Abstract
Background
Individuals with drug addictions report increased willingness to approach rewards. Approach behaviors are thought to involve executive control processes and are more strongly represented in the left compared to right prefrontal cortex. A direct link between approach tendencies and left hemisphere activity has not been shown in the resting brain. We hypothesized that compared to controls, substance dependent individuals (SDI) would have greater left hemisphere activity in the left executive control network (ECN) at rest.
Methods
Twenty-five SDI and 25 controls completed a Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) questionnaire and underwent a resting-state fMRI scan. Group independent component analysis was performed. We used template matching to identify the left and right ECN separately and compared the corresponding components across groups. Across group, BAS scores were correlated with signal fluctuations in the left ECN and BIS scores with right ECN.
Results
BAS scores were higher in SDI compared to controls (p<.003) and correlated with signal fluctuation in the left ECN. SDI showed significantly more activity than controls in the left prefrontal cortex of the left ECN. Conversely, SDI showed less activity than controls in the right prefrontal cortex of the right ECN.
Conclusions
Results from this study suggest that approach tendencies are related to the left ECN, even during rest. Higher resting-state signal in the left ECN may play a role in heightened approach tendencies that contribute to drug-seeking behavior.
Keywords: Substance Dependence, Approach, Avoidance, Resting-State, Executive Control Network, Laterality, BIS/BAS
1. INTRODUCTION
Personality traits such as impulsivity, sensation-seeking, and heightened willingness to approach rewarding events may predispose individuals to initiate drug use (Hanson et al., 2008; Teichman et al., 1989). Gray’s theory of personality proposes two opposing constructs affecting motivation: the behavioral activation system (BAS) and the behavioral inhibition system (BIS) (Gray, 1987). The BAS is sensitive to positive or appetitive outcomes (i.e., approach), while the BIS inhibits behavior that may lead to negative or aversive outcomes (i.e., avoidance) (Carver and White, 1994). Carver and White (1994) developed a psychometric instrument to measure such traits: the BIS/BAS scales. This measure has repeatedly shown differences between healthy individuals and those with psychological disorders. For example, individuals with substance abuse or dependence disorders (Franken et al., 2006; Knyazev, 2004; Simons et al., 2009; van Toor et al., 2011), score higher than controls on BAS, suggesting that these individuals are more likely to approach what they deem to be rewarding, even if those rewards are associated with negative consequences (e.g., seeking drugs because they lead to a “high” even though it can lead to loss of friend or a job). In contrast, individuals with anxiety disorders (Johnson et al., 2003; Torrubia and Tobena, 1984), depression (Johnson et al., 2003), and anorexia nervosa (Harrison et al., 2010) score higher than controls on BIS, suggesting that these individuals are motivated to avoid negative outcomes (e.g., not eating food to avoid gaining weight). Together, these studies suggest that personality differences in the tendency to approach rewards or the tendency to avoid negative outcomes may be linked to vulnerabilities for specific psychopathologies such as substance dependence.
Functional neuroimaging investigations into the neural correlates of approach behaviors have mainly implicated the striatum and left dorsolateral prefrontal cortex (DLPFC; Barros-Loscertales et al., 2006b; 2010; Spielberg et al., 2011) where those of avoidance behaviors suggest that right DLPFC, hippocampal formation, amygdala, and anterior cingulate are involved (Amodio et al., 2008; Barros-Loscertales et al., 2006a; Spielberg et al., 2011; Torrubia and Tobena, 1984). While the DLPFC is involved in both approach and avoidance, these studies suggest hemispheric asymmetry with the left DLPFC more strongly associated with approach and the right DLPFC with avoidance (Heller, 1993; Spielberg et al., 2011).
The neural correlates of the approach and avoidance systems have been predominantly studied using task-based fMRI. Here we extend these studies by examining the relationship between these traits and resting-state brain activity in substance dependent individuals (SDI). The term “activity” used here refers to the strength or amplitude of the signal corresponding to the spatially independent network of interest. Recognizing that the meaning of the term may differ depending on the context (i.e., task-based or non-task based) “activity” is preferred over “connectivity” because the latter is often, although not exclusively, used in the context of seed-based analyses.
