Abstract
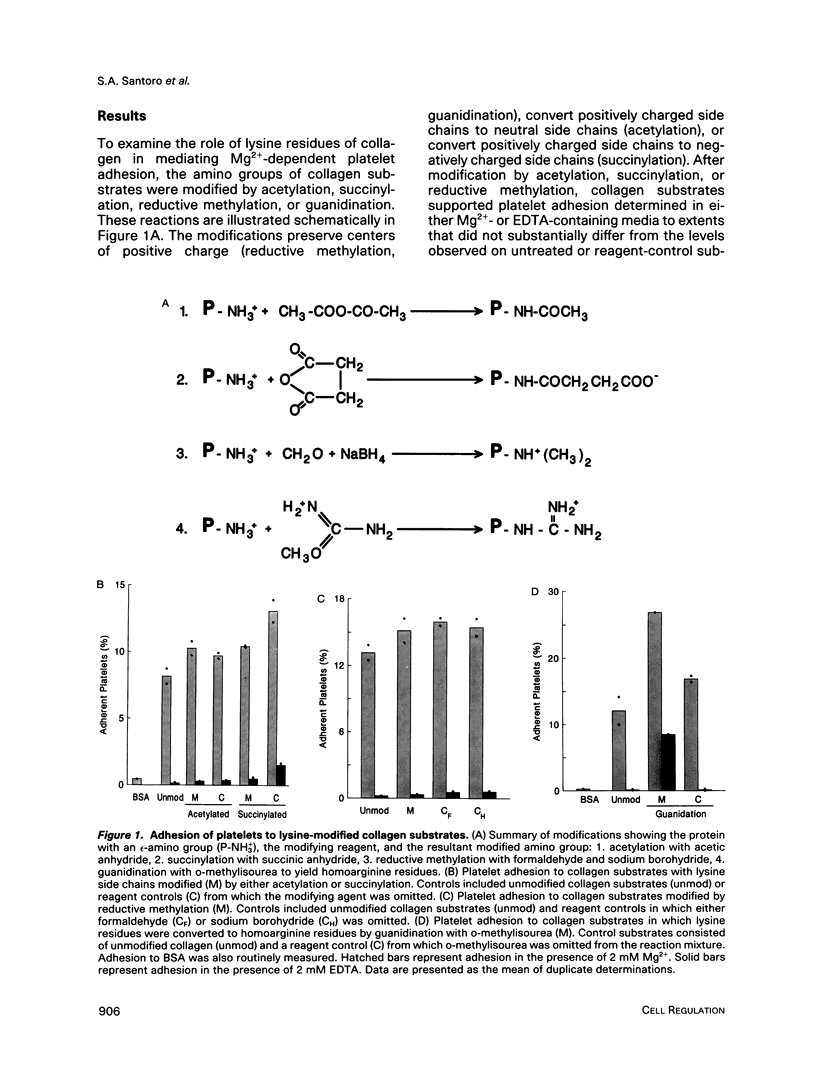
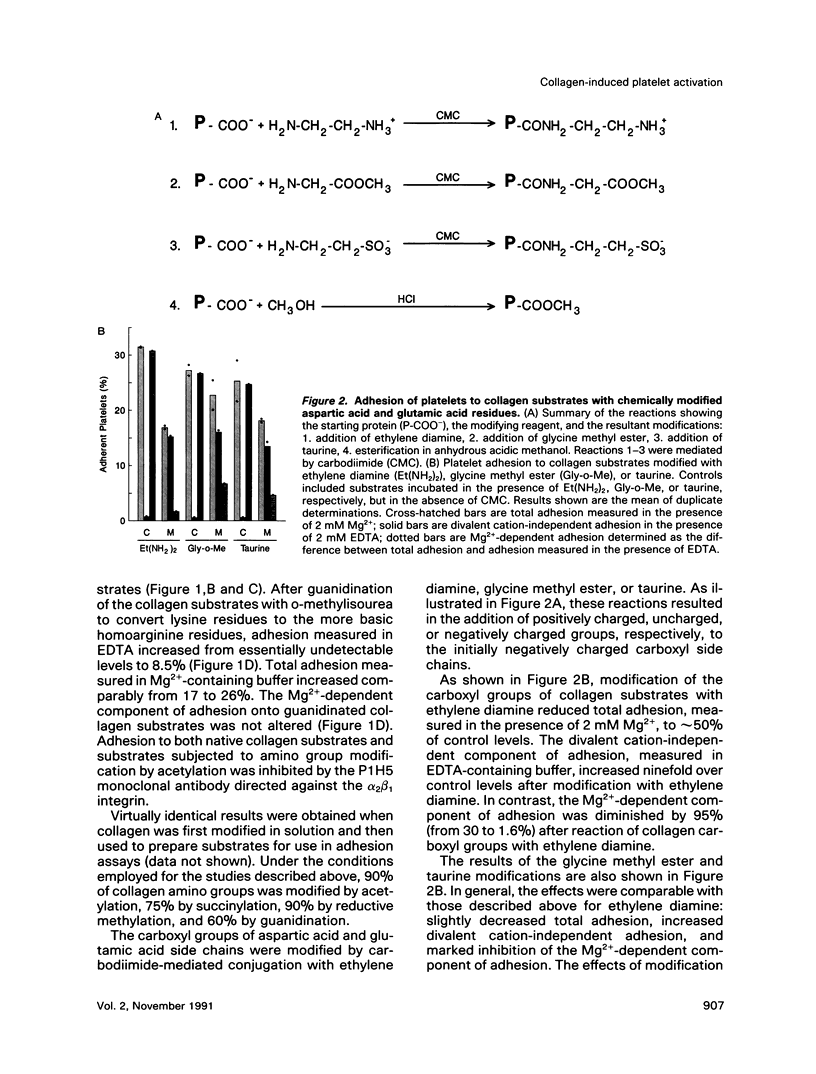
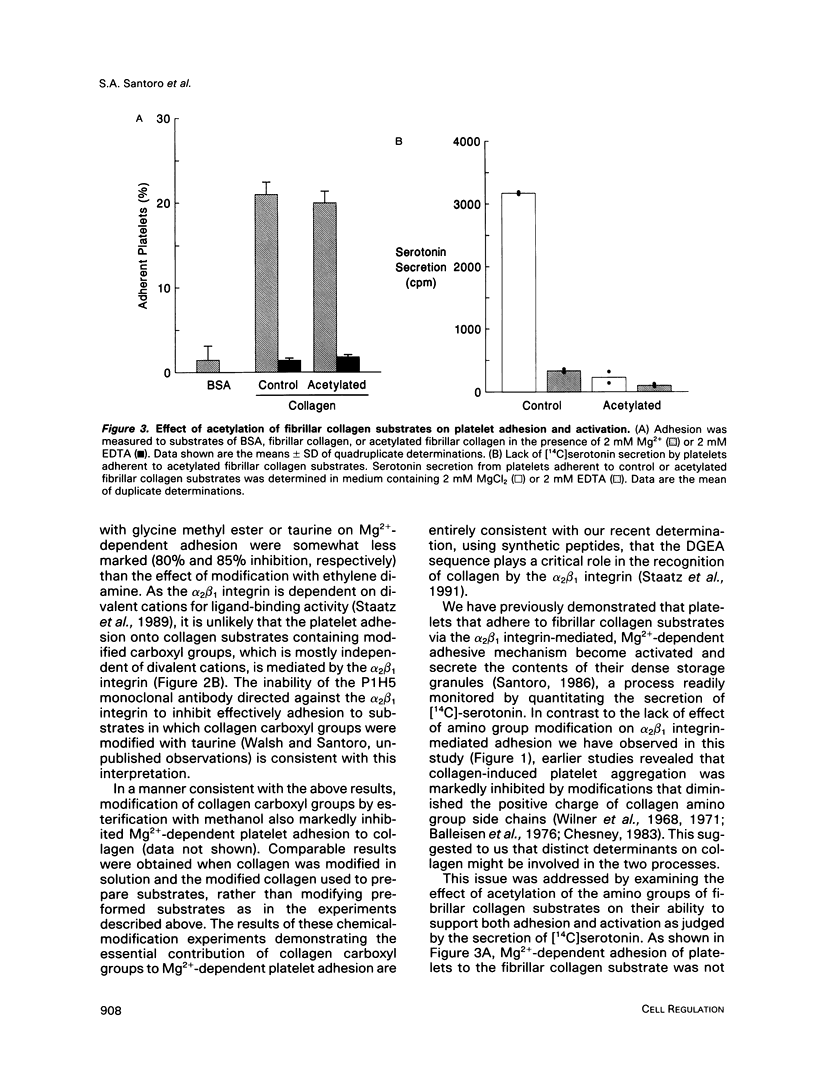
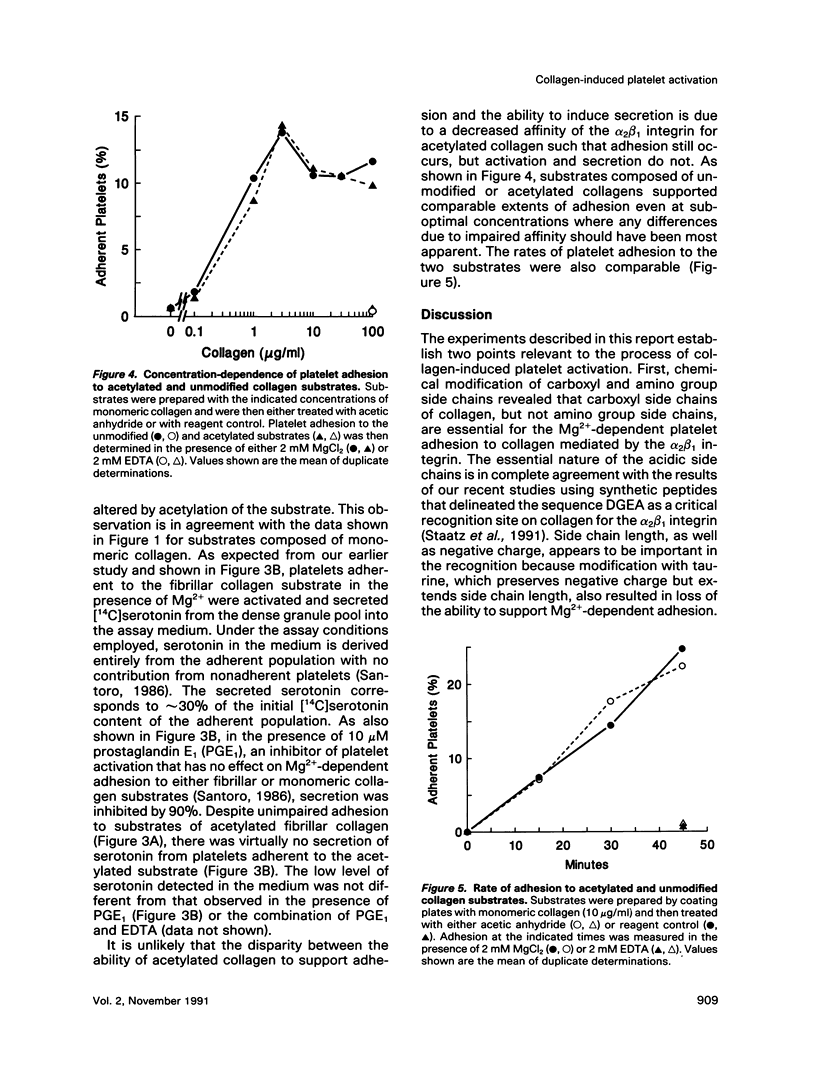
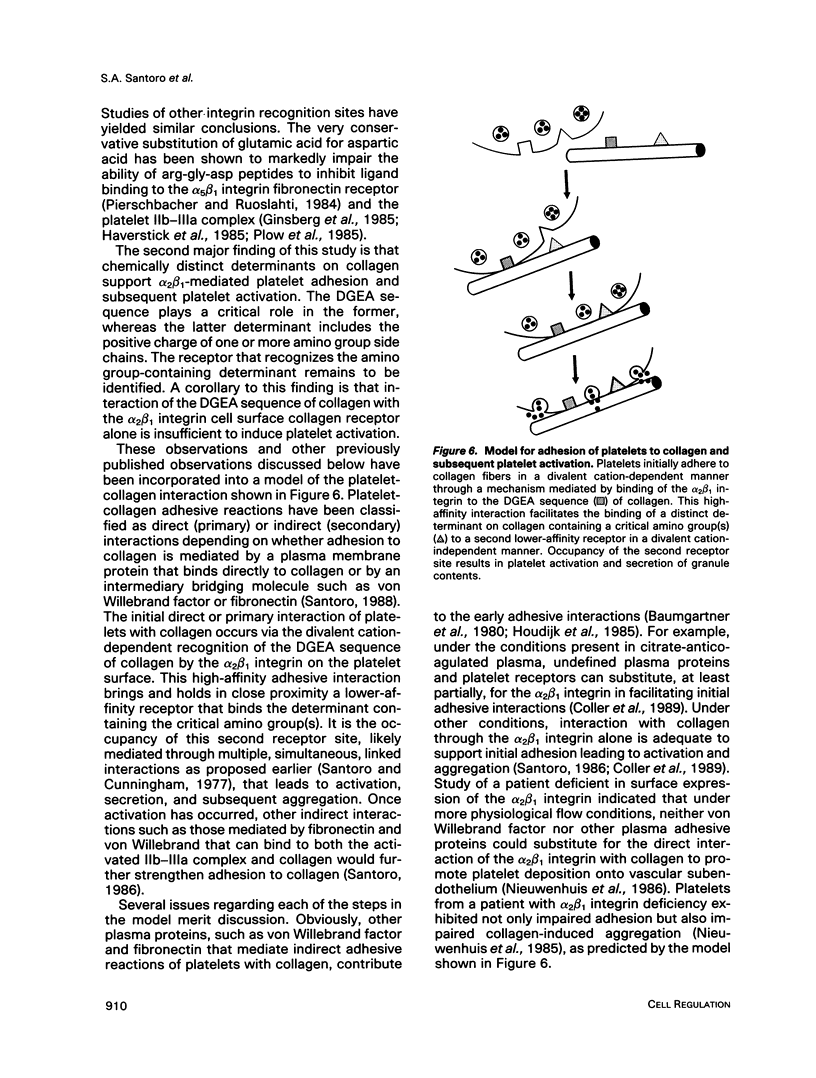
Recent studies have revealed that the sequence of amino acids asp-gly-glu-ala represents an essential determinant of the site within the alpha 1(I)-CB3 fragment of collagen recognized by the alpha 2 beta 1 integrin cell surface collagen receptor (Staatz et al., 1991). Studies employing chemical modifications of collagen amino acid side chains confirm both the essential nature of the acidic side chains of aspartic acid and glutamic acid residues and the nonessentiality of lysine epsilon-amino groups in supporting adhesion mediated by the alpha 2 beta 1 integrin. The approach also indicates the presence of a distinct determinant on collagen separate from the alpha 2 beta 1 recognition site that contains essential lysine side chains and that is necessary for subsequent interactions with the platelet surface that give rise to collagen-induced platelet activation and secretion. The two-step, two-site model for cellular signaling involving both an integrin and a signal-transducing coreceptor suggested by these data may be common to other integrin-mediated processes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balleisen L., Marx R., Kühn K. Platelet-collagen interaction. The influence of native and modified collagen (Type I) on the aggregation of human platelets. Haemostasis. 1976;5(3):155–164. doi: 10.1159/000214131. [DOI] [PubMed] [Google Scholar]
