Abstract
Aims
To evaluate long-term all-cause risk of mortality in women and men hospitalized for the first time with atrial fibrillation (AF) compared with matched controls.
Methods and results
A total of 272 186 patients (44% women) ≤85 years at the time of hospitalization with incidental AF 1995–2008 and 544 344 matched controls free of in-hospital diagnosis of AF were identified. Patients were followed via record linkage of the Swedish National Patient Registry and the Cause of Death Registry. Using Cox regression models, the long-term relative all-cause mortality risk, adjusted for concomitant diseases, in women vs. controls was 2.15, 1.72, and 1.44 (P < 0.001) in the age categories ≤65, 65–74, and 75–85 years, respectively. The corresponding figures for men were 1.76, 1.36, and 1.24 (P < 0.001). Among concomitant diseases, neoplasm, chronic renal failure, and chronic obstructive pulmonary disease contributed most to the increased all-cause mortality vs. controls. In patients with AF as the primary diagnosis, the relative risk of mortality was 1.63, 1.46, and 1.28 (P < 0.001) in women and 1.45, 1.17, and 1.10 (P < 0.001) in men.
Conclusion
Atrial fibrillation was an independent risk factor of all-cause mortality in patients with incident AF. The concomitant diseases that contributed most were found outside the thromboembolic risk scores. The highest relative risk of mortality was seen in women and in the youngest patients compared with controls, and the differences between genders in each age category were statistically significant.
Keywords: Atrial fibrillation, Mortality, Gender, Age, Long term
See page 1027 for the editorial comment on this article (doi:10.1093/eurheartj/eht044)
Introduction
Atrial fibrillation (AF) is a progressive condition associated with increased morbidity and mortality, and an increasing incidence and prevalence will continue to impose a considerable burden on the health-care system.1–4 There is an association between AF, increasing age, and concomitant diseases such as hypertension, ischaemic heart diseases, and heart failure.5–7
Population-based studies have indicated AF and atrial flutter to be independent predictors of increased late mortality.8 Data from the Framingham study demonstrated a 1.5- to 1.9-fold risk of mortality in patients with AF in both men and women across a wide range of ages after adjustment for pre-existing cardiovascular disease.9 In another study, the adjusted relative risk of mortality was about 20% higher in patients with AF in all of six age–sex strata during each of the 3 years of follow-up.4
In Sweden, all inhabitants have a unique personal identification number and equal access to health care and hospital services. This provides the possibility to record and retrieve patients in different registers and thus gives a unique opportunity to analyse morbidity and mortality in the entire patient population. Such nation-wide patient registers are well suited for epidemiological studies, such as an evaluation of the long-term mortality risk in patients with hospitalized incident AF.
The aim of this study was to estimate the risk of mortality in patients hospitalized with incident AF and to determine the role of age, gender, and concomitant diseases in the largest-ever cohort compared with matched controls.
Methods
National registries
The Swedish National Patient Registry (NPR) was started in 1964 and attained full national coverage in 1987, including all patients discharged from hospitals regardless of the cause of hospitalization. These registry data have high validity.10,11 Patients were identified in the NPR from the epidemiological centre at the Swedish National Board of Health and Welfare. Controls were identified in the General Population Registry (GPR) using the personal identification number. The matching procedure for finding controls was carried out by Statistics Sweden. Mortality or emigration was identified through record linkage of the NPR, the GPR, and the Cause of Death Registry. The Cause of Death Registry has a log that goes 2 years back; thus, our analyses were limited to an end date of 31 December 2009. All previous diagnoses from the NPR were added by linking registries to construct the study cohort. Before databases were sent to the research group, the data were made anonymous for all personal identification information.
This study complied with the Declaration of Helsinki, and the study protocol was approved by the Regional Ethical Review Board in Uppsala, Sweden (Dnr 2009/273).
