Cell Biol. 1989 Mar;108(3):797–810. doi: 10.1083/jcb.108.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. L., Tai P. C. Evidence for the involvement of ATP in co-translational protein translocation. Nature. 1987 Jul 9;328(6126):164–166. doi: 10.1038/328164a0. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Connolly T., Gilmore R. Formation of a functional ribosome-membrane junction during translocation requires the participation of a GTP-binding protein. J Cell Biol. 1986 Dec;103(6 Pt 1):2253–2261. doi: 10.1083/jcb.103.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T., Gilmore R. The signal recognition particle receptor mediates the GTP-dependent displacement of SRP from the signal sequence of the nascent polypeptide. Cell. 1989 May 19;57(4):599–610. doi: 10.1016/0092-8674(89)90129-3. [DOI] [PubMed] [Google Scholar]
- Connolly T., Rapiejko P. J., Gilmore R. Requirement of GTP hydrolysis for dissociation of the signal recognition particle from its receptor. Science. 1991 May 24;252(5009):1171–1173. doi: 10.1126/science.252.5009.1171. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Eilers M., Oppliger W., Schatz G. Both ATP and an energized inner membrane are required to import a purified precursor protein into mitochondria. EMBO J. 1987 Apr;6(4):1073–1077. doi: 10.1002/j.1460-2075.1987.tb04860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. A., Berrios M., Blobel G. Isolation and characterization of a proteinaceous subnuclear fraction composed of nuclear matrix, peripheral lamina, and nuclear pore complexes from embryos of Drosophila melanogaster. J Cell Biol. 1982 Mar;92(3):674–686. doi: 10.1083/jcb.92.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge U. I., Hinz G. Energy dependence of protein translocation into chloroplasts. Eur J Biochem. 1986 Nov 3;160(3):563–570. doi: 10.1111/j.1432-1033.1986.tb10075.x. [DOI] [PubMed] [Google Scholar]
- Garcia P. D., Walter P. Full-length prepro-alpha-factor can be translocated across the mammalian microsomal membrane only if translation has not terminated. J Cell Biol. 1988 Apr;106(4):1043–1048. doi: 10.1083/jcb.106.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Hartmann E., Herz J., Otto A., Kraft R., Wiedmann M., Knespel S., Dobberstein B., Rapoport T. A. The signal sequence receptor has a second subunit and is part of a translocation complex in the endoplasmic reticulum as probed by bifunctional reagents. J Cell Biol. 1990 Dec;111(6 Pt 1):2283–2294. doi: 10.1083/jcb.111.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen W., Garcia P. D., Walter P. In vitro protein translocation across the yeast endoplasmic reticulum: ATP-dependent posttranslational translocation of the prepro-alpha-factor. Cell. 1986 May 9;45(3):397–406. doi: 10.1016/0092-8674(86)90325-9. [DOI] [PubMed] [Google Scholar]
- Hartmann E., Wiedmann M., Rapoport T. A. A membrane component of the endoplasmic reticulum that may be essential for protein translocation. EMBO J. 1989 Aug;8(8):2225–2229. doi: 10.1002/j.1460-2075.1989.tb08346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Kassenbrock C. K., Kelly R. B. Interaction of heavy chain binding protein (BiP/GRP78) with adenine nucleotides. EMBO J. 1989 May;8(5):1461–1467. doi: 10.1002/j.1460-2075.1989.tb03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg U. C., Johnson A. E., Walter P. Protein translocation across the endoplasmic reticulum membrane: identification by photocross-linking of a 39-kD integral membrane glycoprotein as part of a putative translocation tunnel. J Cell Biol. 1989 Nov;109(5):2033–2043. doi: 10.1083/jcb.109.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg U. C., Walter P., Johnson A. E. Photocrosslinking of the signal sequence of nascent preprolactin to the 54-kilodalton polypeptide of the signal recognition particle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8604–8608. doi: 10.1073/pnas.83.22.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R., Cunningham K., Brundage L. A., Ito K., Oliver D., Wickner W. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 1989 Mar;8(3):961–966. doi: 10.1002/j.1460-2075.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M., Lodish H. F. Post-translational insertion of a fragment of the glucose transporter into microsomes requires phosphoanhydride bond cleavage. Nature. 1986 Aug 7;322(6079):549–552. doi: 10.1038/322549a0. [DOI] [PubMed] [Google Scholar]
