Introduction
Although reflux disease is the most common cause of esophageal chest pain, esophageal manometry is often done in the course of its evaluation, and manometric abnormalities indicative of DES are often reported. The identified abnormalities, however, are rarely the cause of chest pain and most investigators would agree that the clinical diagnosis of DES is overused. It was that observation that led to a classic reappraisal of DES by Richter and Castell,1 conceived during the renaissance of esophageal manometry in the early 1980s. Arguing for a more restrictive use of the diagnosis, those investigators proposed 2 required manometric criteria for DES: (1) simultaneous contractions in greater than 10% of wet swallows and (2) intermittent normal peristalsis. Other associated features were also described and some minor modifications were subsequently made, but it was these 2 criteria that became part of the lore of (conventional) manometry.
A lot has changed with respect to esophageal motility testing since 1984. Clinical studies are now commonly done with high-resolution systems using in excess of 30 closely spaced pressure transducers, and esophageal contractile patterns are displayed and analyzed in terms of pressure topography rather than as line tracings. Merging these concepts, current motility studies are more accurately termed, HRM imaged with EPT. Although these innovations had their roots in the early 1990s with the pioneering studies of Clouse and colleagues,2–4 it was not until recently that commercial units became available, facilitating widespread adoption of EPT by the clinical community. The advantages of EPT compared with conventional manometry are several: (1) high-quality studies can be obtained that simultaneously image the entire esophagus, (2) standardized objective metrics have been developed for interpretation,5–8 and (3) topographic patterns of contractility are easily learned and recognized with great reproducibility.9,10 EPT also presented challenges, however, not the least of which was the need to reconsider the classification of esophageal motility developed for conventional manometric systems.11 That classification effort led to improved understanding of achalasia subtypes12 and hypomotility patterns.13 Headway has also been made in the domain of hypercontractile conditions, including DES.14,15 This work led to a conclusion, however, that the 2 essential criteria identified by Richter and Castell were suboptimal for defining DES as imaged in EPT and identified a heterogeneous group of patients, most of whom did not have DES.14 Hence, the aims of this synopsis are to update the understanding of esophageal spastic disorders in the era of EPT.
What are the Spastic Disorders of the Esophagus?
Spastic disorders of the esophagus might be conceived of as hyperactive conditions of the esophagus due to contractions of either abnormal propagation (premature contractions) or extreme vigor. In the current iteration of the Chicago classification of esophageal motility disorders,16 the relevant diagnoses are spastic (type III) achalasia, DES, and hypercontractile (jackhammer) esophagus. Despite differences in pathophysiology, which are discussed, these disorders share many similarities, including their clinical presentation: dysphagia, chest pain, regurgitation, and/or heartburn. The identification of these spastic disorders is based on the contractile pattern observed using HRM with EPT. The current Chicago classification criteria for identification of the spastic disorders are summarized in Tables 1 and 2.16
Table 1. EPT patterns of spastic disorders.
| Spastic Disorders | EGJ Relaxation | Esophageal Contractions |
|---|---|---|
| Distal esophageal spasm | Normal (mean IRP <15 mm Hg) | ≥20% Premature contractions (DL <4.5 s) |
| Spastic (type III) achalasia | Impaired (mean IRP ≥15 mm Hg) | ≥20% Premature contractions (DL <4.5 s) |
| Jackhammer esophagus | Normal or impaireda | At least 1 swallow with DCI >8000 mm Hg · s · cm |
EGJ relaxation is assessed using IRP, which corresponds to the 4-s period of the lowest EGJ pressure within the deglutitive window.
EGJ outflow obstruction (defined as mean IRP ≥ 15 mm Hg in association with some instances of peristalsis) may be associated with hypercontractile swallow.
Table 2. EPT metrics used in the Chicago classification.
| Abbreviation | Metric | Description | Normal |
|---|---|---|---|
| IRP | Integrated relaxation pressure | Mean EGJ pressure during the 4 s of maximal relaxation (contiguous or noncontiguous) in the 10-s window after UES relaxation | <15 mm Hg |
| DCI | Distal contractile integral | Amplitude × time × duration (mm Hg · s · cm) of the distal esophageal contraction >20 mm Hg from the proximal pressure through (transition zone) to the EGJ | <5000 mm Hg · s · cm |
| CDP | Contractile deceleration point | The inflection point along the 30–mm Hg isobaric contour where propagation velocity slows demarcating the tubular esophagus from the phrenic ampulla | — |
| CFV | Contractile front velocity | Slope of the tangent approximating the 30–mm Hg between the proximal pressure trough (transition zone) and the CDP | <9 cm/s |
| DL | Distal latency | Interval between UES relaxation and the CDP | >4.5 s |
All pressures are referenced to atmospheric pressure except the IRP, which is referenced to gastric pressure.
Distal Esophageal Spasm and Spastic Achalasia
Definition
DES is an uncommon disorder characterized by an impairment of ganglionic inhibition in the distal esophagus. Using conventional manometry, DES was defined by the presence of simultaneous contractions.11 Using HRM with EPT, however, the higher-resolution recordings demonstrated that propagation velocity normally varies greatly along the length of the esophagus and finding regions of rapid propagation is common. A consequence of this finding is that the finding of rapidly propagated contractions is nonspecific for esophageal spasm.14 Alternatively, premature contractions, defined by reduced distal latency (DL), measured as the interval between upper sphincter relaxation and the onset of contraction in the distal esophagus, are more specific for spasm. Physiologically, the DL is likely a manifestation of inhibitory myenteric neuron activity that determines the timing of contraction in the distal esophagus. Premature contractions with normal EGJ relaxation define DES whereas premature contractions with impaired EGJ relaxation are defining criteria for spastic achalasia (also termed, type III achalasia).12
Impairment of neural inhibition?
DES and spastic achalasia share a common pathophysiology characterized by loss of inhibitory ganglionic neuron function in the distal esophagus. The impairment of inhibitory innervation leads to both premature, rapidly propagated, or simultaneous contractions in the distal esophagus and to incomplete deglutitive EGJ relaxation. Unlike the proximal esophagus, where sequencing of the peristaltic contraction is directly programmed from motor neurons in the medulla, the timing of peristalsis in the distal smooth muscle esophagus is mediated via excitatory (cholinergic) and inhibitory (nitric oxide [NO]) myenteric plexus neurons. Furthermore, a neural gradient exists such that there is an increasing proportion of inhibitory ganglionic neurons progressing distally to the lower esophageal sphincter. The deglutitive response begins with a period of quiescence (deglutitive inhibition) in the distal esophagus that is progressively prolonged approaching the EGJ as a consequence of that neural gradient. Behar and Biancani17 qualified this period of quiescence as contractile latency and suggested that patients with spasm could be characterized by a reduction in contractile latency. Thus, distal contractile latency, measured from the onset of the pharyngeal swallow to the onset of the contraction in the distal esophagus, was shorter in patients with simultaneous contractions than in those with normal peristaltic propagation. NO is the dominant inhibitory neurotransmitter in the esophageal myenteric plexus.18 Experimentally scavenging NO with free hemoglobin in control subjects induces simultaneous esophageal contraction and inhibits deglutitive EGJ relaxation.19 This demonstrates the role of inhibitory innervation in the genesis of DES and impaired EGJ relaxation.