Patients with incident atrial fibrillation
Atrial fibrillation was defined according to the International Classification of Diseases (ICD): 427 D (DA, DB, DC, DD, and DW) in ICD 9 (1987–96) and I 48, I48.9, and I 48.9 (A, B, C, D, E, F, P, and X) in ICD 10 (1997–). Patients were eligible if they had a diagnosis of incident AF 1995–2008 but no AF diagnosis 1987–94, thus making it more likely that the AF was truly incident. No distinction was made between paroxysmal, persistent or permanent AF, and atrial flutter, and the AF diagnosis could be primary or secondary. There were 272 186 patients with a diagnosis of incident AF between 1995 and 2008.
Patients with incident atrial fibrillation as primary diagnosis
In the NPR, every hospital admission is assigned one primary diagnosis and up till nine secondary diagnoses. The primary diagnosis describes the main cause of hospitalization. There were 119 631 patients with a primary diagnosis of incident AF between 1995 and 2008.
Concomitant diseases
The impact of concomitant diseases on mortality risk was studied. These diseases were heart failure, hypertension, diabetes mellitus, stroke/TIA, chronic obstructive pulmonary disease used in the CHADS2, CHA2DS2-VASc, and HATCH scores.12–14 In addition, neoplasm and chronic renal failure were added due to their impact on mortality.15 Concomitant diseases were recorded between 1987 and immediately after inclusion of an AF patient or a control subject.
Comparison cohort
For each AF patient, two controls with no hospital record of AF between 1987 and 2009 were selected and matched for age, gender, and calendar year of the AF diagnosis by linkage with the GPR. All controls were alive on 1 January the year of the AF diagnosis of the index patient. If a control person had died earlier relative to the index patient during the first year, the time at risk was set to 1 day. The risk of all-cause mortality was assessed from the date of diagnosis to the date of death, emigration, or end of follow-up on 31 December 2009.
Statistical analysis
Unadjusted Kaplan–Meier plots are used to illustrate mortality. Mortality rates were defined as number of deaths divided by number of person-years at risk. Cox regression models were used to compare AF patients with controls adjusted by age at diagnosis categorized in 5-year age bands, with the first category younger than 40 years, and modelled as a categorical variable. Adjustments for concomitant diseases modelled as dummy variables. Separate regression models were estimated for men and women combined with categories of age at diagnosis: younger than 65, 65–74, and 75–85 years of age. Because of non-proportional hazards, risk times were split at 1 year after diagnosis and time-dependent models were estimated. Only individuals who survived the first year were included in the analysis of time since diagnosis after 1 year. The same analysis strategy was used to compare AF patients and controls with a primary AF diagnosis and their matched controls. Measures of associations are hazard ratios as an estimate of the relative risk complemented with 95% confidence intervals. All statistical calculations were made with STATA release 11 (StataCorp, College Station, TX, USA), and two-sided P values of <0.05 were considered statistically significant.
Results
Baseline characteristics
In total, 272 186 patients with a mean age of 72.3 ± 10.9 years were identified with incident AF diagnosed in a hospital setting (Table 1). The proportion was 44% women, and their mean age was higher than that of the men, 74.8 ± 9.3 vs. 70.4 ± 11.7 years, respectively. There were 544 344 matched controls. Incident AF was almost three times more common at age 75–85 years (n = 143 172) than at age <65 years (n = 55 118) and twice as common as at age 65–74 (n = 73 896). The proportion of women was 28% at an age younger 65 years, 40% at age 65–74 years, and 52% at age 75–85 years. The incidence rates of AF per 1000 person-years in women were 0.3, 5.2, and 14.9, and the corresponding figures for men were 0.8, 8.7, and 19.3.
Table 1.
