Abstract
An active 66-year-old diabetic woman presented with a 5-day history of vomiting and abdominal pain, refractory shock and acute kidney injury (AKI). There was concomitant ACE inhibitor (ACEi) use and metformin toxicity with severe lactic acidosis. She suffered a pulseless electrical activity (PEA) cardiac arrest within 30 min of arrival to the Medical Admissions Unit. Despite a serum pH of 6.57 she was successfully resuscitated. She remained haemodynamically unstable even with fluid resuscitation, inotropic support and haemodiafiltration, yet made a full and rapid recovery following the introduction of a methylene blue infusion.
Background
We present an extreme case of metformin-associated lactic acidosis mixed with sepsis leading to a cardiac arrest with a nadir serum pH of 6.57 in a patient who was successfully resuscitated with both haemodiafiltration and intravenous methylene blue. This novel treatment allowed the patient to make a complete recovery and return to an active life. We hope to highlight an innovative treatment and encourage further research into the possibility of using methylene blue in cases of refractory shock associated with severe metformin toxicity. The combination of associated factors in this case are together unusual but all represent common presentations and prescriptions. The increasing recognition of severe sepsis along with the growing use of metformin and ACE inhibitor (ACEi) therapy may lead to rise in similar cases.
Case presentation
A 66-year-old woman presented to her general practitioner (GP) with a 5-day history of diarrhoea, vomiting and severe abdominal pain. She had been severely oliguric for 3 days. Prior to this illness she was fit and active, working full time in a local shop. She was a type 2 diabetic managed on 1 g twice a day of metformin who suffered from mild hypertension, treated with ramipril 10 mg once a day and bendroflumethiazide 2.5 mg once a day. She also took 40 mg simvastatin at night.
Following a review by the GP she was transported by ambulance to the Medical Admissions Unit. During the transfer she was noted to be hypothermic and hypotensive at 80/50 mm Hg and hypoglycaemic with a blood sugar level of 1.7 mmol/l. The ambulance crew administered 100 ml of 0.9% saline and 100 ml of 10% glucose.
The patient was reviewed immediately by the medical registrar upon arrival and a blood gas revealed a pH of 6.57, base excess of −35.6 mEq/l and a potassium of 7.4 mmol/l. An ECG at this time demonstrated features consistent with hyperkalaemia including a lack of p waves, irregular broad complexes and tall T waves. She became critically bradycardic and progressed into a pulseless electrical activity (PEA) cardiac arrest despite atropine and epinephrine boluses. Following 2 min of cardiopulmonary resuscitation and a further 1 mg of epinephrine there was a return of spontaneous circulation. She was treated with 20 ml of 10% calcium gluconate and 500 ml of 1.26% sodium bicarbonate infusion. However, she remained unresponsive and was intubated, ventilated and transferred to the intensive care unit (ICU). Postarrest blood tests revealed a pH of 6.61, lactate of 17.0 mmol/l and serum bicarbonate of 3.4 mmol/l, base excess of −34 mEq/l, sodium 127 mmol/l potassium 8.0 mmol/l, urea 45.8 mmol/l and creatine 929 mmol/l. Upon arrival to the ICU she was more hypothermic at 31.8°C but was actively maintained between 32°C and 34°C following local postarrest cooling guidelines. Haemodiafiltration was started within 2 h of hospital admission at a rate of 30 ml/kg/h exchange.
Over the next 12 h the patient remained cardiovascularly unstable despite a norepinephrine (norepinephrine) infusion reaching a maximum of 1.55 μg/kg/min. A bedside echocardiogram revealed reasonable myocardial function. Vasopressin was avoided given the considered possibility of ischaemic bowel. Addition of an epinephrine infusion had a limited benefit. The patient could not tolerate higher haemodiafiltration exchange rates owing to haemodynamic instability. A continuous infusion of 8.4% sodium bicarbonate at 25 ml/h alongside a 5% dextrose infusion at 125 ml/h with a variable rate insulin infusion was started. The patient received a broad-spectrum antibiotic treatment with intravenous cefuroxime 1.5 g, metronidazole 500 mg and gentamicin 360 mg following local guidelines, and was also given hydrocortisone 200 mg and vitamin K 10 mg. She remained intubated and ventilated and was allowed to be passively hypothermic on haemodiafiltration. After 24 h she was actively warmed and sedation with propofol and alfentanil infusions, for comfort, were introduced as her neurological status improved.