Some structural changes have been observed in the esophageal muscularis propria of patients with DES. These are inconsistent and nonspecific, however. Recent observations by Pehlivanov and colleagues20 using high-frequency intraluminal ultrasound suggest increased esophageal smooth muscle thickness in DES patients. Even in the absence of esophageal contractions, the muscularis propria in DES patients was thicker than in controls or patients with nonspecific motor disorders. Finally, a study in knockout mice suggested that lack of inhibitory innervation might result in increased muscularis propria thickness.21
Jackhammer Esophagus
Definition
The term, nutcracker esophagus, was coined in conventional manometry for a novel disorder associated with noncardiac chest pain and characterized by hypertensive but normally propagated peristaltic contractions.22 Unlike the case of spasm, there were no characteristic fluoroscopic abnormalities. The manometric criterion for nutcracker esophagus were initially an average peristaltic amplitude of greater than 180 mm Hg in the distal esophagus. Subsequently, this threshold value was increased to 220 mm Hg in hopes of improving specificity. With the era of HRM and EPT, peristaltic amplitude was replaced by the distal contractile integral (DCI) as the summary metric of the vigor of the distal esophageal contraction. If the entire distal esophageal contraction is envisioned as a solid with the height of the peaks corresponding to peristaltic amplitude and the footprint corresponding to the length of the involved esophagus and the duration of the contraction, the DCI, expressed as mm Hg · s · cm, is the volume of that solid above a 20–mm Hg minimum. A DCI mean value of 5000 mm Hg (hypertensive peristalsis in the Chicago classification) approximately corresponds to nutcracker esophagus in conventional manometry. Even that value is seen in up to 5% of normal subjects, however, making it inherently nonspecific. Alternatively, an extreme phenotype of hypertensive contractions was described based on the occurrence of at least one contraction with a DCI greater than 8000 mm Hg · s · cm, a value never observed in controls.15 This was termed, esophageal hypercontractility or the jackhammer esophagus. Although still somewhat heterogeneous (the pattern is sometimes seen with EGJ outflow obstruction), this extreme phenotype is likely more clinically relevant than hypertensive peristalsis (nutcracker esophagus). Hypercontractility is commonly associated with multipeaked contractions, sometimes resulting in DCI values in excess of 50,000 mm Hg · s · cm.
Excess of cholinergic stimulation?
The pathophysiology of esophageal hypercontractility likely involves an excess of cholinergic drive. Temporal asynchrony between the contractions of circular and longitudinal muscle layers of the muscularis propria have been observed with high-frequency intraluminal ultrasound in patients with nutcracker esophagus.23 This asynchrony was reversed with atropine.24 The observations of Loo and colleagues25 in diabetics with autonomic neuropathy are also an indirect argument for an excess of cholinergic stimulation. Multipeaked contractions occurred more frequently in diabetics with neuropathy than in control subjects or diabetics without neuropathy. Multipeaked contractions became single-peak contraction after atropine injection. Multipeaked contractions are also a common finding in jackhammer esophagus.15 It remains to be determined if atropine may change the multipeaked pattern in such patients. Finally,as with DES, increased muscle thickness has been observed in patients with nutcracker esophagus26 and with esophageal hypercontractility (Kahrilas, 2011, unpublished observations).
Esophageal Spastic Disorders: A Consequence of EGJ Obstruction?
Both DES and jackhammer esophagus can be associated with EGJ outflow obstruction, an association supported by experimental models. For instance, Mittal and colleagues27 observed esophageal muscle hypertrophy and hyperexcitability by placing calibrated ligatures around the EGJ in cats. In humans, esophageal hypercontractility has been observed with mechanical EGJ obstruction induced by fundoplication or gastric lap band.28 Gyawali and Kushnir29 expanded on this observation, reporting that patients with EGJ outflow obstruction exhibited a motor pattern characterized by multipeaked contractions, high distal esophageal amplitude, and prolonged contraction duration. Finally, as previously defined, impaired EGJ relaxation in association with premature contractions constitutes spastic achalasia.12
Given the relationship between EGJ outflow obstruction and hypercontractility, some investigators have speculated that esophageal spastic disorders can progress to achalasia. Supporting this contention, among a series of 35 patients diagnosed with DES on conventional manometry, 5 (14%) progressed to achalasia, 4 (12%) reverted to normal manometry, and 26 (74%) had persistent DES at a mean follow-up of 2.1 years.30 Progression from nutcracker esophagus to achalasia also was observed.31,32 The number of patients reported to undergo such progression, however, is extremely limited (only case reports of nutcracker esophagus), leaving open the possibility that the type of spastic disorder might have been misdiagnosed with either the initial or the follow-up conventional manometry study. EGJ pseudorelaxation secondary to esophageal shortening commonly leads to an erroneous diagnosis of DES instead of spastic achalasia.4
Association with Other Conditions: Gastroesophageal Reflux Disease and Eosinophilic Esophagitis
Manometric findings consistent with primary spastic motility disorders can also occur in conjunction with, or as a consequence of, other conditions, notably gastroesophageal reflux disease (GERD). In a series of 108 patients with DES, GERD was documented by either pH-metry or endoscopy in 38%.33 Furthermore, in some instances, esophageal acid perfusion can induce spasm.34 In a series of 45 patients with nutcracker esophagus (conventional manometry), 47% had abnormal acid exposure time on pH-metry, 4% had endoscopic esophagitis, and 16% positive symptom index.35 Finally, reflux esophagitis has also been observed in patients with jackhammer esophagus and the hypercontractile pattern can resolve with of proton pump inhibitor (PPI) therapy.15
Similar overlap exists between spastic disorders and eosinophilic esophagitis. In a retrospective study of patients who underwent Heller myotomy for achalasia, mucosal eosinophilia was reported in 8%.36 A case of achalasia with eosinophilic infiltrate responding to steroid therapy has been reported.37 Jackhammer esophagus was also associated with eosinophilic esophagitis in 3 of 41 patients (7%) who underwent endoscopy with mucosal biopsies.15
Diagnosis of Esophageal Spastic Disorders
Dysphagia, chest pain, regurgitation, and heartburn are all symptoms potentially associated with esophageal spastic disorders. All of these are nonspecific, however, and esophageal spastic disorders are rare.15,38 Hence, the clinical evaluation needs to prioritize identifying more morbid conditions and more prevalent conditions before pursuing these rare, nonfatal conditions. When chest pain is among the presenting symptoms, the evaluation should first prioritize excluding cardiovascular disease owing to its potentially life-threatening nature. Even within the realm of esophageal chest pain, reflux is a more common cause than spastic disorders. Using the liberal definitions put forth by Richter and Castell, DES accounted for fewer than 5% of patients referred for dysphagia or chest pain in a motility laboratory.39 With the more refined criteria of the Chicago classification, the combined prevalence of DES, spastic achalasia, and jackhammer is even lower, approximately 2%.12,14,15,40 Consequently, evaluation of suspected esophageal spastic disorders requires a thorough evaluation to first identify or exclude other potential causes of esophageal chest pain.
