Abstract
Atherosclerotic plaques may rupture without warning and cause acute cardiovascular events such as heart attack and stroke. Current clinical screening tools are insufficient to identify those patients with risks early and prevent the adverse events from happening. Medical imaging and image-based modeling have made considerable progress in recent years in identifying plaque morphological and mechanical risk factors which may be used in developing improved patient screening strategies. The key steps and factors in image-based models for human carotid and coronary plaques were illustrated, as well as grand challenges facing the researchers in the field to develop more accurate screening tools.
Index Terms: Atherosclerosis, Fluid-structure interaction, IVUS-based modeling, MRI-based modeling, vulnerable plaques
I. Introduction
Atherosclerotic plaques may rupture without warning and cause acute cardiovascular syndromes such as heart attack and stroke [1–4]. Considerable progress has been made in medical imaging and computational models to develop better patient screening and plaque assessment schemes [5–8]. However, the grand challenge for vulnerable plaque research is to identify and validate risk factors, use them to predict plaque rupture and associated clinical events such as stroke and heart attack and recommend proper treatment plan to prevent those clinical events from happening. Currently, plaque stenosis severity is still widely used as a main guidance for revascularization decisions. Results from 6 major clinical trials indicated that compared to patients who received carotid endarterectomies (CEA), only one out of 8–83 patients (this number from the six trials was: 8; 8; 20; 67; 48; 83) patients from the control groups would actually have a stroke (1.2%–12.5%) within two years of the surgery [9]. All other CEAs were actually not necessary and were performed only because available screening methods could not provide more accurate assessment and predictions. It is very desirable to develop more accurate noninvasive (or minimally invasive) predictive screening methods so that future plaque rupture can be predicted early and proper treatment can be recommended to prevent actual drastic clinical events and reduce the number of unnecessary surgeries.
Atherosclerotic plaque rupture is a complex process which involves many factors including mechanical forces (plaque stress, flow shear stress, blood pressure), plaque morphology (thin cap, lipid-rich necrotic core, calcification, hemorrhage, ulcer, etc.), biological processes (inflammation, remodeling), blood conditions (cholesterol level, injury-initiated blood changes), exercise, emotional stress, and various genomic and cellular activities. The mechanisms causing plaque rupture and subsequent clinical events are not fully understood [1–4].
Going from the current stenosis-based patient screening schemes, the following steps may lead to potential screening and diagnosis improvements in the coming years:
Use of medical images that can more accurately quantify plaque morphology, components, lumen dimensions, plaque surface characteristics, and plaque inflammation;
Use of image-based modeling to establish the association of mechanical risk factors (plaque stress/strain, flow shear stress) with biological/clinical events such as heart attack and stroke;
Use of patient follow-up and actual clinical data to identify risk factors with predictive power, quantify their prediction accuracy, and develop predictive methods for plaque rupture and related clinical events;
Use large-scale clinical trial approaches to validate the risk predictors identified above and their prediction ability;
Automation of modeling and data analysis processes for potential software development and commercialization.
The purpose of this paper is to briefly illustrate the key steps and features of image-based model development and the potential contributions image-based modeling could bring in vulnerable plaque assessment and clinical applications. Sections II-III present some important factors and techniques for in vivo image-based carotid and coronary plaque model constructions. Section IV explains how to use those models to identify mechanical and morphological risk factors for plaque assessment. Section V lists some grand challenges facing researchers, engineers, and clinicians in this field about the ways to bring research closer to clinical applications, in particular, identifying risk factors and developing predictive methods for better patient screening schemes. Section VI covers some important emerging imaging modalities.
II. Patient-Specific In Vivo MRI-Based Models for Carotid Atherosclerotic Plaques
Some basic questions should be asked: a) Can modeling provide information useful for plaque assessment? b) Do we have the data needed for modeling? c) Are there special techniques needed for in vivo image-based plaque modeling with multi-components and fluid-structure interactions (FSI)?
Mechanical forces play an important role in plaque progression and rupture. It is well accepted that plaque initiation and early progression correlate negatively with flow shear stress [10–13]. Plaque rupture happens when the mechanical stress in the plaque exceeds the ultimate strength of the plaque cap material. Therefore, accurate calculation of mechanical forces in the plaque is of fundamental importance for our understanding and prediction of plaque progression and rupture processes. Early artery models included simplified 1D, 2D models or 3D models with idealized geometries [7]. Development of medical image technologies made image-based models with realistic plaque geometries possible [6–8]. For carotid plaques, magnetic resonance imaging (MRI) is currently the best image modality providing plaque morphology and component differentiations (lipid-rich necrotic core, calcification, hemorrhage, ulceration, etc.) [5–6]. In vivo MRI-based 3D FSI models integrate plaque morphology, components, fluid and structural forces together and has the potential to provide more accurate flow shear stress and plaque stress/strain calculations for plaque assessment, compared to fluid- and structure-only models [14–15]. Fig. 1 demonstrates an example where critical plaque wall stress (CPWS) was able to predict site of rupture [4]. CPWS is the maximum of all plaque maximum principal stress values at vulnerable sites such as a site where a thin fibrous cap covers a large lipid core. [14].
Fig. 1.
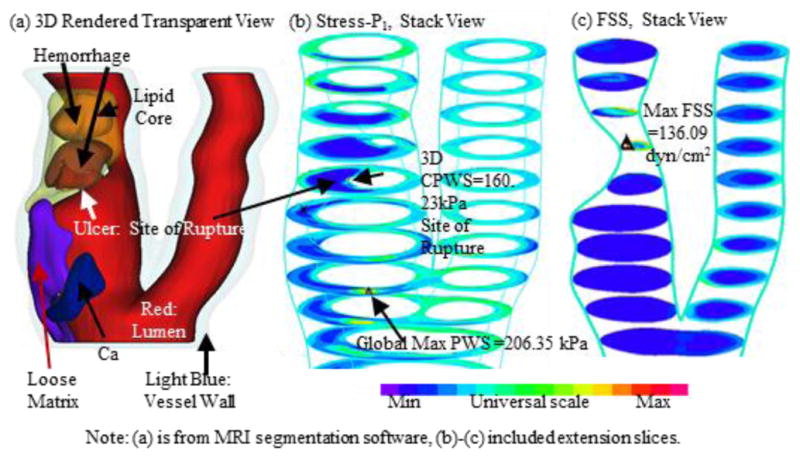
Modeling results from one plaque sample showing CPWS was able to predict the actual rupture site while CFSS failed to do so.
A. Data Requirement and Challenges
Construction of patient-specific 3D FSI models requires the following data: a) 3D in vivo MRI data of the plaque with plaque components; b) on-site pressure and blood flow data; c) vessel and plaque material properties; d) data to determine residual stress in the plaque. While researchers have developed techniques to provide high-resolution MRI data, machine resolution used in most publications is at 0.6×0.6 mm2 level. After interpolation, the in-plane resolution is 0.31×0.31 mm2 [14, 16]. It has been reported that the threshold cap thickness for plaque rupture is 65 μm based on histological data of human coronary plaques [17]. The corresponding threshold cap thickness value for carotid could be at 100–150 μm [18], which means the current MRI resolution needs to be improved to provide accurate cap thickness data and capture plaques which are more prone to rupture in the near future.