- Balleisen L., Marx R., Kühn K. Uber die stimulierende Wirkung von Kollagen und Kollagenderivaten auf die Ausbreitung und Folien-adhäsion von Thrombozyten in defibrinogenisiertem Menschencitratplasma und in tierischen Citratplasmen. Blut. 1975 Aug;31(2):95–106. doi: 10.1007/BF01633725. [DOI] [PubMed] [Google Scholar]
- Baumgartner H. R. Platelet interaction with collagen fibrils in flowing blood. I. Reaction of human platelets with alpha chymotrypsin-digested subendothelium. Thromb Haemost. 1977 Feb 28;37(1):1–16. [PubMed] [Google Scholar]
- Baumgartner H. R., Tschopp T. B., Meyer D. Shear rate dependent inhibition of platelet adhesion and aggregation on collagenous surfaces by antibodies to human factor VIII/von Willebrand factor. Br J Haematol. 1980 Jan;44(1):127–139. doi: 10.1111/j.1365-2141.1980.tb01190.x. [DOI] [PubMed] [Google Scholar]
- Bruns R. R., Gross J. High-resolution analysis of the modified quarter-stagger model of the collagen fibril. Biopolymers. 1974 May;13(5):931–941. doi: 10.1002/bip.1974.360130509. [DOI] [PubMed] [Google Scholar]
- Chesney C. M., Pifer D. D., Crofford L. J., Huch K. M. Reevaluation of the role of the polar groups of collagen in the platelet-collagen interaction. Am J Pathol. 1983 Aug;112(2):200–206. [PMC free article] [PubMed] [Google Scholar]
- Chiang T. M., Jin A., Kang A. H. Platelet-collagen interaction. Inhibition by a monoclonal antibody raised against collagen receptor. J Immunol. 1987 Aug 1;139(3):887–892. [PubMed] [Google Scholar]
- Chiang T. M., Kang A. H. Isolation and purification of collagen alpha 1(I) receptor from human platelet membrane. J Biol Chem. 1982 Jul 10;257(13):7581–7586. [PubMed] [Google Scholar]