Differential diagnosis
Supportive management was now in place in the ICU following a cardiac arrest. The likely causes for this included severe acidosis (pH 6.57), hyperkalaemia (8.0 mmol/l) and refractory hypotension resulting from a combination of dehydration, diuretic use, drug toxicity and sepsis. The cause of the severe abdominal pain, diarrhoea and vomiting was later revealed at laparoscopy to be a phlegmon arising from the sigmoid colon with areas of frank pus and localised peritonitis. This had caused the onset of sepsis which had lead to acute kidney injury (AKI) over the 5 days prior to admission, culminating in anuria for the preceding 24 h. During this time, the patient had, allegedly under advice from the out-of-hours primary care team, continued to take 1 g of metformin twice a day and 10 mg of ramipril once a day. The severe lactic acidosis and the extremely low pH on arrival probably represent a mixture of metformin toxicity, ACEi use, AKI and sepsis. Also, it is worth noting that the hypoglycaemia seen on admission is another feature of metformin toxicity, and one that is exacerbated by ACEi use.1
Treatment
A surgical review was requested owing to the history of severe abdominal pain and diarrhoea and a CT scan was arranged which revealed a localised perforation in the sigmoid colon with free fluid and thickening. However, at this stage the patient was considered too unstable for a surgical intervention.
At 15 h postcritical care admission, the patient's pH had risen to 7.00, bicarbonate to 10 mmol/l, base excess to −20.9 mEq/l and lactate to 13.9 mmol/l following haemodiafiltration and a bicarbonate infusion. There was a persistent cardiovascular instability despite large doses of epinephrine and norepinephrine infusions with a mean arterial pressure between 50 and 70 mm Hg and an ongoing lactataemia owing to what was considered persistent vasodilatory shock. Given maximal treatment with fluid optimisation, inotrope therapy and steroid supplementation, a methylene blue intravenous infusion was started at a loading dose of 2 mg/kg and a maintenance rate of 2 mg/kg/h.
Figure 1 shows the infusion rates of norepinephrine before and after the onset of methylene blue treatment. A rapid fall in the inotrope dose required is noted almost immediately following the methylene blue and clinically a rapid improvement was seen. Colour, capillary refill time and other markers all improved steadily. Methylene blue was continued for 12 h only, owing to the concerns of accumulation in the setting of AKI.
Figure 1.
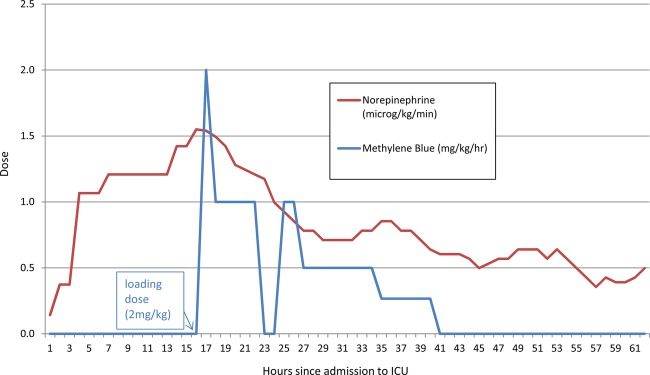
Required infusion rates of norepinephrine versus the dose of methylene blue.
By day 3 her lactate levels had fallen to 3.4 mmol/l; native urine output had resumed and pH had normalised. On day 4 she was taken to the operation theatre for a laparoscopy. As the acute perforation was limited it was treated conservatively. Of note, there was no evidence of gut ischaemia or infarction to explain the lactate levels seen.
Outcome and follow-up
The patient was extubated after 6 days in the ICU and norepinephrine support and haemofiltration were discontinued on day 7. The patient proceeded to make an uncomplicated recovery with no residual neurological deficit. She was discharged from critical care on day 10 and discharged home on day 18. She regained a full level of activity with no residual organ dysfunction and returned to work. Following a review by the endocrinology team her antihypertensive and antidiabetic medications were held temporarily for several weeks and restarted cautiously by her own GP. Two months later, to address the laparoscopy findings, the patient underwent colonoscopy which identified an area of sigmoid high-grade dysplasia. Elective anterior resection was undertaken for a T3N1M0 adenocarcinoma from which the patient made a full recovery.
Discussion
We present this case as it contains a number of interesting points. First, there is an increasing prevalence of diabetes and an increasing use of metformin in the UK population. Therefore, it is reasonable to expect an increase in accidental and intentional metformin toxicity, although to date this remains uncommon.2 The treatment for this has traditionally been supportive therapy, with bicarbonate and high-dose haemofiltration.1 However, this report highlights a possible additional treatment in extreme cases.