The limitation on MRI resolution has another implication: the calculated plaque stress at the cap tends to be lower than the actual stress because the cap thickness given by MRI may be greater than the actual thickness. Caution should be used when one tries to claim “threshold stress value” for plaque rupture since calculated stress values depend on the data and model used in their calculations. In vivo data and model predictions have much higher uncertainties, compared to data and studies based on ex vivo image and histological data.
Other than plaque morphology, on-site blood pressure, patient-specific vessel and plaque component material properties and residual stress conditions are often unavailable and researchers need to use data from the literature in their in vivo MRI models. Phase-contrast MRI has been used to obtain flow information and used as boundary conditions in plaque models. Cine MRI and 3D MRI were combined to quantify patient-specific vessel material properties with limited success [19]. The study used arm cuff pressure in lieu of on-site pressure, which was a severe limitation.
Residual stress has considerable effect on stress/strain calculations. People often relate residual stress in arteries to the opening angle when the artery is cut open, as it was first discovered by YC Fung and his group [20]. Actually, a circular artery segment under in vivo condition goes through three stages to get its open-up zero-stress shape: axial shrinkage (up to 30–50%, could be smaller for atherosclerotic plaques), circumferential shrinkage (5–20% based on our data [21]), and final opening-up with an opening angle (63.5°, n=5) [22]. Omitting any of the three stages would lead to stress/strain prediction errors in the order of 50%–100% or even more [19,21,23]. To take into account of axial/circumferential shrinkages, a shrink-stretch process is needed (see Fig. 2) [24] to: a) shrink the in vivo geometry from MRI to obtain a starting no-load geometry; and b) apply pressure and axial stretch to recover original in vivo geometry with residual stress/strain computed. The shrinking rate in the axial direction, the shrinkage rates of lumen and outer wall were determined so that: (1) mass conservation law was followed; and (2) the contours of the plaque and its components after pressurization and axial stretch had the best match with the in vivo geometry from MRI.
Fig. 2.
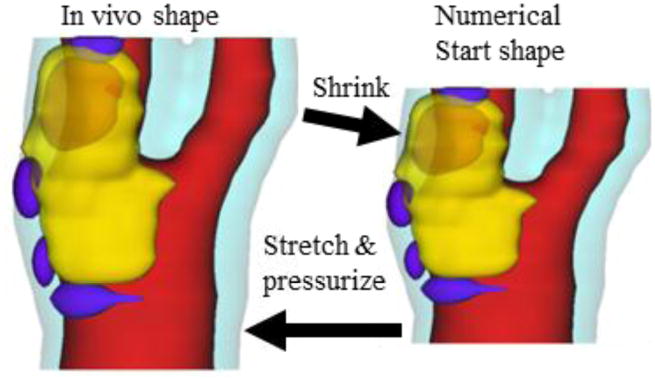
Illustration of the Shrink-Stretch process. The total plaque volume should be conserved. Yellow: lipid; Blue: calcification; light blue: outer wall; Red: lumen.
B. 3D Mesh Construction and Material Model Selection
Because plaques have complex irregular geometries with component inclusions which are challenging for mesh generation, a component-fitting mesh generation technique was developed for our models [25]. Fig. 3 gives an illustration of the method. Each slice was divided into component-fitting areas called “surfaces” (Fig. 3(a,b)). Then the neighboring slices were stacked to form volumes (Fig. 3(c)). Four types of volumes (hexahedron, prism, pyramid, and tetrahedron) were used as bricks to curve-fit the complex plaque geometry. Using this technique, the 3D plaque domain was divided into hundreds of small volumes to fit the irregular plaque geometry with component inclusions. A mesh for each small volume obtained above was then generated by ADINA or other software. Mesh analysis was performed by decreasing mesh size by 10% (in each dimension) until solution differences were less than 2%. The mesh was then chosen for our simulations. Our models were solved by ADINA (ADINA R & D, Watertown, MA, USA), a commercial FE package suitable for a wide range of applications.
Fig. 3.
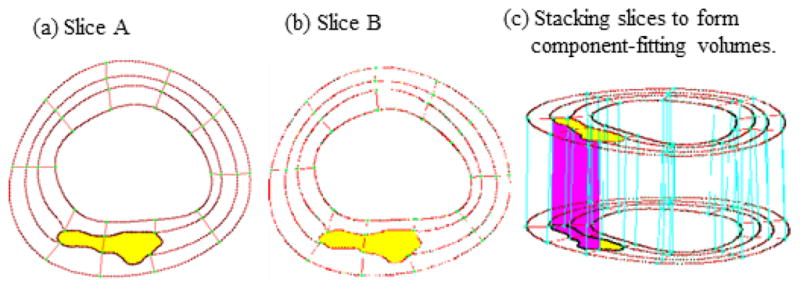
The component-fitting mesh generation process. (a)–(b): two slices with a lipid core inclusion (yellow) and numerically-generated component-fitting curves and surfaces; (c) component-fitting volumes formed by connection corresponding areas from stacking adjacent slices. Slice distance not to scale.
Most in vivo MRI-based plaque models used isotropic material models for vessel wall. However, it is known that arterial walls demonstrate anisotropic properties and consist of three layers: intima, media, and adventitia. Holzapfel et al. introduced anisotropic multi-layer atherosclerotic plaque models and demonstrated that anisotropic material properties and multi-layer structure features had a great effect (up to 400% difference) on stress predictions [26]. Identifying plaque layer structures and quantifying vessel anisotropic material properties non-invasively under in vivo condition remain to be challenges for medical imaging researchers.
III. Patient-Specific In vivo IVUS-Based Modeling for Coronary Plaques
While coronary diseases affect far more people, image-based modeling studies for coronary plaques are more difficult to perform because of the lack of high-resolution imaging tools that can characterize these lesions non-invasively. Coronary arteries are smaller, harder to access, and are constantly in motion. In vivo intravascular ultrasound (IVUS) is a catheter-based invasive approach currently used for direct in vivo imaging of coronary, carotid, and peripheral arteries [27]. IVUS-VH (virtual histology), a segmentation process based on known tissue types, sorts the raw IVUS data into four discrete atherosclerotic plaque components: fibrotic, fibro-fatty, necrotic, and dense calcified tissue [28]. This novel technique has been validated on ex vivo coronary plaques and is increasingly used for the differentiation of coronary plaque components with lipid and fibrous tissue in vivo [29–30].
Attempts at using ultrasound and IVUS techniques have been made to quantify vessel motion, plaque development, mechanical properties, and vessel wall structure, even to predict rupture locations [31–33]. Liang, Zhu and Friedman developed techniques to estimate transverse strain tensors in the artery wall using IVUS image registration [34]. In a multi-patient IVUS-based study, Samady et al. found that coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease [13]. Using patient follow-up data, Gijsen et al. found that high shear stress induces a strain increase in human coronary plaques over a 6-month period [35].