Baseline characteristics
| Women |
Men |
All |
||||
|---|---|---|---|---|---|---|
| AF patients | Controls | AF patients | Controls | AF patients | Controls | |
| AF patients and matched controls | ||||||
| (n = 119 916) | (n = 239 818) | (n = 152 270) | (n = 304 526) | (n = 272 186) | (n = 544 344) | |
| Age, mean (SD) | 74.8 (9.3) | 74.8 (9.3) | 70.4 (11.7) | 70.4 (11.7) | 72.3 (10.9) | 72.3 (10.9) |
| <65 (%) | 13 | 13 | 26 | 26 | 20 | 20 |
| 65–74 (%) | 25 | 25 | 29 | 29 | 27 | 27 |
| 75–85 (%) | 62 | 62 | 45 | 45 | 53 | 53 |
| Gender, women (%) | 44 | 44 | ||||
| Concomitant diseases | ||||||
| Any | 70.6 | 27.8 | 68.6 | 26.7 | 69.5 | 27.2 |
| Ischaemic heart disease (%) | 22.8 | 6.2 | 28.6 | 9.5 | 26.0 | 8.0 |
| Acute myocardial infarction (%) | 10.3 | 2.4 | 13.6 | 4.4 | 12.2 | 3.6 |
| Heart failure (%) | 24.5 | 2.7 | 25.0 | 3.0 | 24.8 | 2.9 |
| Stroke/TIA (%) | 15.2 | 4.1 | 13.5 | 4.7 | 14.2 | 4.4 |
| Stroke (%) | 12.3 | 3.0 | 10.8 | 3.6 | 11.5 | 3.3 |
| Hypertension (%) | 27.7 | 7.3 | 23.5 | 6.5 | 25.4 | 6.8 |
| COPD (%) | 4.8 | 1.3 | 5.0 | 1.4 | 4.9 | 1.4 |
| Diabetes mellitus (%) | 12.8 | 4.8 | 13.8 | 5.5 | 13.4 | 5.2 |
| Neoplasm (any) (%) | 18.5 | 13.6 | 15.1 | 10.1 | 16.6 | 11.6 |
| Chronic renal failure (%) | 1.2 | 0.2 | 2.0 | 0.4 | 1.6 | 0.3 |
| Patients with a primary diagnosis of AF and matched controls | ||||||
| n = 54 022 | n = 108 038 | n = 65 609 | n = 131 213 | n = 119 631 | n = 239 251 | |
| Age, mean (SD) | 72.6 (10.2) | 72.6 (10.2) | 66.4 (13.0) | 66.4 (13.0) | 69.2 (12.2) | 69.2 (12.2) |
| <65 (%) | 19 | 19 | 39 | 39 | 30 | 30 |
| 65–74 (%) | 29 | 29 | 30 | 30 | 29 | 29 |
| 75–85 (%) | 52 | 52 | 31 | 31 | 41 | 41 |
| Gender, women (%) | 45 | 45 | ||||
| Concomitant diseases | ||||||
| Any | 57.0 | 26.3 | 50.9 | 22.6 | 53.6 | 24.3 |
| Ischaemic heart disease (%) | 15.5 | 5.5 | 18.3 | 7.9 | 17.0 | 6.8 |
| Acute myocardial infarction (%) | 5.0 | 2.1 | 6.9 | 3.6 | 6.0 | 3.0 |
| Heart failure (%) | 16.7 | 2.2 | 16.1 | 2.3 | 16.3 | 2.3 |
| Stroke/TIA (%) | 6.8 | 3.6 | 5.7 | 3.8 | 6.2 | 3.7 |
| Stroke (%) | 4.7 | 2.6 | 3.9 | 2.9 | 4.3 | 2.7 |
| Hypertension (%) | 24.4 | 6.7 | 19.4 | 5.5 | 21.6 | 6.0 |
| COPD (%) | 3.0 | 1.2 | 3.0 | 1.1 | 3.0 | 1.2 |
| Diabetes mellitus (%) | 8.5 | 4.5 | 9.0 | 4.8 | 8.8 | 4.7 |
| Neoplasm (any) (%) | 15.8 | 13.4 | 10.3 | 8.5 | 12.8 | 10.7 |
| Chronic renal failure (%) | 0.7 | 0.2 | 1.1 | 0.3 | 0.9 | 0.3 |
AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; SD, standard deviation; TIA, transient ischaemic attack.
All predefined concomitant diseases were more common in patients with AF than in controls, regardless of whether AF was the primary diagnosis (Table 1). Patients with AF as the primary diagnosis were younger and had fewer concomitant diseases than the entire AF population.
