Abstract
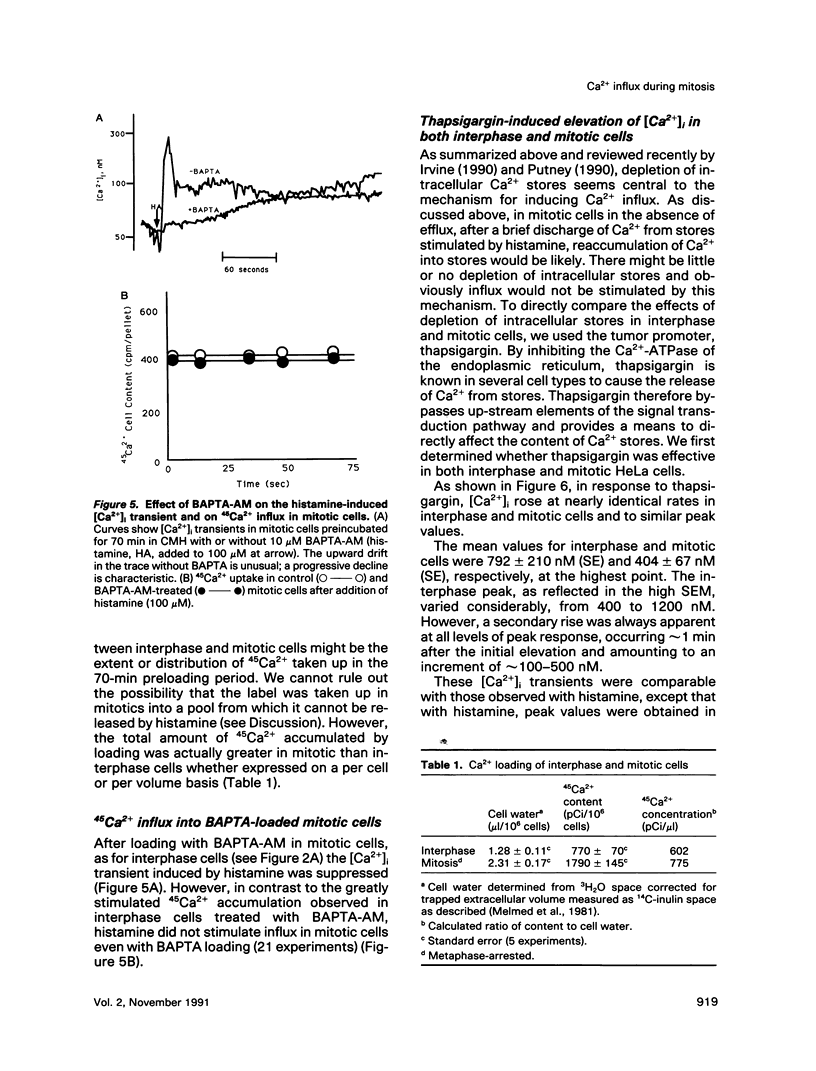
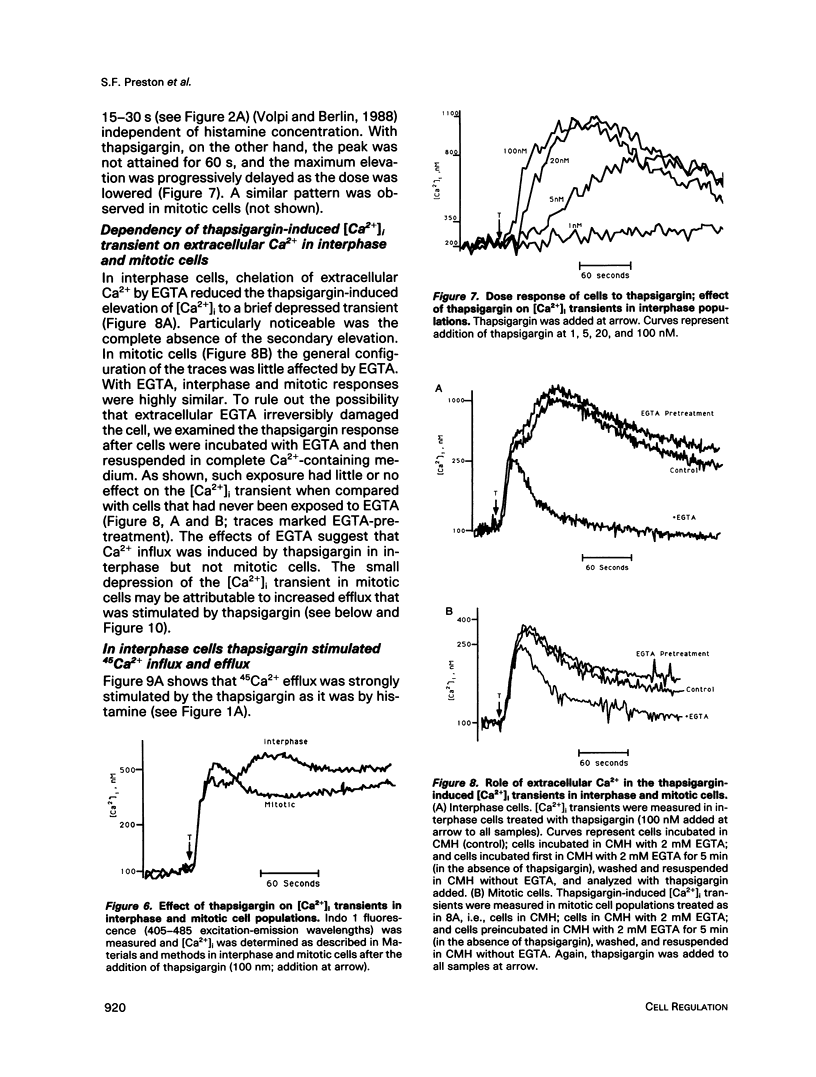
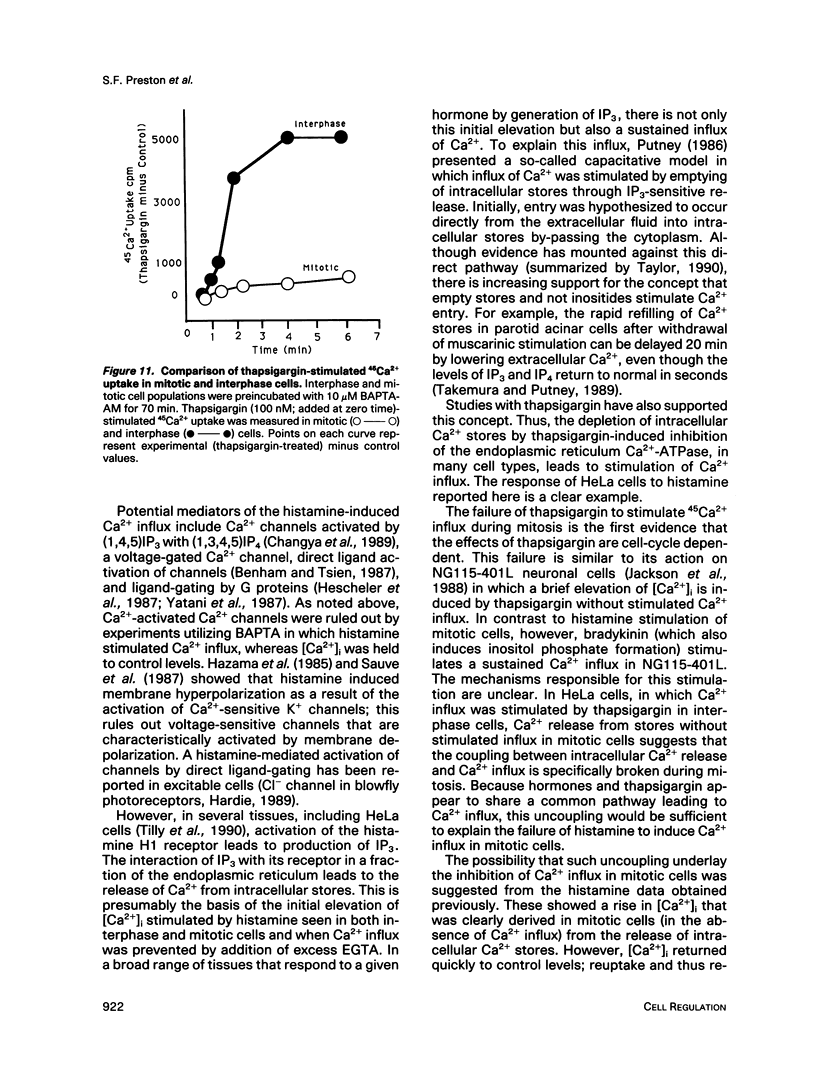
Activation of a wide variety of membrane receptors leads to a sustained elevation of intracellular Ca2+ ([Ca2+]i) that is pivotal to subsequent cell responses. In general, in nonexcitable cells this elevation of [Ca2+]i results from two sources: an initial release of Ca2+ from intracellular stores followed by an influx of extracellular Ca2+. These two phases, release from intracellular stores and Ca2+ influx, are generally coupled: stimulation of influx is coordinated with depletion of Ca2+ from stores, although the mechanism of coupling is unclear. We have previously shown that histamine effects a typical [Ca2+]i response in interphase HeLa cells: a rapid rise in [Ca2+]i followed by a sustained elevation, the latter dependent entirely on extracellular Ca2+. In mitotic cells only the initial elevation, derived by Ca2+ release from intracellular stores, occurs. Thus, in mitotic cells the coupling of stores to influx may be specifically broken. In this report we first provide additional evidence that histamine-stimulated Ca2+ influx is strongly inhibited in mitotic cells. We show that efflux is also strongly stimulated by histamine in interphase cells but not in mitotics. It is possible, thus, that in mitotics intracellular stores are only very briefly depleted of Ca2+, being replenished by reuptake of Ca2+ that is retained within the cell. To ensure the depletion of Ca2+ stores in mitotic cells, we employed the sesquiterpenelactone, thapsigargin, that is known to affect the selective release of Ca2+ from intracellular stores by inhibition of a specific Ca(2+)-ATPase; reuptake is inhibited. In most cells, and in accord with Putney's capacitative model (1990), thapsigargin, presumably by depleting intracellular Ca2+ stores, stimulates Ca2+ influx. This is the case for interphase HeLa cells. Thapsigargin induces an increase in [Ca2+]i that is dependent on extracellular Ca2+ and is associated with a strong stimulation