Abstract
An otherwise healthy 17-year-old boy presented to the paediatric emergency department with acute severe epigastric pain. An admission abdominal radiograph demonstrated gastric dilation, associated with an elevated left hemidiaphragm. Subsequent barium contrast imaging confirmed the diagnosis of organoaxial acute gastric volvulus (AGV). Emergent exploratory laparoscopy revealed AGV with migration of the stomach, spleen, pancreatic tail, splenic flexure, left kidney and adrenal through a left-sided Bochdalek diaphragmatic hernia. Following careful mobilisation of the displaced structures, a mesh closure of the diaphragmatic defect was performed. The patient's postoperative chest radiograph was unremarkable, and he was discharged on the sixth postoperative day after an uneventful recovery. At 2 months the patient was well and asymptomatic, with normal barium contrast imaging results.
Background
Acute gastric volvulus (AGV) is a rare condition which may be associated with Bochdalek diaphragmatic hernia (BDH). Although the vast majority of BDHs are detected within the first year of life, they must be considered in otherwise healthy children and adolescents who present with features of AGV. This case study aims to raise awareness on a surgical emergency and its underlying cause, which can be successfully managed with a totally laparoscopic approach.
Case presentation
A 17-year-old boy presented to the paediatric emergency department with acute severe epigastric pain which occurred a few minutes after meals. The patient denied nausea, vomiting and dyspnoea. His medical history was unremarkable. General physical examination revealed a young and otherwise healthy boy with normal vital signs and an isolated finding of epigastric tenderness.
Investigations
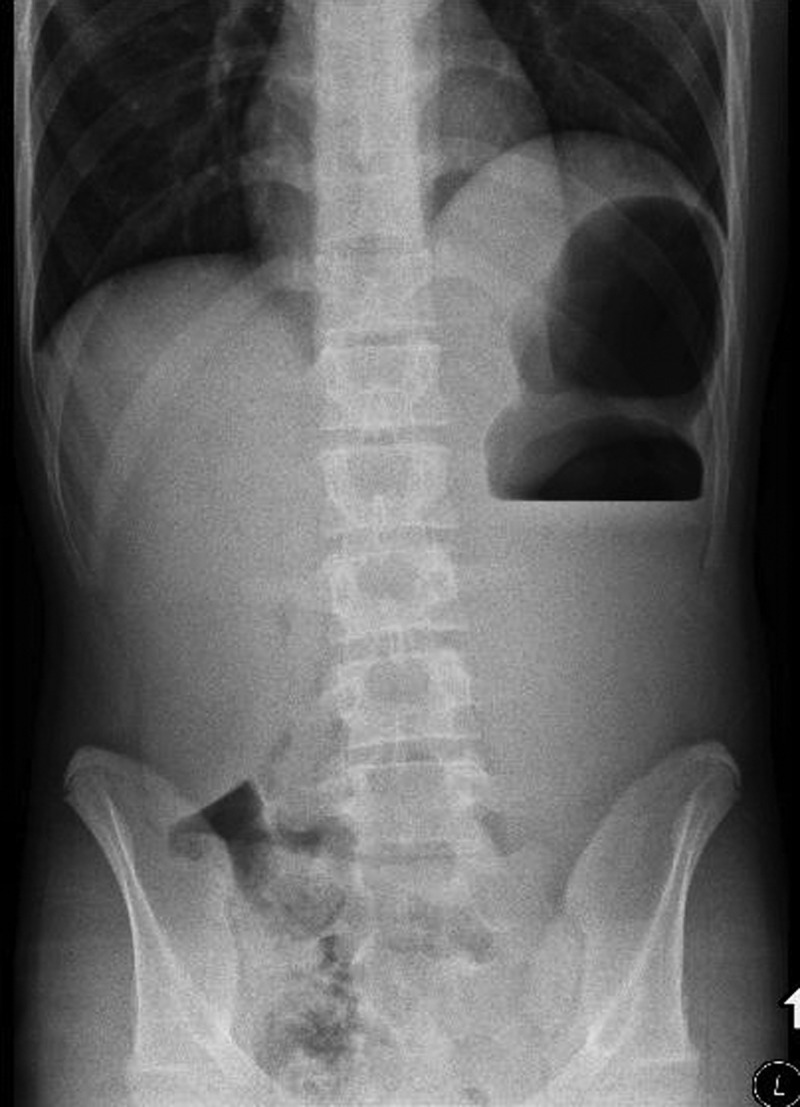
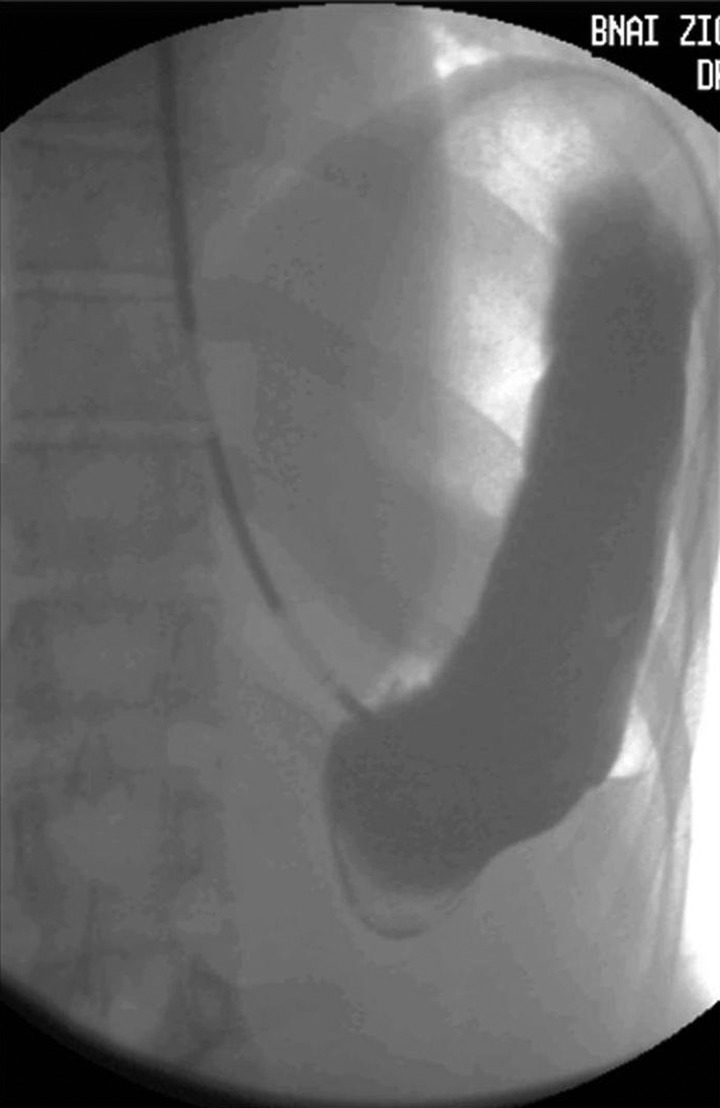
An admission abdominal radiograph demonstrated a dilated stomach measuring 9×26 cm, along with an elevated left hemidiaphragm (figure 1). A nasogastric tube was inserted and barium contrast imaging was performed (figure 2), confirming the diagnosis of organoaxial AGV which was apparent as incomplete gastric filling with luminal obstruction.
Figure 1.
Admission abdominal radiograph demonstrating a dilated stomach under an elevated left hemidiaphragm.
Figure 2.
Barium contrast study, diagnostic of acute organoaxial gastric volvulus.