All-cause mortality by gender and age
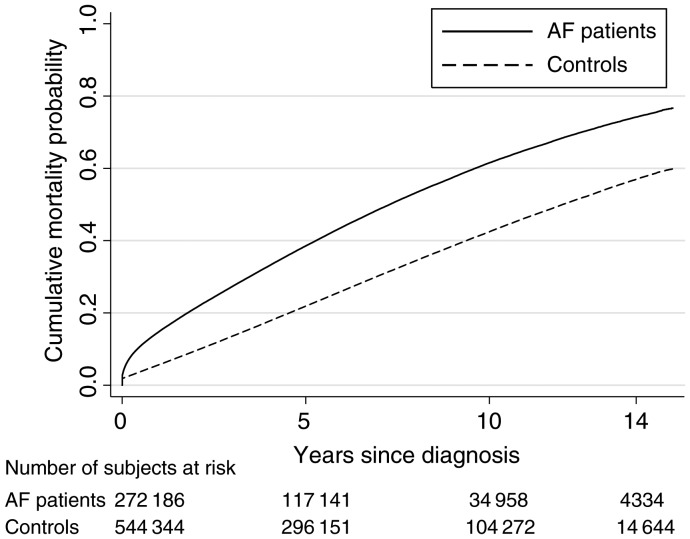
The all-cause mortality, unadjusted for concomitant diseases, was higher in AF patients compared with controls, especially during the first year (Figure 1). Patients and controls were divided by gender and according to the three age categories. At the end of the 14-year follow-up period, the mortality rates per 1000 person-years in the three age categories were 25.0, 63.5, and 152.1; and 27.5, 80.0, and 185.4 in women and men with AF, respectively. The corresponding rates in controls were 7.0, 24.9, and 78.6 in women, and 9.8, 39.4, and 107.8 in men. Thus, the actual mortality rate was consistently lower in women than men, both in the index AF patients and in controls. However, the age-adjusted relative mortality risk in AF patients compared with controls was higher in women than men in all three age categories both during the first year and 1–14 years of follow-up (Table 2).
Figure 1.
All-cause mortality in patients with a diagnosis of primary or secondary atrial fibrillation vs. controls.
Table 2.
Adjusted relative risk (95% confidence interval) for all-cause mortality in patients with a primary or secondary diagnosis of atrial fibrillation vs. controls
| Women |
Men |
|||
|---|---|---|---|---|
| Age-adjusteda HR (95% CI) | Age- and concomitant disease-adjustedb HR (95% CI) | Age-adjusteda HR (95% CI) | Age- and concomitant disease-adjustedb HR (95% CI) | |
| <65 years at AF diagnosis | ||||
| First year after diagnosis | ||||
| Controls | 1.0 | 1.0 | 1.0 | 1.0 |
| AF patients | 7.67 (6.58–8.94) | 4.88 (4.17–5.72) | 4.99 (4.59–5.42) | 3.07 (2.82–3.35) |
| 1–14 years after diagnosis | ||||
| Controls | 1.0 | 1.0 | 1.0 | 1.0 |
| AF patients | 2.94 (2.73–3.15) | 2.15 (1.99–2.32) | 2.50 (2.41–2.60) | 1.76 (1.69–1.84) |
| Concomitant diseases | ||||
| Ischaemic heart disease | 1.10 (0.99–1.22) | 1.23 (1.18–1.30) | ||
| Heart failure | 2.15 (1.96–2.37) | 2.22 (2.11–2.34) | ||
| Stroke | 1.72 (1.49–1.99) | 1.64 (1.51–1.78) | ||
| Transient ischaemic attack | 0.98 (0.74–1.30) | 1.18 (1.02–1.37) | ||
| Hypertension | 0.89 (0.81–0.98) | 0.98 (0.93–1.04) | ||
| COPD | 3.06 (2.68–3.49) | 2.22 (2.01–2.45) | ||
| Diabetes mellitus | 2.14 (1.94–2.37) | 2.00 (1.90–2.12) | ||
| Neoplasm (any) | 2.45 (2.29–2.62) | 3.39 (3.22–3.57) | ||
| Chronic renal failure | 4.31 (3.58–5.18) | 2.45 (2.17–2.77) | ||
| 65–74 years at AF diagnosis | ||||
| First year after diagnosis | ||||