Metformin is a biguanide antidiabetic agent used in the management of type 2 diabetes mellitus, polycystic ovarian syndrome and of metabolic ‘syndrome X’. It is not hepatically metabolised and the bio-available portion is eliminated entirely in the urine; its removal is dependent on glomerular filtration rate. It can also be eliminated via haemofiltration. Metformin acts by inhibiting hepatic glucose production and increasing peripheral sensitivity to insulin.3 Signs of metformin toxicity are non-specific but include gastrointestinal dysfunction, change in consciousness, hypotension and hypothermia—all of which were present in our patient on admission.4 Lactic acidosis is a rare but a well-known consequence of metformin toxicity. Lactic acidosis in patients on metformin therapy can be referred to as ‘metformin-induced’ or ‘metformin-associated’. Metformin-induced implies the absence of another cause of lactic acidosis which was not the case here. Metformin-associated lactic acidosis (MALA) has a mortality of around 30%2 and the underlying cause must also be addressed.
A further action of metformin which has been noted is the increase in peripheral perfusion and glucose uptake in diabetes. Recent studies have suggested the mechanism for this may be increased activation of endothelial nitric oxide synthase (eNOS).5 Moreover, eNOS activation also has a key role in the haemodynamic disturbances seen in sepsis. It is possible that these two factors acted together to produce the dramatic haemodynamic instability seen in our patient. Having seen no benefit from standard supportive therapies, high doses of steroids and in the face of increasing inotropes’ requirements we instigated a further treatment aimed at reducing the profound vasodilatation and hypotension. While the improvement in clinical and physiological markers which followed the introduction of methylene blue may be coincidental we speculate that, in combination with the other treatments, this leads to a rapid recovery which seems to be similar to other case reports of its use in this setting.6
Ramipril inhibits the action of ACEi and thus reduces the presence of angiotensin II and may increase the presence of bradykinins. In a case with AKI and on going ACEi use, it would not be unreasonable to suspect that excessive bradykinin activity may contribute to refractory hypotension.7 Further, bradykinin acts on the endothelium B2 receptors thereby releasing prostacyclin and nitric oxide causing vasodilation.8
Methylene blue (methylthionium chloride) is used across a range of clinical settings, however its use in critical care for the treatment of shock has never fully been established.9 It is an inhibitor of guanylate cyclase which is the mechanism of the smooth muscle relaxation seen with nitric oxide.10 Trials have demonstrated some benefits for use in sepsis in critical care settings,11 although the largest dedicated trial for its use in sepsis (SMURF trial) was recently withdrawn prior to enrolment.12 Methylene blue is used in the treatment of methaemaglobinaemia and overdose of other agents through different mechanisms.13 There are also early experimental data to suggest that it may be a potential neuroprotective agent.14
It should be noted that its use in the critical care setting can be alarming to relatives and staffs as it leads to a rapid and dramatic blue discolouration of the skin and urine, which can affect the interpretation of clinical signs and monitoring such as pulse oximetry. Administration should ideally be via central venous access given its potential to cause tissue necrosis upon extravasation.15 Methylene blue infusion use is not without risk. It is a potent monoamine oxidase inhibitor. Recent case reports have highlighted the potential for serotoninergic crises especially during concomitant administration with antidepressant therapy, and use in this setting must be carefully considered.16 It is only renally excreted and its accumulation can occur despite renal replacement therapy. Large bolus dosages may potentially worsen pulmonary function. Common to many critical care conditions are the presence of acute lung injury or acute respiratory distress syndrome (ARDS), thus the risk–benefit balance needs to be carefully assessed. Methylene blue is contraindicated in glucose-6-phosphate dehydrogenase (G6PD) deficiency as it increases the risk of haemolytic anaemia in this population.12
Second, we present a case of a patient making a complete recovery despite a documented arterial pH of 6.57. Following a literature review, the lowest previously recorded17 pH in a patient who made a complete recovery was 6.59 and highlights the extremes from which survival is possible in metformin overdose. A recent case report by Timbrell et al18 highlighted the importance of aggressively treating metformin overdose despite extremes of lactataemia and metabolic acidosis, and emphasised the poor prognosis normally associated with these parameters.
Third, we highlight the risks of biguanides and ACEi in acute illness. In this case the patient was not aware of the potential need to omit this medication during acute illness and this was apparently not highlighted in conversations with the out-of-hours GP service in the 5 days prior to admission despite ongoing symptoms of dehydration, sepsis and oligo-anuria.