Several resting state networks have been identified, the most extensively studied being the Default Mode Network (DMN), shown to deactivate during task performance and activate during internal mentation (Broyd et al., 2009; Raichle et al., 2001; Vincent et al., 2008). Another resting-state network which might be viewed as particularly pertinent to the field of substance dependence is the Executive Control Network (ECN), thought to be involved in goal-directed behavior and cognitive control (Seeley et al., 2007; Spreng et al., 2010; Sutherland et al., 2012; Vincent et al., 2008). The enhanced motivation to seek and take drugs combined with an inability to inhibit drug-related behaviors are thought to represent a failure of executive control (Barros-Loscertales et al., 2011; Goldstein and Volkow, 2002; Volkow et al., 2011). We propose that the executive control resting-state network allows an investigation of neural mechanisms of approach and avoidance in SDI. Like the approach and avoidance systems, the ECN has been shown to have separable right and left hemisphere components (Damoiseaux et al., 2006; Habas et al., 2009; Seeley et al., 2007; Shirer et al., 2012). Since the approach system is associated with the left DLPFC and SDI typically demonstrate increased willingness to approach rewards, we hypothesized that SDI would have greater activity than controls in the left ECN. Second, we hypothesized that across all subjects approach ratings would be associated with higher left ECN and BIS with higher right ECN activity.
2. MATERIALS AND METHODS
2.1. Participants
Twenty-five drug abstinent SDI were recruited from the University of Colorado Denver’s Addiction Research and Treatment Services (ARTS) program, a gender-specific long-term residential treatment program. Drug abstinence was monitored by observation and random urine screens at the treatment center. The mean duration of self-reported abstinence for all drugs was 1.43 years across all SDI participants. The inclusion criterion was dependence on stimulants (methamphetamine, cocaine or crack) according to the DSM-IV. Most SDI were also dependent on other drugs, most commonly tobacco, alcohol, and cannabis (Table 1). Twenty-five controls were recruited from the community through newspaper ads, flyers, a marketing company, and a database of community members interested in participating in research. Controls were excluded if they met criteria for dependence on any drug or alcohol. Nicotine dependence was not exclusionary. Exclusions were history of head trauma with loss of consciousness exceeding 15 minutes, neurological illness, schizophrenia, bipolar disorder, current major depression (within the last 2 months). Handedness was determined through self-report. All participants provided written informed consent approved by the Colorado Multiple Institutional Review Board.
Table 1.
Drug use for all participants.
| Controls | SDI | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Drug | Number Dependent (% of total) | Duration (years) | Last use (years) | Number Dependent (% of total) | Duration (years) | Last use (years) |
| Stimulants | 0 | - | - | 25 (100%) | 12.4 (7.9) | 4.0 (4.7) |
| Tobacco | 4 (16%) | 16.5 (9.9) | 3.8 (4.8) | 19 (76%) | 19.7 (8.9) | 0.7 (1.9) |
| Alcohol | 0 | - | - | 15 (60%) | 18.8 (8.7) | 2.6 (2.7) |
| Cannabis | 0 | - | - | 10 (40%) | 15.2 (9.5) | 3.2 (2.6) |
| Opioids | 0 | - | - | 6 (24%) | 6.2 (6.4) | 4.0 (4.7) |
| Club Drugs | 0 | - | - | 3 (12%) | 5.7 (3.2) | 5.1 (4.1) |
| Hallucinogens | 0 | - | - | 2 (8%) | 8.0 (4.2) | 11.0 (4.2) |
2.2. Structured Interviews
2.2.1. Composite International Diagnostic Interview-Substance Abuse Module (CIDI-SAM)
This computerized structured interview (Cottler et al., 1989, 1995) was administered to characterize the substance dependence diagnoses of SDI and to ensure that controls did not meet criteria for dependence diagnoses on substances other than tobacco. Results from the interview provide DSM-IV diagnoses for eleven substances: amphetamines, cocaine, marijuana, alcohol, tobacco, hallucinogens, opioids, inhalants, sedatives, club drugs, and PCP.
2.2.2. Diagnostic Interview Schedule – Version IV (CDIS-IV)
This computerized structured interview provides diagnostic and symptom information about psychiatric diagnoses according to the DSM-IV (Robins et al., 2000). Three modules were administered to exclude participants with schizophrenia, bipolar disorder, or current major depression.
2.3. Questionnaire
2.3.1. Behavioral Inhibition and Activation Scale (BIS/BAS)
This 20 item self-report scale measures approach (BAS) and avoidance (BIS) personality traits (Carver and White, 1994). The BAS is divided into three subscales: a persistent pursuit of desired goals (drive), a desire for new rewards and willingness to approach a potentially rewarding event on the spur of the moment (fun-seeking), and a positive response to the occurrence or anticipation of reward (reward responsiveness). A higher score indicates a greater level of that particular trait. BIS is not subdivided, but the questions reference reactions to the anticipation of a punishment.