One potential consequence of spastic contractions is an impairment of esophageal bolus transit that may explain the perception of dysphagia. As evident by the fluoroscopic appearance of DES as a corkscrew or rosary bead esophagus, long segments of simultaneous contractions might occur. In such instances, the bolus becomes trapped in the spastic segment because the distal portion contracts prematurely with insufficient time to allow for bolus transit.17 Paradoxically, Tutuian and Castell41 reported that 55% of patients with DES defined with conventional manometry and 97% of patients with nutcracker esophagus exhibited complete bolus transit when tested with multichannel intraluminal impedance. Although their findings with respect to nutcracker are consistent with understanding of its physiology, the observation regarding DES are not and speak to the overdiagnosis of the condition using conventional manometry and diagnostic criteria. DES patients with dysphagia exhibited more frequently abnormal bolus transit than DES patients with chest pain.42
The mechanism by which spastic disorders induce chest pain is not well understood. The amplitude of contractions might be relevant. Tutuian and colleagues42 demonstrated that DES patients with chest pain had greater amplitude esophageal contractions than DES patients with dysphagia or GERD. Hypersensitivity might also play a role. Using stepwise balloon distension, Mujica and colleagues43 observed a lower chest pain threshold in patients with nutcracker esophagus compared with controls. Hypersensitivity might also explain the perception of heartburn in patients without demonstrable evidence of reflux.
Finally, epiphrenic diverticula might occur as a consequence of spastic disorders. The majority of patients with epiphrenic diverticula are found to have an esophageal motility disorder. In a surgical series of 21 patients with epiphrenic diverticula, 24% were diagnosed as DES before surgery, 24% as nutcracker esophagus, and 9% as achalasia.44 The presence of a diverticulum might also explain the symptoms of dysphagia or regurgitation.
Upper Endoscopy
Upper endoscopy should be performed as initial evaluation of esophageal symptoms consistent with spastic disorders. It allows exclusion of mechanical obstruction, esophageal stenosis, or esophagitis. Systematic esophageal biopsies should be obtained to rule out eosinophilic esophagitis, especially when dysphagia is a prominent symptom. Usually no specific endoscopic abnormality is revealed, but disordered esophageal contractions might be observed by the endoscopist. In some cases of achalasia, increased resistance at the EGJ might be perceived.
Esophageal Manometry
The diagnosis of spastic disorders is established by esophageal manometry and recent developments suggest that HRM with EPT is superior to conventional manometry for several reasons. First, the diagnosis of EGJ relaxation is more reliable with HRM compared with conventional manometry, which is essential in distinguishing DES from spastic achalasia.4,45 A major factor leading to the failure to detect impaired EGJ relaxation with conventional manometry is esophageal shortening that occurs during peristalsis that may be accentuated with spasm. Correct evaluation of EGJ relaxation is of cardinal importance because spastic contractions with normal EGJ relaxation constitute DES but spastic contractions with impaired EGJ relaxation diagnose spastic achalasia, and treatment then focuses on alleviating EGJ obstruction. Moreover, the use of the integrated relaxation pressure (IRP),5 DL,14 and DCI8 measurements in HRM (see Table 2) more accurately diagnose spastic disorders than the metrics used in conventional manometry. The EPT definitions of spastic disorders are summarized in Table 1.
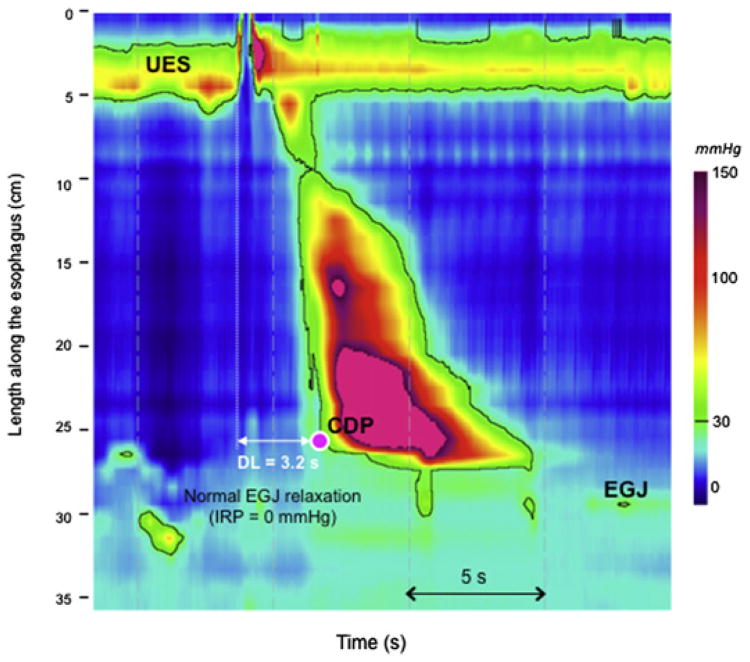
DES was initially defined using conventional manometry by the presence of at least 20% simultaneous contractions with minimum amplitude of 30 mm Hg.11 A simultaneous contraction was defined by a propagation velocity greater than 8 cm/s measured between 3 cm and 8 cm above the EGJ. Associated, but not essential, criteria for DES were spontaneous, repetitive, or multipeaked contractions and intermittent normal peristalsis. With HRM and EPT, DES is defined as at least 20% premature contractions in the context of normal EGJ relaxation.16 Premature contractions exhibit a reduced (<4.5 s) DL defined as the interval between upper esophageal sphincter (UES) relaxation and onset of the contraction at the contractile deceleration point (CDP) (Fig. 1). Recently, Pandolfino and colleagues14 demonstrated that DL was much more specific than the contractile front velocity (CFV) for detecting spastic disorders. Among 1070 patients, 91 exhibited rapid contractions (defined as CFV >9 cm/s). In 24 of them, these contractions were also premature. All of the patients with premature contractions were ultimately managed as either DES or spastic achalasia. In contrast, the 67 patients with rapid contractions but normal DL were more likely to have nonspastic disorders, in particular, weak peristalsis. Finally, in the Chicago classification, there is no requirement of any normal contractions in the diagnosis of DES.
Fig. 1.
Distal esophageal spasm in EPT. EGJ assessed with the IRP is normal. Premature contractions are observed for at least 20% of swallows. A reduced DL (<4.5 s), measured from UES relaxation to CDP (pink dot), defines premature contraction. The CDP corresponds to an abrupt slowing of the contraction wavefront representing the transition from esophageal clearance to the formation of the phrenic ampulla. (Data from Pandolfino JE, Leslie E, Luger D, et al. The contractile deceleration point: an important physiologic landmark on esophageal pressure topography. Neurogastroenterol Motil 2010;22(4):395–400.)