Treatment
With the diagnosis of AGV, our patient was taken to the operating room for emergent exploratory surgery. Laparoscopy was initiated with a 30° 10 mm scope, a 12 mm working port and two 5 mm ports which allowed for traction and provided additional working channels. The procedure was performed under a 14 mm Hg CO2 pneumoperitoneum. As the stomach was reduced, a left-sided diaphragmatic hernia was discovered. The large defect permitted migration and volvulus of the stomach, as well as migration of the spleen and the pancreatic tail. The splenic flexure was fixed in the left chest as were the left kidney and adrenal. Another 10 mm port was inserted to produce good traction and exposure, allowing delicate adhesiolysis and thus enabling mobilisation of these structures into the abdomen and assessment of the diaphragmatic defect. Apart from a small anterior rim, the entire left hemidiaphragm was absent (figure 3). We used a 15 × 20 cm Physiomesh (Ethicon, Inc) to bridge the defect, fixing it to the diaphragmatic rim anteriorly, to the ribs posteriorly and to the left diaphragmatic crux medially using non-absorbable tacks (Covidien), continuous Ethibond Excel 2-0 (Ethicon, Inc) sutures and interrupted Ethibond Excel 2-0 (Ethicon, Inc) sutures (figure 4). The operative time was 120 min with negligible intraoperative blood loss.
Figure 3.
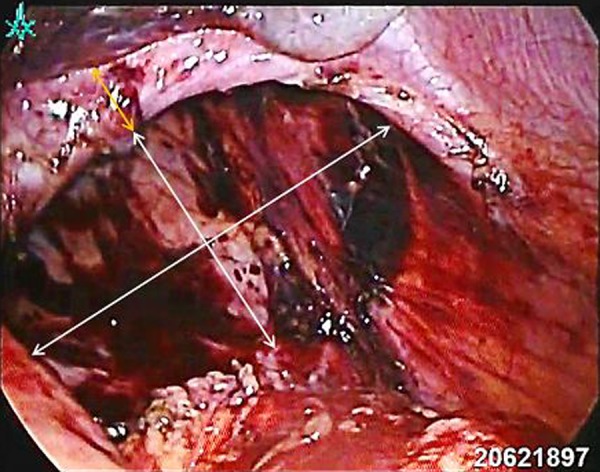
Laparoscopic view of a left-sided Bochdalek diaphragmatic hernia (white arrows) with a small anterior diaphragmatic rim (orange arrows).
Figure 4.
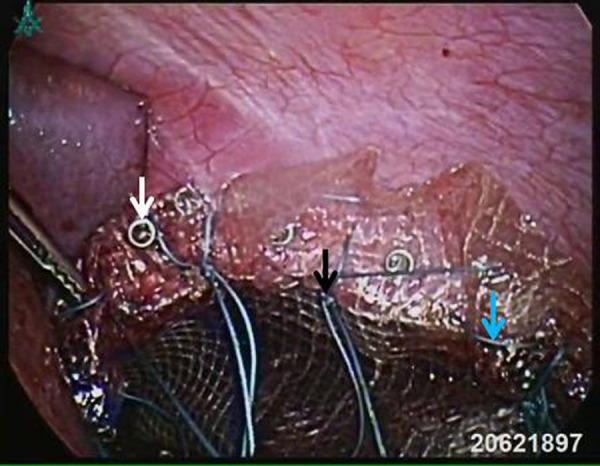
Laparoscopic view after mesh closure using non-absorbable tacks, as well as continuous and interrupted sutures (white, blue and black arrows, respectively).