We introduced the first patient-specific in vivo IVUS-based coronary plaque model with a) fluid-structure interaction; b) anisotropic vessel material properties; c) cyclic bending of the coronary caused by heart motion [25]. Figure 4 used one coronary sample to demonstrate IVUS data, segmented contours and re-constructed 3D vessel geometry of a coronary segment. Figure 5 shows how angiography was used to determine vessel curvature and curvature variations. Four models were used to investigate the effect of a)-c) above on flow shear stress and plaque stress/strain calculations: Model 1 (M1), anisotropic model with cyclic bending and pulsating pressure; Model 2 (M2), same as Model 1, but no bending; Model 3 (M3), isotropic model, no bending; Model 4 is the same as Model 1, with 10% axial stretch. Figure 6 gives plots of the maximum principal stress (Stress-P1) on a cut surface at maximum/minimum curvature conditions. Taking M3 as the base model, maximum Stress-P1 values on the cut-surface (at maximum bending where applicable) from M2, M1 and M4 was 63%, 126%, and 345% higher, respectively. The increases for Strain-P1 were even higher at 104%, 278%, and 391%, respectively. Figure 7 compares flow maximum principal shear stress (FMSS) and flow velocity from M1 and M2. Cyclic bending caused a modest 15% decrease in maximum FMSS, 5% decrease in maximum velocity, and 8.7% decrease in flow rate (99.0 ml/min from M1 vs. 108.4 ml/min from M2).
Fig. 4.
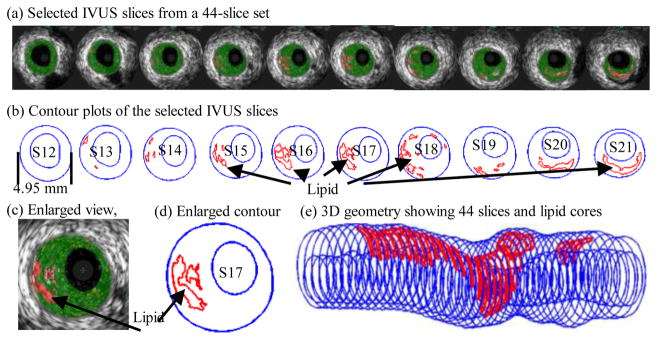
IVUS model construction process: selected 10 slices from a 44-slice IVUS data set with IVUS-VH, contour plots, enlarged view, and 3D plaque geometry showing lipid core locations.
Fig. 5.
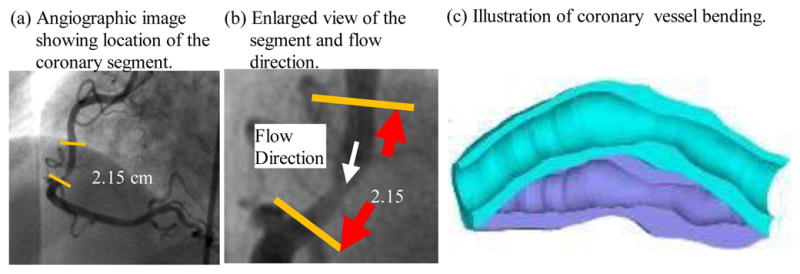
Angiography was used to determine location of the myocardium and vessel curvature variations.
Fig. 6.
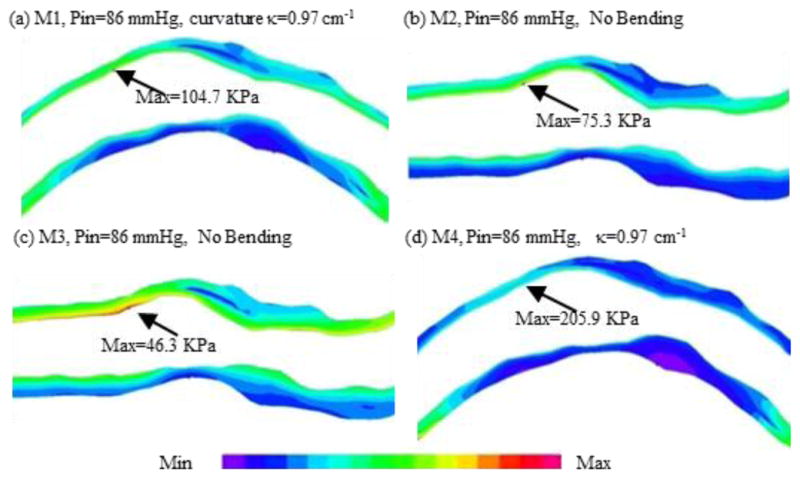
Maximum principal stress (Stress-P1) plots from 4 models showing vessel bending, anisotropic vessel properties and axial stretch have considerable effect on stress/strain distributions.
Fig. 7.
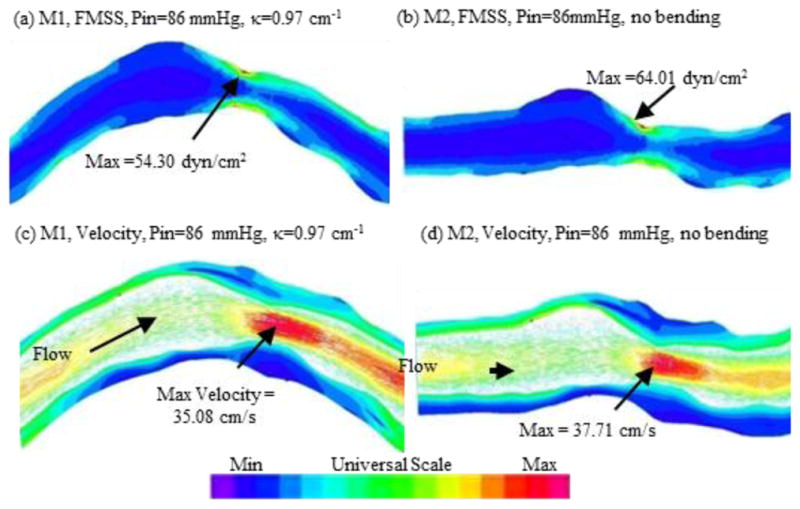
Curvature variation had only mild effect on flow velocity and shear stress values.
IV. Identifying Risk factors and Biomarkers Using Image-based Modeling
The fundamental challenges for biomechanical research are: a) provide in vivo evidence that mechanical risk factors (plaque stress, strain, flow shear stress) may be associated with plaque progression and rupture; b) identify representative values of those factors to be used for more accurate plaque vulnerability assessment and classification; c) develop predictive methods for patient screening and clinical applications.