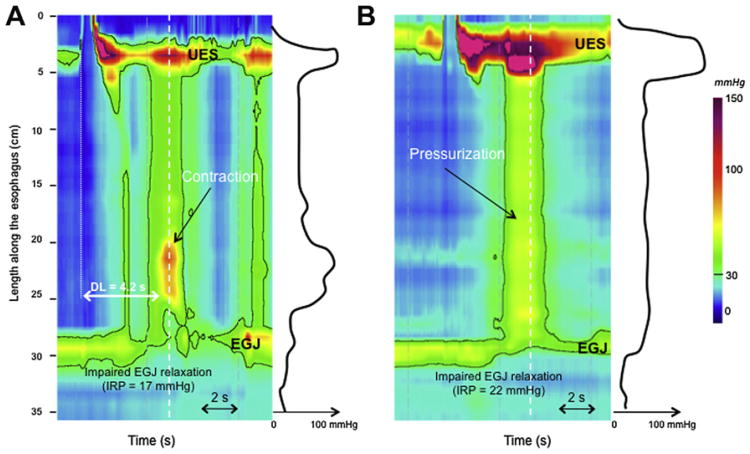
The only differentiating feature between DES and spastic achalasia (also named type III in the Chicago classification) is the adequacy of EGJ relaxation. Using conventional manometry, spastic achalasia was included in the concept of vigorous achalasia. No distinction was made, however, between bolus pressurization from simultaneous contractions, leading to the likely inclusion of many type II achalasia patients in the vigorous group. Using HRM and EPT, it is easy to differentiate panesophageal pressurization from simultaneous contractions by comparing their respective spatial pressure variation plots, which illustrate the instantaneous longitudinal pressure profile within the esophagus. The spatial pressure variation plot between UES and EGJ is flat in instances of panesophageal pressurization whereas it exhibits peaks and valleys in instances of simultaneous contractions (Fig. 2). Thus, using EPT, spastic achalasia is defined as impaired EGJ relaxation (IRP ≥15 mm Hg) associated with at least 20% premature contractions.
Fig. 2.
Spastic (type III) achalasia is characterized as impaired EGJ relaxation associated with at least 20% of premature contractions (A). The premature contraction exhibits a reduced DL (<4.5 s). EGJ relaxation is assesses using the IRP. Simultaneous (premature) contractions might be differentiated from pressurization (B) using the spatial pressure variation plot represented on the right of each EPT plots. Each spatial pressure variation pressure plot was obtained at the time, identified by the white dashed line. In the instance of an esophageal contraction (A), pressure variations are obvious along the esophageal body. In instances of pressurization (B), intraesophageal pressure did not vary between UES and the EGJ. (B) This corresponds to type II achalasia (achalasia with compression).
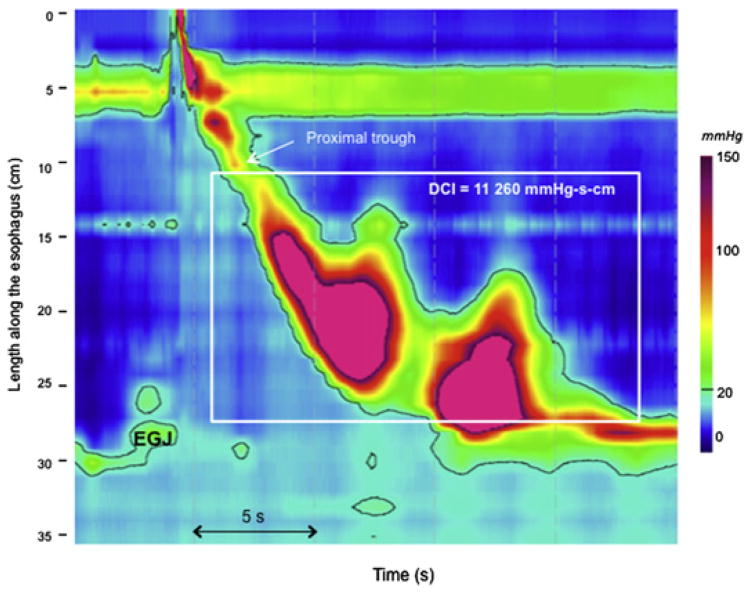
Jackhammer esophagus is an extreme pattern of hypercontractility. Using conventional manometry, nutcracker esophagus was defined as a mean distal esophageal peristaltic amplitude (measured 3 cm and 8 cm above the EGJ) greater than 180 mm Hg in the context of normal LES relaxation.11 Subsequently, some investigators proposed increasing the threshold to 260 mm Hg, a value suggested as having greater clinical relevance.46 Similarly, seeking a more clinically relevant definition of hypercontractility, the diagnosis of jackhammer esophagus was proposed in EPT, defined as at least 1 swallow with a DCI (the metric of contractile vigor) greater than 8000 mm Hg · s · cm (Fig. 3).15 Distinguishing jackhammer from hypertensive peristalsis or nutcracker esophagus, this pattern was never observed in control subjects. Jackhammer may be associated with EGJ outflow obstruction or obstruction. Multipeaked contractions are frequent in patients with jackhammer esophagus but are not mandatory to diagnose this disorder.
Fig. 3.
Jackhammer esophagus is defined as at least 1 swallow with a DCI greater than 8000 mm Hg · s · cm. DCI is the EPT metric summarizing contractile vigor and is calculated as the product of the amplitude (>20 mm Hg) times the duration times the length ofthe contraction between the proximal pressure trough (also known as the transition zone) and the EGJ (white box).
Because symptoms of spastic disorders are intermittent, ambulatory 24-hour manometry has been proposed as a way to increase the diagnostic yield. In 1 such series of 390 patients referred with esophageal motility disorders, 16 (4%) were diagnosed with DES on 24-hour manometry, 14 of whom were missed by laboratory manometry.47 Likely because of the scarcity of suitable recording devices, however, 24-hour manometry is rarely done and the implications of such an examination on patient management have yet to be defined.