2.4. MRI acquisition and preprocessing
2.4.1. MRI acquisition
Images were acquired using a 3T whole body MR scanner (General Electric, Milwaukee, WI, USA) with an 8-channel head coil. A high-resolution 3D T1-weighted anatomic scan was collected. One-hundred fifty resting-state functional scans were acquired with the following parameters: TR 2,000 ms, TE 30 ms, FOV 220 mm2, matrix 64×64, voxel size 3.44×3.44×4 mm, slice thickness 3 mm, gap 1 mm, interleaved, flip angle 70°. Resting fMRI scan duration was five minutes. Participants were instructed to close their eyes, not think of anything in particular, and not fall asleep. Head motion was minimized using a VacFix head-conforming vacuum cushion (Par Scientific A/S, Odense, Denmark).
2.4.2 MRI data analysis
fMRI data were pre-processed using SPM8 (Wellcome Dept. of Imaging Neuroscience, London, UK) running on Matlab R2011a. The first four images were excluded for saturation effects. Images were realigned to the first volume, normalized to the Montreal Neurological Institute (MNI) space, and spatially smoothed with a 6-mm FWHM Gaussian kernel.
Spatial independent component analysis (ICA) was performed using GIFT software v1.3i (http://icatb.sourceforge.net; Calhoun et al., 2001). ICA is a model-free technique that identifies spatially independent sources of blood-oxygen-level-dependent signal variations. ICA is a robust method that can investigate overall brain organization and has been particularly useful in task-free settings. The identified sources, or components, can then be compared across different groups. Group ICA was conducted separately for the SDI and control group, as has been performed previously in the literature (Gao et al., 2009; Sorg et al., 2007; Tregellas et al., 2011). The dimensionality of the data from each subject was reduced using principal component analysis and concatenated into an aggregate data set, then back-reconstructed (Calhoun et al., 2001). A minimum description length (MDL) algorithm was used to identify the number of spatially independent sources.
2.4.3. Component selection
Group ICA identified 35 independent components for controls and 35 for SDI. A left executive control network (ECN) and right executive control network (ECN) template were downloaded from Stanford’s Functional Imaging in Neuropsychiatric Disorders (FIND) lab (http://findlab.stanford.edu/functional_ROIs.html; Shirer et al., 2012). Within each group the 35 components were then spatially correlated with the left ECN and right ECN templates. The component having the highest spatial correlation to the template was selected for further analysis. The spatial correlations to the templates were similar across group for the left ECN (controls r=0.57, SDI r=0.57) and right ECN (controls r=0.53, SDI r =0.52). In addition, to ensure that the same component was selected in each group, all components were visually inspected by 3 researchers. Previous studies have shown that visually identified component selection was the same or better than spatial template matching (Franco et al., 2009).
2.4.4. Statistical comparison of images
A second level, whole brain analysis was conducted to examine group differences in the amplitude of the component associated with the right ECN and that of the left ECN, respectively, using a 2-sample t-test. Contrast maps were set at a threshold of p<0.005, uncorrected, with an extent threshold of 35 voxels, corresponding to a whole-brain cluster-corrected level of p<0.01, based on 10,000 Monte Carlo simulations using AlphaSim in AFNI (http://afni.nimh.nih.gov/afni/). To restrict results to the network of interest, the group difference contrast maps were masked with the map of within-network brain regions demonstrating significant activity for all subjects (i.e., a one-sample T-test, set at uncorrected threshold of p<0.001). Masking ensured that group difference results were restricted to the network of interest by decreasing the chances of extraneous results outside of the ECN.
2.4.5. Regression Analysis
To examine the relationship between the level of approach and avoidance ratings and resting-state activity in each of the ECNs, we conducted a regression analysis on all subjects. BAS scores were regressed against the fMRI signal fluctuations in the left ECN component, while BIS scores were regressed against the fMRI signal fluctuations in the right ECN component. Statistical maps were set at a threshold of p<0.005, uncorrected with an extent threshold of 35 voxels, corresponding to a whole-brain cluster-corrected level of p<0.01, based on 10,000 Monte Carlo simulations using AlphaSim in AFNI (http://afni.nimh.nih.gov/afni/). In an exploratory subanalysis we regressed the BAS subscales with the left ECN.
3. RESULTS
3.1. Participants’ characteristics
There were no differences in age (controls 30.3±8.5, SDI 34.2±8.7, p=0.11), gender (controls 11M/14F, SDI 14M/11F, χ2=0.40), or handedness (controls 23R/2L, SDI 24R/1L, χ2=0.55).
3.2. Substance Dependence
All SDI met DSM-IV dependence criteria for stimulants. Drug characteristics are summarized in Table 1. Four controls were dependent on tobacco. No controls were dependent on drugs or alcohol.