of 45Ca2+ influx. In mitotic cells thapsigargin also induces a [Ca2+]i elevation that is initially comparable in magnitude and largely independent of extracellular Ca2+. However, unlike interphase cells, in mitotic cells the elevation of [Ca2+]i is not sustained and 45Ca2+ influx is not stimulated by thapsigargin. Thus, the coupling between depletion of intracellular stores and Ca2+ influx is specifically broken in mitotic cells. Uncoupling could account for the failure of histamine to stimulate Ca2+ influx during mitosis and would effectively block all stimuli whose effects are mediated by Ca2+ influx and sustained elevations of [Ca2+]i.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M., Walter R. J. Surface functions during Mitosis I: phagocytosis, pinocytosis and mobility of surface-bound Con A. Cell. 1978 Oct;15(2):327–341. doi: 10.1016/0092-8674(78)90002-8. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Potter B. V., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J Membr Biol. 1989 Jul;109(1):85–93. doi: 10.1007/BF01870793. [DOI] [PubMed] [Google Scholar]
- Duddy S. K., Kass G. E., Orrenius S. Ca2(+)-mobilizing hormones stimulate Ca2+ efflux from hepatocytes. J Biol Chem. 1989 Dec 15;264(35):20863–20866. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hardie R. C. A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature. 1989 Jun 29;339(6227):704–706. doi: 10.1038/339704a0. [DOI] [PubMed] [Google Scholar]
- Hazama A., Yada T., Okada Y. HeLa cells have histamine H1-receptors which mediate activation of the K+ conductance. Biochim Biophys Acta. 1985 May 30;845(2):249–253. doi: 10.1016/0167-4889(85)90183-1. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Beaven M. A., Rogers J., Burke B., Warren G. B. Stimulated release of histamine by a rat mast cell line is inhibited during mitosis. J Cell Biol. 1984 Jun;98(6):2250–2254. doi: 10.1083/jcb.98.6.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth E. B., De la Cruz R. A., Daly J. W. Accumulations of inositol phosphates and cyclic AMP in brain slices: synergistic interactions of histamine and 2-chloroadenosine. Eur J Pharmacol. 