A. Critical plaque wall stress (CPWS) is associated with plaque rupture and may be used for plaque assessment
3D plaque stress/strain distributions include huge numbers of data points and one representative value for each risk factor must be identified for vulnerability assessment and plaque classification. One might think that the maximum of maximum principal stress (Stress-P1) values should be the natural candidate. The fact is, as Fig. 1(b) shows, maximum Stress-P1 often appears at sites where the vessel wall is healthy and rupture is not possible. Critical plaque wall stress was defined as the largest local maxima of maximum principal stresses from all possible vulnerable sites of the plaque [14,36]. Possible vulnerable sites of a plaque include all sites with local stress/strain maxima, especially where a thin cap was covering a lipid core, but exclude healthy sites where rupture is unlikely, even if a local stress maximum occurred there [14]. We have published papers reporting that plaque rupture is related to higher critical plaque stress conditions based on in vivo MRI data from human carotid plaques with and without rupture [14]. Our stress-based plaque assessment had 85% agreement rate with histopathological plaque vulnerability index (HPVI) for coronary plaques (34 2D samples, Figures 8–9) and 80.1% agreement rate with in vivo morphological plaque vulnerability index (MPVI) for carotid plaques (206 2D samples) [36–37]. HPVI and MPVI were defined based on factors related to plaque vulnerability including cap thickness, lipid core size, hemorrhage and inflammation, and other factors [36–37].
Fig. 8.
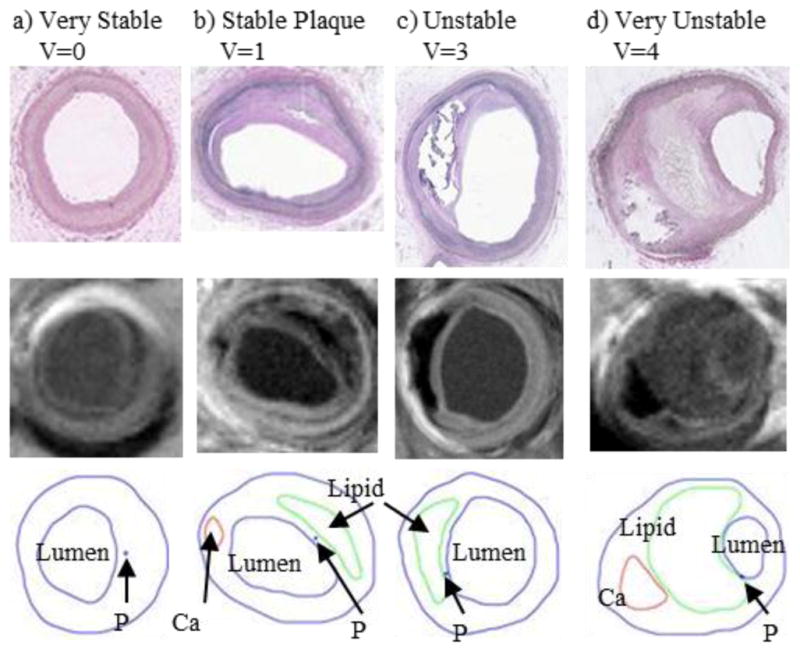
Selected plaque samples with different vulnerability classified by histopathological analysis. Histological images, MR images and segmented plaque with contour lines drawn for each component are shown [36].
Fig. 9.
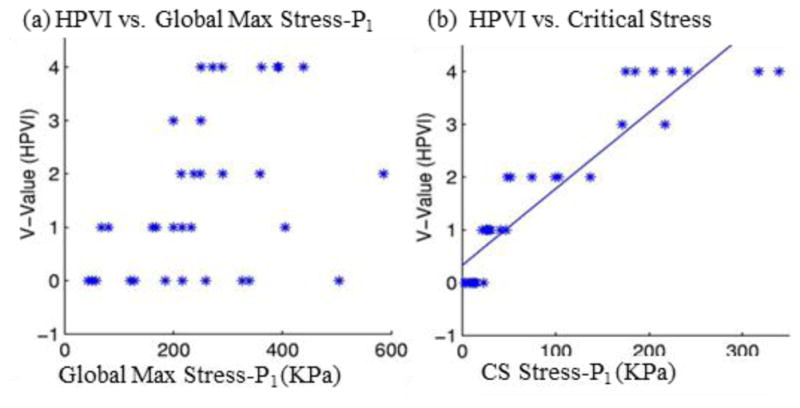
Critical stress shows much better correlation with HPVI than global maximum Stress-P1 from 34 coronary 2D plaque samples. p < 0.0001 [36].
With evidence that higher plaque stresses are linked to plaque rupture and the selection of critical stress value as the representative value, critical stress values have been used in several studies concerning plaque rupture and assessment. Bluestein et al. investigated the effect of microcalcifications on vulnerable plaque [38]. Their results suggested that microcalcifications increased the plaque vulnerability [38]. Gao et al. studied carotid plaques and found that critical stress values from symptomatic patients were higher that from asymptomatic patients [39]. Intraplaque hemorrhage has been found to be closely related to potential plaque rupture [6]. Huang et al performed a multi-patient modeling study and found that locations of intraplaque hemorrhages corresponded to higher stress values than non-hemorrhage locations [40].
B. Mechanisms governing advanced carotid and coronary plaque progression
It has been well accepted that atherosclerosis initiation and progression correlate negatively with low and oscillating flow wall shear stresses (FSS) [10–13]. However, this mechanism cannot explain why advanced plaques continue to grow under elevated FSS conditions. Using patient-specific in vivo carotid MRI data from 14 patients and 32 scan pairs (baseline–follow up, see Fig. 10) and 3D FSI models, we found that 21 out of the 32 scan pairs showed a significant positive correlation between plaque progression and FSS, and 26 out of 32 scan pairs showed a significant negative correlation between plaque progression and plaque wall stress (PWS) [41–42].
Fig. 10.
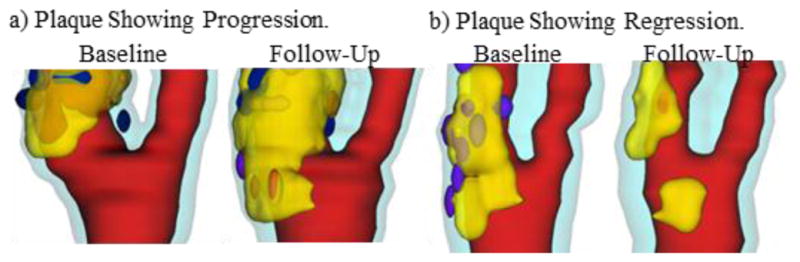
3D human carotid plaque samples re-constructed from in vivo MR images showing progression and regression. Time interval: 18 months. Red: lumen; Yellow: lipid; Dark blue: calcification; light blue: outer wall.
For coronary plaque progression, Samady et al. used IVUS data from 20 patients in a 6-month follow-up study and 3D CFD models to investigate possible association between FSS and plaque progression and remodeling [13]. All IVUS segments (n=2249) were divided into low, intermediate, and high FSS groups. Compared with intermediate-FSS coronary segments, low-FSS segments developed greater plaque and necrotic core progression and constrictive remodeling; and high-FSS segments developed greater necrotic core and calcium progression, regression of fibrous and fibro-fatty tissue, and excessive expansive remodeling, suggestive of transformation to a more vulnerable phenotype.