Barium Swallow
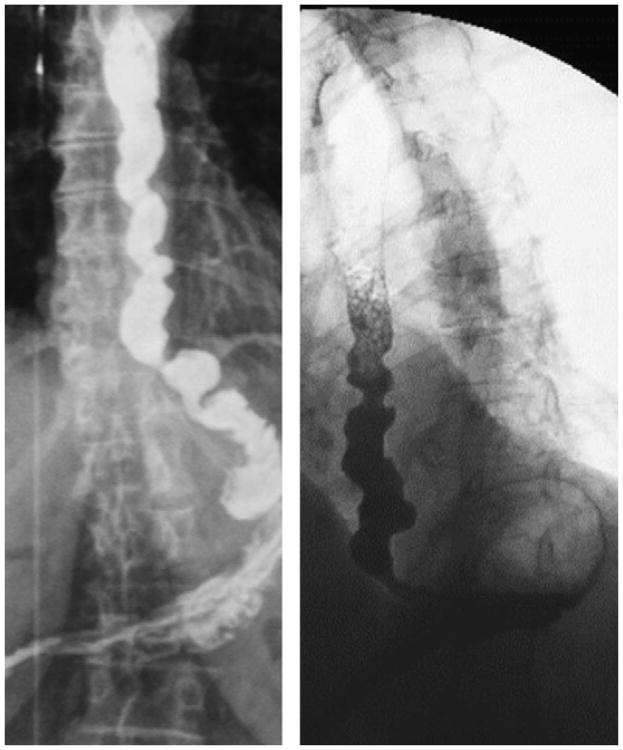
The classic appearance of esophageal spasm is the corkscrew or rosary bead esophagus (Fig. 4). In a series of 14 patients with DES on barium swallow, Prabhakar and colleagues48 observed a classical radiologic pattern of corkscrew esophagus in only 2 patients; the others exhibited nonperistaltic contractions that were not lumen obliterating. Lower esophageal sphincter dysfunction was suspected in 9 patients on barium swallow. Alternatively, using conventional manometry, these 14 patients were classified as DES with normal LES function in 2 cases, DES with LES dysfunction in 4 cases, and spastic achalasia in 8 cases. In total, 13 of the 14 patients had LES dysfunction evident either on barium swallow or manometry, suggesting that the 2 techniques were complementary in differentiating DES from spastic achalasia. Consequently, when spastic disorders are suspected on manometry, a barium swallow might be performed to appreciate the consequences of motility disorders on esophageal bolus transit, such as barium stasis or retrograde barium movement in the esophagus. It may also detect an epiphrenic diverticulum to support the diagnosis of spastic disorders.
Fig. 4.
Barium swallow of 2 patients with distal esophageal spasm. Note the typical pattern of rosary beads or corkscrew esophagus.
Other Examinations
CT scan and endoscopic ultrasonography
Esophageal muscle thickening can be observed in patients with spastic disorders. This thickening is sometimes profound (as much as 1 cm) and identifiable on CT scan.49 CT scan is not routinely indicated in patients with spastic disorders, however, unless there is a suspicion of extrinsic esophageal compression. Alternatively, endoscopic ultrasonography can quantify esophageal thickening and also reveal mediastinal or intramural abnormalities making it useful in atypical cases. The clinical detection of esophageal thickening favors the diagnosis of spastic disorders. Endoscopic ultrasound also allows for the exclusion of intramural esophageal tumor that might potentially induce abnormal contractility.
24-Hour pH monitoring
Because of the potential overlap between DES and GERD, 24-hour pH monitoring should be considered in patients having chest pain, regurgitation, or heartburn to exclude pathologic reflux. The yield of pH impedance in this indication is not known. Wireless pH monitoring has been evaluated in patients with noncardiac chest pain.50 As pH recording was extended to 48 hours with the wireless pH capsule; a diagnostic gain of 9.7% was observed in detecting pathologic esophageal acid exposure compared with 24-hour recording. Two-thirds of patients, however, reported severe chest pain during wireless pH monitoring in this indication. Consequently, wireless pH monitoring should be used with caution in patients with noncardiac chest pain.
Impedance manometry
Impedance manometry allows for a direct concurrent assessment of bolus transit and motility.41 Compared with barium swallow, impedance monitoring has the advantage of avoiding radiation exposure. Recently, it has been suggested that impedance might be as accurate as barium swallow to evaluate bolus transit in patients with dysphagia.51 The concordance was high for severe barium stasis and incomplete bolus transit on impedance (97%) and for normal barium transit and complete bolus transit (96%). Instances of complete bolus transit on impedance and mild barium stasis on fluoroscopy, however, were observed in patients who had been treated for DES or achalasia, making the claim of equivalency between the examinations questionable.
Treatment of Esophageal Spastic Disorders
The first treatment strategy of spastic disorders depends on whether or not there is an accompanying EGJ outflow obstruction (Table 3). If EGJ relaxation is impaired, the initial treatment should be directed at alleviating EGJ obstruction. Otherwise, the goal of treatment is to reduce the vigor of the abnormal esophageal contractions.
Table 3. Therapeutic options for spastic disorders.
| Therapeutic option | Impaired EGJ relaxation (spastic [type III] achalasia) | Normal EGJ relaxation (distal esophageal spasm, jackhammer esophagus) |
|---|---|---|
| Pharmacologic | Phosphodiesterase-5 inhibitors? | Nitrates Phosphodiesterase-5 inhibitors Calcium channel blockers Peppermint oil Low-dose antidepressants PPIs |
| Endoscopic | Pneumatic dilation Toxin botulinum at the EGJ level POEM |
Botulinum toxin in the esophageal body Extended POEM? |
| Surgical | Myotomy | Extended myotomy? |
Pharmacologic Treatment
Smooth muscle relaxants, such as nitrates, NO donors, and calcium channel blockers, have been proposed for treating esophageal spastic disorders. These drugs both reduce LES pressure and esophageal contraction amplitude. Placebo-controlled crossover trials report only minimal benefit in achalasia, however.52 In DES, nitrates may improve manometric findings and chest discomfort.53,54 They also prolong the DL and decrease the distal contraction amplitude in patients with DES.55 Nitrates have not been tested in controlled trials, however, in DES or in nutcracker esophagus.
Phosphodiesterase-5 inhibitors (eg sildenafil) represent a new therapeutic option. Phosphodiesterase-5 inhibitors block the degradation of NO, enhancing its effect and resulting in more prolonged smooth muscle relaxation. Sildenafil reduces both contractile amplitude and propagation velocity in controls and in patients with motility disorders. Preliminary data also suggest it is effective in relieving esophageal symptoms and improving manometric findings in patients with spastic motility disorders.56,57 Practical limitations of this treatment, however, are side effects (dizziness and headache) and cost. Because its main approved indication, erectile dysfunction in men, is viewed as recreational, most insurers do not cover the cost for patients.
Another smooth muscle relaxant, peppermint oil, has also been reported by one group of investigators58 to eliminate simultaneous contractions. No other study, however, has yet confirmed these data.
Low-dose antidepressants can improve patients' reaction to pain without objectively improving motility function.59 A controlled trial showing efficacy for this strategy was with the anxiolytic, trazadone (serotonin uptake inhibitor), suggesting that reassurance and control of anxiety are important therapeutic goals.59 Also consistent with that conclusion, success has been reported using behavioral modification and biofeedback.60
Finally, due to the potential overlap between GERD and spastic disorders, a trial of PPIs may be beneficial, especially in the setting of esophagitis or abnormal pH-metry.