3.3. Behavioral Inhibition System/Behavioral Activation System (BIS/BAS)
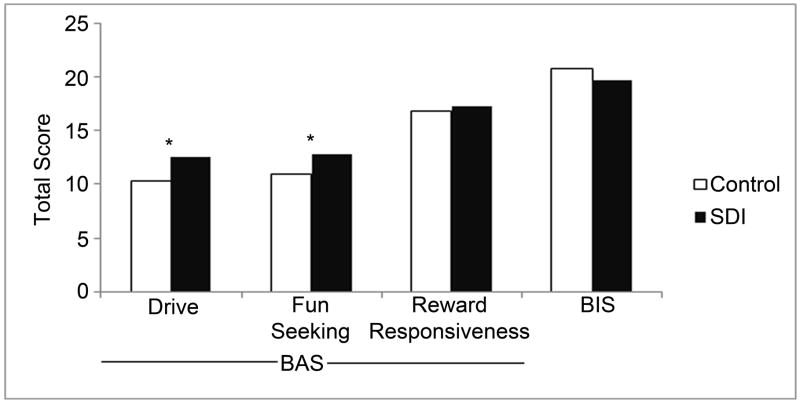
A significant group difference on the BAS was observed, with SDI showing higher scores than controls (controls 38.3±5.2, SDI 42.8±4.9, p=0.003). Further analysis of the BAS subscales showed that SDI had higher scores on “drive” (controls 10.4±2.4, SDI 12.6±2.6, p=0.002) and “fun-seeking” (controls 11.0±2.1, SDI 12.9± 2.0, p=0.002), but not “reward-responsiveness” (controls 16.9±1.9, SDI 17.3±2.0, p=0.47). No group difference on the BIS was observed (controls 20.8±2.4, SDI 19.6±3.1, p=0.12). Data are presented in Figure 1.
Figure 1.
Group differences for BIS/BAS. BAS is subdivided into drive, fun-seeking, and reward responsiveness.
* = significant at p=0.002
3.4. Imaging
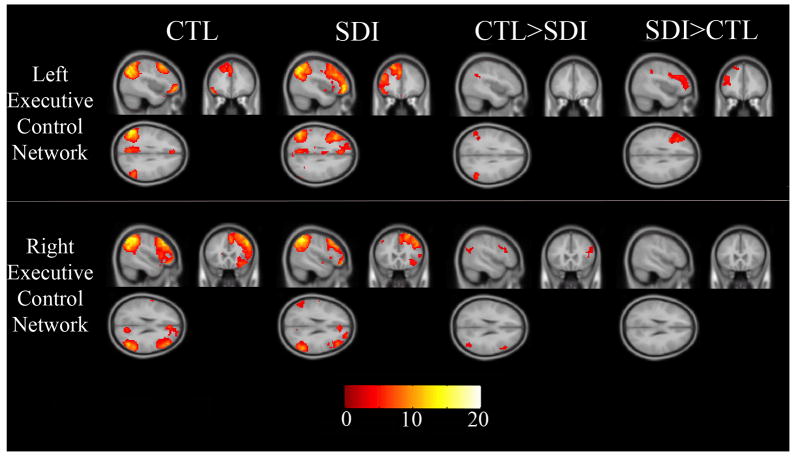
Figure 2 shows the left ECN and right ECN for controls and SDI separately and group differences. Table 2 lists brain regions yielding group differences.
Figure 2.
Left and right executive control networks at rest for controls, SDI, and group differences. Images are in neurological orientation. Color bar is t-score.
Table 2.
Significant Clusters and MNI coordinates for 2 contrasts (where controls showed more activation than SDI and where the SDI showed more activation than controls) for 3 networks of interests. Clusters are based on a p-value of 0.005 excluding clusters smaller than 35 voxels.
| BA | Extent | x | y | z | t | |
|---|---|---|---|---|---|---|
| Left Executive Control Network | ||||||
| SDI > Controls | ||||||
| Left Dorsolateral Prefrontal Cortex | 8,9,10,46 | 486 | −39 | 23 | 28 | 6.32 |
| 156 | −27 | 20 | 61 | 6.94 | ||
| Left Inferior Parietal Cortex | 40 | 112 | −30 | −46 | 40 | 4.44 |
| Controls > SDI | ||||||
| Bilateral Lateral Parietal Cortex | 40 | 150 | 54 | −52 | 31 | 5.76 |
| 95 | −54 | −58 | 25 | 5.05 | ||
| Right Executive Control Network | ||||||
| Controls > SDI | ||||||
| Right Dorsolateral Prefrontal Cortex | 9,46 | 121 | 42 | 26 | 16 | 5.10 |
| Right Inferior Parietal Cortex | 39 | 128 | 45 | −67 | 25 | 4.29 |
| SDI> Controls | ||||||
| None | ||||||
3.4.1. Left executive control network
Compared to controls, SDI had significantly greater activity in the left DLPFC. This area of increased activity covered a substantial portion of the DLPFC (extent = 486 voxels). When excluding the three left handed individuals this result became more significant (MNI −39 23 28, T-score increased from 6.32 to 6.70). SDI also showed greater activity than controls in the left inferior parietal cortex. However, SDI had less activity than controls in bilateral lateral parietal cortex (Figure 2, top row).