| Controls | 1.0 | 1.0 | 1.0 | 1.0 |
| AF patients | 4.44 (4.15–4.74) | 2.88 (2.69–3.09) | 3.14 (3.00–3.27) | 2.07 (1.97–2.16) |
| 1–14 years after diagnosis | ||||
| Controls | 1.0 | 1.0 | 1.0 | 1.0 |
| AF patients | 2.33 (2.26–2.40) | 1.72 (1.67–1.78) | 1.86 (1.82–1.90) | 1.36 (1.33–1.40) |
| Concomitant diseases | ||||
| Ischaemic heart disease | 1.13 (1.09–1.18) | 1.06 (1.04–1.09) | ||
| Heart failure | 1.74 (1.67–1.81) | 1.89 (1.84–1.95) | ||
| Stroke | 1.69 (1.60–1.77) | 1.56 (1.50–1.62) | ||
| Transient ischaemic attack | 1.11 (1.01–1.22) | 1.09 (1.02–1.16) | ||
| Hypertension | 0.96 (0.92–1.00) | 0.96 (0.93–0.99) | ||
| COPD | 2.71 (2.55–2.88) | 2.13 (2.03–2.23) | ||
| Diabetes mellitus | 2.01 (1.92–2.09) | 1.75 (1.70–1.80) | ||
| Neoplasm (any) | 1.82 (1.76–1.89) | 2.15 (2.09–2.21) | ||
| Chronic renal failure | 3.25 (2.90–3.64) | 2.49 (2.31–2.69) | ||
| 75–85 years at AF diagnosis | ||||
| First year after diagnosis | ||||
| Controls | 1.0 | 1.0 | 1.0 | 1.0 |
| AF patients | 2.81 (2.74–2.88) | 2.09 (2.04–2.15) | 2.33 (2.28–2.39) | 1.72 (1.68–1.76) |
| 1–14 years after diagnosis | ||||
| Controls | 1.0 | 1.0 | 1.0 | 1.0 |
| AF patients | 1.83 (1.81–1.86) | 1.44 (1.42–1.46) | 1.57 (1.55–1.60) | 1.24 (1.22–1.26) |
| Concomitant diseases | ||||
| Ischaemic heart disease | 1.10 (1.08–1.11) | 1.04 (1.02–1.05) | ||
| Heart failure | 1.56 (1.53–1.59) | 1.62 (1.60–1.65) | ||
| Stroke | 1.54 (1.51–1.58) | 1.44 (1.41–1.46) | ||
| Transient ischaemic attack | 1.10 (1.06–1.14) | 1.13 (1.09–1.16) | ||
| Hypertension | 0.96 (0.95–0.98) | 0.96 (0.94–0.98) | ||
| COPD | 1.94 (1.88–2.00) | 1.71 (1.66–1.76) | ||
| Diabetes mellitus | 1.63 (1.60–1.66) | 1.50 (1.47–1.53) | ||
| Neoplasm (any) | 1.38 (1.36–1.40) | 1.64 (1.62–1.67) | ||
| Chronic renal failure | 2.37 (2.22–2.52) | 2.10 (2.00–2.19) | ||
AF, atrial fibrillation; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
aAdjusted for age at diagnosis in 5-year categories from <40 years up to 80–85 years.
bAdjusted for age at diagnosis and concomitant diseases.
Patients with AF as the primary diagnoses showed the same pattern between genders and in the three age categories, although the relative and absolute mortality risk were lower (Table 3). In women, the mortality rates per 1000 person-years in the three age categories were 15.2, 42.6, and 106.9 vs. 17.0, 54.8, and 129.7 in men. The corresponding rates in controls were 6.9, 24.2, and 74.8; and 8.9, 38.1, and 103.4 in women and men, respectively.
Table 3.