V. Grand Challenges: Data, Model, Validation, and Patient Screening
A. Patient-Specific Modeling with Incomplete and Inaccurate Data, Multi-Modality Approach, Data Sharing
Patient-specific image-based plaque modeling faces many challenges. One of them is that we often have to use incomplete data with limited accuracy. Some examples are: a) image resolution may not be enough to reveal critical plaque features such as thin plaque cap close to threshold value and lumen surface erosion and inflammation; b) patient-specific residual stress condition, vessel and plaque component material properties are not available in vivo; c) on-site (at the site of the lesion) pressure and flow information; d) data at cellular and molecular level may not be available. Researchers have been using multi-modality approaches to gather data from different channels to complete the loop in modeling. IVUS and angiography are combined to get 3D coronary geometry, on-site pressure and vessel curvature variations [13,25]. Chiu et al. are using 3D ultrasound as a prescreening tool before MRI to reduce cost and making the diagnosis-preventive care more practical [43].
Because patient data are hard and expensive to collect, the need for data sharing has been recognized by the research community. The benefit is certainly clear. However, many issues remain to be solved: resources, data format, maintenance, method to share, credits, etc..
B. Modeling Limitation and Interpretation of Modeling Results
Due to the fact data a given model uses may be incomplete and inaccurate because of the difficulty of obtaining data in vivo, it is important to be cautious when interpreting modeling results and predictions. Researchers make best effort to include the most important factors in their models for the biological and clinical problems they are modeling. However, all models must ignore many factors so that the models are solvable and analysis is possible. Sensitivity analysis of these models should be performed to assess the uncertainties and errors that models bring due to those omissions and limitations. Errors may be introduced from a) inaccuracy of data; b) data not available (so generic data from the literature are used); c) model assumptions and simplifications; d) numerical algorithms. With proper assessment, model predictions could be better understood and applied in potential applications.
It should be emphasized that, even with these limitations and uncertainties, model predictions including stress/strain calculations are still useful because most applications are concerned with relative differences compared to either prior readings of the same patient, or the normal range of a population group that the patient is compared to. It is with that reason that simpler models could be used, as long as their predictions are reasonably validated by patient studies.
C. Risk Indicators, Predictions, and Precision Medicine
Most investigations for atherosclerotic plaque progression and rupture have focused on correlation studies between risk factors and potential events (progression, rupture, stroke, and heart attack). However, it is far more important and of more practical significance that we develop methods which can predict the critical clinical events before their actual occurrence. In our recent paper [44], a predictive method was introduced where 3D FSI models were constructed based on patient data with follow-up scan showing plaque rupture. Plaque wall stress (PWS) and strain (PWSn) and Flow Maximum Shear Stress (FSS) were extracted from all 600 matched nodal points (100 points per matched slice, baseline matching follow-up) on the lumen surface for analysis. Each of the 600 points was marked “ulcer” or “non-ulcer” using follow-up scan. Predictive statistical models for each of the 7 combinations of PWS, PWSn and FSS were trained using the follow-up data and applied to the baseline data to assess their sensitivity and specificity using the 600 data points for ulcer predictions. Using probability 0.3 as a threshold to infer ulcer occurrence at the prediction stage, the combination of PWS and PWSn provided the best predictive accuracy with (sensitivity, specificity) = (0.97, 0.958). The method could be applied at population level to identify the optimal predictor(s) for plaque rupture or selected specific clinical observations.
The time has come that predictive methods based on in vivo data with patient follow-up be developed to a) identify risk indicators which have predictive power; b) quantify the prediction accuracy of those risk indicators; c) identify the optimal predictors (or combination of several indicators) for plaque rupture and other clinical events; d) validate those findings by large-scale patient studies and implement patient screening schemes for actual applications.
One important point worth noting is that, with so much effort invested in plaque mechanical analysis and some evidence that critical stress may be related to plaque rupture, it has not been established that adding mechanical stress/strain factors into our consideration would lead to more accurate predictions of potential rupture. That remains to be a great challenge in the coming years.
D. Plaque Classifications, Plaque Vulnerability Score, Precision Medicine
The American Heart Association has introduced plaque classifications based primarily on histopathological features. With risk indicators identified, more quantitative plaque classifications can be introduced based on morphological features, critical stress and strain, flow shear stress or other predictor. Going in the direction of “Precision Medicine”, several plaque vulnerability indices (PVI) could be introduced, each would link a given risk predictor (such as cap thickness or critical stress) to a numerical score called cap-PVI or stress-PVI, which should be validated by clinical events in large-scale patient studies. There would be Cap-PVI, Stress-PVI, FSS-PVI, or others, as what research would lead us to. The PVI values could be converted from the corresponding risk predictor values, such as cap thickness and critical stress, to numerical values easy to understand by physicians and the general public. We have suggested using stress-PVI values from 0 to 4 with 0 for most stable and 4 for highly vulnerable [36]. Composite PVIs made of combination of several predictors with proper weighting are also possible if they provide optimal predicting accuracies.
Precision medicine includes several aspects: personalized quantification of risk factors, disease state assessment, personalized medication and even surgical treatment plan, among others. Atherosclerosis is a complicated disease process. Plaque vulnerability assessment is a challenging task because validation by large-scale patient study with sufficient clinical events is extremely difficult. However, the potential for improvement to the current assessment schemes does exist.
E. Validations
It should be made clear that there are different kinds and levels of validation, and each requires special techniques and procedures: image segmentation validation; image reproducibility validation; computational model validation; numerical method validation; model output validation; risk factor validation; risk factor prediction accuracy validation; PVI-based patient screening or plaque classification scheme validation.
One approach is cross validation. To compare a PVI-based plaque classification scheme with the stenosis based scheme, we could simply apply the two schemes to a patient pool. Each scheme will come up with surgical treatment recommendation for each patient analyzed. If a large pool of patient data with follow-up is available, we compare the recommendations from the two methods to actual occurrences of plaque rupture, stroke, or other clinical events which require surgical interventions. If the PVI-based scheme has a better prediction success rate, it will be a successful validation of the scheme.
F. Transforming Research to Clinical Application
If research progressed to the point of validating the above mentioned schemes, transforming them to clinical applications could be even more challenging. First of all, the involved procedures, including image data processing, model construction, data analysis and final screening results would need to be automated with minimum operator interactions. Then software development, FDA approval, clinical trials testing, manufacturing, and commercialization would be required. Those tasks are beyond the scope of this paper.
VI. Emerging Engineering Imaging Techniques for Plaque Identification and Tissue Differentiation
This paper aims to provide a perspective review for image-based vulnerable plaque modeling and its potential for patient screening and translational diagnostic applications. However, the accuracy and success of any modeling effort are based on availability and accuracy of the desired or required data. Several imaging techniques have emerged with the potential to improve over the available modalities. X-ray computed tomography (CT) could be very useful for carotid and coronary plaque characterization, given the considerable progress made recently. A study of spectral CT [45–47] shows a possibility of four-material decomposition for assessing the vulnerability of plaques, in terms of plaque’s inflammation and spotty calcification. Techniques using ultrasound-induced thermal strain imaging (TSI) [48], Rayleigh mixture model [49], intracoronary optical coherence tomography (OCT) [50], 3-D ultrasound [51], and magnetic nanoparticulate probes for molecular MRI of atherosclerosis [52] all show promises for future improved medical imaging of carotid and coronary plaques with potential applications. Development of new imaging techniques will provide better data which will lead to improved modeling accuracy and predictions.