- Chu F. S., Crary E., Bergdoll M. S. Chemical modification of amino groups in staphylococcal enterotoxin B. Biochemistry. 1969 Jul;8(7):2890–2896. doi: 10.1021/bi00835a030. [DOI] [PubMed] [Google Scholar]
- Coller B. S., Beer J. H., Scudder L. E., Steinberg M. H. Collagen-platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood. 1989 Jul;74(1):182–192. [PubMed] [Google Scholar]
- Deckmyn H., Chew S. L., Vermylen J. Lack of platelet response to collagen associated with an autoantibody against glycoprotein Ia: a novel cause of acquired qualitative platelet dysfunction. Thromb Haemost. 1990 Aug 13;64(1):74–79. [PubMed] [Google Scholar]
- Elices M. J., Hemler M. E. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9906–9910. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M., Pierschbacher M. D., Ruoslahti E., Marguerie G., Plow E. Inhibition of fibronectin binding to platelets by proteolytic fragments and synthetic peptides which support fibroblast adhesion. J Biol Chem. 1985 Apr 10;260(7):3931–3936. [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Haverstick D. M., Cowan J. F., Yamada K. M., Santoro S. A. Inhibition of platelet adhesion to fibronectin, fibrinogen, and von Willebrand factor substrates by a synthetic tetrapeptide derived from the cell-binding domain of fibronectin. Blood. 1985 Oct;66(4):946–952. [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- Houdijk W. P., Sakariassen K. S., Nievelstein P. F., Sixma J. J. Role of factor VIII-von Willebrand factor and fibronectin in the interaction of platelets in flowing blood with monomeric and fibrillar human collagen types I and III. J Clin Invest. 