1986 Mar 11;122(1):45–50. doi: 10.1016/0014-2999(86)90156-1. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed R. N., Karanian P. J., Berlin R. D. Control of cell volume in the J774 macrophage by microtubule disassembly and cyclic AMP. J Cell Biol. 1981 Sep;90(3):761–768. doi: 10.1083/jcb.90.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Khademazad M., Sachs G. The route of Ca2+ entry during reloading of the intracellular Ca2+ pool in pancreatic acini. J Biol Chem. 1990 Feb 5;265(4):2011–2016. [PubMed] [Google Scholar]
- Nasmith P. E., Grinstein S. Are Ca2+ channels in neutrophils activated by a rise in cytosolic free Ca2+? FEBS Lett. 1987 Aug 31;221(1):95–100. doi: 10.1016/0014-5793(87)80359-9. [DOI] [PubMed] [Google Scholar]
- Preston S. F., Regula C. S., Sager P. R., Pearson C. B., Daniels L. S., Brown P. A., Berlin R. D. Glycosaminoglycan synthesis is depressed during mitosis and elevated during early G1. J Cell Biol. 1985 Sep;101(3):1086–1093. doi: 10.1083/jcb.101.3.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Sager P. R., Brown P. A., Berlin R. D. Analysis of transferrin recycling in mitotic and interphase HeLa cells by quantitative fluorescence microscopy. Cell. 1984 Dec;39(2 Pt 1):275–282. doi: 10.1016/0092-8674(84)90005-9. [DOI] [PubMed] [Google Scholar]
- Sauvé R., Simoneau C., Parent L., Monette R., Roy G. Oscillatory activation of calcium-dependent potassium channels in HeLa cells induced by histamine H1 receptor stimulation: a single-channel study. J Membr Biol. 1987;96(3):199–208. doi: 10.1007/BF01869302. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Takemura H., Putney J. W., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989 Mar 1;258(2):409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W. Receptor-regulated Ca2+ entry: secret pathway or secret messenger? Trends Pharmacol Sci. 1990 Jul;11(7):269–271. doi: 10.1016/0165-6147(90)90003-q. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Dawson A. P., Scharff O., Foder B., Cullen P. J., Drøbak B. K., Bjerrum P. J., Christensen S. B., Hanley M. R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989 Apr;27(1-2):17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Tilly B. C., Tertoolen L. G., Lambrechts A. C., Remorie R., de Laat S. W., Moolenaar W. H. Histamine-H1-receptor-mediated phosphoinositide hydrolysis, Ca2+ signalling and membrane-potential oscillations in human HeLa carcinoma cells. Biochem J. 1990 Feb 15;266(1):235–243. doi: 10.1042/bj2660235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tuomikoski T., Felix M. A., Dorée M., Gruenberg J. Inhibition of endocytic vesicle fusion in vitro by the cell-cycle control protein kinase cdc2. Nature. 1989 Dec 21;342(6252):942–945. doi: 10.1038/342942a0. [DOI] [PubMed] [Google Scholar]
- Volpi M., Berlin R. D. Intracellular elevations of free calcium induced by activation of histamine H1 receptors in interphase and mitotic HeLa cells: hormone signal transduction is altered during mitosis. J Cell Biol. 1988 Dec;107(6 Pt 2):2533–2539. doi: 10.1083/jcb.107.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Davoust J., Cockcroft A. Recycling of transferrin receptors in A431 cells is inhibited during mitosis. EMBO J. 1984 Oct;3(10):2217–2225. doi: 10.1002/j.1460-2075.1984.tb02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Featherstone C., Griffiths G., Burke B. Newly synthesized G protein of vesicular stomatitis virus is not transported to the cell surface during mitosis. J Cell Biol. 1983 Nov;97(5 Pt 1):1623–1628. doi: 10.1083/jcb.97.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley D. N., Glenesk A., Inglis M. S. Protein synthesis and mitosis. 1. Influence of anti-tubulins on protein synthetic activity in synchronous M-phase and asynchronous populations of HeLa S-3 cells. Cytobios. 1988;55(220):41–50. [PubMed] [Google Scholar]
- Yatani A., Codina J., Imoto Y., Reeves J. P., Birnbaumer L., Brown A. M. A G protein directly regulates mammalian cardiac calcium channels. Science. 1987 Nov 27;238(4831):1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]