Acknowledgments
This work was supported in part by NIH R01 EB004759 and NSF DMS-0540684. Chun Yang’s research is supported by National Sciences Foundation of China grant 11171030.
Biographies

Dalin Tang received the Ph. D. degree in applied mathematics from University of Wisconsin-Madison in 1988. He is currently a professor of mathematics and biomedical engineering at Worcester Polytechnic Institute. He is a member of ASME, BMES, AHA (Fellow), and AMS. His research interests include computational biomechanics and modeling, image-based modeling for cardiovascular diseases, vulnerable plaques, ventricle models, fluid-structure interactions, computer-aided surgical design, computational fluid dynamics, and scientific computing.

Chun Yang received her B.A. (1988) and M.S. (1991) degrees in computational mathematics from Beijing University. She is currently Associate Professor of Mathematics in School of Mathematics, Beijing Normal University. Her research interest includes nonlinear analysis, numerical analysis, biomathematics, partial differential equations, nonlinear geometry, computational modeling for cardiovascular systems, ventricle models, vulnerable plaques, atherosclerosis progression.

Jie Zheng received the B.S. degree in Electrical Engineering department from Beijing University, China, in 1986. He then received Ph.D. degree in Medical Physics from University of Cincinnati in 1994. He is currently an Associate Professor in the Mallinckrodt Institute of Radiology, at Washington University School of Medicine, USA. His current research interests include myocardial oxygenation imaging and atherosclerotic plaque imaging in vivo.

Gador Canton received her B.S. in Chemical Engineering from the Complutense University of Madrid, Spain, in 1998, and her M.S. and Ph.D. degrees in Mechanical Engineering from the University of California, San Diego (UCSD), in 2002 and 2004, respectively. She joined the faculty of the Mechanical Engineering Department at the University of Washington as a Research Assistant Professor in 2012 after holding postdoctoral positions both at UCSD and UW. Professor Canton’s research focuses on the interaction between the behavior of biological fluids and pathological processes. In particular, she is interested on the role that mechanical stresses play in the etiology of diseases in the circulatory system. Over the years she has developed expertise in experimental and computational techniques to characterize blood flow and identify flow conditions associated with cardiovascular diseases such as intracranial aneurysms, carotid atherosclerosis and abdominal aortic aneurysms. Her honors include a fellowship from the UW School of Medicine Cardiovascular Research Training Program and a R.B. Woolley Jr. Graduate Leadership Fellowship granted by the University of California, San Diego’s Jacobs School of Engineering.

Richard G. Bach received his M.D. degree from New York University School of Medicine in 1984. He is a Fellow of the American College of Cardiology and of the Society for Cardiac Angiography and Interventions, and a member of the American Heart Association Scientific Councils on Basic Science and Clinical Cardiology. He is currently Professor of Medicine at Washington University School of Medicine and Medical Director of the Cardiac Intensive Care Unit at Barnes-Jewish Hospital in St. Louis, Missouri. His research interests focus on acute coronary syndromes, advanced coronary imaging techniques and coronary physiology.

Thomas Hatsukami received his B.A. degree in human biology at Stanford University and M.D. degree at the University of California Los Angeles. He is a Professor of Vascular Surgery at the University of Washington and co-directs the Vascular Imaging Laboratory with Dr. Chun Yuan, Professor of Radiology. His research has focused on the histological validation of novel MRI techniques for in vivo characterization of human atherosclerosis, prospective studies examining risk factors and mechanisms of atherosclerosis progression, observational studies to identify carotid plaque features associated with an increased risk for stroke, and clinical trials using MRI to directly assess changes in plaque structure and composition in response to pharmacological therapy.

Liang Wang received his B.S in School of Mathematic Science at Beijing Normal University in 2011. He is currently a graduate student in School of Mathematic Science at Beijing Normal University.

Deshan Yang received the BSEE degree in 1992 from the Tsinghua University, Beijing, China, the M.S. degree in computer science from Illinois Institute of Technology, Chicago, in 2001, and PhD degree from the University of Wisconsin-Madison in 2005. He is an assistant professor in the department of radiation oncology, school of medicine, Washington University in Saint Louis. In the field of medical physics, his research covers medical image reconstruction and processing, computer intelligence and applications in radiation oncology clinics.

Kristen Billiar received his B.S. in Mechanical and Aerospace Engineering at Cornell University in 1991 and his Ph.D. in Bioengineering from the University of Pennsylvania in 1998. He is currently an Associate Professor in the Department of Biomedical Engineering at WPI and an Adjunct Associate
Chun Yuan received the BS degree in Physics from Beijing Normal University, China, in 1982. He then received his Ph.D. degree in Medical Physics from University of Utah in 1988. He is currently a professor of radiology in the Department of Radiology, University of Washington School of Medicine, Seattle, WA. His research interests include medical images, MRI, ultrasound, biomechanics, and vulnerable plaques.
Contributor Information
Dalin Tang, Email: dtang@wpi.edu, Southeast University, Nanjing, China. Worcester Polytechnic Institute, MA 01609, USA, 508-831-5332; fax: 508-831-5824.
Chun Yang, Email: yangchun@bnu.edu.cn, Beijing Normal University, Beijing, China.
Jie Zheng, Email: zhengj@mir.wustl.edu, Washington University, St. Louis, MO 63110, USA.
Gador Canton, Email: gcanton@u.washington.edu, University of Washington, Seattle, WA 98195.
Richard Bach, Email: RBACH@im.wustl.edu, Washington University, St. Louis, MO 63110, USA.
Thomas S. Hatsukami, Email: tomhat@u.washington.edu, University of Washington, Seattle, WA 98195
Liang Wang, Email: wangliang@mail.bnu.edu.cn, Beijing Normal University, Beijing, China.
Deshan Yang, Email: dyang@radonc.wustl.edu, Washington University, St. Louis, MO 63110, USA.
Kristen L. Billiar, Email: kbilliar@wpi.edu, Worcester Polytechnic Institute, MA 01609, USA, 508-831-5332; fax: 508-831-5824
Chun Yuan, Email: cyuan@u.washington.edu, University of Washington, Seattle, WA 98195.
