Abstract
Disseminated herpes simplex virus (HSV) infection may lead to acute liver failure (ALF) and the need for emergency liver transplantation (LT). The primary aim of this study was to determine the utility of HSV serological testing and HSV DNA testing by polymerase chain reaction (PCR) in the diagnosis and management of indeterminate, pregnancy-related, and known HSV-related ALF. Stored sera obtained on study day 1 or 2 from patients enrolled in the United States ALF Study Group with indeterminate (n = 51), pregnancy-related (n = 12), and HSV-related (n = 4) ALF were screened for HSV DNA by PCR and serology. While 7 of the indeterminate and pregnant patients had positive anti-HSV immunoglobulin M, none had detectable HSV DNA. The 4 known HSV cases all had high-titer HSV DNA on presentation (range: 3.5 to 36 × 108 copies/mL). Two HSV patients underwent LT but developed posttransplant extrahepatic HSV infection despite suppression of HSV DNA with acyclovir treatment, and one of them eventually died. The 2 other fulminant HSV patients died within 48 hours of presentation. In conclusion, serum HSV DNA indicative of occult HSV infection was not detected in 51 indeterminate and 12 pregnancy-related ALF patients. The 4 patients with known HSV-related ALF all had high HSV DNA levels at presentation, and despite the rapid use of antiviral therapy and emergency LT, substantial morbidity and mortality were encountered, highlighting the poor prognosis with severe disseminated HSV infection.
Herpes simplex virus (HSV) hepatitis is an uncommon cause of acute liver failure (ALF) and is usually not identified until after liver transplantation (LT) or death. HSV-associated ALF typically occurs as reactivation in pregnancy or with immunosuppression, although disease in immunocompetent patients has been reported. Of great concern is the fact that only a fraction of cases are diagnosed soon after presentation, and this results in a high rate of multiorgan involvement and death.1-3 Success with transplantation has been reported, mostly in neonates and children, although the mortality is high in adult recipients.3-11
Currently, 17% of adult ALF patients are considered to have indeterminate etiology.12 Prior studies have failed to demonstrate an identifiable, causal viral infection in indeterminate ALF.13-15 Some authors have suggested that occult HSV infection may account for cases of pregnancy-related or indeterminate ALF and that polymerase chain reaction (PCR) for HSV DNA should be performed on presentation.16,17 However, it is unknown whether PCR testing is more sensitive and specific than serological assays for detecting HSV as a cause of ALF or is useful as a quantitative measure in the management of patients with known HSV ALF. The aim of our study was to search for cases of occult HSV hepatitis in an indeterminate and pregnancy-related ALF group (US ALF registry), as measured by HSV PCR, and determine the diagnostic utility of HSV PCR compared to standard serological assays in this population. In addition, we set out to determine the utility of serial quantitative HSV PCR assays in relation to outcomes of patients with known HSV ALF before and after LT.
PATIENTS AND METHODS
Acute Liver Failure Study Group (ALFSG) Subjects
The ALFSG is a 24-center consortium funded by the National Institute of Diabetes and Digestive and Kidney Diseases to determine the etiology, clinical features, and outcomes of adult patients with ALF.12 Enrollment requires a written informed consent document approved by the local institutional review board and signed by the patient’s next of kin. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee. All data are collected at the clinical sites and sent to the University of Texas Southwestern Medical Center for central review and analysis.
The ALFSG database (n = 1033) was searched for all patients with ALF due to indeterminate causes, pregnancy, and HSV. ALF was defined as the onset of coagulopathy and encephalopathy within 26 weeks of illness onset in a patient without preexisting liver disease. An indeterminate ALF case was defined as a patient with no identifiable cause of ALF after exclusion of known infectious, metabolic, immune, malignant, and vascular etiologies of ALF at the local site using standard serological, radiological, and histological criteria. Day 1 or 2 stored sera from all 12 ALFSG patients with pregnancy-related ALF (6 were presumed to have an acute fatty liver, and 6 were presumed to have HELLP syndrome) and from 51 randomly selected indeterminate ALF patients (from the 152 indeterminate ALFSG patients overall) were used for testing. All of the identified HSV ALF (n = 4) cases had available daily serum specimens assayed for HSV DNA with quantitative PCR until the time point of death or LT. Any available quantitative HSV DNA levels post-transplant were recorded, if they were performed by the centers. Case forms were reviewed to determine the clinical presentation, diagnosis, management, and outcomes of ALF. The explanted liver histology of the PCR-negative, immunoglobulin M (IgM)–positive patients was reviewed for any evidence of HSV infection.