Adjusted relative risk (95% confidence interval) for all-cause mortality in patients with a primary diagnosis of atrial fibrillation vs. controls
| Women |
Men |
|||
|---|---|---|---|---|
| Age-adjusteda HR (95% CI) | Age- and concomitant disease-adjustedb HR (95% CI) | Age-adjusteda HR (95% CI) | Age and concomitant disease-adjustedb HR (95% CI) | |
| <65 years at AF diagnosis | ||||
| First year after diagnosis | ||||
| Controls | 1.0 | 1.0 | 1.0 | 1.0 |
| AF patients | 2.99 (2.42–3.70) | 2.11 (1.70–2.62) | 2.36 (2.09–2.67) | 1.65 (1.45–1.88) |
| 1–14 years after diagnosis | ||||
| Controls | 1.0 | 1.0 | 1.0 | 1.0 |
| AF patients | 2.10 (1.91–2.31) | 1.63 (1.47–1.80) | 1.87 (1.77–1.97) | 1.45 (1.37–1.53) |
| 65–74 years at AF diagnosis | ||||
| First year after diagnosis | ||||
| Controls | 1.0 | 1.0 | 1.0 | 1.0 |
| AF patients | 1.91 (1.71–2.12) | 1.44 (1.29–1.61) | 1.55 (1.44–1.68) | 1.18 (1.09–1.28) |
| 1–14 years after diagnosis | ||||
| Controls | 1.0 | 1.0 | 1.0 | 1.0 |
| AF patients | 1.79 (1.71–1.87) | 1.46 (1.39–1.53) | 1.44 (1.39–1.49) | 1.17 (1.13–1.22) |
| 75–85 years at AF diagnosis | ||||
| First year after diagnosis | ||||
| Controls | 1.0 | 1.0 | 1.0 | 1.0 |
| AF patients | 1.45 (1.38–1.52) | 1.20 (1.14–1.26) | 1.23 (1.17–1.29) | 1.01 (0.96–1.06) |
| 1–14 years after diagnosis | ||||
| Controls | 1.0 | 1.0 | 1.0 | 1.0 |
| AF patients | 1.50 (1.46–1.53) | 1.28 (1.25–1.31) | 1.28 (1.25–1.31) | 1.10 (1.07–1.13) |
AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio.
aAdjusted for age at diagnosis in 5-year categories from <40 years up to 80–85 years.
bAdjusted for age at diagnosis and concomitant diseases.
All-cause mortality and concomitant diseases
When adjusting for concomitant diseases and age, the relative risk of mortality remained increased in all three age categories and during both time periods of follow-up when compared with controls, with a statistically significant difference between women and men (Table 2). Although the relative risk declined in older age categories, it remained statistically significantly increased compared with controls, and statistically significantly different between women and men. Chronic renal failure, neoplasm, and chronic obstructive pulmonary disease contributed most to the adjusted elevated risk of mortality.
Patients with AF as their primary diagnosis, when adjusting for concomitant diseases and age, showed significant differences in all age categories compared with controls except in men 75–85 years of age during the first year after the diagnosis (Table 3). The significant differences between genders remained, except in the age category younger than 65 years of age.
Discussion
Patients with an incident AF diagnosis between 1995 and 2008 were associated with a significant increase of all-cause mortality as compared with controls, and the risk remained increased after adjustment for concomitant diseases. The actual risk of mortality was consistently lower in women, both in patients with AF and in matched controls, but, owing to the lower actual mortality in controls, the relative risk of mortality was higher in women and highest in the youngest patients.
Role of gender and age
A report from the Framingham Heart Study on the impact of AF on the risk of death, based on a long-term follow-up of 325 women and 296 men with incident AF and matched subjects without AF showed, after adjustment for concomitant diseases, an odds ratio for death of 1.9 in women and 1.5 in men, without any changes across four age categories.9 Wolf et al.4 observed no significant difference between age categories or genders during a 3-year follow-up between 1989 and 1991. A Danish report showed a statistically significant difference in the relative risk of mortality between genders in age categories older than 70 years during 14 years of follow-up between 1980 and 1993.16 Consistent with these reports, our results, based on a much larger AF population, demonstrated a significant difference between genders and the three age categories. In addition, we found a difference in both the actual and the relative risk of mortality between women and men in all age categories in patients with AF, even when compared with matched controls.