Endoscopic Treatment
Pneumatic dilation has been proposed for treating spastic disorders and some success has been reported.61 A caveat to this success is that it is unclear whether or not the patients benefited by pneumatic dilation would not be more properly categorized as having spastic achalasia or achalasia with esophageal compression, emphasizing the need for accurate manometric classification. Pandolfino and colleagues12 observed that pneumatic dilation was associated with a lower treatment response in patients with spastic achalasia compared with patients with achalasia with esophageal compression.
Botulinum toxin injection is a pathophysiologically attractive approach to treating patients with spastic disorders, and therapeutic trials suggest it can reduce chest pain.62 The technique has not been standardized in this application with some reports injection of botulinum toxin only at the level of the EJG and others also injecting the distal esophagus.62 Some efficacy is noted in a majority of achalasia patients with injection in the EGJ. Achalasia subtypes, however, were not defined in these trials and effects were temporary with a fall-off in success rates from 80% to 90% after 1 month to 53% to 54% after 1 year.63 In a sham-controlled trial of 22 patients with DES or nutcracker esophagus, thus far reported only in abstract form, injection of toxin botulinum in the distal esophagus was superior to placebo in improving dysphagia.64
Recently, peroral endoscopic myotomy (POEM) has been introduced to treat achalasia65 and a case of DES successfully treated by extended POEM has been reported.66 Short-term results with this technique are promising but larger and longer-term studies are required to determine its place in the management of patients with spastic disorders.
Surgical Treatment
Heller myotomy is an established treatment of achalasia. As with pneumatic dilation, however, a lower response rate has been observed in patients with spastic achalasia.12 This might be explained by the disease involving not only the LES but also the esophageal body. Long myotomy extending from the LES proximally onto the esophageal body has been used to treat patients with spastic disorders. The extent of the myotomy may be guided by manometric findings.67,68 In a series of 20 patients with extended myotomy (14 cm on the esophagus and 2 cm below the EGJ) and anterior fundoplication for DES, dysphagia and chest pain were significantly improved after a median follow-up of 50 months.69 Functional results seemed to be stable with time in that series. An uncontrolled study suggested that surgical treatment might be more effective than the medical treatment of DES.68 Choice of treatment, however, was based on physician preference, patient choice, and access to a referral center for treatment. Controlled trials are required to determine if surgical management is more effective than endoscopic or medical treatment.
Summary
Largely as a consequence of refined classification made possible with HRM and EPT, the current concept of esophageal spastic disorders has evolved to encompass spastic achalasia, DES, and jackhammer esophagus. These are conceptually distinct in that spastic achalasia and DES are characterized by a loss of neural inhibition, whereas jackhammer esophagus is associated with hypercontractility, presumably by activation of the cholinergic pathway. Esophageal spastic disorders can present with dysphagia, chest pain, regurgitations, and/or heartburn. Because the defining endoscopic features may also occur in the setting of EGJ obstruction, endoscopic examination is required when esophageal spastic disorders are suspected to evaluate for mechanical obstruction. Esophageal biopsies should also be performed because of the possible association with eosinophilic esophagitis. The key examination, however, is high-resolution esophageal manometry, which facilitates a specific definition of each spastic disorder. HRM with EPT is preferred to conventional manometry because these disorders have not been reliably distinguished from one another with the older technology. Finally, other examinations, such as barium swallow and esophageal pH-metry, might be useful to assess bolus transit and esophageal acid exposure, respectively. Therapeutic management depends on the presence of EGJ outflow obstruction. Alleviating EGJ outflow obstruction is achieved with either pneumatic dilation, endoscopic botulinum injection, or myotomy. Pharmacologic treatment (nitrates and phosphodiesterase-5 inhibitors) may reduce esophageal contractions as effectively as botulinum toxin injection. Extensive myotomy using the POEM technique might have a role in cases of treatment failure.
Key Points.
Largely as a consequence of refined classification made possible with high-resolution manometry (HRM) and esophageal pressure topography (EPT), the current concept of esophageal spastic disorders has evolved to encompass spastic achalasia, distal esophageal spasm (DES), and jackhammer esophagus.
These esophageal spastic disorders are conceptually distinct in that spastic achalasia and DES are characterized by a loss of neural inhibition, whereas jackhammer esophagus is associated with hypercontractility, presumably by activation of the cholinergic pathway.
Because the defining endoscopic features may also occur in the setting of esophagogastric junction (EGJ) obstruction, endoscopic examination is required when esophageal spastic disorders are suspected to evaluate for mechanical obstruction.
Therapeutic management depends on the presence of EGJ outflow obstruction.
Extensive myotomy using the POEM technique might have a role in cases of treatment failure.
Acknowledgments
This work was supported by Grant No. R01DK56033 from the National Institutes of Health. Conflict of interest: SR has served as consultant for Given Imaging.
References
- 1.Richter JE, Castell DO. Diffuse esophageal spasm: a reappraisal. Ann Intern Med. 1984;100(2):242–5. doi: 10.7326/0003-4819-100-2-242. [DOI] [PubMed] [Google Scholar]
- 2.Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol. 1991;261(4 Pt 1):G677–84. doi: 10.1152/ajpgi.1991.261.4.G677. [DOI] [PubMed] [Google Scholar]
- 3.Clouse RE, Staiano A, Alrakawi A. Development of a topographic analysis system for manometric studies in the gastrointestinal tract. Gastrointest Endosc. 1998;48(4):395–401. doi: 10.1016/s0016-5107(98)70010-0. [DOI] [PubMed] [Google Scholar]