3.4.2. Right executive control network
Compared to controls, SDI had significantly less activity in the right DLPFC. However, this area of decreased activity in the right ECN was not nearly as large (extent = 121 voxels) as group differences in the left ECN noted above. SDI showed significantly less activity than controls in the right inferior parietal cortex (Figure 2, bottom row).
3.4.3. Regression Analysis
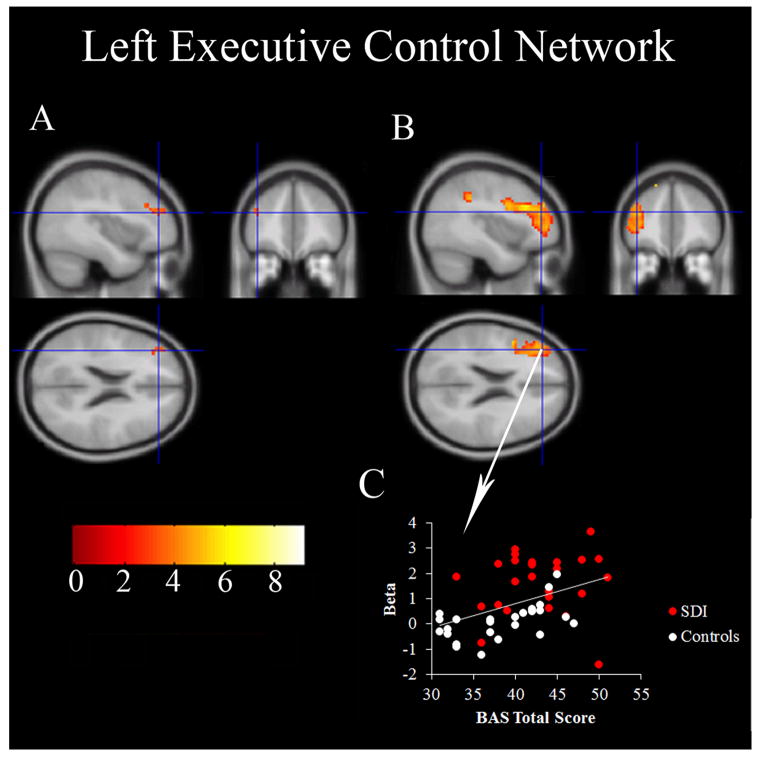
Across both groups, BAS scores positively correlated with signal fluctuations in the left DLPFC of the left ECN. Moreover, this area of the DLPFC overlapped with the same region demonstrating group differences in the left ECN wherein SDI showed greater activity than controls (Figure 3). Further analysis revealed no correlation between ECN signal and the BAS subscales. No correlations were observed between BIS scores and the right ECN.
Figure 3.
Part A shows the BAS regression model where the voxels in the left executive control network correlate with total BAS scores. Part B shows the group difference in the left executive control network where SDI had greater activity than controls. The crosshairs show the same location on both parts A and B. Part C shows the correlation (r=0.43, p=0.002) between activation and the total BAS scores at the point of the crosshairs (MNI −39, 41, 22). This figure is presented in neurologic view (right is right, left is left).
4. DISCUSSION
Three main findings are reported in this study investigating brain signal fluctuations in SDI at rest. First, higher approach scores on the BIS/BAS scales were observed in SDI, consistent with prior studies of approach/avoidance systems in substance users. Second, SDI had increased activity in the left ECN and decreased activity in the right ECN compared to controls. Third, BAS scores correlated with the signal fluctuations in the left ECN across both groups. Throughout this paper, the term “activity” is used to indicate the strength or amplitude of the signal fluctuations corresponding to the spatially independent network of interest. We recognize that the term “activity” is imprecise and its meaning may differ depending on the context (i.e., task-based or non-task based).