1985 Feb;75(2):531–540. doi: 10.1172/JCI111729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEE W. A., RICHARDS F. M. The reaction of O-methylisourea with bovine pancreatic ribonuclease. J Biol Chem. 1957 Nov;229(1):489–504. [PubMed] [Google Scholar]
- Kirchhofer D., Languino L. R., Ruoslahti E., Pierschbacher M. D. Alpha 2 beta 1 integrins from different cell types show different binding specificities. J Biol Chem. 1990 Jan 15;265(2):615–618. [PubMed] [Google Scholar]
- Kotite N. J., Cunningham L. W. Specific adsorption of a platelet membrane glycoprotein by human insoluble collagen. J Biol Chem. 1986 Jun 25;261(18):8342–8347. [PubMed] [Google Scholar]
- Kunicki T. J., Nugent D. J., Staats S. J., Orchekowski R. P., Wayner E. A., Carter W. G. The human fibroblast class II extracellular matrix receptor mediates platelet adhesion to collagen and is identical to the platelet glycoprotein Ia-IIa complex. J Biol Chem. 1988 Apr 5;263(10):4516–4519. [PubMed] [Google Scholar]
- Languino L. R., Gehlsen K. R., Wayner E., Carter W. G., Engvall E., Ruoslahti E. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. J Cell Biol. 1989 Nov;109(5):2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means G. E., Feeney R. E. Reductive alkylation of amino groups in proteins. Biochemistry. 1968 Jun;7(6):2192–2201. doi: 10.1021/bi00846a023. [DOI] [PubMed] [Google Scholar]