Learning points.
Metformin and ACE inhibitors (ACEi) are potentially lethal in concomitant acute illness, including sepsis and thus apply to all specialties.
Patients should be advised to stop ACEi, diuretics and metformin when faced with a dehydrating illness such as diarrhoea and vomiting.
The aggressive and successful treatment of metformin-associated lactic acid (MALA) despite an arterial serum pH of 6.5.
The potential use of methylene blue as a specific treatment for MALA.
The need for more research and investigation into the use of methylene blue and the role of endothelial nitric oxide synthase in metformin toxicity.
Footnotes
Contributors: BP the lead author and AP the coauthor were involved in the clinical care of the patient. PW, the coauthor and lead clinician was responsible for patient's care in the intensive care unit.
Patient consent: Obtained.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Toxbase—UK National Poisons Information Service. http://www.toxbase.org/Poisons-Index-A-Z/M-Products/Metformin (accessed Jan 2013). [Google Scholar]
- 2.Peters N, Jay N, Barraud D, et al. Metformin associated lactic acidosis in an intensive care unit. Crit Care 2008;12:R149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med 2002;137:25–33 [DOI] [PubMed] [Google Scholar]
- 4.Wu M-P, Liao H-C, Liaw S-J. Metformin associated high anion gap metabolic acidosis: a case report. J Emerg Crit Care Med 2007;18:173–8 [Google Scholar]
- 5.Davies BJ, Xie Z, Viollet B, et al. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 2006;55:496–505 [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal N, Kupfer Y, Seneviratne C, et al. Methylene blue reverses recalcitrant shock in β-blocker and calcium channel blocker overdose. BMJ Case Rep 2013; pii:bcr2012007402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sivieri DO, Jr, Bispo-da-Silva LB, Oliveira EB, et al. Potentiation of bradykinin effect by angiotensin-converting enzyme inhibition does not correlate with angiotensin-converting enzyme activity in the rat mesenteric arteries Hypertension 2007;50:110–15 [DOI] [PubMed] [Google Scholar]
- 8.Murphey L, Vaughan D, Brown N. Contribution of bradykinin to the cardiovascular protective effects of ACE inhibitors Eur Heart J Suppl 2003;5(Suppl A):A37–41 [Google Scholar]
- 9.Donati A, Conti G, Loggi S, et al. Does methylene blue administration to septic shock patients affect vascular permeability and blood volume? Crit Care Med 2002;30:2271–7 [DOI] [PubMed] [Google Scholar]
- 10.Ginimuge PR, Jyothi SD. Methylene blue: revisited. J Anaesthesiol Clin Pharmacol 2010;26:517–20 [PMC free article] [PubMed] [Google Scholar]
- 11.Heemskerk S, van Haren FMP, Foudraine NA, et al. Short-term beneficial effects of methylene blue on kidney damage in septic shock patients Intensive Care Med 2008;34:350–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US NIH trials registry http://clinicaltrials.gov/ct2/show/NCT00486174 (accessed Jan 2013).
- 13.Rizvi I, Zaman S, Zaidi N, et al. Acute life-threatening methaemaglobinaemia following ingestion of chloroquine. BMJ Case Rep 2012. doi:10.1136/bcr.12.2011.5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miclescu A, Sharma HS, Martijn C, et al. Methylene blue protects the cortical blood–brain barrier against ischemia/reperfusion-induced disruptions. Crit Care Med 2010;38:2199–206 [DOI] [PubMed] [Google Scholar]
- 15.Dumbarton TC, Gorman SK, Minor S, et al. Local cutaneous necrosis secondary to a prolonged peripheral infusion of methylene blue in vasodilatory shock. Ann Pharmacother 2012;46:e6. [DOI] [PubMed] [Google Scholar]
- 16.Rowley M, Riutort K, Shapiro D, et al. Methylene blue-associated serotonin syndrome: a ‘green’ encephalopathy after parathyroidectomy. Neurocrit Care 2009;11;88–93 [DOI] [PubMed] [Google Scholar]
- 17.Dell'Aglio DM, Perino LJ, Todino JD, et al. Metformin overdose with a resultant serum pH of 6.59: survival without sequalae. J Emerg Med 2010;39;e77–80 [DOI] [PubMed] [Google Scholar]
- 18.Timbrell S, Wilbourn G, Harper J, et al. Lactic acidosis secondary to metformin overdose: a case report. J Med Case Rep 2012;6;230. [DOI] [PMC free article] [PubMed] [Google Scholar]