Testing Procedures
Pretransplant qualitative and quantitative HSV DNA by PCR (HSV-1 and HSV-2) by TaqMan Assay was measured at the University of Alabama at Birmingham with the referenced techniques.18-20 Viral DNA was extracted from 200 μL of serum with the Qiagen BioRobot EZ1 and blood reagent cartridge. An initial volume of 200 μL was extracted and eluted in 200 μL of water. Type-common HSV polymerase TaqMan primers and probe were selected from type-common regions of the HSV polymerase gene using Primer Express software (Applied Biosystems, Foster City, CA). The forward primer was ACC GCC GAA CTG AGC AGA C, the reverse primer was TGA GCT TGT AAT ACA CCG TCA GGT, and the probe was 6FAM-CGC GTA CAC CAA CAA GCG CCT G-TAMRA. The assay conditions were optimized in an ABI 7300 SDS (Applied Biosystems, Foster City, CA). Samples were run in triplicate reactions containing 5 μL of target in a final volume of 25 μL containing 1 × TaqMan universal master mix and 900 nM of each primer and 200 nM probe. Thermal cycling was performed with an initial step of 50°C for 2 minutes and 95°C for 10 minutes followed by 40 cycles of denaturation at 95°C for 15 seconds and a combined annealing-extension step at 60°C for 1 minute. Fluorescence data were collected during each annealing-extension step and analyzed with ABI-Prism SDS software version 1.2.3. A plasmid containing HSV DNA polymerase was used to construct the standard curve. A linear 5-point standard curve and an NTC (no target control) were run with each plate. The standard curve ranged from 50 to 5 × 105 copies/5 μL and resulted in Ct values of 36.7 to 22.4 respectively. The lower limit of detection for the qualitative assay was between 1 and 5 copies/reaction. Posttransplant quantitative HSV DNA by PCR for patients 3 and 4 was performed at ViraCor Laboratories (Lee’s Summit, MO); as such, the values are not equally comparable to the pretransplant assays performed at the University of Alabama at Birmingham.
All sera were tested at the University of Texas Southwestern Medical Center for type-specific HSV-1 and HSV-2 antibody by an enzyme-linked immunosorbent assay, the Captia-type specific immunoglobulin G test, using antigens and reagents manufactured by Trinity Biotech (United States). All sera were tested for IgM indirect fluorescent antibody against HSV-1 and HSV-2 after serum absorption with goat anti-human immunoglobulin G and a reaction with infected cells and an anti-human IgM fluoresceinated conjugate.21 The HSV antigens were obtained from Hemagen Diagnostics, Inc. (Columbia, MD).
RESULTS
Screening of ALF Groups for HSV
The demographics and clinical presentation of the indeterminate and pregnancy ALF groups are described in Table 1. None of the 51 patients with indeterminate ALF or any of the 12 women with pregnancy-related ALF had detectable serum HSV DNA by qualitative PCR. However, 6 of the indeterminate ALF patients and 1 of the pregnancy ALF patients had positive anti-HSV-1 or anti-HSV-2 IgM (all 1:20; PCR-negative), while only 2 of the 4 with known HSV had positive anti-HSV-1 or anti-HSV-2 IgM (patient 3, 1:80, and patient 4, 1:20,480; Tables 1 and 2). None of the PCR-negative, IgM-positive patients had evidence of HSV infection on histological review of the liver explants.