- Moroi M., Jung S. M., Okuma M., Shinmyozu K. A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. J Clin Invest. 1989 Nov;84(5):1440–1445. doi: 10.1172/JCI114318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton L. F., Peachey A. R., Barnes M. J. Platelet-reactive sites in collagens type I and type III. Evidence for separate adhesion and aggregatory sites. Biochem J. 1989 Feb 15;258(1):157–163. doi: 10.1042/bj2580157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis H. K., Akkerman J. W., Houdijk W. P., Sixma J. J. Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature. 1985 Dec 5;318(6045):470–472. doi: 10.1038/318470a0. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis H. K., Sakariassen K. S., Houdijk W. P., Nievelstein P. F., Sixma J. J. Deficiency of platelet membrane glycoprotein Ia associated with a decreased platelet adhesion to subendothelium: a defect in platelet spreading. Blood. 1986 Sep;68(3):692–695. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro S. A., Cunningham L. W. Collagen-mediated platelet aggregation. Evidence for multivalent interactions of intermediate specificity between collagen and platelets. J Clin Invest. 1977 Nov;60(5):1054–1060. doi: 10.1172/JCI108856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro S. A. Differential effects of concanavalin A and succinyl concanavalin A on the macromolecular events of platelet activation. Biochim Biophys Acta. 1983 May 4;757(1):101–110. doi: 10.1016/0304-4165(83)90157-5. [DOI] [PubMed] [Google Scholar]
- Santoro S. A. Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell. 1986 Sep 12;46(6):913–920. doi: 10.1016/0092-8674(86)90073-5. [DOI] [PubMed] [Google Scholar]
- Santoro S. A. Molecular basis of platelet adhesion to collagen. Prog Clin Biol Res. 1988;283:291–314. [PubMed] [Google Scholar]