- Nicchitta C. V., Blobel G. Assembly of translocation-competent proteoliposomes from detergent-solubilized rough microsomes. Cell. 1990 Jan 26;60(2):259–269. doi: 10.1016/0092-8674(90)90741-v. [DOI] [PubMed] [Google Scholar]
- Nunnari J. M., Zimmerman D. L., Ogg S. C., Walter P. Characterization of the rough endoplasmic reticulum ribosome-binding activity. Nature. 1991 Aug 15;352(6336):638–640. doi: 10.1038/352638a0. [DOI] [PubMed] [Google Scholar]
- Perara E., Rothman R. E., Lingappa V. R. Uncoupling translocation from translation: implications for transport of proteins across membranes. Science. 1986 Apr 18;232(4748):348–352. doi: 10.1126/science.3961485. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. Transport of F1-ATPase subunit beta into mitochondria depends on both a membrane potential and nucleoside triphosphates. FEBS Lett. 1986 Dec 15;209(2):152–156. doi: 10.1016/0014-5793(86)81101-2. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Tropschug M., Neupert W. Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell. 1987 Jun 19;49(6):815–823. doi: 10.1016/0092-8674(87)90619-2. [DOI] [PubMed] [Google Scholar]
- Potter R. L., Haley B. E. Photoaffinity labeling of nucleotide binding sites with 8-azidopurine analogs: techniques and applications. Methods Enzymol. 1983;91:613–633. doi: 10.1016/s0076-6879(83)91054-6. [DOI] [PubMed] [Google Scholar]
- Prehn S., Herz J., Hartmann E., Kurzchalia T. V., Frank R., Roemisch K., Dobberstein B., Rapoport T. A. Structure and biosynthesis of the signal-sequence receptor. Eur J Biochem. 1990 Mar 10;188(2):439–445. doi: 10.1111/j.1432-1033.1990.tb15421.x. [DOI] [PubMed] [Google Scholar]
- Rapoport T. A. Protein transport across the ER membrane. Trends Biochem Sci. 1990 Sep;15(9):355–358. doi: 10.1016/0968-0004(90)90076-n. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Meyer D. I. Secretion in yeast: translocation and glycosylation of prepro-alpha-factor in vitro can occur via an ATP-dependent post-translational mechanism. EMBO J. 1986 May;5(5):1031–1036. doi: 10.1002/j.1460-2075.1986.tb04318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz A. J., Meyer D. I. Identification of a ribosome receptor in the rough endoplasmic reticulum. Nature. 1990 Aug 9;346(6284):540–544. doi: 10.1038/346540a0. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Blobel G. A protein-conducting channel in the endoplasmic reticulum. Cell. 1991 May 3;65(3):371–380. doi: 10.1016/0092-8674(91)90455-8. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Walter P., Jackson R. C., Marcus M. M., Lingappa V. R., Blobel G. Tryptic dissection and reconstitution of translocation activity for nascent presecretory proteins across microsomal membranes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1795–1799. doi: 10.1073/pnas.76.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. G., Blobel G. Secretory protein translocation in a yeast cell-free system can occur posttranslationally and requires ATP hydrolysis. J Cell Biol. 1986 May;102(5):1543–1550. doi: 10.1083/jcb.102.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann M., Goerlich D., Hartmann E., Kurzchalia T. V., Rapoport T. A. Photocrosslinking demonstrates proximity of a 34 kDa membrane protein to different portions of preprolactin during translocation through the endoplasmic reticulum. FEBS Lett. 1989 Nov 6;257(2):263–268. doi: 10.1016/0014-5793(89)81549-2. [DOI] [PubMed] [Google Scholar]
- Wiedmann M., Kurzchalia T. V., Hartmann E., Rapoport T. A. A signal sequence receptor in the endoplasmic reticulum membrane. 1987 Aug 27-Sep 2Nature. 328(6133):830–833. doi: 10.1038/328830a0. [DOI] [PubMed] [Google Scholar]
- Yu Y. H., Zhang Y. Y., Sabatini D. D., Kreibich G. Reconstitution of translocation-competent membrane vesicles from detergent-solubilized dog pancreas rough microsomes. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9931–9935. doi: 10.1073/pnas.86.24.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D. L., Walter P. Reconstitution of protein translocation activity from partially solubilized microsomal vesicles. J Biol Chem. 1990 Mar 5;265(7):4048–4053. [PubMed] [Google Scholar]