Outcome and follow-up
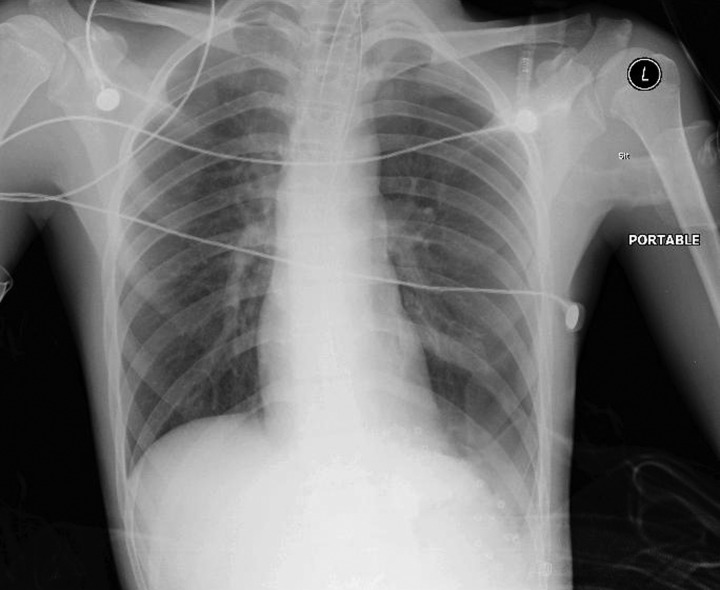
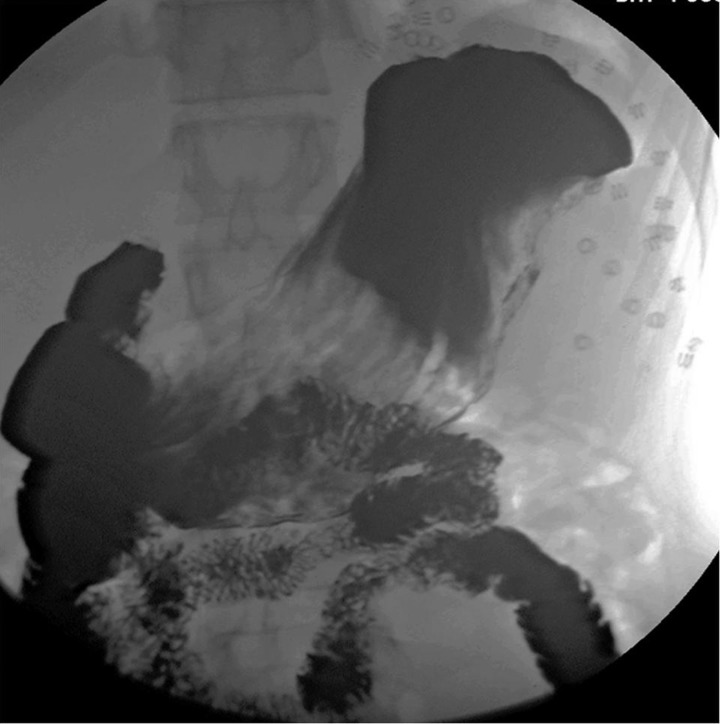
The patient's postoperative course was uneventful. He started drinking on the following day and had resumed a full diet by the second postoperative day. A chest radiograph performed on the first postoperative day demonstrated a flat left hemidiaphragm, and was otherwise reported as normal (figure 5). The patient was discharged home on the sixth postoperative day on a normal diet and oral omeprazole. At 2 months he was well and asymptomatic, with normal barium contrast imaging results (figure 6).
Figure 5.
Postoperative chest radiograph demonstrating a flat left hemidiaphragm.
Figure 6.
Follow-up barium contrast imaging, confirming normal gastric position and emptying, with non-absorbable tacks in situ.
Discussion
Gastric volvulus (GV) in infants, children and adolescents is a rare condition which describes the revolution of the stomach by at least 180° about an axis, causing foregut obstruction. A recent review identified 581 published cases of paediatric GV, 43% of which presented acutely. Over half of the patients presented as neonates or infants, with only 4% presenting between the ages of 13 and 18 years, as in our case.1
GV can be classified anatomically or aetiologically. The former classification, which was proposed by Singleton, is used more frequently and includes organoaxial, mesenteroaxial and combined-unclassified GV.2 In organoaxial GV (59% of cases) the stomach rotates around its longitudinal axis, whereas in mesenteroaxial GV (29% of cases) the stomach rotates around an axis which bisects the greater and lesser curvatures. In the remaining 12% of cases, elements of both these types are seen and GV is termed ‘combined-unclassified’.3
According to the aetiological classification on the other hand, GV can be divided into primary and secondary types. Primary GV results from the absence or abnormal laxity of the four gastric ‘anchors’: the gastrocolic, gastrohepatic, gastrophrenic and gastrosplenic ligaments. Finally, secondary GV is usually due to diaphragmatic defects, but may also be caused by anatomical variations such as abnormal adhesions, asplenism, gastrointestinal malformations or by gastroesophageal surgery.4
GV classically presents with Borchardt's triad, which was described more than a century ago. It consists of severe epigastric pain, non-productive retching and difficulty or inability to pass a nasogastric tube into the stomach.5 In 1980, Carter et al6 proposed three additional important features: minimal abdominal findings when the stomach is in the thorax, a gas-filled viscus in the lower chest or upper abdomen shown by chest radiography and obstruction at the site of volvulus shown by emergency upper gastrointestinal series.