- Santoro S. A., Rajpara S. M., Staatz W. D., Woods V. L., Jr Isolation and characterization of a platelet surface collagen binding complex related to VLA-2. Biochem Biophys Res Commun. 1988 May 31;153(1):217–223. doi: 10.1016/s0006-291x(88)81211-7. [DOI] [PubMed] [Google Scholar]
- Staatz W. D., Rajpara S. M., Wayner E. A., Carter W. G., Santoro S. A. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J Cell Biol. 1989 May;108(5):1917–1924. doi: 10.1083/jcb.108.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staatz W. D., Walsh J. J., Pexton T., Santoro S. A. The alpha 2 beta 1 integrin cell surface collagen receptor binds to the alpha 1 (I)-CB3 peptide of collagen. J Biol Chem. 1990 Mar 25;265(9):4778–4781. [PubMed] [Google Scholar]
- Sugiyama T., Okuma M., Ushikubi F., Sensaki S., Kanaji K., Uchino H. A novel platelet aggregating factor found in a patient with defective collagen-induced platelet aggregation and autoimmune thrombocytopenia. Blood. 1987 Jun;69(6):1712–1720. [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Tandon N. N., Kralisz U., Jamieson G. A. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem. 1989 May 5;264(13):7576–7583. [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilner G. D., Nossel H. L., LeRoy E. C. Aggregation of platelets by collagen. J Clin Invest. 1968 Dec;47(12):2616–2621. doi: 10.1172/JCI105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilner G. D., Nossel H. L., Procupez T. L. Aggregation of platelets by collagen: polar active sites of insoluble human collagen. Am J Physiol. 1971 Apr;220(4):1074–1079. doi: 10.1152/ajplegacy.1971.220.4.1074. [DOI] [PubMed] [Google Scholar]