TABLE 1. Demographics, Clinical Presentation, and HSV Testing of Indeterminate and Pregnancy ALF Groups.
| Indeterminate ALF (n = 51) | Pregnancy ALF (n = 12) | |
|---|---|---|
| Age (years) | 39.3 ± 16.2 | 29.9 ± 3.7 |
| Sex: female | 24 (47.6%) | 12 (100%) |
| Race: white | 36 (70.6%) | 7 (58.3%) |
| Fever | 17 (33.3%) | 1 (8.3%) |
| Rash* | 9 (17.6%) | 1 (8.3%) |
| Symptom onset to admission (days) | 17.2 ± 17.6 | 1.6 ± 0.7 |
| ALT (U/L) | 2079 ± 2711 | 660 ± 2250 |
| Bilirubin (mg/dL) | 20.8 ± 13.1 | 16.4 ± 23.7 |
| INR | 3.3 ± 2.1 | 1.8 ± 0.4 |
| HSV-1 IgM (≥1:20) | 5 (9.8%) | 1 (8.3%) |
| HSV-2 IgM (≥1:20) | 1 (1.9%) | 0 (0%) |
| HSV-1 IgG (≥1:20) | 41 (80.4%) | 11 (91.6%) |
| HSV-2 IgG (≥1:20) | 23 (45.1%) | 6 (50%) |
| HSV PCR | 0 (0%) | 0 (0%) |
| Liver transplant | 19 (37.3%) | 1/12 (8.3%) |
| Overall survival | 31 (60.8%) | 6 (50%) |
NOTE: Values were recorded at the time of initial hospital presentation and are expressed as means ± standard deviation or percentages.
Abbreviations: ALF, acute liver failure; ALT, alanine aminotransferase; HSV, herpes simplex virus; IgG, immunoglobulin G; IgM, immunoglobulin M; INR, international normalized ratio; PCR, polymerase chain reaction.
The appearance and distribution of rash are not described in the case report forms.
TABLE 2. Clinical Characteristics of Known HSV ALF patients.
| Patient | Age | Race/Sex | Hx | Sx | Diagnosis | ALT (U/L) |
Bili (mg/dL) |
INR | HSV-1/2 IgM |
HSV-1/2 IgG |
HSV PCR (copies/mL)* |
Rx | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | WF | UC | RUQ pain, fever |
Autopsy | 3323 | 2.2 | 2.7 | −/− | +/− | 3.6 × 109 | ACV | Death |
| 2 | 39 | WF | Asthma | RUQ pain, fever |
Oral lesion, autopsy |
4065 | 3.4 | 2.2 | −/− | −/+ | 1.2 × 109 | None | Death |
| 3 | 58 | WM | Asthma | RUQ pain, rash, fever |
Serology, skin/liver biopsy |
5670 | 10.5 | 3.3 | +/+ | +/+ | 1.2 × 109 | ACV | LT |
| 4 | 44 | WF | None | Rash, fever |
Skin/liver biopsy |
1899 | 10.8 | 3.1 | +/+ | +/+ | 3.5 × 108 | ACV | LKT, death |
NOTE: All values were recorded at the time of initial hospital presentation and are expressed as means ± standard deviation or percentages.
Abbreviations: ACV, acyclovir: ALF, acute liver failure: ALT, alanine aminotransferase: Bili, total bilirubin: HSV, herpes simplex virus: Hx, history: IgG, immunoglobulin G: IgM, immunoglobulin M: INR, international normalized ratio: LKT, liver-kidney transplantation: LT, liver transplantation: PCR, polymerase chain reaction: RUQ, right upper quadrant: Rx, treatment: Sx, symptoms: UC, ulcerative colitis: WF, white female: WM, white male.
The PCR type was HSV-2 for all except patient 2 (HSV-1).
Evaluation of Known HSV ALF Patients
All 4 known HSV ALF patients had high circulating titers of HSV DNA on presentation (Table 2). Patient 1 was a 28-year-old Caucasian female with ulcerative colitis on chronic prednisone therapy (20 mg/day) who presented with abdominal pain and fever without rash in the 8 days prior to the onset of ALF. Intravenous (IV) acyclovir therapy (500 mg, 10 mg/kg) was administered empirically, but the patient died 1 day after admission. Autopsy revealed massive hepatic necrosis and hemorrhage with HSV viral inclusions.