Role of concomitant diseases
After adjustment for concomitant diseases, the all-cause mortality risk for patients diagnosed with AF remained significantly higher than that of controls in both genders and in all age categories, indicating that AF was an independent risk of mortality. The observed difference between patients with AF as a primary diagnosis and the total study population with an AF diagnosis, primary and secondary, indicates the great influence of concomitant diseases on mortality risk. There was a significantly higher prevalence of hypertension, stroke, and neoplasm in women as compared with men, probably due to the higher mean age at which AF was diagnosed. Several factors might have affected the results. During the observation period between 1995 and 2009, important changes in the management of AF and concomitant diseases occurred that may have influenced morbidity and mortality. Secondary prevention, for instance, after myocardial infarction and stroke/TIA has had a great impact in reducing mortality, as well as new treatment strategies in heart failure.17–19
Antiarrhythmic drugs used as rhythm or rate control agents in the treatment of AF have been associated with a reduction in cardiovascular but not in all-cause mortality, which may seem reasonable considering their mode of action.20 However, rhythm control agents that are recommended in current guidelines are neither sufficiently effective nor sufficiently safe to be commonly used.21 Anticoagulation therapy in AF patients with a risk of stroke has improved survival.22 A recent study indicated that women have an increased relative risk of stroke, which might help to explain the increased relative mortality risk in women with AF.23 However, under-treatment with anticoagulation agents is a great challenge.24,25
Strengths and limitations
Strengths of this study are that the analyses are based on a large and complete consecutive national population of patients hospitalized with incident AF. Each patient included had two matched controls. Furthermore, the exclusion of patients diagnosed 8 years before the study period increases the probability of AF being truly incident in our study population.
We had limited knowledge about AF that was managed only in outpatient care but, when detected through linkage of registries, such patients were excluded. Patients managed on an outpatient basis only probably have fewer symptoms or concomitant diseases than the hospitalized patients and thus might be a healthier population, with less impact on mortality.
The various types of AF, i.e. paroxysmal, persistent, or permanent, might have influenced the results, but the registry data did not allow this differentiation. On the other hand, the registry allowed us to identify incident AF, and thus it is likely that patients were found in an early phase and that some of them progressed to persistent and permanent types during the 14 years of follow-up. In addition, we decided to include atrial flutter, which we found to be justified since it often coexists in patients with AF and the differential diagnosis can be difficult.
Conclusions
Atrial fibrillation was an independent risk factor of all-cause mortality in patients hospitalized with incident AF. The concomitant diseases that contributed most to an increased mortality were neoplasm, chronic renal failure and chronic obstructive pulmonary disease. None of these diseases are included in the scoring schemes used to asses thromboembolic risk. The relative risk of mortality was higher in women than in men in all age categories and was highest in the youngest patients. Patients with AF as the primary diagnosis showed the same pattern, and although the differences were smaller, they remained statistically significant.
Funding
This study was supported by a grant from AstraZeneca R&D, Mölndal, Sweden. T.A. received funding from the Örebro Heart Foundation. Funding to pay the Open Access publication charges for this article was provided by the Örebro Heart Foundation and the Research Committee of Örebro University Hospital.