References
- 1.Fuster V. The Vulnerable Atherosclerotic Plaque: Understanding, Identification, and Modification. In: Fuster Valentin, Fredrick Cornhill J, Dinsmore Robert E, Fallon John T, Insull William, Libby Peter, Nissen Steven, Rosenfeld Michael E, Wagner William D., editors. AHA Monograph series. Futura Publishing; Armonk NY: 1998. [Google Scholar]
- 2.Fuster V, Stein B, Ambrose JA, Badimon L, Badimon JJ, Chesebro JH. Atherosclerotic plaque rupture and thrombosis, evolving concept. Circulation. 1990;82(Supplement II):II-47–II-59. [PubMed] [Google Scholar]
- 3.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108(14):1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 4.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108(15):1772–1778. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 5.Yuan C, Mitsumori LM, Beach KW, Maravilla KR. Special review Carotid atherosclerotic plaque: noninvasive MR characterization and identification of vulnerable lesions. Radiology. 2001;221:285–99. doi: 10.1148/radiol.2212001612. [DOI] [PubMed] [Google Scholar]
- 6.Underhill HR, Hatsukami TS, Fayad ZA, Fuster V, Yuan C. MRI of carotid atherosclerosis: clinical implications and future directions. Nat Rev Cardiol. 2010;7(3):165–73. doi: 10.1038/nrcardio.2009.246. [DOI] [PubMed] [Google Scholar]
- 7.Tang D. Modeling flow in healthy and stenosed arteries. In: Metin Akay Hoboekn., editor. Wiley Encyclopedia of Biomedical Engineering. John Wiley & Sons, Inc; New Jersey: 2006. pp. 1–16. Article 1525. [Google Scholar]
- 8.Friedman MH, Krams R, Chandran KB. Flow interactions with cells and tissues: cardiovascular flows and fluid-structure interactions, (Review) Ann Biomed Eng. 2010;38(3):1178–87. doi: 10.1007/s10439-010-9900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorelick PB. Carotid endarterectomy: where do we draw the line? Stroke. 1990;30:1745–1750. doi: 10.1161/01.str.30.9.1745. [DOI] [PubMed] [Google Scholar]
- 10.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation: positive correlation between plaque location and low and oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 11.Friedman MH, Bargeron CB, Deters OJ, Hutchins GM, Mark FF. Correlation between wall shear and intimal thickness at a coronary artery branch. Atherosclerosis. 1987;68:27–33. doi: 10.1016/0021-9150(87)90090-6. [DOI] [PubMed] [Google Scholar]
- 12.Giddens DP, Zarins CK, Glagov S. The role of fluid mechanics in the localization and detection of atherosclerosis. J Biomech Engng. 1993;115:588–594. doi: 10.1115/1.2895545. [DOI] [PubMed] [Google Scholar]
- 13.Samady H, Eshtehardi P, McDaniel MC, Suo J, Dhawan SS, Maynard C, Timmins LH, Quyyumi AA, Giddens DP. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124(7):779–88. doi: 10.1161/CIRCULATIONAHA.111.021824. [DOI] [PubMed] [Google Scholar]
- 14.Tang D, Teng ZZ, Canton G, Yang C, Ferguson M, Huang X, Zheng J, Woodard PK, Yuan C. Sites of rupture in human atherosclerotic carotid plaques are associated with high structural stresses: an in vivo MRI-based 3D fluid-structure interaction study. Stroke. 2009;40:3258–3263. doi: 10.1161/STROKEAHA.109.558676. Featured article on MDlinx.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang D, Yang C, Zheng J, Woodard PK, Sicard GA, Saffitz JE, Yuan C. 3D MRI-Based Multi-Component FSI Models for Atherosclerotic Plaques a 3-D FSI model. Annals of Biomedical Engineering. 2004;32(7):947–960. doi: 10.1023/b:abme.0000032457.10191.e0. Best Paper Selection, International Medical Informatics Association (IMIA) Year Book 2006. [DOI] [PubMed] [Google Scholar]
- 16.Li ZY, Howarth S, Trivedi RA, U-King-Im JM, Graves MJ, Brown A, Wang LQ, Gillard JH. Stress Analysis of Carotid Plaque Rupture Based on In Vivo High Resolution MRI. J Biomech. 2006;39:2611–2622. doi: 10.1016/j.jbiomech.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262–75. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 18.Virmani R, Ladich ER, Burke AP, Kolodgie FD. Histopathology of carotid atherosclerotic disease. Neurosurgery. 2006;59:S219–S227. doi: 10.1227/01.NEU.0000239895.00373.E4. [DOI] [PubMed] [Google Scholar]
- 19.Liu HF, Canton G, Yuan C, Yang C, Billiar KL, Teng ZZ, Hoffman AH, Tang D. Using in vivo Cine and 3D multi-contrast MRI to determine human atherosclerotic carotid artery material properties and circumferential shrinkage rate and their impact on stress/strain predictions. J Biomech Eng. 2012;134(1):011008. doi: 10.1115/1.4005685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung YC. Biomechanics Mechanical Properties of Living Tissues. 2. Springer; New York: 1993. [Google Scholar]
- 21.Huang X, Yang C, Yuan C, Liu F, Canton G, Zheng J, Woodard PK, Sicard GA, Tang D. Patient-specific artery shrinkage and 3D zero-stress state in multi-component 3D FSI models for carotid atherosclerotic plaques based on in vivo MRI data. Molecular & Cellular Biomech. 2009;6(2):121–134. [PMC free article] [PubMed] [Google Scholar]
- 22.Kural MH, Cai MC, Tang D, Gwyther T, Zheng J, Billiar KL. Planar biaxial characterization of diseased human coronary and carotid arteries for computational modeling. J Biomech. 2012;45(5):790–798. doi: 10.1016/j.jbiomech.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohayon J, Dubreuil O, Tracqui P, Floc’h SL, Rioufol G, Chalabreysse L, Thivolet F, Pettigrew RI, Finet G. Influence of residual stress/strain on the biomechanical stability of vulnerable coronary plaques: potential impact for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol. 2007;293:H1987–H1996. doi: 10.1152/ajpheart.00018.2007. [DOI] [PubMed] [Google Scholar]
- 24.Yang C, Tang D, Yuan C, Hatsukami TS, Zheng J, Woodard PK. In Vivo/Ex Vivo MRI-Based 3D Models with Fluid-Structure Interactions for Human Atherosclerotic Plaques Compared with Fluid/Wall-Only Models. CMES: Comput Model Eng Sci vol. 2007;19(3):233–245. [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C, Bach R, Zheng J, El Naqa I, Woodard PK, Teng ZZ, Billiar KL, Tang D. In vivo IVUS-based 3D fluid structure interaction models with cyclic bending and anisotropic vessel properties for human atherosclerotic coronary plaque mechanical analysis. IEEE Transactions on Biomed Eng. 2009;56(10):2420–2428. doi: 10.1109/TBME.2009.2025658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holzapfel GA, Stadler M, Schulze-Bause CAJ. A layer-specific three-dimensional model for the simulation of balloon angioplasty using Magnetic Resonance Imaging and mechanical testing. Annals of Biomedical Engineering. 2002;30(6):753–767. doi: 10.1114/1.1492812. [DOI] [PubMed] [Google Scholar]