Patient 2 was a 39-year-old Caucasian female with asthma (no corticosteroids) who presented with abdominal pain, fever, and painful swallowing over 20 days before the onset of ALF. Acyclovir was not administered. The patient died 2 days after admission. Autopsy revealed HSV esophagitis and hepatitis with coagulative necrosis.
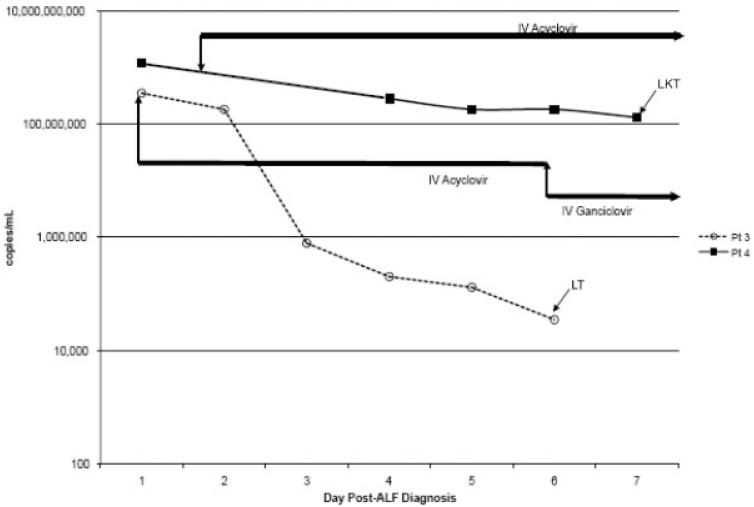
Patient 3 is a 58-year-old Caucasian male with asthma (no corticosteroids) who developed fever and a vesicular rash in the 8 days prior to ALF. Liver biopsy showed hepatic necrosis and intranuclear inclusions consistent with HSV infection. The patient was treated with IV acyclovir (10 mg/kg three times a day; Fig. 1) and received a LT 6 days after admission. Initial immunosuppression included tacrolimus, mycophenolate mofetil (MMF), and 3 days of corticosteroids. IV ganciclovir prophylaxis (5 mg/kg once a day) was given after LT until HSV esophagitis developed on day 36 (HSV PCR–negative on day 23). MMF was discontinued, and IV acyclovir was resumed for 10 days. Except for day 23, no other PCR was performed after LT. Post-LT complications included biliary leak and stricture, pneumonia, renal failure, and Aspergillus fumigatus osteomyelitis. After 2 years, the patient has not developed recurrence of HSV on maintenance valacyclovir (500 mg once a day).
Figure 1.
Herpes simplex virus polymerase chain reaction levels during antiviral therapy prior to liver transplantation (patients 3 and 4). Abbreviations: ALF, acute liver failure; IV, intravenous; LT, liver transplantation; LKT, liver-kidney transplantation.