- 4.Clouse RE, Staiano A, Alrakawi A, et al. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol. 2000;95(10):2720–30. doi: 10.1111/j.1572-0241.2000.03178.x. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh SK, Pandolfino JE, Rice J, et al. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G878–85. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 6.Pandolfino JE, Leslie E, Luger D, et al. The contractile deceleration point: an important physiologic landmark on oesophageal pressure topography. Neurogastroenterol Motil. 2010;22(4):395–400. doi: 10.1111/j.1365-2982.2009.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman S, Lin Z, Pandolfino JE, et al. Distal contraction latency: a measure of propagation velocity optimized for esophageal pressure topography studies. Am J Gastroenterol. 2011;106(3):443–51. doi: 10.1038/ajg.2010.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh SK, Pandolfino JE, Zhang Q, et al. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G988–97. doi: 10.1152/ajpgi.00510.2005. [DOI] [PubMed] [Google Scholar]
- 9.Grubel C, Borovicka J, Schwizer W, et al. Diffuse esophageal spasm. Am J Gastroenterol. 2008;103(2):450–7. doi: 10.1111/j.1572-0241.2007.01632.x. [DOI] [PubMed] [Google Scholar]
- 10.Soudagar AS, Sayuk GS, Gyawali CP. Learners favour high resolution oesophageal manometry with better diagnostic accuracy over conventional line tracings. Gut. 2012;61(6):798–803. doi: 10.1136/gutjnl-2011-301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49(1):145–51. doi: 10.1136/gut.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135(5):1526–33. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roman S, Lin Z, Kwiatek MA, et al. Weak peristalsis in esophageal pressure topography: classification and association with dysphagia. Am J Gastroenterol. 2011;106(2):349–56. doi: 10.1038/ajg.2010.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandolfino JE, Roman S, Carlson D, et al. Distal esophageal spasm in high-resolution esophageal pressure topography: defining clinical phenotypes. Gastroenterology. 2011;141(2):469–75. doi: 10.1053/j.gastro.2011.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roman S, Pandolfino JE, Chen J, et al. Phenotypes and clinical context of hypercontractility in high resolution pressure topography (EPT) Am J Gastroenterol. 2012;107(1):37–45. doi: 10.1038/ajg.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bredenoord AJ, Fox M, Kahrilas PJ, et al. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal esophageal pressure topography (EPT) Neurogastroenterol Motil. 2012;24(Suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behar J, Biancani P. Pathogenesis of simultaneous esophageal contractions in patients with motility disorders. Gastroenterology. 1993;105(1):111–8. doi: 10.1016/0016-5085(93)90016-6. [DOI] [PubMed] [Google Scholar]
- 18.Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008;42(5):610–9. doi: 10.1097/MCG.0b013e31816b444d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray JA, Ledlow A, Launspach J, et al. The effects of recombinant human hemoglobin on esophageal motor functions in humans. Gastroenterology. 1995;109(4):1241–8. doi: 10.1016/0016-5085(95)90584-7. [DOI] [PubMed] [Google Scholar]
- 20.Pehlivanov N, Liu J, Kassab GS, et al. Relationship between esophageal muscle thickness and intraluminal pressure in patients with esophageal spasm. Am J Physiol Gastrointest Liver Physiol. 2002;282(6):G1016–23. doi: 10.1152/ajpgi.00365.2001. [DOI] [PubMed] [Google Scholar]
- 21.Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology. 2000;119(3):766–73. doi: 10.1053/gast.2000.16509. [DOI] [PubMed] [Google Scholar]
- 22.Benjamin SB, Gerhardt DC, Castell DO. High amplitude, peristaltic esophageal contractions associated with chest pain and/or dysphagia. Gastroenterology. 1979;77(3):478–83. [PubMed] [Google Scholar]
- 23.Jung HY, Puckett JL, Bhalla V, et al. Asynchrony between the circular and the longitudinal muscle contraction in patients with nutcracker esophagus. Gastroenterology. 2005;128(5):1179–86. doi: 10.1053/j.gastro.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Korsapati H, Bhargava V, Mittal RK. Reversal of asynchrony between circular and longitudinal muscle contraction in nutcracker esophagus by atropine. Gastroenterology. 2008;135(3):796–802. doi: 10.1053/j.gastro.2008.05.082. [DOI] [PubMed] [Google Scholar]
- 25.Loo FD, Dodds WJ, Soergel KH, et al. Multipeaked esophageal peristaltic pressure waves in patients with diabetic neuropathy. Gastroenterology. 1985;88(2):485–91. doi: 10.1016/0016-5085(85)90511-6. [DOI] [PubMed] [Google Scholar]
- 26.Dogan I, Puckett JL, Padda BS, et al. Prevalence of increased esophageal muscle thickness in patients with esophageal symptoms. Am J Gastroenterol. 2007;102(1):137–45. doi: 10.1111/j.1572-0241.2006.01003.x. [DOI] [PubMed] [Google Scholar]
- 27.Mittal RK, Ren J, McCallum RW, et al. Modulation of feline esophageal contractions by bolus volume and outflow obstruction. Am J Physiol. 1990;258(2 Pt 1):G208–15. doi: 10.1152/ajpgi.1990.258.2.G208. [DOI] [PubMed] [Google Scholar]
- 28.Burton PR, Brown W, Laurie C, et al. The effect of laparoscopic adjustable gastric bands on esophageal motility and the gastroesophageal junction: analysis using high-resolution video manometry. Obes Surg. 2009;19(7):905–14. doi: 10.1007/s11695-009-9845-3. [DOI] [PubMed] [Google Scholar]
- 29.Gyawali CP, Kushnir VM. High-resolution manometric characteristics help differentiate types of distal esophageal obstruction in patients with peristalsis. Neurogastroenterol Motil. 2011;23(6):502–e197. doi: 10.1111/j.1365-2982.2011.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontes LH, Herbella FA, Rodriguez TN, et al. Progression of diffuse esophageal spasm to achalasia: incidence and predictive factors. Dis Esophagus. 2012 doi: 10.1111/j.1442-2050.2012.01377.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Anggiansah A, Bright NF, McCullagh M, et al. Transition from nutcracker esophagus to achalasia. Dig Dis Sci. 1990;35(9):1162–6. doi: 10.1007/BF01537590. [DOI] [PubMed] [Google Scholar]
- 32.Paterson WG, Beck IT, Da Costa LR. Transition from nutcracker esophagus to achalasia. A case report. J Clin Gastroenterol. 1991;13(5):554–8. doi: 10.1097/00004836-199110000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Almansa C, Heckman MG, DeVault KR, et al. Esophageal spasm: demographic, clinical, radiographic, and manometric features in 108 patients. Dis Esophagus. 2012;25(3):214–21. doi: 10.1111/j.1442-2050.2011.01258.x. [DOI] [PubMed] [Google Scholar]
- 34.Crozier RE, Glick ME, Gibb SP, et al. Acid-provoked esophageal spasm as a cause of noncardiac chest pain. Am J Gastroenterol. 1991;86(11):1576–80. [PubMed] [Google Scholar]
- 35.Borjesson M, Pilhall M, Rolny P, et al. Gastroesophageal acid reflux in patients with nutcracker esophagus. Scand J Gastroenterol. 2001;36(9):916–20. doi: 10.1080/003655201750305413. [DOI] [PubMed] [Google Scholar]
- 36.Cools-Lartigue J, Chang SY, McKendy K, et al. Pattern of esophageal eosinophilic infiltration in patients with achalasia and response to Heller myotomy and Dor fundoplication. Dis Esophagus. 2012 doi: 10.1111/j.1442-2050.2012.01385.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Savarino E, Gemignani L, Zentilin P, et al. A case of achalasia with dense eosinophilic infiltrate responding to steroidal treatment. Clin Gastroenterol Hepatol. 2011;9(12):1104–6. doi: 10.1016/j.cgh.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Patti MG, Gorodner MV, Galvani C, et al. Spectrum of esophageal motility disorders: implications for diagnosis and treatment. Arch Surg. 2005;140(5):442–8. discussion: 448–9. [Google Scholar]