4.1. The BIS/BAS Scales
Higher BAS drive and BAS fun-seeking scores were observed in SDI, compared to controls, consistent with prior studies in substance dependence (Franken et al., 2006; Knyazev, 2004; Simons et al., 2009; van Toor et al., 2011; Wardell et al., 2011), suggesting that SDI have strong approach systems and may be more willing to approach a reward, particularly on the spur of the moment, compared to controls. No group differences were observed on BAS reward responsiveness subscale, consistent with Franken and colleagues (2006) who suggested this may mean that SDI are not more sensitive to rewards in general but rather a specific reward (i. e., drugs). In contrast to BAS, SDI did not differ compared with controls on BIS scores, suggesting no difference in their willingness to avoid negative outcomes. These results are also consistent with previous studies investigating substance dependence, though some studies have shown that BIS may be lower in SDI, and has been suggested to influence some aspects of substance dependence such as alcohol abuse (Wardell et al., 2011). Of note, these SDI have been abstinent from drugs and alcohol for over a year (average = 1.43 years), suggesting a persisting proclivity toward approach even after the cessation of drug use. While the causal relationship between these findings and drug exposure is unknown, it may be a factor contributing to high relapse rates among SDI.
4.2. The Left Executive Control Network
Two aspects of our data are consistent with the suggestions that approach behaviors are more strongly associated with the left hemisphere compared with the right (Spielberg et al., 2011). First, SDI participants, who typically manifest strong approach characteristics, showed increased resting-state activity compared to controls in DLPFC regions of the left ECN. The ECN is thought to be engaged during both cognitive control and goal-directed behavior (Cole et al., 2010; Dosenbach et al., 2007; Seeley et al., 2007). The SDI in our study had relatively increased activity only in the left ECN and actually exhibited decreased activity in the right ECN compared to controls. Together, these dissociable findings suggest significant hemispheric differences in the roles of the left- and right-hemisphere components of the ECN.
Second, we found that across both SDI and control individuals, BAS scores were associated with left ECN activity, specifically in the DLPFC. This result is consistent with previous studies that found BAS to be associated with left hemisphere activation (Spielberg et al., 2011) and brain anatomy (Xu et al., 2012), respectively. More specifically, Spielberg et al. (2011) observed this asymmetry in the middle frontal gyrus of healthy controls performing a Stroop task, while Xu et al. (2012) found that white matter fractional anisotropy, mainly on the left side of the brain, correlated with BAS. Our findings are also consistent with EEG studies showing that individuals with higher BAS scores have increased left hemisphere activity at rest (Harmon-Jones and Allen, 1997).
In an exploratory subanalysis to determine if one of the BAS subscales were driving these results, we regressed the subscales with the left ECN. We did not find any correlations, possibly due to the limited range of scores as each BAS subscale contains only 4 or 5 questions.
Our data suggest that the left ECN may be involved in reaching goals through the willingness to approach a reward rather than avoiding a punishment. There are multiple ways to reach a goal and they involve a balance between appetitive type and avoidant behaviors (i.e., one may lose weight by approaching exercise or avoiding food). SDI may be driven more by appetitive type than avoidant behaviors. This is not necessarily problematic. However, when the goal is to take drugs, perhaps due to a dysregulated reward system in SDI, then an over dominant approach system may predispose these individuals to engage in dangerous and risky behaviors to reach their goal. Alternatively, rather than representing a failure of executive control as the name implies, the left resting-state ECN may be involved in maintaining a strong bias towards seeking and taking drugs despite the long-term negative consequences.
Also of interest in our findings is that the group differences were not similarly distributed over the entire left ECN. Rather, the group differences were notable across most of the left DLPFC, while group differences in parietal cortex were much less pronounced. Our study did find minor group differences in the parietal cortex, but most of the parietal lobe showed no group differences. Whereas DLPFC is implicated in goal-directed behavior, parietal cortex is more involved in directing attention towards salient places, objects, or items in working memory (Curtis and D’Esposito, 2003; Seeley et al., 2007; Tamber-Rosenau et al., 2011). This pattern of findings perhaps suggests that at rest SDI and controls do not differ on lower-level attention aspects of the ECN, but instead, groups differ on higher-level goal-direction and abstract reasoning that is more often associated with DLPFC.
4.3. The Right Executive Control Network
SDI showed decreased resting-state activity compared to controls in the right ECN. This result is consistent with a previous study that found decreased activity in a right frontoparietal control network in cocaine users compared to controls (Barros-Loscertales et al., 2011), but under a cognitive challenge, during performance of the Stroop task. We extend these results by showing similar effects at rest, with decreased activity in both right frontal and right parietal cortex of SDI compared to controls.