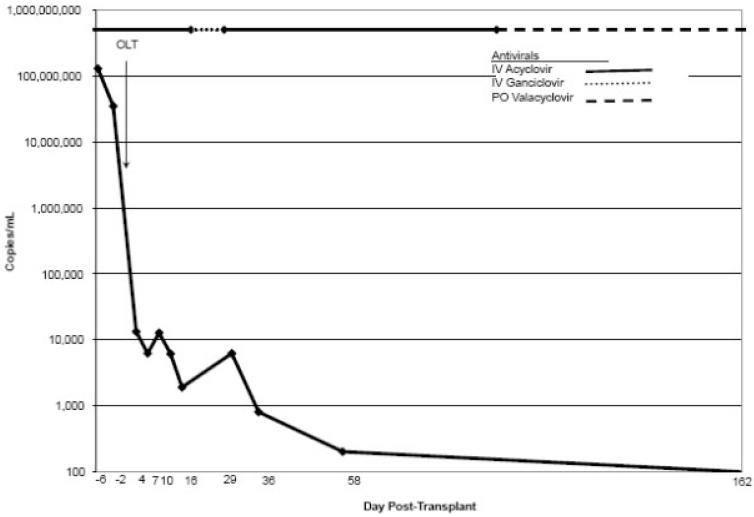
Patient 4 was a healthy 44-year-old Caucasian female who presented with a vesicular eruption and fever 10 days prior to ALF. Liver biopsy revealed HSV hepatitis. IV acyclovir (10 mg/kg three times a day) was administered (Fig. 1), and combined liver/kidney transplantation was performed 7 days later. Initial immunosuppression included tacrolimus, MMF, and corticosteroids. IV acyclovir was continued for 20 days, the patient was switched to IV ganciclovir (5 mg/kg once a day) for 10 days, and then acyclovir was resumed when a vesicular rash occurred on day 30. Prednisone and MMF were discontinued. HSV PCR did not become undetectable until long after LT (Fig. 2). Postoperative complications included Candida fungemia, Staphylococcus aureus and Enterococcus faecium bacteremia, biliary stricture, and prolonged ventilation. While on maintenance valacyclovir, the patient developed an acyclovir-resistant HSV rash (negative serum PCR) on day 250. Foscarnet was administered, but the patient expired on day 365 with no obvious HSV disease or viremia. Autopsy was declined by the family.
Figure 2.
Herpes simplex virus polymerase chain reaction levels during antiviral therapy after liver transplantation (patient 4). Abbreviations: IV, intravenous; OLT, orthotopic liver transplantation; PO, per os.
DISCUSSION
We focused on the utility of screening blood samples for evidence of occult HSV infection with a highly sensitive qualitative PCR assay. Our results support previous findings that HSV is an unlikely culprit in indeterminate and pregnancy ALF.22,23 For the 4 ALFSG patients with known HSV, quantitative HSV DNA on presentation was uniformly high. The 2 patients who underwent LT had experienced a preoperative decline in HSV DNA with acyclovir. While hepatic recurrence of HSV after LT was not seen in either, the hospital courses were complicated by extrahepatic HSV infection and numerous complications, despite a significant reduction in HSV DNA immediately after hepatectomy and eventual clearance of viremia. While the data are limited, these findings suggest that a decline in quantitative HSV DNA may not correlate with a reduction in posttransplant extrahepatic HSV or other morbidity.
Interestingly, for 7 of the 63 pregnant/indeterminate negative PCR patients, anti-HSV-1 or anti-HSV-2 IgM was detectable at a titer of 1:20, likely representing false positivity, persistent low-level IgM antibody after prior infection, or mild serological reactivation in these acutely ill patients, which can be seen with other herpesviruses. A positive serology without viremia would be highly unlikely to represent actual infection in the vascularized organs where high-titer viremia is universal. In support of this, a review of available liver tissue from these 7 subjects failed to demonstrate evidence of intranuclear inclusions. In contrast, 2 of the 4 patients with known HSV infection had negative anti-HSV IgM levels, which reflected either the inaccuracy of serological testing or the fact that the patients were too ill or too early in the course of infection to manufacture IgM. However, the other 2 HSV patients had higher titers of IgM (1:80, 1:20,480). These data highlight concerns regarding the diagnostic capability of serological testing for HSV and emphasize the importance of PCR as the definitive confirmatory test.
When the 2 known HSV cases presented for LT evaluation, there was concern that active viremia might be associated with adverse post-LT outcomes, particularly recurrent HSV. However, the viral kinetics of HSV have not been studied in ALF, and the available data suggest that recurrent HSV hepatitis is rare after LT.3-5,7-11 The 2 patients did not have recurrent hepatitis despite the presence of viral replication at the time of transplantation. Merely removing the infected liver with transplantation dramatically reduced the HSV viral load, and this likely reflected the fact that the liver, being a highly vascularized organ, was a significant reservoir for HSV. The viral load of one of the patients did not become undetectable until 3 months after LT, while the other cleared more quickly. Interestingly, extrahepatic HSV disease occurred in both despite declining or undetectable HSV DNA. While our data are limited to these patients, they provide the first insight into the viral kinetics and associated outcomes of HSV ALF.