- 39.Dalton CB, Castell DO, Hewson EG, et al. Diffuse esophageal spasm. A rare motility disorder not characterized by high-amplitude contractions. Dig Dis Sci. 1991;36(8):1025–8. doi: 10.1007/BF01297441. [DOI] [PubMed] [Google Scholar]
- 40.Pandolfino JE, Ghosh SK, Rice J, et al. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103(1):27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 41.Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am J Gastroenterol. 2004;99(6):1011–9. doi: 10.1111/j.1572-0241.2004.30035.x. [DOI] [PubMed] [Google Scholar]
- 42.Tutuian R, Mainie I, Agrawal A, et al. Symptom and function heterogenicity among patients with distal esophageal spasm: studies using combined impedance-manometry. Am J Gastroenterol. 2006;101(3):464–9. doi: 10.1111/j.1572-0241.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 43.Mujica VR, Mudipalli RS, Rao SS. Pathophysiology of chest pain in patients with nutcracker esophagus. Am J Gastroenterol. 2001;96(5):1371–7. doi: 10.1111/j.1572-0241.2001.03791.x. [DOI] [PubMed] [Google Scholar]
- 44.Tedesco P, Fisichella PM, Way LW, et al. Cause and treatment of epiphrenic diverticula. Am J Surg. 2005;190(6):891–4. doi: 10.1016/j.amjsurg.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Fox M, Hebbard G, Janiak P, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16(5):533–42. doi: 10.1111/j.1365-2982.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 46.Agrawal A, Hila A, Tutuian R, et al. Clinical relevance of the nutcracker esophagus: suggested revision of criteria for diagnosis. J Clin Gastroenterol. 2006;40(6):504–9. doi: 10.1097/00004836-200607000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Barham CP, Gotley DC, Fowler A, et al. Diffuse oesophageal spasm: diagnosis by ambulatory 24 hour manometry. Gut. 1997;41(2):151–5. doi: 10.1136/gut.41.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prabhakar A, Levine MS, Rubesin S, et al. Relationship between diffuse esophageal spasm and lower esophageal sphincter dysfunction on barium studies and manometry in 14 patients. AJR Am J Roentgenol. 2004;183(2):409–13. doi: 10.2214/ajr.183.2.1830409. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg MF, Levine MS, Torigian DA. Diffuse esophageal spasm: CT findings in seven patients. AJR Am J Roentgenol. 2008;191(3):758–63. doi: 10.2214/AJR.07.3747. [DOI] [PubMed] [Google Scholar]
- 50.Prakash C, Clouse RE. Wireless pH monitoring in patients with non-cardiac chest pain. Am J Gastroenterol. 2006;101(3):446–52. doi: 10.1111/j.1572-0241.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 51.Cho YK, Choi MG, Oh SN, et al. Comparison of bolus transit patterns identified by esophageal impedance to barium esophagram in patients with dysphagia. Dis Esophagus. 2012;25(1):17–25. doi: 10.1111/j.1442-2050.2011.01212.x. [DOI] [PubMed] [Google Scholar]
- 52.Traube M, Hongo M, Magyar L, et al. Effects of nifedipine in achalasia and in patients with high-amplitude peristaltic esophageal contractions. JAMA. 1984;252(13):1733–6. [PubMed] [Google Scholar]
- 53.Orlando RC, Bozymski EM. Clinical and manometric effects of nitroglycerin in diffuse esophageal spasm. N Engl J Med. 1973;289(1):23–5. doi: 10.1056/NEJM197307052890106. [DOI] [PubMed] [Google Scholar]
- 54.Swamy N. Esophageal spasm: clinical and manometric response to nitroglycerine and long acting nitrites. Gastroenterology. 1977;72(1):23–7. [PubMed] [Google Scholar]
- 55.Konturek JW, Gillessen A, Domschke W. Diffuse esophageal spasm: a malfunction that involves nitric oxide? Scand J Gastroenterol. 1995;30(11):1041–5. doi: 10.3109/00365529509101604. [DOI] [PubMed] [Google Scholar]
- 56.Eherer AJ, Schwetz I, Hammer HF, et al. Effect of sildenafil on oesophageal motor function in healthy subjects and patients with oesophageal motor disorders. Gut. 2002;50(6):758–64. doi: 10.1136/gut.50.6.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bortolotti M, Mari C, Lopilato C, et al. Effects of sildenafil on esophageal motility of patients with idiopathic achalasia. Gastroenterology. 2000;118(2):253–7. doi: 10.1016/s0016-5085(00)70206-x. [DOI] [PubMed] [Google Scholar]
- 58.Pimentel M, Bonorris GG, Chow EJ, et al. Peppermint oil improves the manometric findings in diffuse esophageal spasm. J Clin Gastroenterol. 2001;33(1):27–31. doi: 10.1097/00004836-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Clouse RE, Lustman PJ, Eckert TC, et al. Low-dose trazodone for symptomatic patients with esophageal contraction abnormalities. A double-blind, placebo-controlled trial. Gastroenterology. 1987;92(4):1027–36. doi: 10.1016/0016-5085(87)90979-6. [DOI] [PubMed] [Google Scholar]
- 60.Latimer PR. Biofeedback and self-regulation in the treatment of diffuse esophageal spasm: a single-case study. Biofeedback Self Regul. 1981;6(2):181–9. doi: 10.1007/BF00998868. [DOI] [PubMed] [Google Scholar]
- 61.Irving JD, Owen WJ, Linsell J, et al. Management of diffuse esophageal spasm with balloon dilatation. Gastrointest Radiol. 1992;17(3):189–92. doi: 10.1007/BF01888544. [DOI] [PubMed] [Google Scholar]
- 62.Storr M, Allescher HD, Rosch T, et al. Treatment of symptomatic diffuse esophageal spasm by endoscopic injections of botulinum toxin: a prospective study with long-term follow-up. Gastrointest Endosc. 2001;54(6):754–9. [PubMed] [Google Scholar]
- 63.Boeckxstaens GE. Achalasia. Best Pract Res Clin Gastroenterol. 2007;21(4):595–608. doi: 10.1016/j.bpg.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Vanuytsel T, Bisschops R, Holvoet L, et al. A sham-control study of injection of botulinum toxin in non achalasia esophageal hypermotility disorder. Gastroenterology. 2009;136(5 Suppl 1):A–152. [Google Scholar]
- 65.Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42(4):265–71. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 66.Shiwaku H, Inoue H, Beppu R, et al. Successful treatment of diffuse esophageal spasm by peroral endoscopic myotomy. Gastrointest Endosc. 2012 doi: 10.1016/j.gie.2012.02.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 67.Ellis FH., Jr Esophagomyotomy for noncardiac chest pain resulting from diffuse esophageal spasm and related disorders. Am J Med. 1992;92(5A):129S–31S. [PubMed] [Google Scholar]
- 68.Patti MG, Pellegrini CA, Arcerito M, et al. Comparison of medical and minimally invasive surgical therapy for primary esophageal motility disorders. Arch Surg. 1995;130(6):609–15. doi: 10.1001/archsurg.1995.01430060047009. discussion: 615–6. [DOI] [PubMed] [Google Scholar]
- 69.Leconte M, Douard R, Gaudric M, et al. Functional results after extended myotomy for diffuse oesophageal spasm. Br J Surg. 2007;94(9):1113–8. doi: 10.1002/bjs.5761. [DOI] [PubMed] [Google Scholar]