Although we hypothesized that BIS would be related to the right ECN, no correlation between BIS and the right ECN resting network was observed. One explanation for this null result could be due to limitations in the BIS/BAS scales. Carver and White’s BIS (avoidant behavior) scale may be relatively insensitive compared to the BAS scale (approach behavior). For example, there are 13 questions for BAS compared to 7 for BIS. Consistent with this idea of relative insensitivity, differences in BAS are reported in substance dependent populations while few studies have demonstrated group differences in BIS (Simons et al., 2009; Wardell et al., 2011). An alternative future means of testing the hypothesis that the right ECN is involved in avoidant traits might be conducted in a population where BIS scores are consistently reported as higher than control scores (e.g., individuals with depression or anxiety).
4.4. Lateralization of the Executive Control Network
Although our results suggest differences between the right and left ECN, it is important to recognize that there is some divergence in the literature over the validity of splitting the ECN into left and right components (Vincent et al., 2008). Consistent with other groups that have found separate left ECN and right ECNs (Damoiseaux et al., 2006; Habas et al., 2009; Shirer et al., 2012; Sridharan et al., 2008), we adopted this method based on a hypothesis of hemispheric specialization for approach versus avoidance. Overall, our results do suggest that SDI utilize the left and right component of the ECN differently. It is also important to note that our definition of the ECN is arbitrary. We defined the ECN based on a network mask from Stanford’s FIND lab (Shirer et al., 2012). Studies define the ECN differently and may use different nomenclature for the ECN (e.g., the frontoparietal network; Spreng et al., 2010; Vincent et al., 2008). It is important to keep in mind that variability exists across studies with regard to the name and exact locations of the ECN.
4.5. Limitations
There are limitations to this study. First, the SDI group was dependent on multiple drugs, as is very common in this population. Therefore, we cannot isolate the effects to a single drug class. We cannot determine if these results were caused by drugs or if alterations in the ECN preceded or predisposed individuals to develop drug use. Given our interest in hemispheric differences, a possible limitation is that three left-handed individuals were included in this study. However, a separate analysis excluding these three participants yielded the same results. By performing ICA on the groups separately there is a possibility of increasing the chance of false positive results. The argument against splitting the groups, however, would be the possibility of increasing the chance of false negative results. That is, if the components truly differ across group, then ICA over all subjects may not identify the correct component. To help guard against false positives, we visually inspected the component maps to insure that the same component was being selected for each group. Third, inferences about the functional significance are limited because participants are not engaged in a specific task making the interpretation of the resting-state activity more ambiguous. Finally, networks that have been implicated in reward processing, such as the basal ganglia network, were not investigated. Given the possible relevance to approach motivations, future studies of other resting networks in SDI may be useful.
4.6 Conclusion
SDI show increased activity in the left ECN and decreased activity in the right ECN compared to controls at rest. These results suggest that the left ECN, which is associated with the approach system, may be involved in the persistent, even unrelenting, approach towards drugs in SDI despite the long-term negative consequences.
Acknowledgments
Role of Funding Source
Funding for this study was provided by NIDA grants R01 DA024104 and DA027748; NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors would like to acknowledge the staff at Addiction Research Treatment Services (ARTS), Debra Singel, RT, and Robert Perry, MD.
Footnotes
Contributors
Krmpotich: analysis, data interpretation, manuscript; Tregellas: design, analysis, interpretation, manuscript; Thompson: data interpretation, manuscript; Banich: data interpretation, manuscript; Klenk: analysis, manuscript; Tanabe: design, analysis, interpretation manuscript
Conflict of Interest
All authors declared they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: implications for theories of self-regulation. Psychophysiology. 2008;45:11–19. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Barros-Loscertales A, Bustamante JC, Ventura-Campos N, Llopis JJ, Parcet MA, Avila C. Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry Res. 2011;194:111–118. doi: 10.1016/j.pscychresns.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Barros-Loscertales A, Meseguer V, Sanjuan A, Belloch V, Parcet MA, Torrubia R, Avila C. Behavioral Inhibition System activity is associated with increased amygdala and hippocampal gray matter volume: a voxel-based morphometry study. Neuroimage. 2006a;33:1011–1015. doi: 10.1016/j.neuroimage.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Barros-Loscertales A, Meseguer V, Sanjuan A, Belloch V, Parcet MA, Torrubia R, Avila C. Striatum gray matter reduction in males with an overactive behavioral activation system. Eur J Neurosci. 2006b;24:2071–2074. doi: 10.1111/j.1460-9568.2006.05084.x. [DOI] [PubMed] [Google Scholar]