- 27.Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS) J Am Coll Cardiol. 2001;37:1478–92. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 28.Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106:2200–2206. doi: 10.1161/01.cir.0000035654.18341.5e. [DOI] [PubMed] [Google Scholar]
- 29.Nair A, Margolis MP, Kuban BD, Vince DG. Automated coronary plaque characterization with intravascular ultrasound backscatter: ex vivo validation. EuroIntervention. 2007;3:113–20. [PubMed] [Google Scholar]
- 30.Nasu K, Tsuchikane E, Katoh O, Vince DG, Virmani R, Surmely JF, Murata A, Takeda Y, Ito T, Ehara M, Matsubara T, Terashima M, Suzuki T. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006;47:2405–2412. doi: 10.1016/j.jacc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 31.Chandran KB, Wahle A, Vigmostad SC, Olszewski ME, Rossen JD, Sonka M. Coronary arteries: imaging, reconstruction, and fluid dynamic analysis. Crit Rev Biomed Eng. 2006;34(1):23–103. doi: 10.1615/critrevbiomedeng.v34.i1.20. [DOI] [PubMed] [Google Scholar]
- 32.Wahle A, Lopez JJ, Olszewski ME, Vigmostad SC, Chandran KB, Rossen JD, Sonka M. Plaque development, vessel curvature, and wall shear stress in coronary arteries assessed by X-ray angiography and intravascular ultrasound. Med Image Anal. 2006;10(4):615–31. doi: 10.1016/j.media.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohayon J, Pierre T, Finet G, Rioufol G. In-vivo prediction of human coronary plaque rupture location using intravascular ultrasound and the Finite Element Method. Coronary Artery Disease. 2001;12:655–663. doi: 10.1097/00019501-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Liang Y, Zhu H, Friedman MH. Estimation of the transverse strain tensor in the arterial wall using IVUS image registration. Ultrasound Med Biol. 2008;34(11):1832–45. doi: 10.1016/j.ultrasmedbio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Gijsen FJ, Mastik F, Schaar JA, Schuurbiers JC, van der Giessen WJ, de Feyter PJ, Serruys PW, van der Steen AF, Wentzel JJ. High shear stress induces a strain increase in human coronary plaques over a 6-month period. EuroIntervention. 2011;7(1):121–7. doi: 10.4244/EIJV7I1A20. [DOI] [PubMed] [Google Scholar]
- 36.Tang D, Yang C, Zheng J, Woodard PK, Saffitz JE, Petruccelli JD, Sicard GA, Yuan C. Local maximal stress hypothesis and computational plaque vulnerability index for atherosclerotic plaque assessment. Ann Biomed Eng. 2005;33(12):1789–1801. doi: 10.1007/s10439-005-8267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang D, Teng ZZ, Canton G, Hatsukami TS, Dong L, Huang X, Yuan C. Local Critical Stress Correlates Better than Global Maximum Stress with Plaque Morphological Features Linked to Atherosclerotic Plaque Vulnerability: An In Vivo Multi-Patient Study. BioMedical Engineering OnLine. 2009;8:15. doi: 10.1186/1475-925X-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bluestein D, Alemu Y, Avrahami I, Gharib M, Dumont K, Ricotta JJ, Einav S. Influence of microcalcifications on vulnerable plaque mechanics using FSI modeling. J Biomech. 2008;41(5):1111–1118. doi: 10.1016/j.jbiomech.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 39.Gao H, Long Q, Kumar Das S, Halls J, Graves M, Gillard JH, Li ZY. Study of carotid arterial plaque stress for symptomatic and asymptomatic patients. J Biomech. 2011;44(14):2551–7. doi: 10.1016/j.jbiomech.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Huang X, Teng ZZ, Canton G, Ferguson M, Yuan C, Tang D. Intraplaque hemorrhage is associated with higher structural stresses in human atherosclerotic plaques: an in vivo MRI-based 3D fluid-structure interaction study. Biomed Eng Online, Paper #. 2010;9:86. doi: 10.1186/1475-925X-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang C, Canton G, Yuan C, Ferguson M, Hatsukami TS, Tang D. Advanced human carotid plaque progression correlates positively with flow shear stress: an in vivo MRI multi-patient 3D FSI study. J Biomechanics. 2010;43(13):2530–2538. doi: 10.1016/j.jbiomech.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerwin WS, Hooker A, Spilker M, Vicini P, Ferguson M, Hatsukami TS, Yuan C. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation. 2003;107(6):851–6. doi: 10.1161/01.cir.0000048145.52309.31. [DOI] [PubMed] [Google Scholar]
- 43.Chiu B, Shamdasani V, Entrekin R, Yuan C, Kerwin WS. Characterization of carotid plaques on 3-dimensional ultrasound imaging by registration with multicontrast magnetic resonance imaging. J Ultrasound Med. 2012;31(10):1567–80. doi: 10.7863/jum.2012.31.10.1567. [DOI] [PubMed] [Google Scholar]
- 44.Wu Z, Yang C, Tang D. In vivo serial MRI-based models and statistical methods to quantify sensitivity and specificity of mechanical predictors for carotid plaque rupture: location and Beyond. J Biomech Eng. 2011;133(6):064503. doi: 10.1115/1.4004189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baturin P, Alivov Y, Molloi S. Spectral CT imaging of vulnerable plaque with two independent biomarkers. Physics in Medicine and Biology. 2012;57:4117–4138. doi: 10.1088/0031-9155/57/13/4117. [DOI] [PubMed] [Google Scholar]
- 46.Gao H, Yu HY, Osher S, Wang G. Multi-energy CT based on a prior rank, intensity and sparsity model (PRISM) Inverse Problems. 2011;27(11):22. doi: 10.1088/0266-5611/27/11/115012. paper # 115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He P, Wei B, Cong W, Wang G. Optimization of K-Edge Imaging with Spectral CT. Med Phys. 2012;39(11):6572–79. doi: 10.1118/1.4754587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim K, Huang SW, Hall TL, Witte RS, Chenevert TL, O’Donnell M. Arterial Vulnerable Plaque Characterization Using Ultrasound-Induced Thermal Strain Imaging (TSI) IEEE Trans Biomed Eng. 2008;55(1):171–180. doi: 10.1109/TBME.2007.900565. [DOI] [PubMed] [Google Scholar]
- 49.Seabra JC, Ciompi F, Pujol O, Mauri J, Radeva P, Sanches J. Rayleigh mixture model for plaque characterization in intravascular ultrasound. IEEE Trans Biomed Eng. 2011;58(5):1314–24. doi: 10.1109/TBME.2011.2106498. [DOI] [PubMed] [Google Scholar]
- 50.Suter MJ, Tearney GJ, Wang-Yuhl O, Bouma BE. Progress in Intracoronary Optical Coherence Tomography. IEEE J of Selected Topics in Quantum Electronics. 2010;16(4):706–714. [Google Scholar]
- 51.Seabra JCR, Pedro LM, Fernandes e Fernandes J, Sanches JM. A 3-D Ultrasound-Based Framework to Characterize the Echo Morphology of Carotid Plaques. IEEE Transactions on Biomedical Engineering. 2009;56(5):1442–1453. doi: 10.1109/TBME.2009.2013964. [DOI] [PubMed] [Google Scholar]
- 52.Kanwar RK, Chaudhary R, Tsuzuki T, Kanwar JR. Emerging engineered magnetic nanoparticulate probes for molecular MRI of atherosclerosis: how far have we come? Nanomedicine (Lond) 2012;7(6):899–916. doi: 10.2217/nnm.12.57. [DOI] [PubMed] [Google Scholar]