The outcomes of the 2 transplanted patients in this study do not settle the controversy of whether LT for HSV should be performed in adults. Only 11 adult patients (9 in the literature and 2 from this report) with HSV ALF have been reported to have undergone LT; 6 (55%) died post-transplant (the deaths were often not directly related to recurrent HSV), and most had significant morbidity.3,5,8-11 It is uncertain if the high morbidity and mortality after LT were due to indirect effects of the virus itself or other factors, such as the severity of illness before and after LT or undiagnosed immunodeficiency states that predisposed these patients to HSV ALF.
Several important limitations of our study require consideration. First, we screened a randomly selected subgroup of the indeterminate ALFSG patients for HSV in order to determine if this initial investigation warranted testing of the entire cohort. Because HSV DNA was not detected in the serum of any of the 51 indeterminate ALF patients, further screening of the remaining 101 indeterminate patients was not undertaken. However, since the presenting clinical features, demographics, and outcomes of the randomly selected indeterminate patients were similar to those of the untested indeterminate patients (data not shown), the subgroup of tested patients appears to be representative of the overall indeterminate group. While it is theoretically possible that HSV DNA could have been detected in 1 or more of the unscreened patients, our data are consistent with other studies and suggest that HSV infection is not only an exceedingly uncommon cause of ALF in general (4 of 1033 consecutive adult patients) but also unlikely to account for a significant percentage of indeterminate ALF cases.22,23 For the known HSV cases, our serial quantitative PCR analysis was limited by the small number of patients with HSV ALF in this large database. Only 2 patients survived long enough to have daily pre-LT sera available for serial PCR analysis, and only 1 had serial PCRs performed after LT. The timing of PCR monitoring was not predetermined and was therefore not uniform. Therefore, our understanding of the viral kinetics still remains limited, and further studies are needed.
In summary, the detection of HSV DNA by PCR appeared to be more discriminating than serological testing for diagnosing or excluding HSV as a cause of ALF. Unsuspected HSV infection was not identified in any of the indeterminate/pregnant ALF patients selected. On the basis of the small number of known HSV ALF cases in our study, firm conclusions cannot be made regarding the usefulness of quantitative PCR measurement before and after LT. However, all HSV hepatitis cases had high DNA levels, supporting the use of HSV PCR as a screening test for indeterminate ALF to formulate a rapid management plan versus other inaccurate and invasive tests, such as serology and liver biopsy. Given the poor outcomes of LT for HSV ALF, prospective data are needed to determine if quantitative HSV DNA testing is useful in predicting morbidity and mortality after LT and in determining the optimal type and length of antiviral regimen to use post-transplant.
ACKNOWLEDGMENTS
The members and institutions participating in the US Acute Liver Failure Study Group are as follows: W.M. Lee, M.D. (principal investigator), Julie Polson, M.D., and Carla Pezzia, University of Texas Southwestern, Dallas, TX; Anne Larson, M.D., University of Washington, Seattle, WA; Timothy Davern, M.D., University of California, San Francisco, CA; Paul Martin, M.D., Mount Sinai School of Medicine, New York, NY; Timothy McCashland, M.D., University of Nebraska, Omaha, NE; J. Eileen Hay, M.D., Mayo Clinic, Rochester, MN; Natalie Murray, M.D., Baylor University Medical Center, Fort Worth, TX; A. Obaid S. Shaikh, M.D., University of Pittsburgh, Pittsburgh, PA; Andres Blei, M.D., Northwestern University, Chicago, IL; Atif Zaman, M.D., University of Oregon, Portland, OR; Steven Han, M.D., University of California, Los Angeles, CA; Robert Fontana, M.D., University of Michigan, Ann Arbor, MI; Brendan McGuire, M.D., University of Alabama, Birmingham, AL; Ray Chung, M.D., Massachusetts General Hospital, Boston, MA; Alastair Smith, M.B., Ch.B., Duke University Medical Center, Durham, NC; Michael Schilsky, M.D., Cornell/Columbia University, New York, NY; Adrian Reuben, M.B.B.S., Medical University of South Carolina, Charleston, SC; Santiago Munoz, M.D., Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, M.D., University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, M.D., Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, M.D., University of California at Davis, Sacramento, CA; Raj Satyanarayana, M.D., Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, M.D., University of California, San Diego, CA.