A working knowledge of the aforementioned classifications is useful in anticipating anatomical challenges which may occur during surgery, since the majority of AGV cases (69%) are secondary, whereas the majority of chronic GV cases (74%) are of the primary type.1 Furthermore, an awareness of Borchardt's triad and Carter's features enables prompt diagnosis and treatment of a condition which, left untreated, confers a mortality risk as high as 80%.7 In this case, AGV was diagnosed on the basis of acute epigastric pain, a large gas-filled stomach shown by abdominal radiography, inability to pass a nasogastric tube and the contrast study findings. The presence of a diaphragmatic hernia, however, was confirmed intraoperatively.
Congenital diaphragmatic hernias (CDHs) have a prevalence of 1 in 2000–3000 newborns, the majority (70%) of which are BDHs, with the remainder consisting of Morgagni (27%) and central (2–3%) hernias.8 BDH, by definition, occurs when there is herniation through a persistent pleuroperitoneal cavity in the posterolateral diaphragm, known as the foramen of Bochdalek. There is a high left-sided predilection, and most BDHs (75–95%) are diagnosed perinatally by ultrasonography and/or MRI, and may be associated with other congenital malformations. In the commoner early-presenting cases, morbidity and mortality arises from respiratory distress, which is in turn due to pulmonary hypoplasia and hypertension.8 9 These young patients require postnatal resuscitation with ventilatory support, as well as an appropriately timed surgical correction of the diaphragmatic defect.10
In contrast to the neonatal presentation, a retrospective study based on approximately 13 000 abdominal CT scans identified only 22 adult patients with incidental BDH (an incidence of 0.17%), all of whom were asymptomatic. Symptomatic, late-presenting BDHs are considered rare and usually present with chronic pulmonary disease, bowel incarceration or intra-abdominal organ dysfunction as in the case of AGV.11
Although the pathogenesis of BDH and of CDHs in general is poorly understood, current speculation is that it arises from genetic defects which lead to abnormal heart, lung and diaphragm development and especially of the pleuroperitoneal folds. Recent studies have implicated recessive mutations in the FREM1 gene, which normally influences diaphragmatic development, in the causation of CDHs. Furthermore, a recent case–control study has found that newborns with this condition were more likely to have lower retinol and retinol-binding protein levels in cord blood samples, thus reinforcing the long-held theory that CDH can be caused by disturbances in the retinoid signalling pathway.12 13
Definitive treatment of a BDH involves reducing any herniated structures and repairing the diaphragmatic defect. Brown et al14 recently examined 173 cases of Bochdalek hernia and identified five different approaches: laparotomy (38%), thoracotomy (32%), laparoscopy (12%), thoracoscopy (3%) and combined (13%). Although the most commonly used approach is currently laparotomy, minimally invasive techniques such as laparoscopy are increasingly gaining ground, due to their favourable complication, mortality and recovery profiles.15 In the presence of AGV, laparoscopic exploration can be utilised to visualise the abdomen, identify the gastric position, reduce the stomach, repair the diaphragmatic defect and fix the stomach if necessary. One should keep in mind that younger patients with AGV might present with a Bochdalek-type defect rather than a paraesophageal hernia. Abdominal organs might therefore be found fixed in the chest from birth, creating difficulty in understanding the anatomy, and mobilising structures. In this case, dealing with the extremely displaced splenic flexure as well as the fixed left kidney and adrenal required the expertise of an experienced laparoscopic surgeon.