- Barros-Loscertales A, Ventura-Campos N, Sanjuan-Tomas A, Belloch V, Parcet MA, Avila C. Behavioral activation system modulation on brain activation during appetitive and aversive stimulus processing. Soc Cogn Affect Neurosci. 2010;5:18–28. doi: 10.1093/scan/nsq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral-inhibition, behavioral activation, and affective responses to impending reward and punishment - the BIS BAS Scales. J Person Soc Psychol. 1994;67:319–333. [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: a comprehensive substance abuse interview. Br J Addict. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Schuckit MA, Helzer JE, Crowley T, Woody G, Nathan P, Hughes J. The DSM-IV field trial for substance use disorders: major results. Drug Alcohol Depend. 1995;38:59–69. doi: 10.1016/0376-8716(94)01091-x. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AR, Pritchard A, Calhoun VD, Mayer AR. Interrater and intermethod reliability of default mode network selection. Hum Brain Mapp. 2009;30:2293–2303. doi: 10.1002/hbm.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, Muris P, Georgieva I. Gray’s model of personality and addiction. Addict Behav. 2006;31:399–403. doi: 10.1016/j.addbeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, Lin W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci U S A. 2009;106:6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of emotion and personality. In: Stahl SM, Iverson SD, Goodman EC, editors. Cognitive Neurochemistry. Oxford University Press; New York: 1987. pp. 171–190. [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Luciana M, Sullwold K. Reward-related decision-making deficits and elevated impulsivity among MDMA and other drug users. Drug Alcohol Depend. 2008;96:99–110. doi: 10.1016/j.drugalcdep.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. J Abnorm Psychol. 1997;106:159–163. doi: 10.1037//0021-843x.106.1.159. [DOI] [PubMed] [Google Scholar]
- Harrison A, O’Brien N, Lopez C, Treasure J. Sensitivity to reward and punishment in eating disorders. Psychiatry Res. 2010;177:1–11. doi: 10.1016/j.psychres.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Heller W. Neuropsychological mechanisms of indivdual differences in emotion, personality, and arousal. Neuropsychology. 1993;7:476–489. [Google Scholar]
- Johnson SL, Turner RJ, Iwata N. BIS/BAS levels and psychiatric disorder: an epidemiological study. J Psychopathol Behav Assess. 2003;25:25–36. [Google Scholar]
- Knyazev GG. Behavioural activation as predictor of substance use: mediating and moderating role of attitudes and social relationships. Drug Alcohol Depend. 2004;75:309–321. doi: 10.1016/j.drugalcdep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) Washington University; St. Louis: 2000. [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Dvorak RD, Lau-Barraco C. Behavioral inhibition and activation systems: differences in substance use expectancy organization and activation in memory. Psychol Addict Behav. 2009;23:315–328. doi: 10.1037/a0015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Miller GA, Engels AS, Herrington JD, Sutton BP, Banich MT, Heller W. Trait approach and avoidance motivation: lateralized neural activity associated with executive function. Neuroimage. 2011;54:661–670. doi: 10.1016/j.neuroimage.2010.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamber-Rosenau BJ, Esterman M, Chiu YC, Yantis S. Cortical mechanisms of cognitive control for shifting attention in vision and working memory. J Cogn Neurosci. 2011;23:2905–2919. doi: 10.1162/jocn.2011.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichman M, Barnea Z, Rahav G. Sensation seeking, state and trait anxiety, and depressive mood in adolescent substance users. Int J Addict. 1989;24:87–99. doi: 10.3109/10826088909047277. [DOI] [PubMed] [Google Scholar]
- Torrubia R, Tobena A. A scale for the assessment of “susceptibility to punishment” as a measure of anxiety: preliminary results. Person Individ Diff. 1984;5:371–375. [Google Scholar]
- Tregellas JR, Wylie KP, Rojas DC, Tanabe J, Martin J, Kronberg E, Cordes D, Cornier MA. Altered default network activity in obesity. Obesity (Silver Spring) 2011;19:2316–2321. doi: 10.1038/oby.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Toor D, Roozen HG, Evans BE, Rombout L, Van de Wetering BJ, Vingerhoets AJ. The effects of psychiatric distress, inhibition, and impulsivity on decision making in patients with substance use disorders: a matched control study. J Clin Exp Neuropsychol. 2011;33:161–168. doi: 10.1080/13803395.2010.493300. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell JD, O’Connor RM, Read JP, Colder CR. Behavioral approach system moderates the prospective association between the behavioral inhibition system and alcohol outcomes in college students. J Stud Alcohol Drugs. 2011;72:1028–1036. doi: 10.15288/jsad.2011.72.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kober H, Carroll KM, Rounsaville BJ, Pearlson GD, Potenza MN. White matter integrity and behavioral activation in healthy subjects. Hum Brain Mapp. 2012;33:994–1002. doi: 10.1002/hbm.21275. [DOI] [PMC free article] [PubMed] [Google Scholar]