The US Acute Liver Failure Study Group is funded by National Institutes of Health grant U-01 DK58369. Additional funding has been provided by the Stephen B. Tips Memorial Fund of Northwestern Memorial Hospital and the Jeanne Roberts Fund of the University of Texas Southwestern Medical Foundation.
Abbreviations
- ACV
acyclovir
- ALF
acute liver failure
- ALFSG
Acute Liver Failure Study Group
- ALT
alanine aminotransferase
- Bili
total bilirubin
- HSV
herpes simplex virus
- Hx
history
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- INR
international normalized ratio
- IV
intravenous
- LT
liver transplantation
- LKT
liver-kidney transplantation
- MMF
mycophenolate mofetil
- OLT
orthotopic liver transplantation
- PCR
polymerase chain reaction
- PO
per os
- RUQ
right upper quadrant
- Rx
treatment
- Sx
symptoms
- UC
ulcerative colitis
- WF
white female
- WM
white male
REFERENCES
- 1.Flewett T, Parker RG, Philip WM. Acute hepatitis due to herpes simplex in an adult. J Clin Pathol. 1969;22:60–66. doi: 10.1136/jcp.22.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufman B, Gandhi SA, Louie E. Herpes simplex virus: case report and review. Clin Inf Dis. 1997;24:334–338. doi: 10.1093/clinids/24.3.334. [DOI] [PubMed] [Google Scholar]
- 3.Ichai P, Roque Afonso AM, Sebagh M, Gonzalez ME, Codés L, Azoulay D, et al. Herpes simplex virus-associated acute liver failure: a difficult diagnosis with a poor prognosis. Liver Transpl. 2005;11:1550–1555. doi: 10.1002/lt.20545. [DOI] [PubMed] [Google Scholar]
- 4.Egawa H, Inomata Y, Nakayama S, Matsui A, Yamabe H, Uemoto S, et al. Fulminant hepatic failure secondary to herpes simplex virus infection in a neonate: a case report of successful treatment with liver transplantation and perioperative acyclovir. Liver Transpl Surg. 1998;4:513–515. doi: 10.1002/lt.500040601. [DOI] [PubMed] [Google Scholar]
- 5.Shanley CJ, Braun DK, Brown K, Turcotte JG, Greenson JK, Beals TF, et al. Fulminant hepatic failure secondary to herpes simplex virus hepatitis. Successful outcome after orthotopic liver transplantation. Transplantation. 1995;59:145–149. doi: 10.1097/00007890-199501150-00028. [DOI] [PubMed] [Google Scholar]
- 6.Twagira M, Hadzic N, Smith M, Ramaswamy M, Verma A, Dhawan A, et al. Disseminated neonatal herpes simplex virus (HSV) type 2 infection diagnosed by HSV DNA detection in blood and successfully managed by LT. Eur J Pediatr. 2004;163:166–169. doi: 10.1007/s00431-003-1383-8. [DOI] [PubMed] [Google Scholar]
- 7.Longerich T, Eisenbach C, Penzel R, Kremer T, Flechtenmacher C, Helmke B, et al. Recurrent herpes simplex virus hepatitis after liver retransplantation despite acyclovir therapy. Liver Transpl. 2005;11:1289–1294. doi: 10.1002/lt.20567. [DOI] [PubMed] [Google Scholar]
- 8.Montalbano M, Slapak-Green GI, Neff GW. Fulminant hepatic failure from herpes simplex virus: post liver transplantation acyclovir therapy and literature review. Transplant Proc. 2005;37:4393–4396. doi: 10.1016/j.transproceed.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 9.Biancofiore G, Bisà M, Bindi LM, Urbani L, Tascini C, Menichetti F, Filipponi F. Liver transplantation due to herpes simplex virus-related sepsis causing massive hepatic necrosis after thoracoscopic thymectomy. Minerva Anestesiol. 2007;73:319–322. [PubMed] [Google Scholar]