In conclusion, this case adds to the existing evidence that laparoscopic surgery for AGV and BDH is feasible and safe, allowing patients to quickly resume their daily activities. In addition, it highlights the importance of considering AGV as a cause of epigastric pain, and suspecting concurrent diaphragmatic hernias to ensure an appropriate surgical approach. In our view, the technique described is the correct option when performed by an experienced laparoscopic surgeon.
Learning points.
Laparoscopic treatment of acute gastric volvulus (AGV) and Bochdalek diaphragmatic hernia is feasible and safe in experienced hands.
Consider AGV as a cause of epigastric pain.
Suspect a diaphragmatic hernia in patients with AGV.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cribbs RK, Gow KW, Wulkan ML. Gastric volvulus in infants and children. Pediatrics 2008;122:e752–62 [DOI] [PubMed] [Google Scholar]
- 2.Singleton AC. Chronic gastric volvulus. Radiology 1940;34:53–61 [Google Scholar]
- 3.Milne LN, Hunter JJ. Gastric volvulus: 2 cases and review of the literature. J Emerg Med 1994;12:299–306 [DOI] [PubMed] [Google Scholar]
- 4.Miller DL, Pasquale MD, Seneca RP, et al. Gastric volvulus in the pediatric population. Arch Surg 1991;126:1146–9 [DOI] [PubMed] [Google Scholar]
- 5.Borchardt M. Zun pathologie and therapy des magnevolvulus. Arch Klin Chir 1904;74:243–8 [Google Scholar]
- 6.Carter R, Brewer LA, Hinshaw DB. Acute gastric volvulus: a study of 25 cases. Am J Surg 1980;140:99–106 [DOI] [PubMed] [Google Scholar]
- 7.Palanivelu C, Rangarajan M, Shetty AR, et al. Laparoscopic suture gastropexy for gastric volvulus: a report of 14 cases. Surg Endosc 2007;21:863–6 [DOI] [PubMed] [Google Scholar]
- 8.Van Loenhout RB, Tibboel D, Post M, et al. Congenital diaphragmatic hernia: comparison of animal models and relevance to the human situation. Neonatology 2009;96:137–49 [DOI] [PubMed] [Google Scholar]
- 9.Rout S, Foo FJ, Hayden JD, et al. Right-sided Bochdalek hernia obstructing in an adult: case report and review of the literature. Hernia 2007;11:359–62 [DOI] [PubMed] [Google Scholar]
- 10.Bohn D. Congenital diaphragmatic hernia. Am J Respir Crit Care Med 2002;166:911–15 [DOI] [PubMed] [Google Scholar]
- 11.Mullins ME, Stein J, Saini SS, et al. Prevalence of incidental Bochdalek's hernia in a large adult population. Am J Roentgenol 2001;177:363–6 [DOI] [PubMed] [Google Scholar]
- 12.Beck TF, Veenma D, Shchelochkov OA, et al. Deficiency of FRAS1-related extracellular matrix 1 (FREM1) causes congenital diaphragmatic hernia in humans and mice. Hum Mol Genet 2013;22:1026–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beurskens LW, Tibboel D, Lindemans J, et al. Retinol status of newborn infants is associated with congenital diaphragmatic hernia. Pediatrics 2010;126:712–20 [DOI] [PubMed] [Google Scholar]
- 14.Brown SR, Horton JD, Trivette E, et al. Bochdalek hernia in the adult: demographics, presentation, and surgical management. Hernia 2011;15:23–30 [DOI] [PubMed] [Google Scholar]
- 15.Vijfhuize S, Deden AC, Costerus SA, et al. Minimal access surgery for repair of congenital diaphragmatic hernia: is it advantageous?—an open review. Eur J Pediatr Surg 2012;22:364–73 [DOI] [PubMed] [Google Scholar]