Conflict of interest: K.M.H. is an employee of AstraZeneca R&D, Mölndal, Sweden.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmstead County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. doi:10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. doi:10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 3.Frost L, Vestergaard P, Mosekilde L, Mortensen LS. Trends in incidence and mortality in the hospital diagnosis of atrial fibrillation or flutter in Denmark, 1980–1999. Int J Cardiol. 2005;103:78–84. doi: 10.1016/j.ijcard.2004.08.024. doi:10.1016/j.ijcard.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Wolf PA, Mitchell JB, Baker CS, Kannel WB, D'Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–234. doi: 10.1001/archinte.158.3.229. doi:10.1001/archinte.158.3.229. [DOI] [PubMed] [Google Scholar]
- 5.Wachtell K, Hornestam B, Lehto M, Slotwiner DJ, Gerdts E, Olsen MH, Aurup P, Dahlöf B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, Nieminen MS, Rokkedal J, Devereux RB. Cardiovascular morbidity and mortality in hypertensive patients with a history of atrial fibrillation: the Losartan Intervention for End point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:705–711. doi: 10.1016/j.jacc.2004.06.080. doi:10.1016/j.jacc.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 6.Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction. A systematic review and meta-analysis. Circulation. 2011;123:1587–1593. doi: 10.1161/CIRCULATIONAHA.110.986661. doi:10.1161/CIRCULATIONAHA.110.986661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van den Berg MP, van Gelder IC, van Veldhuisen DJ. Impact of atrial fibrillation on mortality in patients with chronic heart failure. Eur J Heart Fail. 2002;4:571–575. doi: 10.1016/s1388-9842(02)00094-6. doi:10.1016/S1388-9842(02)00094-6. [DOI] [PubMed] [Google Scholar]
- 8.Vidaillet H, Granada JF, Chyou PH, Massen K, Qrtiz M, Pulido JN, Sharma P, Smith PN, Hayes J. A population-based study of mortality among patients with atrial fibrillation or flutter. Am J Med. 2002;113:365–370. doi: 10.1016/s0002-9343(02)01253-6. doi:10.1016/S0002-9343(02)01253-6. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin EJ, Wolf PA, D'Agostino RB, Silershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. doi:10.1161/01.CIR.98.10.946. [DOI] [PubMed] [Google Scholar]
- 10.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish National Inpatient Register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. doi:10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JG, Platonov PG, Hedblad B, Engström G, Melander O. Atrial fibrillation in the Malmö diet and cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2009;25:95–102. doi: 10.1007/s10654-009-9404-1. doi:10.1007/s10654-009-9404-1. [DOI] [PubMed] [Google Scholar]
- 12.Gage BF, Waterman AD, Shannon W. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. doi:10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 13.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. doi:10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 14.De Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, Allessie MA, Crijns HJ. Progression from paroxysmal to persistent atrial fibrillation. J Am Coll Cardiol. 2010;55:725–731. doi: 10.1016/j.jacc.2009.11.040. doi:10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 15.Official Statistics of Sweden . Statistics—Health and Medical Care. Causes of Death 2009. (2010) 978-91-86585-94-5.
- 16.Frost L, Engholm G, Møller H, Husted S. Decrease in mortality in patients with a hospital diagnosis of atrial fibrillation in Denmark during the period 1980–1993. Eur Heart J. 1999;20:1592–1599. doi: 10.1053/euhj.1999.1713. doi:10.1053/euhj.1999.1713. [DOI] [PubMed] [Google Scholar]
- 17.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. doi:10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 18.Steg G, James SK, Atar D, Badano LP, Blomstrom Lundqvist C, Borger MA, Di Mario C, Dickstein K. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. doi:10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 19.McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. doi:10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 20.Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, Connolly SJ. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–678. doi: 10.1056/NEJMoa0803778. doi:10.1056/NEJMoa0803778. [DOI] [PubMed] [Google Scholar]
- 21.Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Mahé I, Bergmann JF. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch Intern Med. 2006;166:719–728. doi: 10.1001/archinte.166.7.719. doi:10.1001/archinte.166.7.719. [DOI] [PubMed] [Google Scholar]
- 22.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 23.Friberg L, Benson L, Rosenqvist M, Lip GY. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. Br Med J. 2012;344:e3522. doi: 10.1136/bmj.e3522. doi:10.1136/bmj.e3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friberg L, Hammar N, Ringh M, Pettersson H, Rosenqvist M. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort-study on Atrial Fibrillation (SCAF-study) Eur Heart J. 2006;27:1954–1964. doi: 10.1093/eurheartj/ehl146. doi:10.1093/eurheartj/ehl146. [DOI] [PubMed] [Google Scholar]
- 25.Waldo AL, Becker RC, Tapson VF, Colgan KJ. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol. 2005;46:1729–1736. doi: 10.1016/j.jacc.2005.06.077. doi:10.1016/j.jacc.2005.06.077. [DOI] [PubMed] [Google Scholar]