- 10.Ganner A, Lee YM, Busche C, Schmitt-Graeff A, Encke J, Walz G, Gerke P. Successful liver transplantation in a kidney and pancreas allograft recipient with fulminant herpes simplex virus type 2 hepatitis. Nephrol Dial Transplant. 2007;22:3334–3337. doi: 10.1093/ndt/gfm550. [DOI] [PubMed] [Google Scholar]
- 11.Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428–1434. doi: 10.1002/lt.21250. [DOI] [PubMed] [Google Scholar]
- 12.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 13.Umemura T, Tanaka E, Ostapowicz G, Brown KE, Heringlake S, Tassopoulos NC, et al. Investigation of SEN virus infection in patients with cryptogenic acute liver failure, hepatitis-associated aplastic anemia, or acute and chronic non-A-E hepatitis. J Infect Dis. 2003;188:1545–1552. doi: 10.1086/379216. [DOI] [PubMed] [Google Scholar]
- 14.Shang D, Lin YH, Rigopoulou I, Chen B, Alexander GJ, Allain JP. Detection of TT virus DNA in patients with liver disease and recipients of liver transplant. J Med Virol. 2000;61:455–461. doi: 10.1002/1096-9071(200008)61:4<455::aid-jmv7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Sheng L, Soumillion A, Beckers N, Wu CG, Verslype C, Nevens F, et al. Hepatitis G virus infection in acute fulminant hepatitis: prevalence of HGV infection and sequence analysis of a specific viral strain. J Viral Hepat. 1998;5:301–306. doi: 10.1046/j.1365-2893.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 16.Luzar B, Ferlan-Marolt V, Poljak M, Sojar V, Stanisavljevic D, Bukovac T, Markovic S. Acute fatty liver of pregnancy—an underlying condition for herpes simplex type 2 fulminant hepatitis necessitating liver transplantation. Z Gastroenterol. 2005;43:451–454. doi: 10.1055/s-2005-857952. [DOI] [PubMed] [Google Scholar]
- 17.Whitley RJ. Herpes simplex virus infection. Semin Pediatr Infect Dis. 2002;13:6–11. doi: 10.1053/spid.2002.29752. [DOI] [PubMed] [Google Scholar]
- 18.Kimberlin DW, Lakeman FD, Arvin AM, Prober CG, Corey L, Powell DA, et al. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group Application of the polymerase chain reaction to the diagnosis and management of neonatal herpes simplex virus disease. J Infect Dis. 1996;174:1162–1167. doi: 10.1093/infdis/174.6.1162. [DOI] [PubMed] [Google Scholar]
- 19.Taddeo B, Zhang W, Lakeman F, Roizman B. Cells lacking NF-κB or in which NF-κB is not activated vary with respect to ability to sustain herpes simplex virus 1 replication and are not susceptible to apoptosis induced by a replication-incompetent mutant virus. J Virol. 2004;78:11615–11621. doi: 10.1128/JVI.78.21.11615-11621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakeman FD, Whitley RJ, the NIAID Collaborative Antiviral Study Group Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain biopsied patients and correlation with disease. J Infect Dis. 1995;171:857–863. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- 21.Wald A, Ashley-Morrow R. Serological testing for herpes simplex virus (HSV)-1 and HSV-2 infection. Clin Infect Dis. 2002;35:S173–S182. doi: 10.1086/342104. [DOI] [PubMed] [Google Scholar]
- 22.Schiodt FV, Atillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV, et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999;5:29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 23.Mason A, Sallie R, Perrillo R, Rayner A, Lizhe X, Dohner DE, et al. Prevalence of herpesviridae and hepatitis B virus DNA in the liver of patients with non-A, non-B fulminant hepatic failure. Hepatology. 1996;24:1361–1365. doi: 10.1002/hep.510240608. [DOI] [PubMed] [Google Scholar]